A Biological Study of Composites Based on the Blends of Nanohydroxyapatite, Silk Fibroin and Chitosan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of the Scaffolds
2.2. ATR-FTIR Spectroscopy
2.3. Porosity Measurements
2.4. Scanning Electron Microscopy
2.5. In Vitro Degradation Test
2.6. Mechanical Properties
2.7. The Measurements of the Biological Properties
2.8. Statistical Analysis
3. Results and Discussion
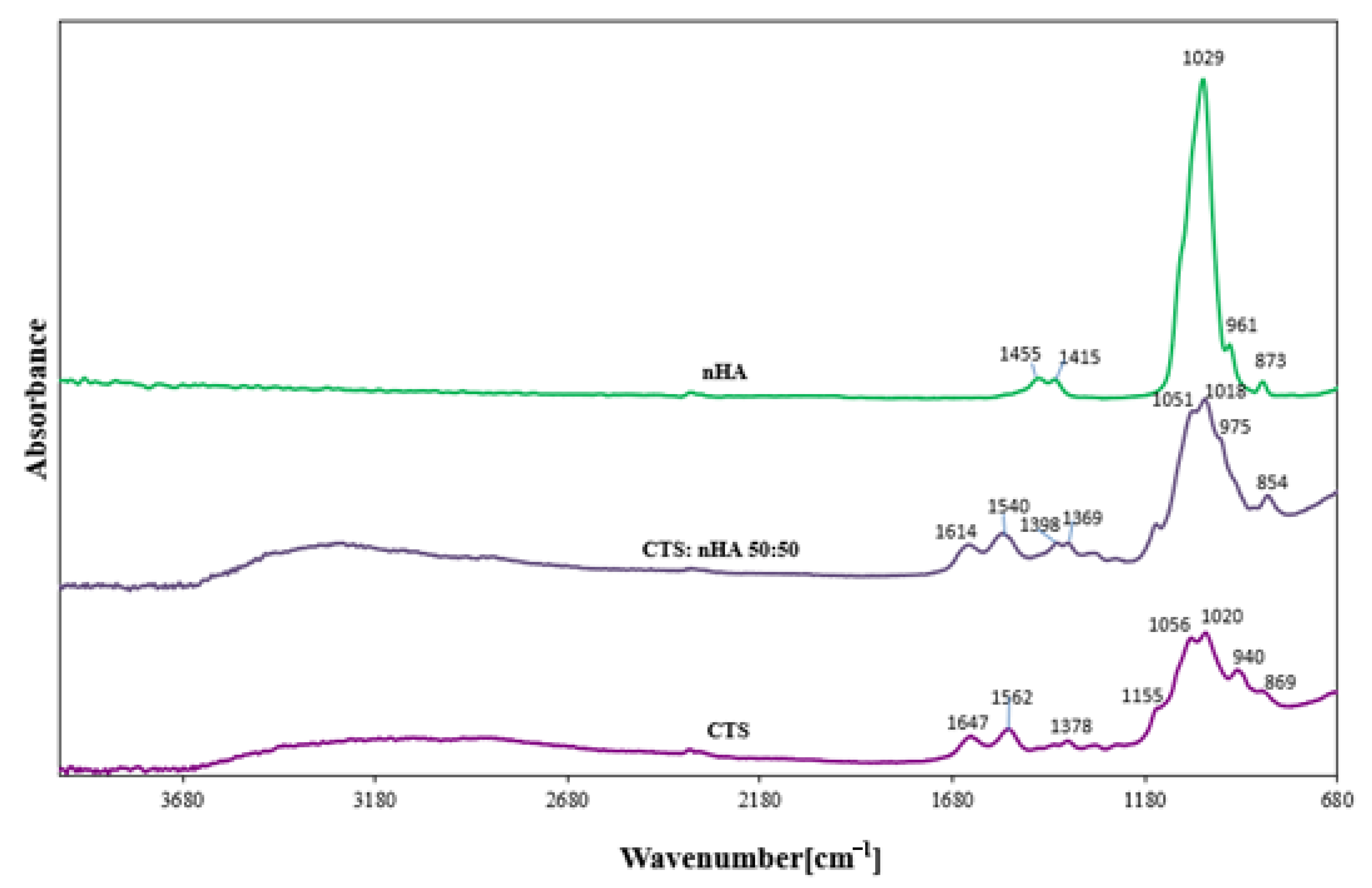
3.1. FTIR Spectroscopy
3.2. Porosity
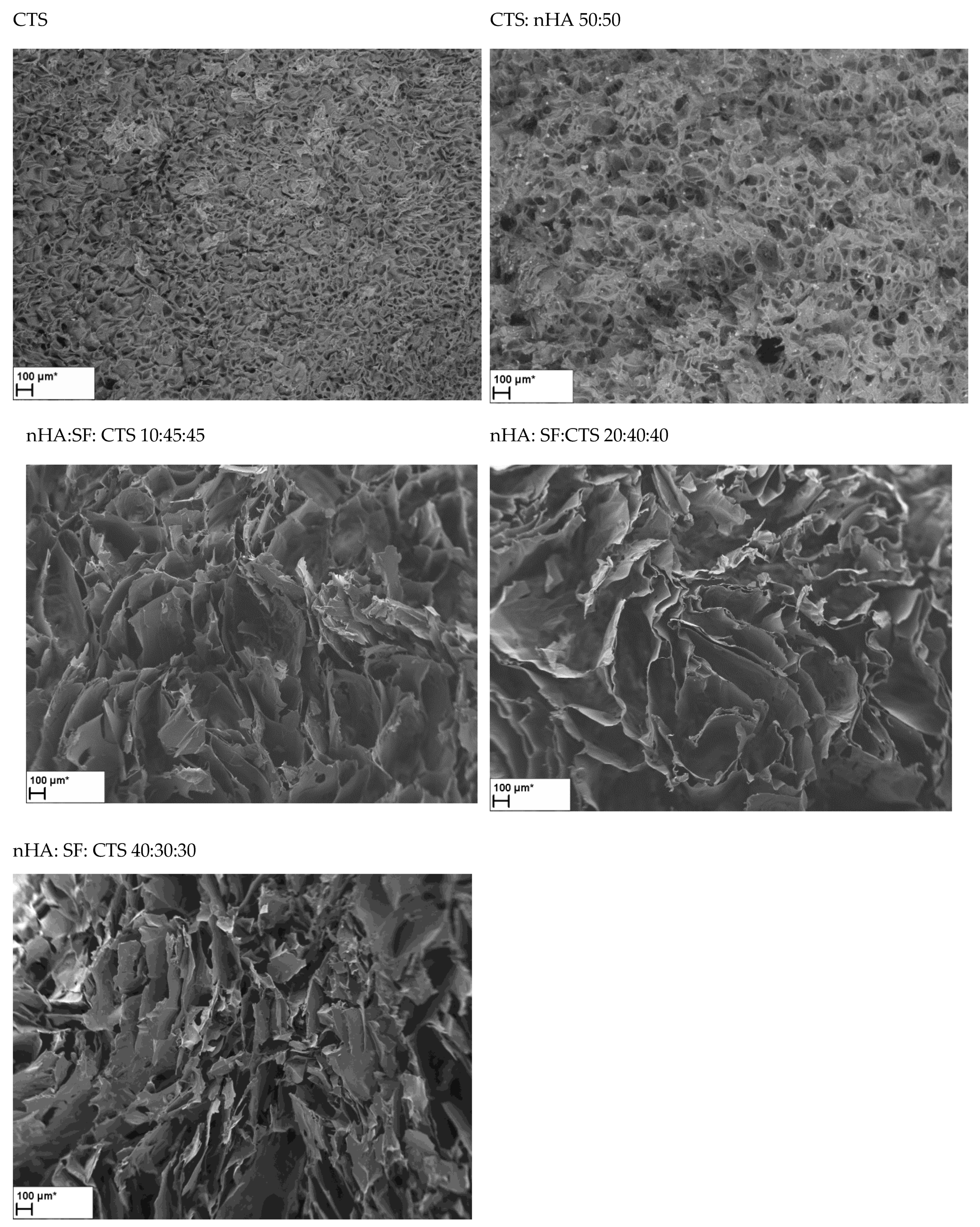
3.3. Scanning Electron Microscopy
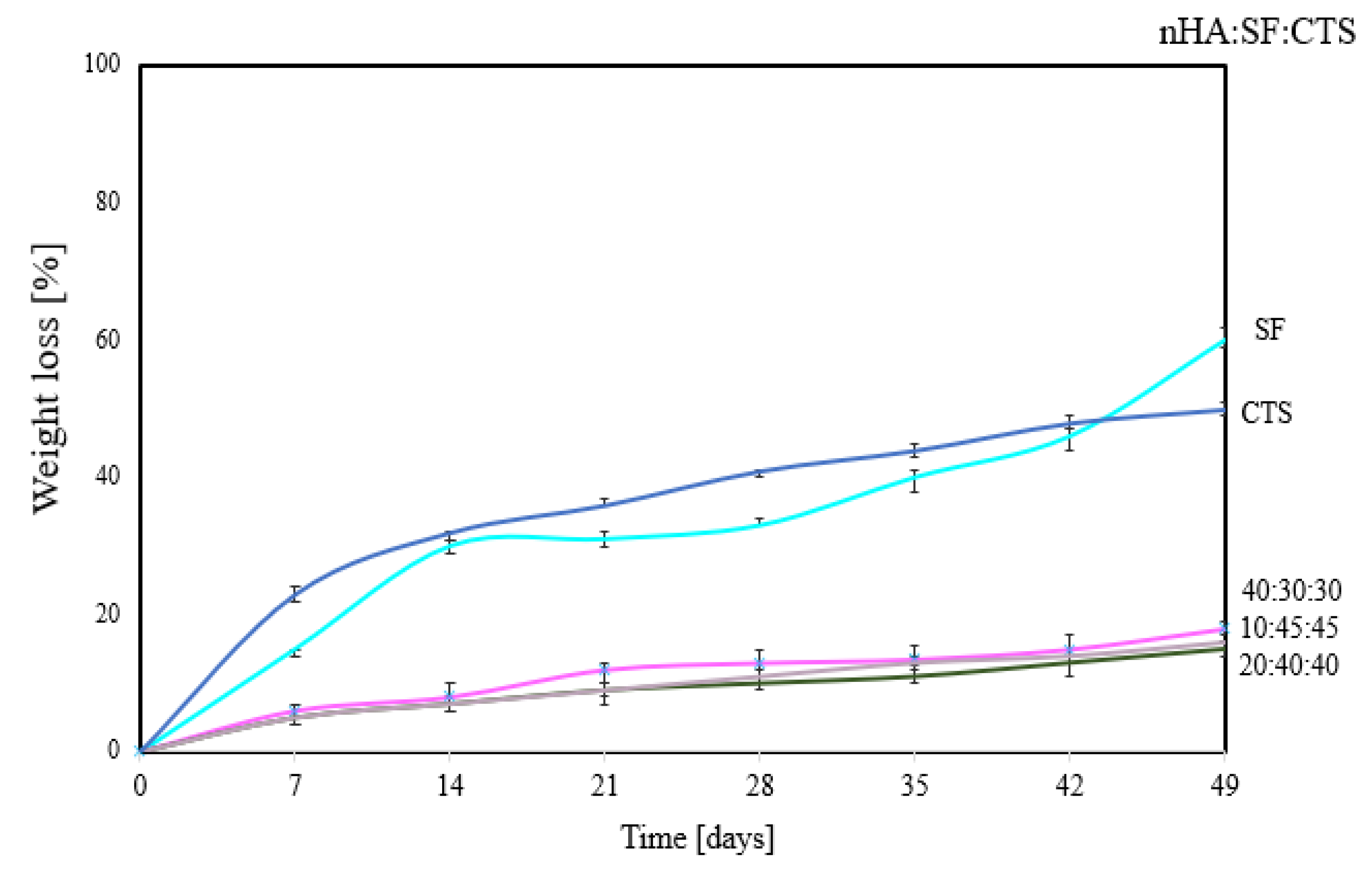
3.4. Degradation Properties
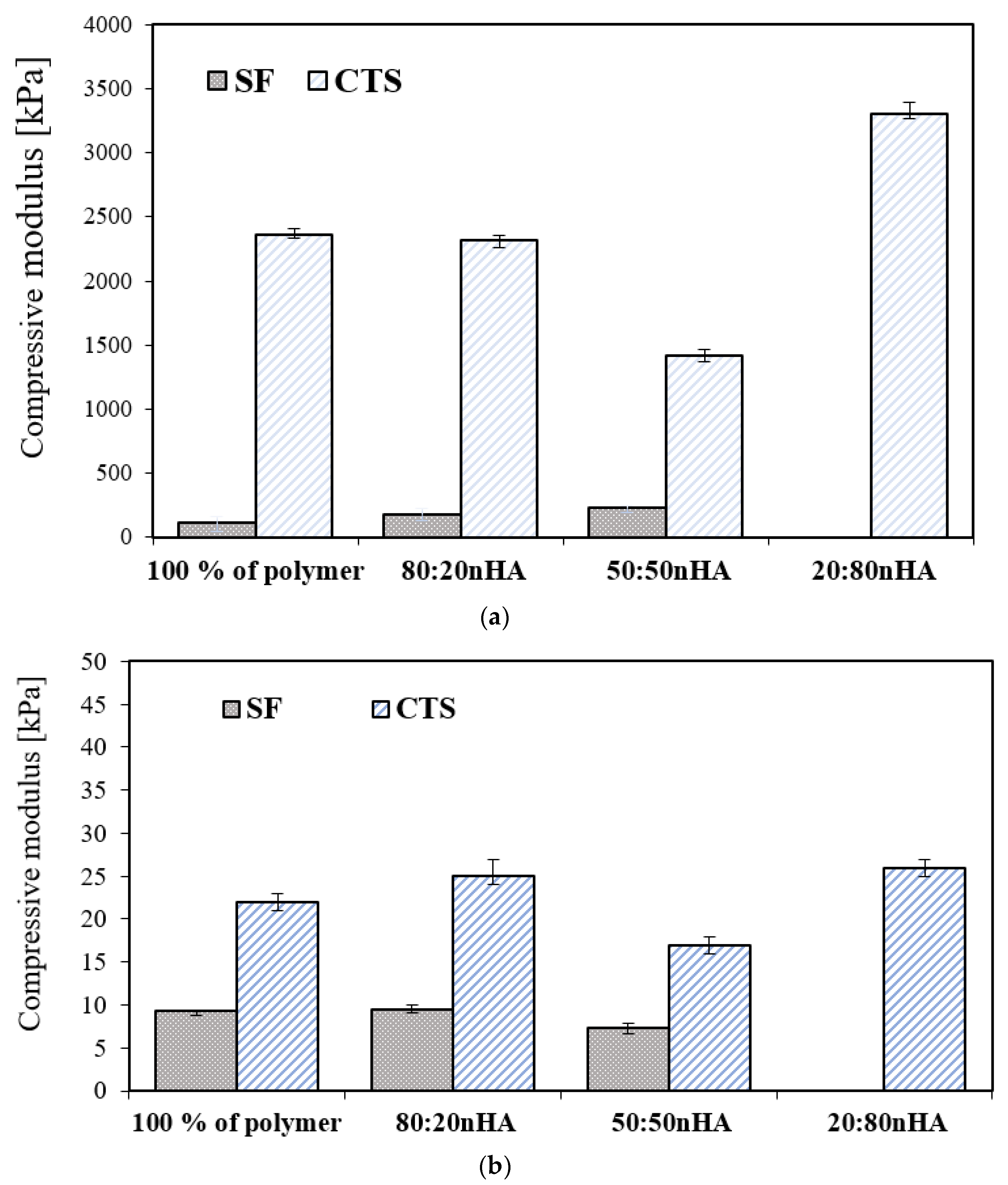
3.5. Mechanical Properties
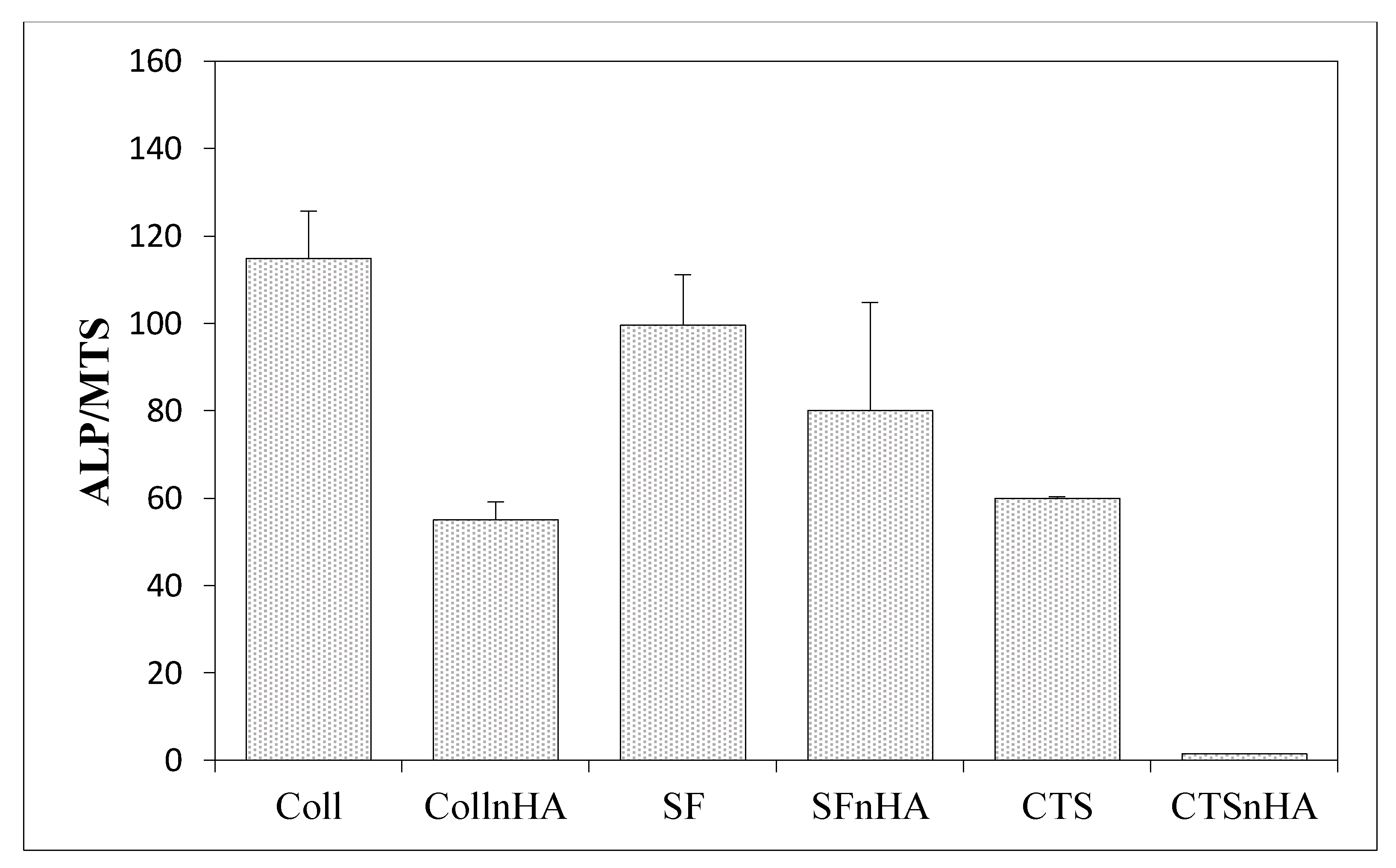
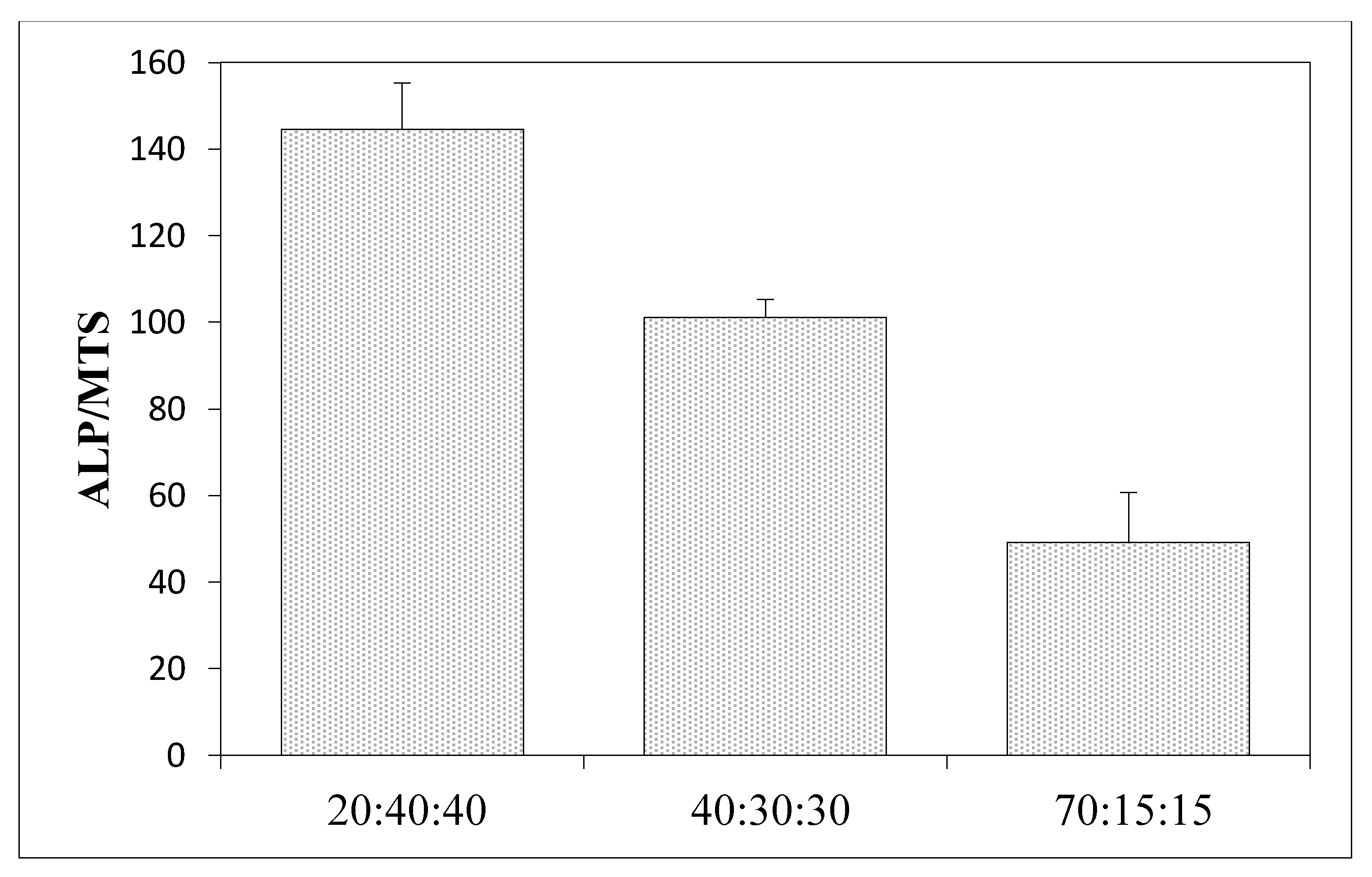
3.6. Results of the Measurements of Biological Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Office of the Surgeon General (US). Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville (MD): Office of the Surgeon General (US); 2004. 2 The Basics of Bone in Health and Disease. Available online: https://www.ncbi.nlm.nih.gov/books/NBK45504/ (accessed on 8 July 2022).
- Wei, S.; Ma, J.-X.; Xu, L.; Gu, X.-S.; Ma, X.-L. Biodegradable materials for bone defect repair. Mil. Med. Res. 2020, 7, 1–25. [Google Scholar] [CrossRef]
- Genasan, K.; Mehrali, M.; Veerappan, T.; Talebian, S.; Malliga Raman, M.; Singh, S.; Swamiappan, S.; Mehrali, M.; Kamarul, T.; Balaji Raghavendran, H.R. Calcium-Silicate-Incorporated Gellan-Chitosan Induced Osteogenic Differentiation in Mesenchymal Stromal Cells. Polymers 2021, 13, 3211. [Google Scholar] [CrossRef]
- Hasany, M.; Talebian, S.; Sadat, S.; Ranjbar, N.; Mehrali, M.; Wallace, G.G.; Mehrali, M. Synthesis, properties, and biomedical applications of alginate methacrylate (ALMA)-based hydrogels: Current advances and challenges. Appl. Mater. Today 2021, 24, 101150. [Google Scholar] [CrossRef]
- Rosa, N.; Moura, M.F.S.F.; Olhero, S.; Simoes, R.; Magalhães, F.D.; Marques, A.T.; Ferreira, J.P.S.; Reis, A.R.; Carvalho, M.; Parente, M. Bone: An Outstanding Composite Material. Appl. Sci. 2022, 12, 3381. [Google Scholar] [CrossRef]
- Almer, J.D.; Stock, S.R. Micromechanical response of mineral and collagen phases in bone. J. Struct. Biol. 2007, 157, 365–370. [Google Scholar] [CrossRef]
- Aksekili, A.M.E.; Polat, Y.; Yüksel, K.; Asiltürk, M.; Uğurlu, M.; Kara, H.; Önder, E.Ö.; Tosun, N. An evaluation of the effect on lower extremity fracture healing of collagen-based fusion material containing 2 different calcium phosphate salts: An experimental rat model. Adv. Clin. Exp. Med. 2018, 27, 1295–1301. [Google Scholar] [CrossRef]
- Filippi, M.; Born, G.; Chaaban, M.; Scherberich, A. Natural Polymeric Scaffolds in Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 474. [Google Scholar] [CrossRef]
- Tuwalska, A.; Grabska-Zielińska, S.; Sionkowska, A. Chitosan/Silk Fibroin Materials for Biomedical Applications—A Review. Polymers 2022, 14, 1343. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, H.-J.; Vunjak-Novakovic, G.; Kaplan, D.L. Stem cell-based tissue engineering with silk biomaterials. Biomaterials 2008, 27, 6064–6082. [Google Scholar] [CrossRef]
- Kadumudi, F.B.; Jahanshahi, M.; Mehrali, M.; Zsurzsan, T.-G.; Taebnia, N.; Hasany, M.; Mohanty, S.; Knott, A.; Godau, B.; Akbari, M.; et al. A Protein-Based, Water-Insoluble, and Bendable Polymer with Ionic Conductivity: A Roadmap for Flexible and Green Electronics. Adv. Sci. 2019, 6, 1801241. [Google Scholar] [CrossRef]
- Dinoro, J.; Maher, M.; Talebian, S.; Jafarkhani, M.; Mehrali, M.; Orive, G.; Foroughi, J.; Lord, M.S.; Dolatshahi-Pirouz, A. Sulfated polysaccharide-based scaffolds for orthopaedic tissue engineering. Biomaterials 2019, 214, 119214. [Google Scholar] [CrossRef]
- Levengood, S.L.; Zhang, M. Chitosan-based scaffolds for bone tissue engineering. J. Mater. Chem. B Mater. Biol. Med. 2014, 2, 3161–3184. [Google Scholar] [CrossRef]
- Czechowska-Biskup, R.; Jarosińska, D.; Rokita, B.; Ulański, P.; Rosiak, J.M. Determination of degree of deacetylation of chitosan—Comparision of methods. Prog. Chem. Appl. Chitin Its Deriv. 2012, 17, 5–20. [Google Scholar]
- Ostrowska-Czubenko, J.; Gierszewska-Drużyńska, M. Effect of ionic crosslinking on the water state in hydrogel chitosan membranes. Carbohydr. Polym. 2009, 77, 590–598. [Google Scholar] [CrossRef]
- Kubasiewicz-Ross, P.; Hadzik, J.; Seeliger, J.; Kozak, K.; Jurczyszyn, K.; Gerber, H.; Dominiak, M.; Kunert-Keil, C. New nano-hydroxyapatite in bone defect regeneration: A histological study in rats. Ann. Anat. 2017, 213, 83–90. [Google Scholar] [CrossRef]
- Sionkowska, A.; Tuwalska, A. Preparation and characterization of new materials based on silk fibroin, chitosan and nanohydroxyapatite. Int. J. Polym. Anal. Charact. 2020, 25, 315–333. [Google Scholar] [CrossRef]
- Jung Park, H.; Lee, J.S.; Lee, O.J.; Sheikh, F.A.; Moon, B.M.; Ju, H.W.; Kim, J.H.; Kim, D.K.; Park, C.H. Fabrication of microporous three-dimensional scaffolds from silk fibroin for tissue engineering. Macromol. Res. 2014, 22, 592–599. [Google Scholar] [CrossRef]
- Ajisawa, A. Dissolution of silk fibroin with calcium chloride/ethanol aqueous solution. J. Seric. Sci. Jpn. 1998, 67, 91–94. [Google Scholar] [CrossRef]
- Vojtová, L.; Pavliňáková, V.; Muchová, J.; Kacvinská, K.; Brtníková, J.; Knoz, M.; Lipový, B.; Faldyna, M.; Göpfert, E.; Holoubek, J.; et al. Healing and Angiogenic Properties of Collagen/Chitosan Scaffolds Enriched with Hyperstable FGF2-STAB(R) Protein: In Vitro, Ex Ovo and In Vivo Comprehensive Evaluation. Biomedicines 2021, 6, 590. Available online: https://pubmed.ncbi.nlm.nih.gov/34067330/ (accessed on 8 July 2022). [CrossRef]
- Slovikova, A.; Vojtova, L.; Jancar, J. Preparation and modification of collagen-based scaffold for tissue engineering. Chem. Pap. 2008, 4, 417–422. Available online: https://www.degruyter.com/document/doi/10.2478/s11696-008-0045-8/html (accessed on 8 July 2022). [CrossRef]
- Sionkowska, A.; Planecka, A. Preparation and characterization of silk fibroin/chitosan composite sponges for tissue engineering. J. Mol. Liq. 2013, 178, 5–14. [Google Scholar] [CrossRef]
- Porstmann, B.; Jung, K.; Schmechta, H.; Evers, U.; Pergande, M.; Porstmann, T.; Kramm, H.J.; Krause, H. Measurement of lysozyme in human body fluids: Comparison of various enzyme immunoassay techniques and their diagnostic application. Clin. Biochem. 1989, 22, 349–355. [Google Scholar] [CrossRef]
- Osyczka, A.M.; Nöth, U.; O’Connor, J.; Caterson, E.J.; Yoon, K.; Danielson, K.G.; Tuan, R.S. Multilineage differentiation of adult human bone marrow progenitor cells transduced with human papilloma virus type 16 E6/E7 genes. Calcif. Tissue Int. 2002, 71, 447–458. [Google Scholar] [CrossRef]
- Hammer, R.; Harper, D.; Ryan, P. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Fernandes Queiroz, M.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the Use of Chitosan Contribute to Oxalate Kidney Stone Formation? Mar. Drugs 2015, 13, 141–158. [Google Scholar] [CrossRef]
- Infrared Spectroscopy: Fundamentals and Applications. Biological Application; Chapter 7. B. Stuart; John Wiley and Sons, Ltd.: Hoboken, NJ, USA, 2004; ISBN 0-470-85427-8 (HB); 0-470-85428-6 (PB).
- Ibrahim, M.; Abdel-Fattah, W.I.; El-Sayed, E.S.M.; Omar, A. A novel model for Chitosan/Hydroxyapatite Interaction. Quantum Matter 2013, 2, 234–237. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [Green Version]
- Sugawara, Y.; Kamioka, H.; Honjo, T.; Tezuka, K.; Takano-Yamamoto, T. Threedimensional reconstruction of chick calvarial osteocytes and their cell processes using confocal microscopy. Bone 2005, 36, 877–883. [Google Scholar] [CrossRef]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.-T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Dev. 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Lim, T.C.; Chian, K.S.; Leong, K.F. Cryogenic prototyping of chitosan scaffolds with controlled micro and macro architecture and their effect on in vivo neovascularization and cellular infiltration. J. Biomed. Mater. Res. 2010, 94, 1303–1311. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Di Luca, A.; Ostrowska, B.; Lorenzo-Moldero, I.; Lepedda, A.J.; Swieszkowski, W.; Van Blitterswijk, C.; Moroni, L. Gradients in pore size enhance the osteogenic differentiation of human mesenchymal stromal cells in three-dimensional scaffolds. Sci. Rep. 2016, 6, 22898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mineralizacja Organizmu Człowieka Żyjącego: (Mineralogia Człowieka) Mineralization of the Living Human Organism Pawlikowski, Maciej, Choroby Wewnętrzne, Minerały Biochemia 1987, Prace Mineralogiczne/Polska Akademia Nauk; Komisja Nauk Minerologicznych: Cracow, Poland, 1987; Volume 79, p. 79-3396.
- Sashina, E.S.; Bochek, A.M.; Noselov, N.P.; Kirichenko, D.A. Structure and solubility of natural silk fibroin. Russ. J. Appl. Chem. 2006, 79, 876–896. [Google Scholar] [CrossRef]
- Krticka, M.; Planka, L.; Vojtova, L.; Nekuda, V.; Stastny, P.; Sedlacek, R.; Brinek, A.; Kavkova, M.; Gopfert, E.; Hedvicakova, V.; et al. Lumbar interbody fusion conducted on a porcine model with a bioresorbable ceramic/biopolymer hybrid implant enriched with Hyperstable Fibroblast Growth Factor 2. Biomedicines 2021, 9, 733. [Google Scholar] [CrossRef] [PubMed]
- Stachewicz, U.; Szewczyk, P.; Kruk, A.; Barber, A.; Czyrska-Filemonowicz, A. Pore shape and size dependence on cell growth into electrospun fiber scaffolds for tissue engineering: 2D and 3D analyses using SEM and FIB-SEM tomography. Mater. Sci. Eng. C 2019, 95, 397–408. [Google Scholar] [CrossRef]
- Han, Y.; Lian, M.; Wu, Q.; Qiao, Z.; Sun, B.; Dai, K. Effect of pore size on cell behavior using melt electrowritten scaffolds. Front. Bioeng. Biotechnol. 2021, 9, 495. [Google Scholar] [CrossRef]
- Spoerke, E.D.; Murray, N.G.; Li, H.; Brinson, L.C.; Dunand, D.C.; Stupp, S.I. Titanium with aligned, elongated pores for orthopedic tissue engineering applications. J. Biomed. Mater. Res. Part A 2008, 84, 402–412. [Google Scholar] [CrossRef]
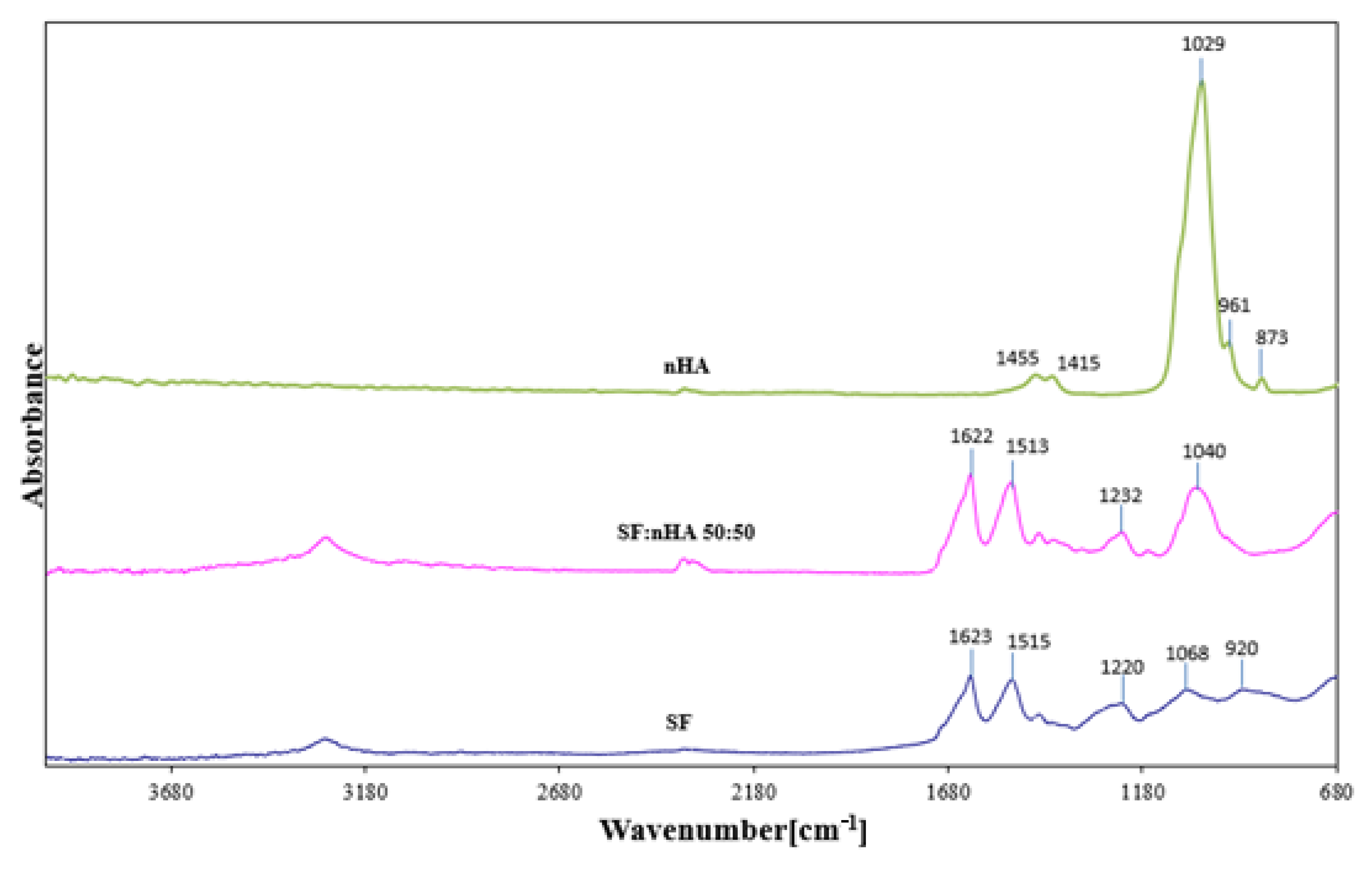



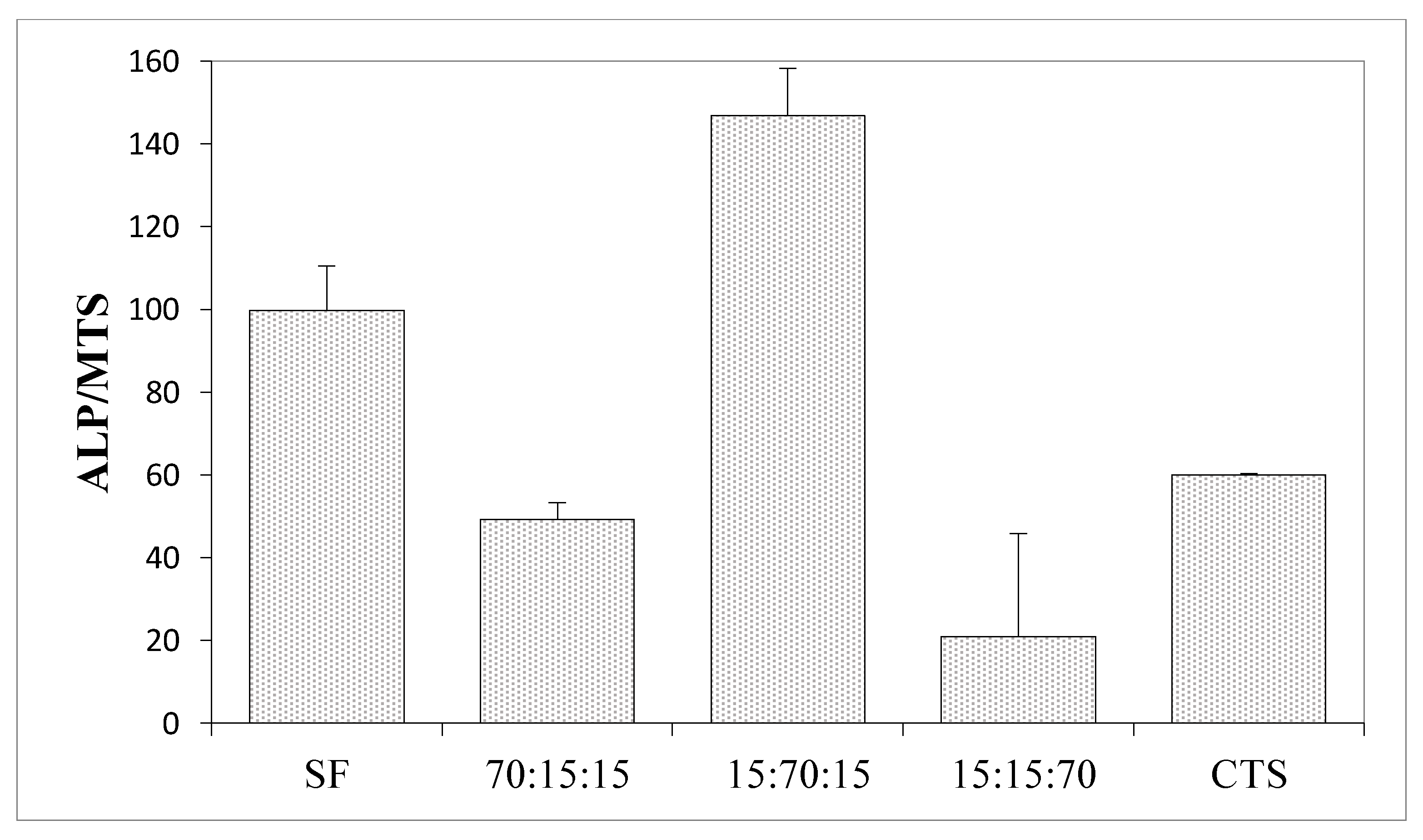
| Assignments | Observed Vibrational Frequencies Wavenumber [cm−1] | ||
|---|---|---|---|
| SF | SF:nHA 50:50 | nHA | |
| H2O absorber., N-H stretching (A amide) | 3285 | 3282 | |
| C-H (B amide) | 3095 | - | |
| C = O stretching (I amide) intermolecular β structure | 1623 | 1622 | |
| 60% N-H bending; 40% C-N stretching (II amide) (after Methanol treatment) | 1515 | 1516 | |
| CH2 scissioring | 1447 | 1446 | 1455 |
| CH2; COO− | 1411 | 1408 | 1415 |
| CH3 wagging; O-H bending (from Serine) | 1340 | 1335 | |
| 30%C-N< stretching; 30% N-H bending, 10% C = O stretching; 10% O = C-N bending; 20% other (III amide) | 1220–1236 | 1232 | |
| -C-O-C stretching asymm. | 1168 | 1167 | |
| PO43− | - | 1040 | 1029 |
| HPO42− | - | - | 961 |
| HPO42−/CO3− | - | 958 | 873 |
| Assignments | Observed Vibrational Frequencies Wavenumber [cm−1] | ||
|---|---|---|---|
| CTS | CTS:nHA 50:50 | nHA | |
| H2O absorber., N-H (A amide) | 3320 | 3296 | |
| C-H (B amide) | 3085 | - | |
| C-H stretching | 2886 | 2873 | |
| C = O stretching (I amide) | 1647 | 1636 | |
| 60% N-H bending; 40% C-N stretching (II amide) | 1562 | 1540 | |
| >CH2 scissioring | - | - | 1455 |
| >CH2 | 1411 | 1407 | 1415 |
| CH3 in amide group | 1378 | 1369; 1398 | |
| -C-O-C stretching asymm. | 1155 | 1167 | |
| =CO stretching skeletal vibration | 1056; 1020 | 1051 | |
| PO43− | - | 1018 | 1029 |
| HPO42− | - | 975 | 961 |
| HPO42−/CO32− | - | 854 | 873 |
| Porosity [%] | |
|---|---|
| SF | 86 |
| SF:nHA 50:50 | 75 |
| CTS:nHA 50:50 | 65 |
| CTS | 70 |
| nHA/SF/CTS 10:45:45 | 85 |
| nHA/SF/CTS 20:40:40 | 80 |
| nHA/SF/CTS 40:30:30 | 75 |
| nHA:SF: CTSRatio | Average Pore Size [μm] | Shape | Wall Thickness [μm] | Surface (Smooth/Rough) |
|---|---|---|---|---|
| SF | 140–220 | Multi-shaped and round | 0.3–1.5 | very rough and extensive surface |
| SF:nHA | 110–200 | Multi-shaped and round | 1 | very rough |
| CTS | 40–80 | round | 1–3 | smooth |
| CTS:nHA | 80 | Multi-shaped and round | 2–5 | rough |
| SF: CTS 80:20 | 50–160 with a predominance of 90 | Multi-shaped | 3–5 | rough |
| SF: CTS 50:50 | 60–160 | Multi-shaped | 1–5 | smooth and rough in places |
| SF: CTS 20:80 | 150 and 325 | Oblong and round | 1–5 | smooth |
| nHA:SF:CTS 20:40:40 | 175–375 | Oblong, spindle-shaped | 0.5–3 | smooth |
| nHA:SF:CTS 40:30:30 | 125–225 and also 600 | Oblong, spindle-shaped, narrow, parallel to each other, and round | 1–4 | smooth |
| nHA:SF:CTS 70:15:15 | 110 | round | Mostly 2, but there are also 7 | smooth |
| nHA:SF:CTS 15:70:15 | 100–300 and 500–600 | Oblong, spindle-shaped | Mostly 5, but there are also 2, 10 | rough |
| nHA:SF:CTS 15:15:70 | 175 | round | Mostly 2, but there are also bigger 10–13 | smooth and rough in places |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuwalska, A.; Sionkowska, A.; Bryła, A.; Tylko, G.; Osyczka, A.M.; Laus, M.; Vojtová, L. A Biological Study of Composites Based on the Blends of Nanohydroxyapatite, Silk Fibroin and Chitosan. Materials 2022, 15, 5444. https://doi.org/10.3390/ma15155444
Tuwalska A, Sionkowska A, Bryła A, Tylko G, Osyczka AM, Laus M, Vojtová L. A Biological Study of Composites Based on the Blends of Nanohydroxyapatite, Silk Fibroin and Chitosan. Materials. 2022; 15(15):5444. https://doi.org/10.3390/ma15155444
Chicago/Turabian StyleTuwalska, Anna, Alina Sionkowska, Amadeusz Bryła, Grzegorz Tylko, Anna Maria Osyczka, Michele Laus, and Lucy Vojtová. 2022. "A Biological Study of Composites Based on the Blends of Nanohydroxyapatite, Silk Fibroin and Chitosan" Materials 15, no. 15: 5444. https://doi.org/10.3390/ma15155444