Synthesis and Characterization of Nanoporous ZnO Films by Controlling the Zn Sublimation by Using ZnO/Zn Precursor Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemical Reagents
2.2. Synthesis of ZnO/Zn Precursor Films
2.3. Synthesis of Nanoporous ZnO Films
2.4. Characterization Techniques
3. Results
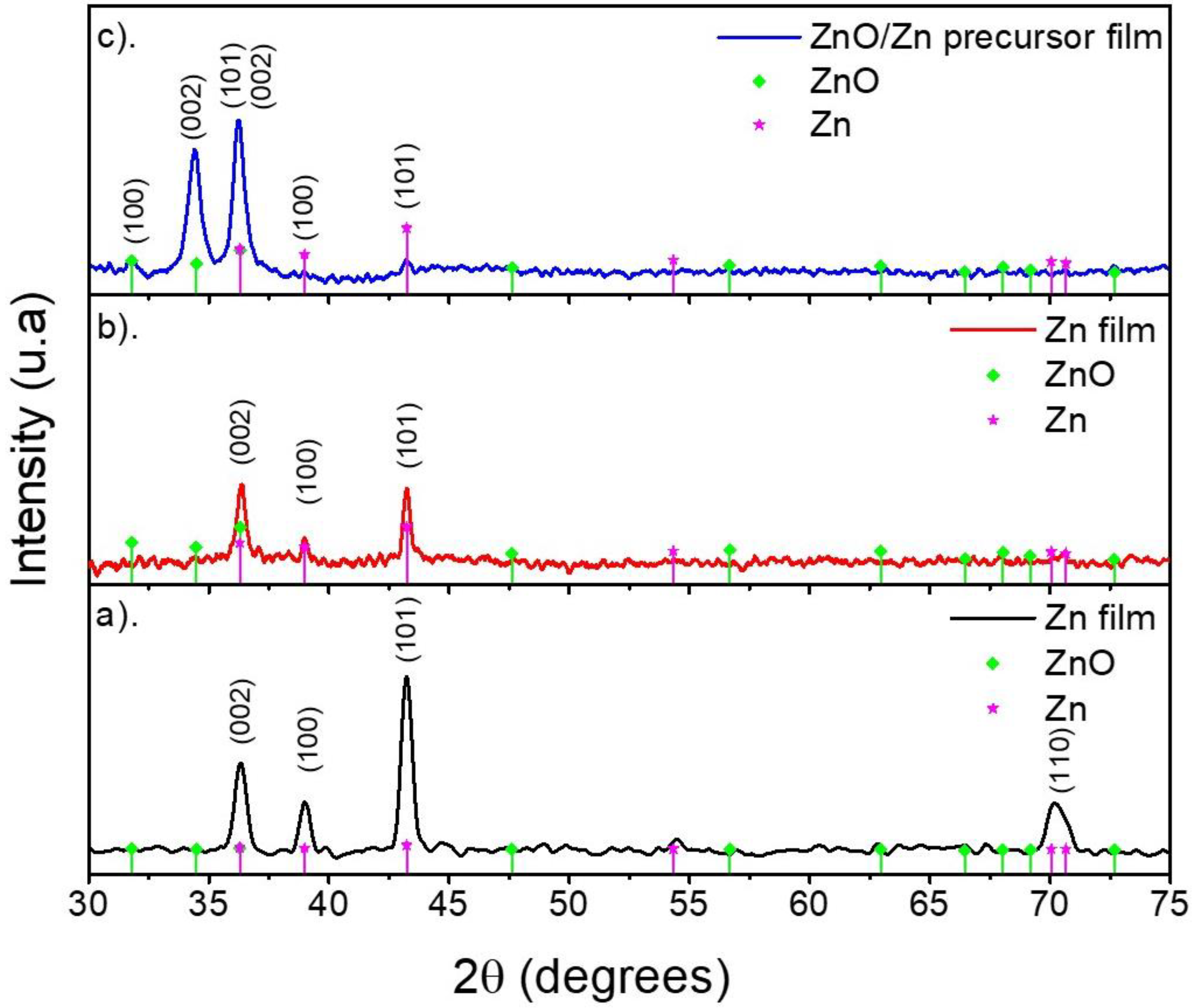
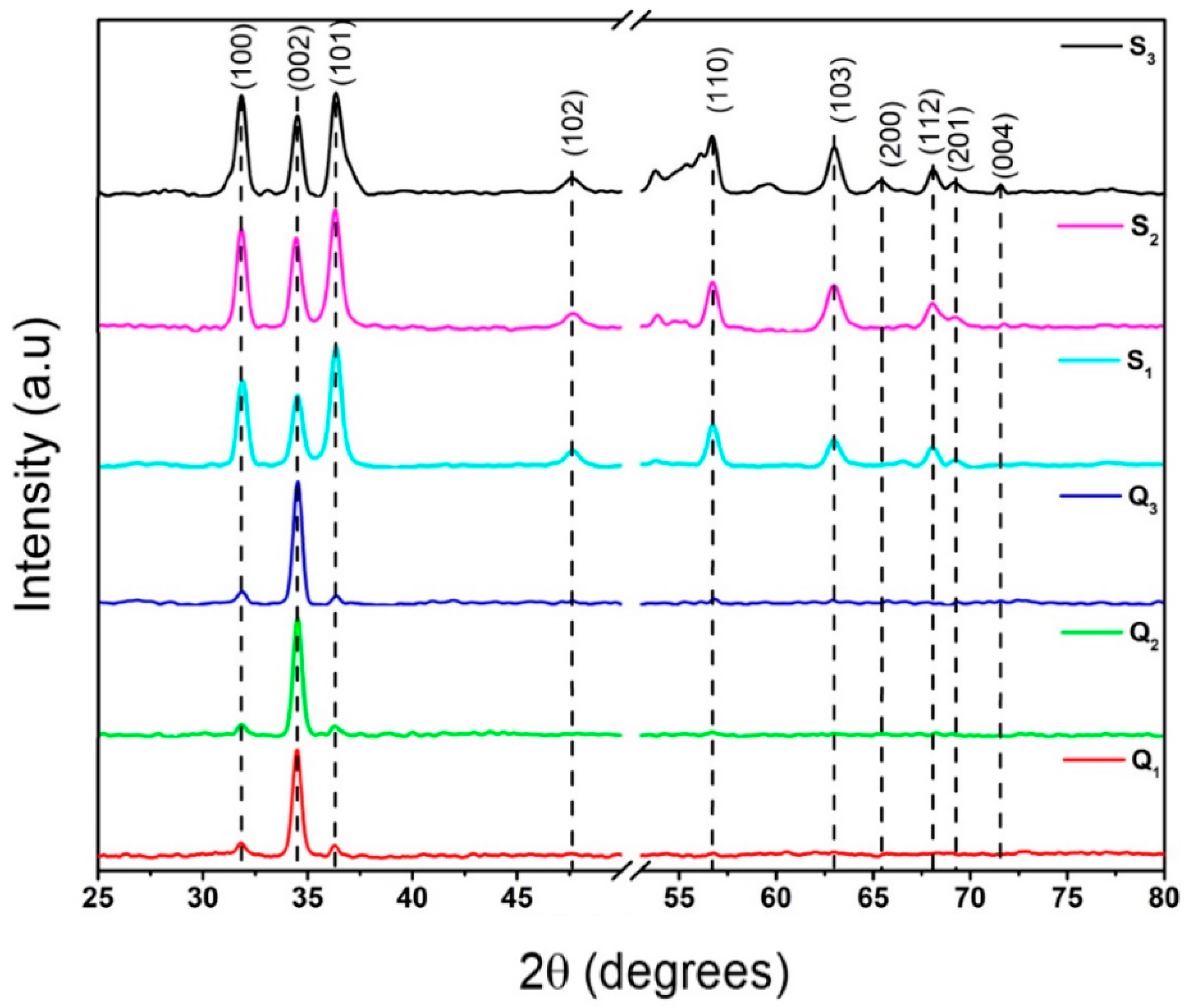
3.1. X-ray Diffraction
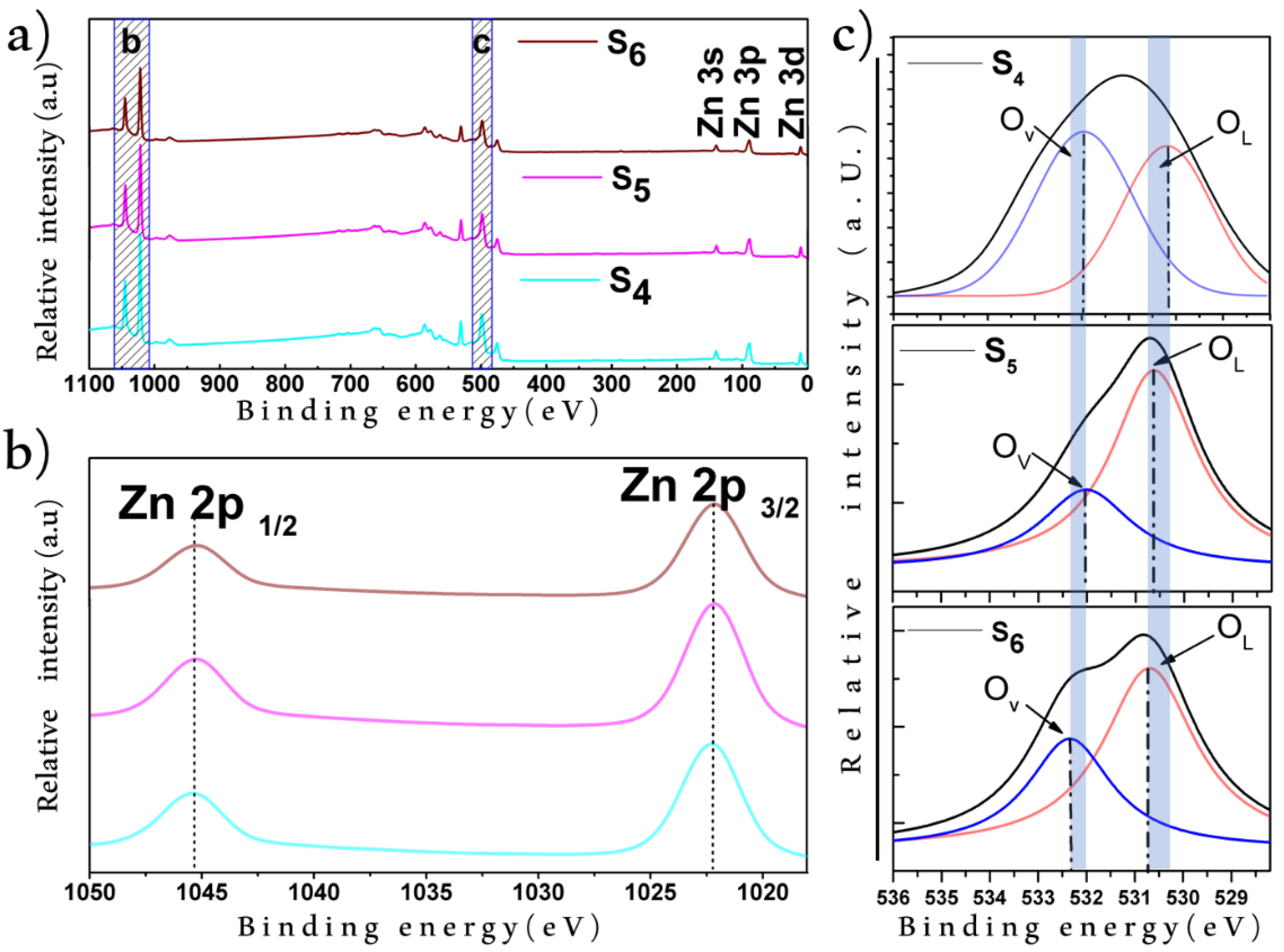
3.2. X-ray Photoelectron Spectroscopy
3.3. Photoluminescence
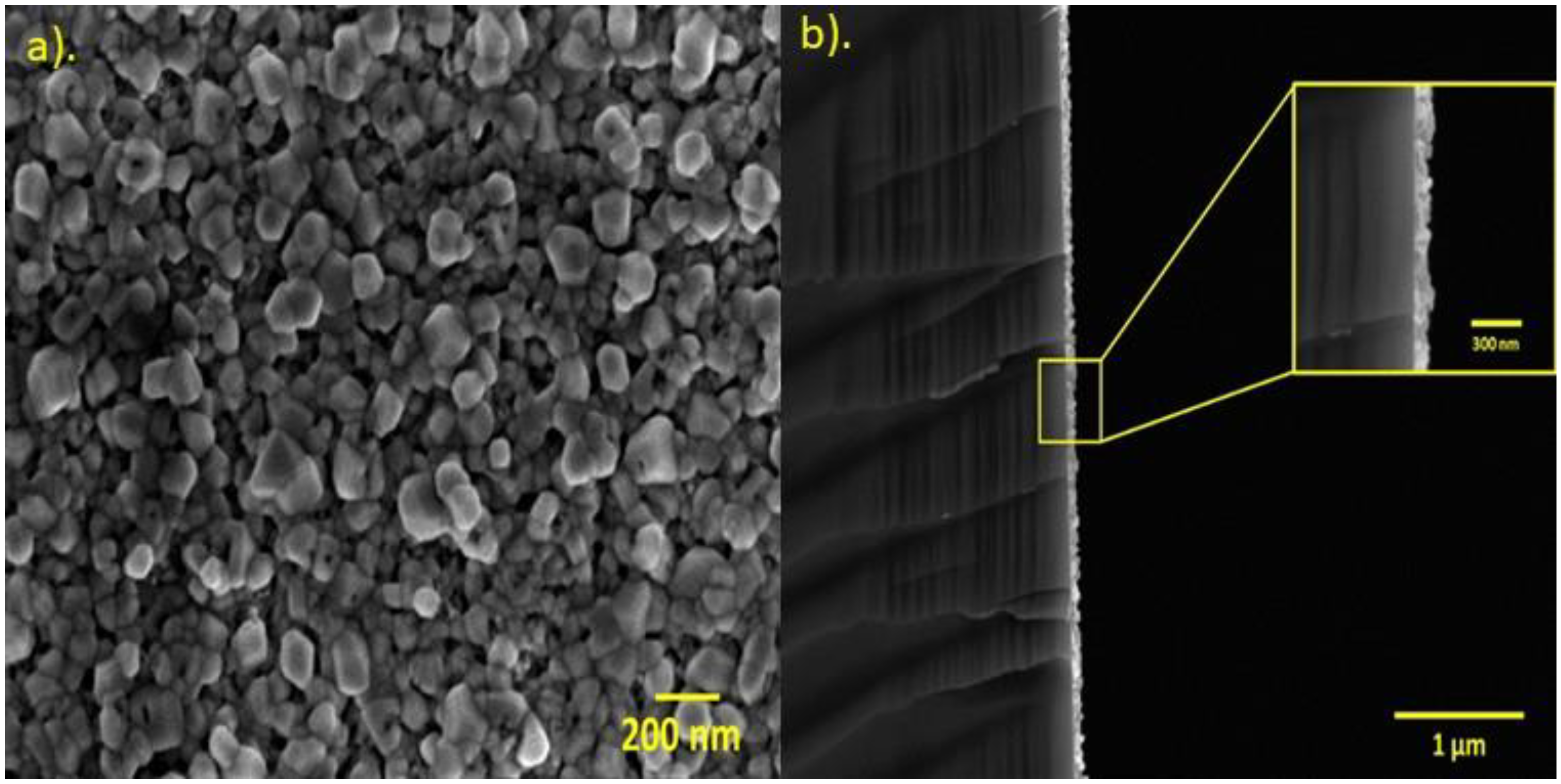
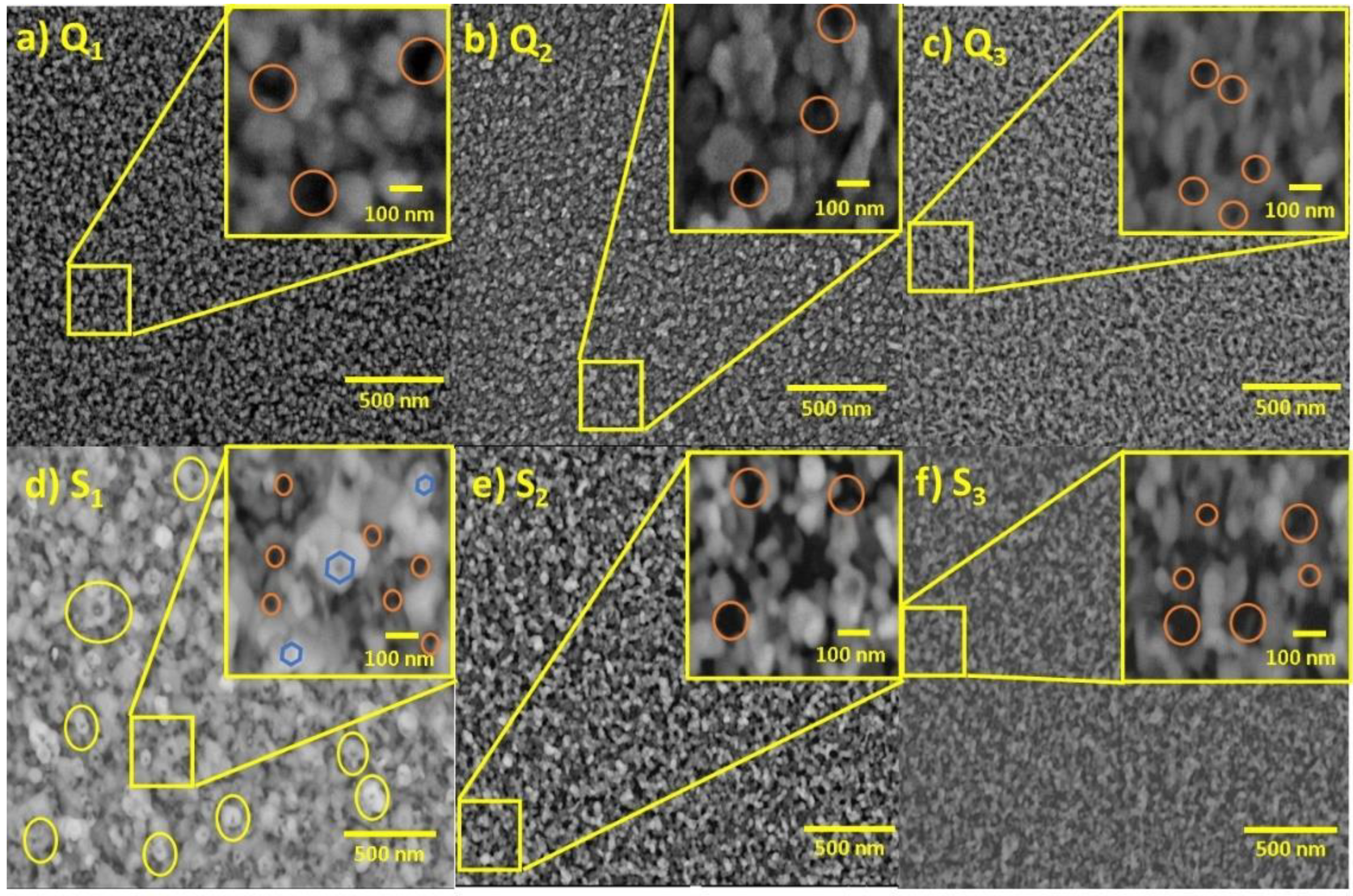
3.4. Field-Emission Scanning Electron Microscopy
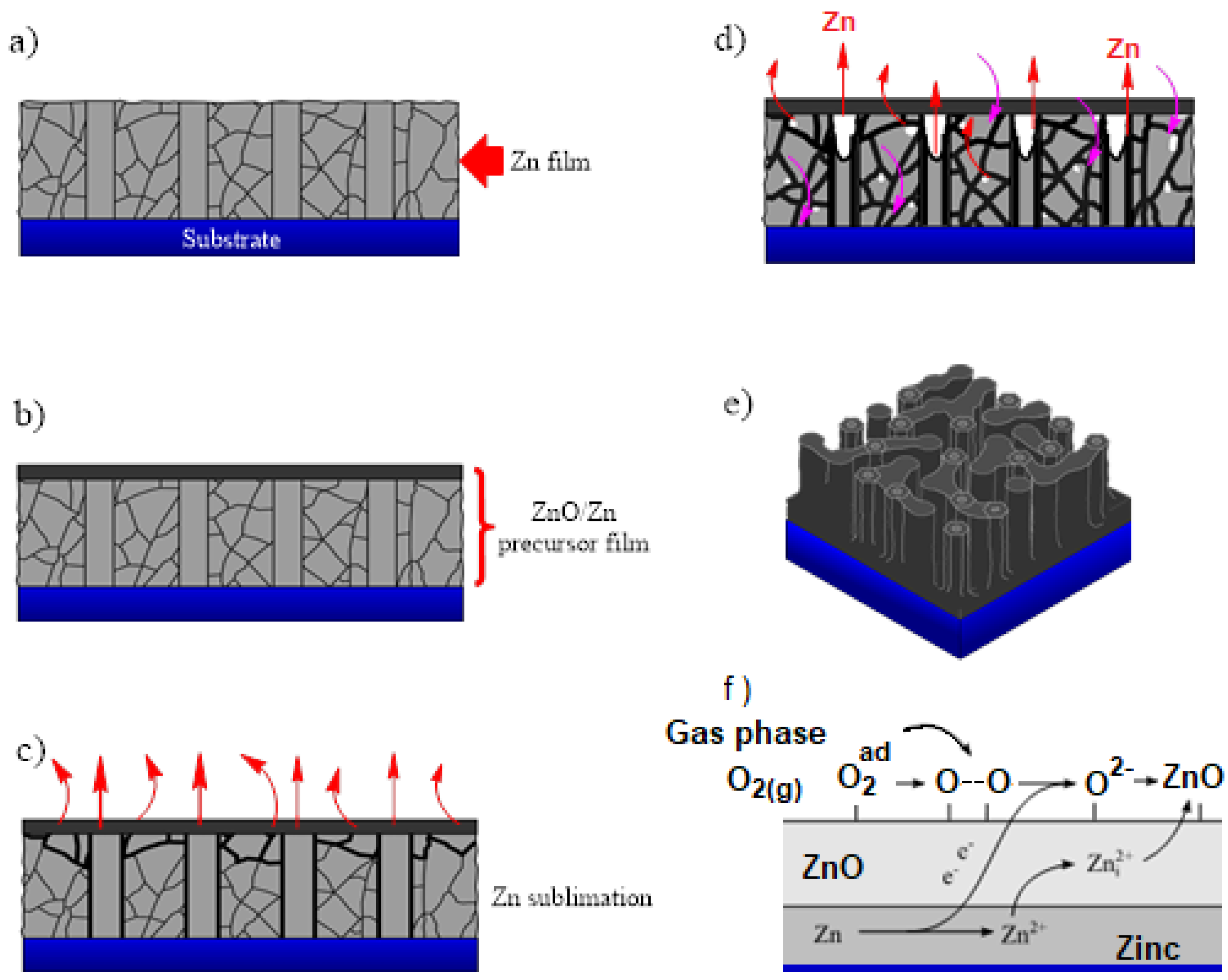
3.5. Mechanism of Nanoporous ZnO Film Formation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lupan, O.; Emelchenko, G.; Ursaki, V.; Chai, G.; Redkin, A.; Gruzintsev, A.; Tiginyanu, I.; Chow, L.; Ono, L.; Cuenya, B.R.; et al. Synthesis and characterization of ZnO nanowires for nanosensor applications. Mater. Res. Bull. 2010, 45, 1026–1032. [Google Scholar] [CrossRef]
- Bai, S.; Sun, C.; Guo, T.; Luo, R.; Lin, Y.; Chen, A.; Sun, L.; Zhang, J. Low temperature electrochemical deposition of nanoporous ZnO thin films as novel NO2 sensors. Electrochim. Acta 2013, 90, 530–534. [Google Scholar] [CrossRef]
- Dikici, T. Temperature-dependent growth of ZnO structures by thermal oxidation of Zn coatings electrodeposited on steel substrates and their photocatalytic activities. Ceram. Int. 2017, 43, 8289–8293. [Google Scholar] [CrossRef]
- Lamberti, A.; Gazia, R.; Sacco, A.; Bianco, S.; Quaglio, M.; Chiodoni, A.; Tresso, E.; Pirri, C.F. Coral-shaped ZnO nanostructures for dye-sensitized solar cell photoanodes. Prog. Photovoltaics: Res. Appl. 2012, 22, 189–197. [Google Scholar] [CrossRef]
- Sacco, A.; Lamberti, A.; Gazia, R.; Bianco, S.; Manfredi, D.; Shahzad, N.; Cappelluti, F.; Ma, S.; Tresso, E. High efficiency dye-sensitized solar cells exploiting sponge-like ZnO nanostructures. Phys. Chem. Chem. Phys. 2012, 14, 16203–16208. [Google Scholar] [CrossRef] [PubMed]
- Ravirajan, P.; Peiró, A.M.; Nazeeruddin, M.K.; Graetzel, M.; Bradley, D.; Durrant, A.J.R.; Nelson, J. Hybrid Polymer/Zinc Oxide Photovoltaic Devices with Vertically Oriented ZnO Nanorods and an Amphiphilic Molecular Interface Layer. J. Phys. Chem. B 2006, 110, 7635–7639. [Google Scholar] [CrossRef]
- Wan, Q.; Lin, C.L.; Yu, X.; Wang, T.H. Room-temperature hydrogen storage characteristics of ZnO nanowires. Appl. Phys. Lett. 2004, 84, 124–126. [Google Scholar] [CrossRef]
- Chu, S.; Olmedo, M.; Yang, Z.; Kong, J.; Liu, J. Electrically pumped ultraviolet ZnO diode lasers on Si. Appl. Phys. Lett. 2008, 93, 181106. [Google Scholar] [CrossRef]
- Vettumperumal, R.; Kalyanaraman, S.; Thangavel, R. Photoconductive UV detectors based heterostructures of Cd and Mg doped ZnO sol gel thin films. Mater. Chem. Phys. 2014, 145, 237–242. [Google Scholar] [CrossRef]
- Wu, B.; Zhuang, S.-W.; Chi, C.; Shi, Z.-F.; Jiang, J.-Y.; Dong, X.; Li, W.-C.; Zhang, Y.-T.; Zhang, B.-L.; Du, G.-T. The growth of ZnO on stainless steel foils by MOCVD and its application in light emitting devices. Phys. Chem. Chem. Phys. 2016, 18, 5614–5621. [Google Scholar] [CrossRef]
- Ahn, C.H.; Kim, Y.Y.; Kim, D.C.; Mohanta, S.K.; Cho, H.K. A comparative analysis of deep level emission in ZnO layers deposited by various methods. J. Appl. Phys. 2009, 105, 013502. [Google Scholar] [CrossRef]
- Janotti, A.; Van de Walle, C.G. Oxygen vacancies in ZnO. Appl. Phys. Lett. 2005, 87, 122102. [Google Scholar] [CrossRef]
- Tseng, Y.-C.; Lin, Y.-J.; Chang, H.-C.; Chen, Y.-H.; Liu, C.-J.; Zou, Y.-Y. Effects of Ti content on the optical and structural properties of the Ti-doped ZnO nanoparticles. J. Lumin. 2012, 132, 491–494. [Google Scholar] [CrossRef]
- Vanheusden, K.; Seager, C.H.; Warren, W.L.; Tallant, D.R.; Voigt, J.A. Correlation between photoluminescence and oxygen vacancies in ZnO phosphors. Appl. Phys. Lett. 1996, 68, 403–405. [Google Scholar] [CrossRef]
- Zhang, S.B.; Wei, S.-H.; Zunger, A. Intrinsic n-type versus p-type doping asymmetry and the defect physics of ZnO. Phys. Rev. B 2001, 63, 075205. [Google Scholar] [CrossRef]
- Zhou, H.; Alves, H.; Hofmann, D.M.; Kriegseis, W.; Meyer, B.K.; Kaczmarczyk, G.; Hoffmann, A. Behind the weak excitonic emission of ZnO quantum dots: ZnO/Zn(OH)2 core-shell structure. Appl. Phys. Lett. 2002, 80, 210–212. [Google Scholar] [CrossRef]
- Li, T.; Herng, T.S.; Liang, H.K.; Bao, N.N.; Chen, T.P.; I Wong, J.; Xue, J.M.; Ding, J. Strong green emission in ZnO films after H2surface treatment. J. Phys. D Appl. Phys. 2012, 45, 185102. [Google Scholar] [CrossRef]
- Pöppl, A.; Völkel, G. ESR and Photo-ESR Investigations of Zinc Vacancies and Interstitial Oxygen Ions in Undoped ZnO Ceramics. Phys. Status Solidi (A) 1991, 125, 571–581. [Google Scholar] [CrossRef]
- Thomas, M.A.; Cui, J.B. Investigations of acceptor related photoluminescence from electrodeposited Ag-doped ZnO. J. Appl. Phys. 2009, 105, 093533. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, B.; Dhar, S.; Gondorf, A.; Spasova, M. Effect of surface groups on the luminescence property of ZnO nanoparticles synthesized by sol–gel route. Surf. Sci. 2012, 606, L13–L17. [Google Scholar] [CrossRef]
- Jankowski, A.F.; Hayes, J.P. Sputter deposition of a spongelike morphology in metal coatings. J. Vac. Sci. Technol. A 2003, 21, 422–425. [Google Scholar] [CrossRef]
- Wang, X.; Ding, Y.; Li, Z.; Song, J.; Wang, Z.L. Single-Crystal Mesoporous ZnO Thin Films Composed of Nanowalls. J. Phys. Chem. C 2009, 113, 1791–1794. [Google Scholar] [CrossRef]
- Yang, W.; Liu, J.; Guan, Z.; Liu, Z.; Chen, B.; Zhao, L.; Li, Y.; Cao, X.; He, X.; Zhang, C.; et al. Morphology, electrical and optical properties of magnetron sputtered porous ZnO thin films on Si(100) and Si(111) substrates. Ceram. Int. 2019, 46, 6605–6611. [Google Scholar] [CrossRef]
- Craciun, V.; Elders, J.; Gardeniers, H.; Boyd, I. Characteristics of high quality ZnO thin films deposited by pulsed laser deposition. Appl. Phys. Lett. 1994, 65, 2963–2965. [Google Scholar] [CrossRef]
- Laurenti, M.; Cauda, V. Porous Zinc Oxide Thin Films: Synthesis Approaches and Applications. Coatings 2018, 8, 67. [Google Scholar] [CrossRef]
- Nasiri, N.; Clarke, C. Nanostructured Gas Sensors for Medical and Health Applications: Low to High Dimensional Materials. Biosensors 2019, 9, 43. [Google Scholar] [CrossRef]
- Xu, Q.; Hong, R.; Chen, X.; Wei, J.; Wu, Z. Synthesis of ZnO nanoporous structure materials by two-step thermal oxidation of Zn film. Ceram. Int. 2017, 43, 16391–16394. [Google Scholar] [CrossRef]
- Gazia, R.; Chiodoni, A.; Bianco, S.; Lamberti, A.; Quaglio, M.; Sacco, A.; Tresso, E.; Mandracci, P.; Pirri, C. An easy method for the room-temperature growth of spongelike nanostructured Zn films as initial step for the fabrication of nanostructured ZnO. Thin Solid Films 2012, 524, 107–112. [Google Scholar] [CrossRef]
- Yuvaraj, D.; Rao, K.N. Selective growth of ZnO nanoneedles by thermal oxidation of Zn microstructures. Mater. Sci. Eng. B 2009, 164, 195–199. [Google Scholar] [CrossRef]
- Gui, Y.; Xie, C.; Zhang, Q.; Hu, M.; Yu, J.; Weng, Z. Synthesis and characterization of ZnO nanostructures by two-step oxidation of Zn nano- and microparticles. J. Cryst. Growth 2006, 289, 663–669. [Google Scholar] [CrossRef]
- Anthrop, D.F.; Searcy, A.W. Sublimation and Thermodynamic Properties of Zinc Oxide. J. Phys. Chem. 1964, 68, 2335–2342. [Google Scholar] [CrossRef]
- Gao, P.X.; Wang, Z.L. Mesoporous Polyhedral Cages and Shells Formed by Textured Self-Assembly of ZnO Nanocrystals. J. Am. Chem. Soc. 2003, 125, 11299–11305. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Chen, N.; Dai, R.; Liu, L.; Zhang, X.; Wang, X.; Wu, J.; Chai, C. On the formation of well-aligned ZnO nanowall networks by catalyst-free thermal evaporation method. J. Cryst. Growth 2007, 305, 296–301. [Google Scholar] [CrossRef]
- Kern, W. The Evolution of Silicon Wafer Cleaning Technology. J. Electrochem. Soc. 1990, 137, 1887–1892. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, Y.M.; Tehuacanero-Cuapa, S.; Peña-Sierra, R.; Paredes, G.R. Synthesis of Porous ZnO Films on Quartz Substrates by Thermal Oxidation and the Oxidant Atmosphere Effect. Key Eng. Mater. 2020, 834, 49–54. [Google Scholar] [CrossRef]
- Rojas-Chávez, H.; Cruz-Martínez, H.; Montejo-Alvaro, F.; Farías, R.; Hernández-Rodríguez, Y.; Guillen-Cervantes, A.; Ávila-García, A.; Cayetano, N.; Medina, D.I.; Cigarroa-Mayorga, O.E. The formation of ZnO structures using thermal oxidation: How a previous chemical etching favors either needle-like or cross-linked structures. Mater. Sci. Semicond. Process. 2019, 108, 104888. [Google Scholar] [CrossRef]
- Rojas-Chávez, H.; Miralrio, A.; Hernández-Rodríguez, Y.; Cruz-Martínez, H.; Pérez-Pérez, R.; Cigarroa-Mayorga, O. Needle- and cross-linked ZnO microstructures and their photocatalytic activity using experimental and DFT approach. Mater. Lett. 2021, 291, 129474. [Google Scholar] [CrossRef]
- Kim, K.S.; Kim, H.W.; Kim, N.H. Structural characterization of ZnO films grown on SiO2 by the RF magnetron sputtering. Phys. B Condens. Matter 2003, 334, 343–346. [Google Scholar] [CrossRef]
- Kim, D.-S.; Park, J.-H.; Lee, S.-J.; Ahn, K.-J.; Lee, M.-S.; Ham, M.-H.; Lee, W.; Myoung, J.-M. Effects of oxygen concentration on the properties of Al-doped ZnO transparent conductive films deposited by pulsed DC magnetron sputtering. Mater. Sci. Semicond. Process. 2013, 16, 997–1001. [Google Scholar] [CrossRef]
- Khun, K.; Ibupoto, Z.H.; AlSalhi, M.S.; Atif, M.; Ansari, A.A.; Willander, M. Fabrication of Well-Aligned ZnO Nanorods Using a Composite Seed Layer of ZnO Nanoparticles and Chitosan Polymer. Materials 2013, 6, 4361–4374. [Google Scholar] [CrossRef]
- Sun, Y.; Seo, J.H.; Takacs, C.J.; Seifter, J.; Heeger, A.J. Inverted Polymer Solar Cells Integrated with a Low-Temperature-Annealed Sol-Gel-Derived ZnO Film as an Electron Transport Layer. Adv. Mater. 2011, 23, 1679–1683. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, X.; Yu, Y.; Pei, Z.; Bai, X.; Sun, C.; Huang, R.; Wen, L. X-ray photoelectron spectroscopy and auger electron spectroscopy studies of Al-doped ZnO films. Appl. Surf. Sci. 2000, 158, 134–140. [Google Scholar] [CrossRef]
- Banger, K.; Yamashita, Y.; Mori, K.; Peterson, R.L.; Leedham, T.L.; A Rickard, J.; Sirringhaus, H. Low-temperature, high-performance solution-processed metal oxide thin-film transistors formed by a ‘sol–gel on chip’ process. Nat. Mater. 2010, 10, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, S.; Caglar, Y.; Ilican, S.; Caglar, M. Sol–gel derived Li–Mg co-doped ZnO films: Preparation and characterization via XRD, XPS, FESEM. J. Alloys Compd. 2012, 512, 171–178. [Google Scholar] [CrossRef]
- Ma, H. Influence of Heat Treatments on the Properties of ZnO Nanorods Prepared by Hydrothermal Synthesis. Semiconductors 2019, 53, 1811–1816. [Google Scholar] [CrossRef]
- Malik, G.; Mourya, S.; Jaiswal, J.; Chandra, R. Effect of annealing parameters on optoelectronic properties of highly ordered ZnO thin films. Mater. Sci. Semicond. Process. 2019, 100, 200–213. [Google Scholar] [CrossRef]
- Gallino, F.; Pacchioni, G.; Di Valentin, C. Transition levels of defect centers in ZnO by hybrid functionals and localized basis set approach. J. Chem. Phys. 2010, 133, 144512. [Google Scholar] [CrossRef] [PubMed]
- McCluskey, M.D.; Jokela, S.J. Defects in ZnO. J. Appl. Phys. 2009, 106, 071101. [Google Scholar] [CrossRef]
- Li, D.; Leung, Y.H.; Djurišić, A.B.; Liu, Z.T.; Xie, M.H.; Shi, S.L.; Xu, S.; Chan, W.K. Different origins of visible luminescence in ZnO nanostructures fabricated by the chemical and evaporation methods. Appl. Phys. Lett. 2004, 85, 1601–1603. [Google Scholar] [CrossRef]
- Pramanik, S.; Mondal, S.; Mandal, A.C.; Mukherjee, S.; Das, S.; Ghosh, T.; Nath, R.; Ghosh, M.; Kuiri, P.K. Role of oxygen vacancies on the green photoluminescence of microwave-assisted grown ZnO nanorods. J. Alloys Compd. 2020, 849, 156684. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Leung, Y.H.; Tam, K.H.; Hsu, Y.F.; Ding, L.; Ge, W.K.; Zhong, Y.; Wong, K.S.; Chan, W.K.; Tam, H.L.; et al. Defect emissions in ZnO nanostructures. Nanotechnology 2007, 18, 095702. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Choy, W.C.; Roy, V.A.L.; Leung, Y.H.; Kwong, C.Y.; Cheah, K.W.; Rao, T.K.G.; Chan, W.K.; Lui, H.F.; Surya, C. Photoluminescence and Electron Paramagnetic Resonance of ZnO Tetrapod Structures. Adv. Funct. Mater. 2004, 14, 856–864. [Google Scholar] [CrossRef]
- Leiter, F.H.; Alves, H.R.; Hofstaetter, A.; Hofmann, D.M.; Meyer, B.K. The Oxygen Vacancy as the Origin of a Green Emission in Undoped ZnO. Phys. Stat. Sol. 2001, 226, R4–R5. [Google Scholar] [CrossRef]
- Hsieh, P.-T.; Chen, Y.-C.; Kao, K.-S.; Wang, C.-M. Luminescence mechanism of ZnO thin film investigated by XPS measurement. Appl. Phys. A 2007, 90, 317–321. [Google Scholar] [CrossRef]
- Janotti, A.; Van de Walle, C.G. Native point defects in ZnO. Phys. Rev. B 2007, 76, 165202. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Wang, W.-C.; Jenq, F.-L.; Liu, C.-C.; Hung, C.-I.; Houng, M.-P. Surface modification of ZnO film by hydrogen peroxide solution. J. Appl. Phys. 2008, 104, 053521. [Google Scholar] [CrossRef]
- Cizek, J.; Valenta, J.; Hruška, P.; Melikhova, O.; Prochazka, I.; Novotný, M.; Bulíř, J. Origin of green luminescence in hydrothermally grown ZnO single crystals. Appl. Phys. Lett. 2015, 106, 251902. [Google Scholar] [CrossRef]
- Liu, X.; Wu, X.; Cao, H.; Chang, R.P.H. Growth mechanism and properties of ZnO nanorods synthesized by plasma-enhanced chemical vapor deposition. J. Appl. Phys. 2004, 95, 3141–3147. [Google Scholar] [CrossRef]
- Reynolds, D.C.; Look, D.C.; Jogai, B. Fine structure on the green band in ZnO. J. Appl. Phys. 2001, 89, 6189–6191. [Google Scholar] [CrossRef]
- Garces, N.Y.; Wang, L.; Bai, L.; Giles, N.C.; Halliburton, L.E.; Cantwell, G. Role of copper in the green luminescence from ZnO crystals. Appl. Phys. Lett. 2002, 81, 622–624. [Google Scholar] [CrossRef]
- Thornton, J.A. High-rate thick-film growth. Annu. Rev. Mater. Sci. 1977, 7, 239–260. [Google Scholar] [CrossRef]
- Li, Z.; Gao, W.; Reeves, R.J. Zinc oxide films by thermal oxidation of zinc thin films. Surf. Coat. Technol. 2005, 198, 319–323. [Google Scholar] [CrossRef]
- Cigarroa-Mayorga, O.E. Tuning the size stability of MnFe2O4 nanoparticles: Controlling the morphology and tailoring of surface properties under the hydrothermal synthesis for functionalization with myricetin. Ceram. Int. 2021, 47, 32397–32406. [Google Scholar] [CrossRef]
- Fan, H.J.; Gösele, U.; Zacharias, M. Formation of Nanotubes and Hollow Nanoparticles Based on Kirkendall and Diffusion Processes: A Review. Small 2007, 3, 1660–1671. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Rho, S.-H.; Lee, H.-S.; Kim, K.-M.; Lee, H.-C. Fabrication of Highly Porous and Pure Zinc Oxide Films Using Modified DC Magnetron Sputtering and Post-Oxidation. Materials 2021, 14, 6112. [Google Scholar] [CrossRef] [PubMed]

| Sample | Sublimation Temperature (°C) | 2θ (°) | FWHM | D (nm) |
|---|---|---|---|---|
| Q1 | 600 | 34.48 | 0.47 ± 0.03 | 17.55 ± 0.02 |
| Q2 | 700 | 34.52 | 0.49 ± 0.06 | 16.82 ± 0.04 |
| Q3 | 800 | 34.52 | 0.49 ± 0.02 | 16.88 ± 0.03 |
| S1 | 600 | 34.52 | 0.61 ± 0.04 | 13.56 ± 0.05 |
| S2 | 700 | 34.48 | 0.59 ± 0.03 | 14.16 ± 0.07 |
| S3 | 800 | 34.50 | 0.51 ± 0.06 | 16.18 ± 0.02 |
| Sample | Thickness (nm) |
|---|---|
| Q1 | 159.65 |
| Q2 | 163.4 |
| Q3 | 153.1 |
| S1 | 162.2 |
| S2 | 166.89 |
| S3 | 164.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Rodríguez, Y.M.; Lopez-Salazar, P.; Juarez-Diaz, G.; Paredes-Rubio, G.R.; Peña-Sierra, R. Synthesis and Characterization of Nanoporous ZnO Films by Controlling the Zn Sublimation by Using ZnO/Zn Precursor Films. Materials 2022, 15, 5509. https://doi.org/10.3390/ma15165509
Hernández-Rodríguez YM, Lopez-Salazar P, Juarez-Diaz G, Paredes-Rubio GR, Peña-Sierra R. Synthesis and Characterization of Nanoporous ZnO Films by Controlling the Zn Sublimation by Using ZnO/Zn Precursor Films. Materials. 2022; 15(16):5509. https://doi.org/10.3390/ma15165509
Chicago/Turabian StyleHernández-Rodríguez, Yazmin Mariela, Primavera Lopez-Salazar, Gabriel Juarez-Diaz, Gabriel Romero Paredes-Rubio, and Ramón Peña-Sierra. 2022. "Synthesis and Characterization of Nanoporous ZnO Films by Controlling the Zn Sublimation by Using ZnO/Zn Precursor Films" Materials 15, no. 16: 5509. https://doi.org/10.3390/ma15165509






