Experimental and Computational Study of Novel Pyrazole Azo Dyes as Colored Materials for Light Color Paints
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Procedure for the Synthesis of the Pyrazole Azo Dyes
2.2. Experimental
2.3. Theoretical Study
2.3.1. Computational Analysis of Dye Structures
2.3.2. UV-VIS, 1H NMR, and 13C NMR Spectra Simulation
2.3.3. Toxicity Simulation
3. Results and Discussions
3.1. Synthesis of the Pyrazole Azo Dyes
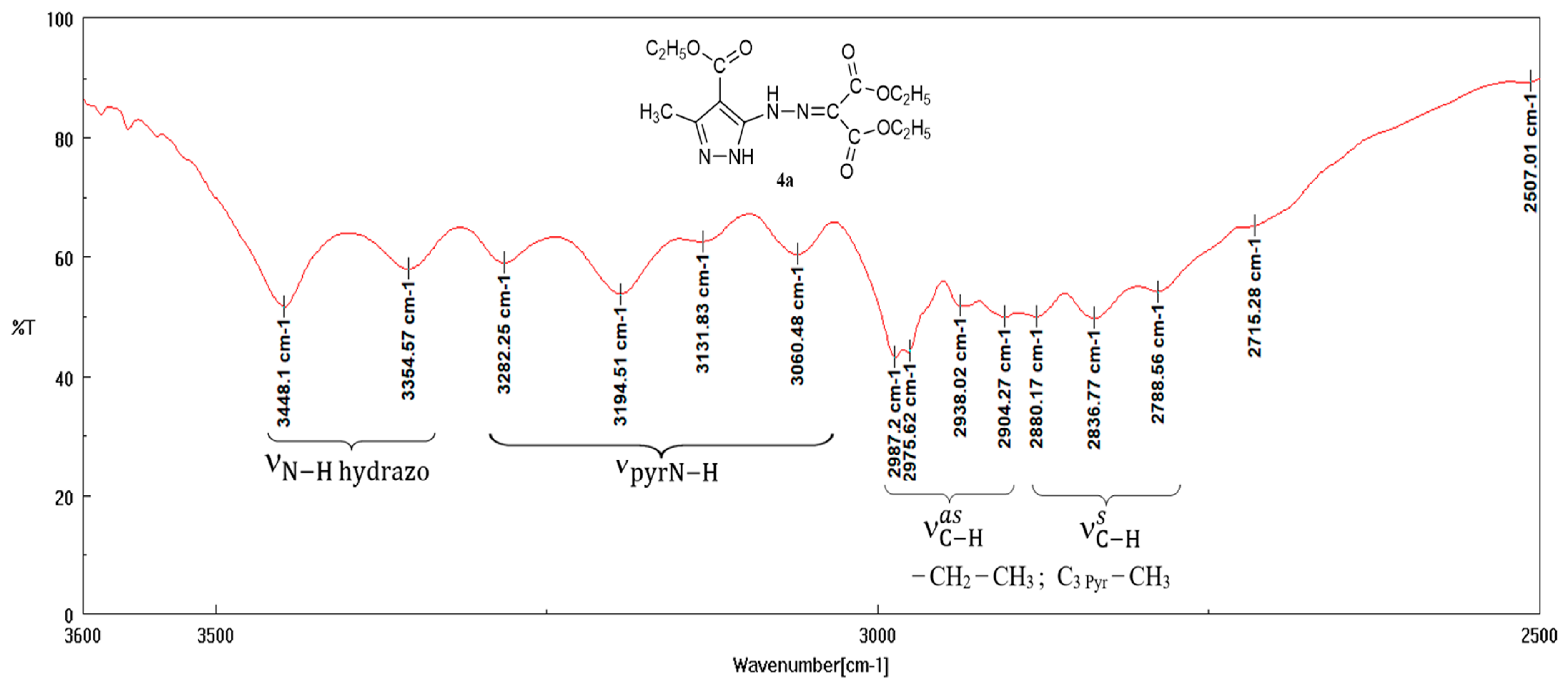
3.2. FT-IR Spectra
3.3. Computational Analysis of Dye Structures
3.4. Experimental and Computed 1H NMR and 13C NMR Spectra
3.5. Experimental and Simulated UV-VIS Spectra
3.6. Experimental Mass Spectra
3.7. Color Analysis
3.8. Dye Toxicity Prediction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geronikaki, A.; Babaev, E.; Dearden, J.; Dehaen, W.; Filimonov, D.; Galaeva, I.; Krajneva, V.; Lagunin, A.; Macaev, F.; Molodavkin, G.; et al. Design, synthesis, computational and biological evaluation of new anxiolytics. Bioorg. Med. Chem. 2004, 12, 6559. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, A.A.; Abdel-Aziem, T. Design, synthesis and biological evaluation of some pyrazole derivatives as anti-inflammatory-antimicrobial agents. Bioorg. Med. Chem. 2004, 12, 1935. [Google Scholar] [CrossRef] [PubMed]
- Wardakhan, W.W.; Louca, N.A. Synthesis of novel pyrazole, coumarin and pyridazine derivatives evaluated as potential antimicrobial and antifungal agents. J. Chil. Chem. Soc. 2007, 52, 1145. [Google Scholar] [CrossRef]
- El-Naggar, M.; Abd El-All, A.S.; El-Naem, S.I.A.; Abdalla, M.M.; Rashdan, H.R.M. New Potent 5α- Reductase and Aromatase Inhibitors Derived from 1,2,3-Triazole Derivative. Molecules 2020, 25, 672. [Google Scholar] [CrossRef] [Green Version]
- Hafez, H.N.; El-Gazzar, A.-R.B.A. Synthesis and Biological Evaluation of N-Pyrazolyl Derivatives and Pyrazolopyrimidine Bearing a Biologically Active Sulfonamide Moiety as Potential Antimicrobial Agent. Molecules 2016, 21, 1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shindy, H.A.; El-Maghraby, M.A.; Eissa, F.M. Synthesis and colour spectrophotometric measurements of some novel merocyanine dyes. Dyes Pigm. 2012, 92, 929. [Google Scholar] [CrossRef]
- Shan, B.; Tong, X.; Xiong, W.; Qiu, W.; Tang, B.; Lu, R.; Ma, W.; Luo, Y.; Zhang, S. A new kind of H-acid monoazo-anthraquinone reactive dyes with surprising colour. Dyes Pigm. 2015, 123, 44. [Google Scholar] [CrossRef]
- Kim, Y.D.; Cho, J.H.; Park, C.R.; Choi, J.-H.; Yoon, C.; Kim, J.P. Synthesis, application and investigation of structure—Thermal stability relationships of thermally stable water-soluble azo naphthalene dyes for LCD red color filters. Dyes Pigm. 2011, 89, 1. [Google Scholar] [CrossRef]
- Sakong, C.; Kim, Y.D.; Choi, J.-H.; Yoon, C.; Kim, J.P. The synthesis of thermally-stable red dyes for LCD color filters and analysis of their aggregation and spectral properties. Dyes Pigm. 2011, 89, 166. [Google Scholar] [CrossRef]
- Bevziu, K.; Chebotarev, A.; Snigur, D.; Bazel, Y.; Fizer, M.; Sidey, V. Spectrophotometric and theoretical studies of the protonation of Allura Red AC and Ponceau 4R. J. Mol. Struct. 2017, 1144, 216. [Google Scholar] [CrossRef]
- Yun, T.Y.; Moon, H.C. Highly stable gel-based electrochromic devices: Effects of molecular structure and concentration of electrochromic chromophores. Org. Electron. 2018, 56, 176. [Google Scholar] [CrossRef]
- Olutas, M.; Sagirli, A. Solvatochromic and solid-state emissive azlactone-based AIEE-active organic dye: Synthesis, photophysical properties and color-conversion LED application. J. Mol. Liq. 2020, 313, 113482. [Google Scholar] [CrossRef]
- Abate, M.T.; Zhou, Y.; Guan, J.; Chen, G.; Ferri, A.; Nierstrasz, V. Colouration and bio-activation of polyester fabric with curcumin insupercritical CO2: Part II—Effect of dye concentration on the colour and functional properties. J. Supercrit. Fluids 2020, 157, 104703. [Google Scholar] [CrossRef]
- Xue, T.; Tang, L.; Tang, R.; Li, Y.; Nie, J.; Zhu, X. Color evolution of a pyrrole-based enone dye in radical photopolymerization formulations. Dyes Pigm. 2021, 188, 109212. [Google Scholar] [CrossRef]
- Rizk, H.F.; Ibrahim, S.A.; El-Borai, M.A. Synthesis, fastness properties, color assessment and antimicrobial activity of some azo reactive dyes having pyrazole moiety. Dyes Pigm. 2015, 112, 86. [Google Scholar] [CrossRef]
- Singh, D.K.; Pradhan, M.; Materny, A. Modern Techniques of Spectroscopy-Basics, Instrumentation, and Applications; Springer: Singapore, 2021; pp. 3–56. [Google Scholar]
- Butler, R.N. Diazotization of heterocyclic primary amines. Chem. Rev. 1975, 75, 241. [Google Scholar] [CrossRef]
- Beyer, H.; Wolter, G.; Lemke, H. Über die Pyrazolbildung aus α-Chlor-acetessigester und Thiocarbohydrazid. Chem. Ber. 1956, 89, 2550. [Google Scholar] [CrossRef]
- Hawkins, P.C.D.; Skillman, A.G.; Warren, G.L.; Ellingson, B.A.; Stahl, M.T. Conformer Generation with OMEGA: Algorithm and Validation Using High Quality Structures from the Protein Databank and Cambridge Structural Database. J. Chem. Inf. Model. 2010, 50, 572. [Google Scholar] [CrossRef]
- Halgren, T.A. MMFF VI. MMFF94s option for energy minimization studies. J. Comput. Chem. 1999, 20, 720. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648. [Google Scholar] [CrossRef] [Green Version]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian-basis sets for molecular calculations. 1. 2nd row atoms, Z=11–18. J. Chem. Phys. 1980, 72, 5639. [Google Scholar] [CrossRef]
- Raghavachari, K.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-Consistent Molecular Orbital Methods. 20. Basis set for correlated wave-functions. J. Chem. Phys. 1980, 72, 650. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 Revision B.01; Gaussian Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Parr, R.G.; Donnelly, R.A.; Levy, M.; Palke, W.E. Electronegativity: The density functional viewpoint. J. Chem. Phys. 1978, 68, 3801. [Google Scholar] [CrossRef]
- Pearson, R.G. Recent advances in the concept of hard and soft acids and bases. J. Chem. Educ. 1987, 64, 561. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpály, L.V.; Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922. [Google Scholar] [CrossRef]
- Yang, W.; Parr, R.G. Hardness, softness, and the fukui function in the electronic theory of metals and catalysis. Proc. Natl. Acad. Sci. USA 1985, 82, 6723. [Google Scholar] [CrossRef] [Green Version]
- Sherrill, C.D.; Schaefer III, H.F. The Configuration Interaction Method: Advances in Highly Correlated Approaches. Adv. Quantum Chem. 1999, 34, 143. [Google Scholar]
- Runge, E.; Gross, E.K.U. Density-Functional Theory for Time-Dependent Systems. Phys. Rev. Let. 1984, 52, 997. [Google Scholar] [CrossRef]
- Ridley, J.; Zerner, M. An Intermediate Neglect of Differential Overlap Technique for Spectroscopy: Pyrrole and the Azines. Theor. Chim. Acta 1973, 32, 111. [Google Scholar] [CrossRef]
- Miertuš, S.; Scrocco, E.; Tomasi, J. Electrostatic Interaction of a Solute with a Continuum. A Direct Utilization of ab initio Molecular Potentials for the Prevision of Solvent Effects. Chem. Phys. 1981, 55, 117. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06 functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215. [Google Scholar] [CrossRef] [Green Version]
- Barich, D.H.; Nicholas, J.B.; Haw, J.F. Gauge-Including Atomic Orbital Proton Chemical Shifts of Strong Hydrogen Bonds: The Importance of Electron Correlation. J. Phys. Chem. A 2001, 105, 4708. [Google Scholar] [CrossRef]
- Wolinski, K.; Hinton, J.F.; Pulay, P. Efficient implementation of the gauge independent atomic orbital method for NMR chemical shift calculations. J. Am. Chem. Soc. 1990, 112, 8251. [Google Scholar] [CrossRef]
- Facelli, J.C. Chemical shift tensors: Theory and application to molecular structural problems. Prog. Nucl. Magn. Reson. Spectrosc. 2011, 58, 176. [Google Scholar]
- Pierens, G.K. 1H and 13C NMR Scaling Factors for the Calculation of Chemical Shifts in Commonly Used Solvents Using Density Functional Theory. J. Comput. Chem. 2014, 35, 1388. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, O.A.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257. [Google Scholar] [CrossRef] [Green Version]
- Drwal, M.N.; Banerjee, P.; Dunkel, M.; Wettig, M.R.; Preissner, R. ProTox: A web server for the in silico prediction of rodent oral toxicity. Nucleic Acids Res. 2014, 42, W53. [Google Scholar] [CrossRef] [Green Version]
- US EPA. Estimation Programs Interface Suite™ for Microsoft Windows; Version 4.11; United States Environmental Protection Agency: Washington, DC, USA, 2012. [Google Scholar]
- Zerbi, G.; Alberti, C. Infrared spectra of pyrazoles—I Pyrazoles mono-alkyl substituted. Spectrochim. Acta 1962, 18, 407–423. [Google Scholar] [CrossRef]
- Singh, S.; Srivastava, S.K.; Singh, D.K. Hydrogen bonding patterns in different acrylamide—Water clusters: Microsolvation probed by micro Raman spectroscopy and DFT calculations. RSC Adv. 2014, 4, 1761–1774. [Google Scholar] [CrossRef]
- Joseph, J.; Jemmis, E.D. Red-, Blue-, or No-Shift in Hydrogen Bonds: A Unified Explanation. J. Am. Chem. Soc. 2007, 129, 4620–4632. [Google Scholar] [CrossRef] [PubMed]
- Pedretti, A.; Mazzolari, A.; Gervasoni, S.; Fumagalli, L.; Vistoli, G. The VEGA suite of programs: A versatile platform for cheminformatics and drug design projects. Bioinformatics 2021, 37, 1174–1175. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, G.A. An Introduction to Hydrogen Bonding; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Grabowski, S.J. Theoretical studies of strong hydrogen bonds. Annu. Rep. Prog. Chem. Sec. C 2006, 102, 131–165. [Google Scholar] [CrossRef]
- Afonin, A.F.; Vashchenko, A.V.; Sigalow, M.V. Estimating the energy of intramolecular hydrogen bonds from 1H NMR and QTAIM calculations. Org. Biomol. Chem. 2016, 14, 11199–11211. [Google Scholar] [CrossRef]
- Grabowski, S.J. π−H•••O Hydrogen Bonds: Multicenter Covalent π−H Interaction Acts as the Proton-Donating System. J. Phys. Chem. A. 2007, 111, 13537. [Google Scholar] [CrossRef]
- Cai, J.; Li, Z.; Qiu, Y.; Ouyang, Z.; Lin, W.; Yang, L.; Feng, W.; Yu, X.; Dong, W. The syntheses, structural and azo-hydrazone tautomeric studies of three triazole/tetrazole azo dyes. New J. Chem. 2016, 11, 9370–9379. [Google Scholar] [CrossRef]
- Deneva, V.; Lyčka, A.; Hristova, S.; Crochet, A.; Fromm, K.M.; Antonov, L. Tautomerism in azo dyes: Border cases of azo and hydrazo tautomers as possible NMR reference compounds. Pigments 2019, 165, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Pearson, R. Chemical hardness and density functional theory. J. Chem. Sci. 2005, 117, 369–377. [Google Scholar] [CrossRef]
- Schüürmann, G. Ecotoxic Modes of Action of Chemical Substances. In Ecotoxicology: Ecological Fundamentals, Chemical Exposure, and Biological Effects; Schüürmann, G., Markert, B., Eds.; John Wiley/Spektrum Akademischer Verlag: New York, NY, USA, 1998; pp. 665–749. [Google Scholar]
- Parthasarathi, R.; Subramanian, V.; Roy, D.R.; Chattaraj, P.K. Electrophilicity index as a possible descriptor of biological activity. Bioorg. Med. Chem. 2004, 12, 5533. [Google Scholar] [CrossRef]
- Padmanabhan, J.; Parthasarathi, R.; Elango, M.; Subramanian, V.; Krishnamoorthy, B.S.; Gutierrez-Oliva, S.; Toro-Labbe, A.; Roy, D.R.; Chattaraj, P.K. Multiphilic Descriptor for Chemical Reactivity and Selectivity. J. Phys. Chem. A 2007, 111, 9130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhide, R.; Jadhav, A.G.; Sekar, N. Light fast monoazo dyes with an inbuilt photostabilizing unit: Synthesis and computational studies. Fibers Polym. 2016, 17, 349. [Google Scholar] [CrossRef]
- Kalinowski, H.O.; Berger, S.; Braun, S. 13C NMR-Spektroskopie; Georg Thieme Verlag: Stuttgart, Germany, 1980. [Google Scholar]
- Aziz, S.G.; Osman, O.I.; Elroby, S.A.; Hassan, W.M.I.; Jedidi, A.; Hilal, R.H. Proton-coupled electron transfer in dye-sensitized solar cells: A theoretical perspective. Struct. Chem. 2018, 29, 983. [Google Scholar] [CrossRef]
- Yum, J.H.; Walter, P.; Huber, S.; Rentsch, D.; Geiger, T.; Nüesch, F.; De Angelis, F.; Grätzel, M.; Nazeeruddin, M.K. Efficient far red sensitization of nanocrystalline TiO2 films by an unsymmetrical squaraine dye. J. Am. Chem. Soc. 2007, 129, 10320. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, G.R.; Garcia, H.C.; Couri, M.R.C.; Dos Santos, H.F.; de Oliveira, L.F.C. On the Azo/Hydrazo Equilibrium in Sudan I Azo Dye Derivatives. J. Phys. Chem. A 2013, 117, 642. [Google Scholar] [CrossRef]
- Zhang, G.; Bailey, A.D.; Bucks, M.E.; Murphy, B.P. A new class of oxazolidinone- and phthalimide-based oxidation dye couplers and their effect on azomethine dye color. Dyes Pigm. 2018, 149, e176. [Google Scholar] [CrossRef]
- Kim, T.-H.; Lee, B.-J.; An, S.-O.; Lee, J.-H.; Choi, J.-H. The synthesis of red dyes based on diketo-pyrrolo-pyrrole chromophore to improve heat stability and solubility for colour filter fabrication. Dyes Pigm. 2020, 174, 108053. [Google Scholar] [CrossRef]
- Abe, A. Conformational analysis and dipole moments of dialkyl esters of some simple dicarboxylic acids. J. Am. Chem. Soc. 1984, 106, 14–19. [Google Scholar] [CrossRef]
- Dean, J.A. Lange’s Handbook of Chemistry, 15th ed.; McGraw Hill Education: New York, NY, USA, 1998; pp. 5105–5134. [Google Scholar]
- Charles, S.W.; Jones, G.I.L.; Owen, N.L. Vibrational spectra and rotational isomerism of methyl and ethyl cyanoacetate. J. Chem. Soc. Faraday Trans. 2 1973, 69, 1454. [Google Scholar] [CrossRef]













Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nitu, S.; Milea, M.S.; Boran, S.; Mosoarca, G.; Zamfir, A.D.; Popa, S.; Funar-Timofei, S. Experimental and Computational Study of Novel Pyrazole Azo Dyes as Colored Materials for Light Color Paints. Materials 2022, 15, 5507. https://doi.org/10.3390/ma15165507
Nitu S, Milea MS, Boran S, Mosoarca G, Zamfir AD, Popa S, Funar-Timofei S. Experimental and Computational Study of Novel Pyrazole Azo Dyes as Colored Materials for Light Color Paints. Materials. 2022; 15(16):5507. https://doi.org/10.3390/ma15165507
Chicago/Turabian StyleNitu, Sabina, Marius Silviu Milea, Sorina Boran, Giannin Mosoarca, Alina D. Zamfir, Simona Popa, and Simona Funar-Timofei. 2022. "Experimental and Computational Study of Novel Pyrazole Azo Dyes as Colored Materials for Light Color Paints" Materials 15, no. 16: 5507. https://doi.org/10.3390/ma15165507
APA StyleNitu, S., Milea, M. S., Boran, S., Mosoarca, G., Zamfir, A. D., Popa, S., & Funar-Timofei, S. (2022). Experimental and Computational Study of Novel Pyrazole Azo Dyes as Colored Materials for Light Color Paints. Materials, 15(16), 5507. https://doi.org/10.3390/ma15165507









