Analysing the Implications of Charging on Nanostructured Li2MnO3 Cathode Materials for Lithium-Ion Battery Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Potential Model and Simulation Codes
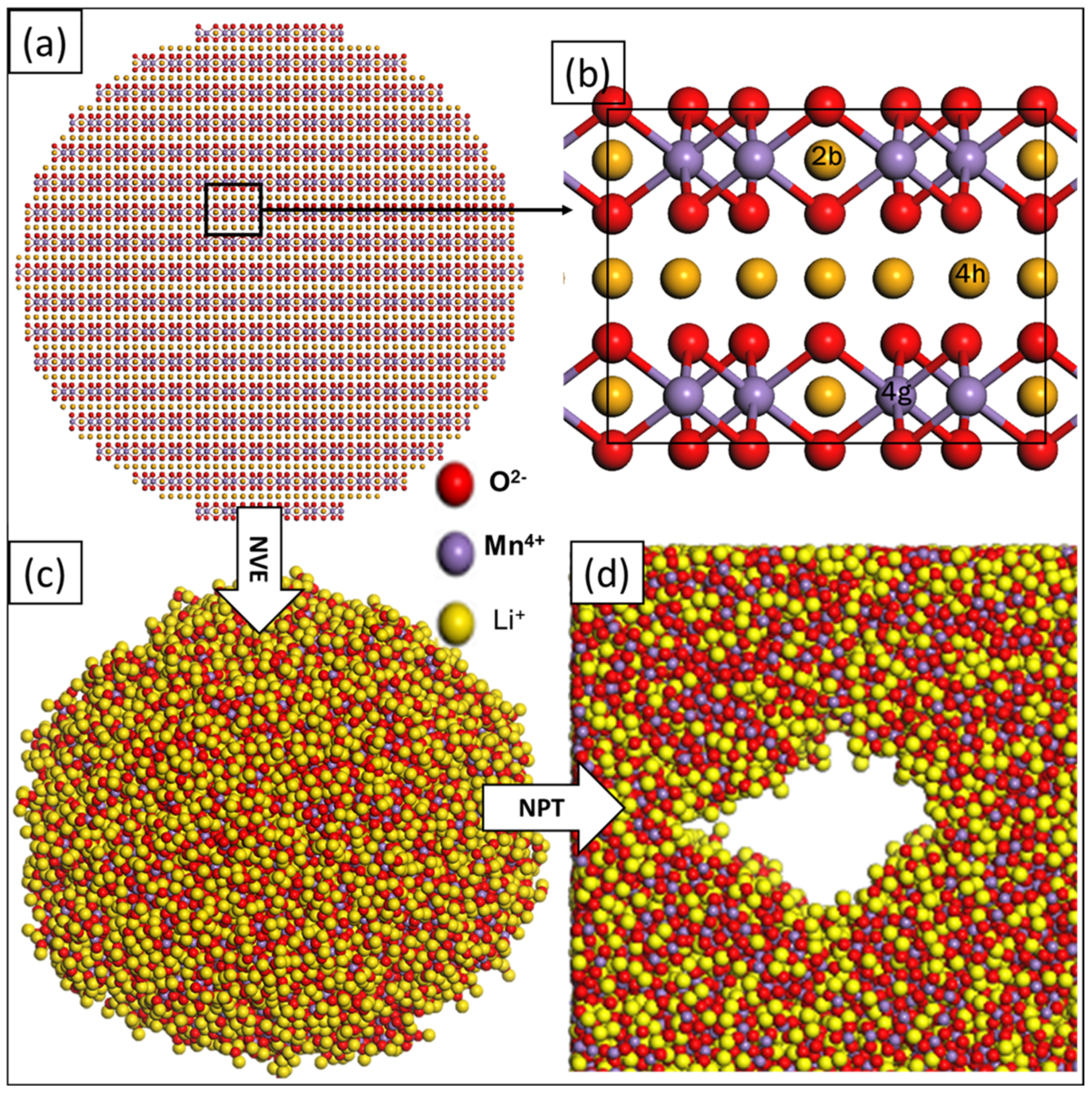
2.2. Generation of the Li2MnO3 Atomistic Models
3. Results and Discussions
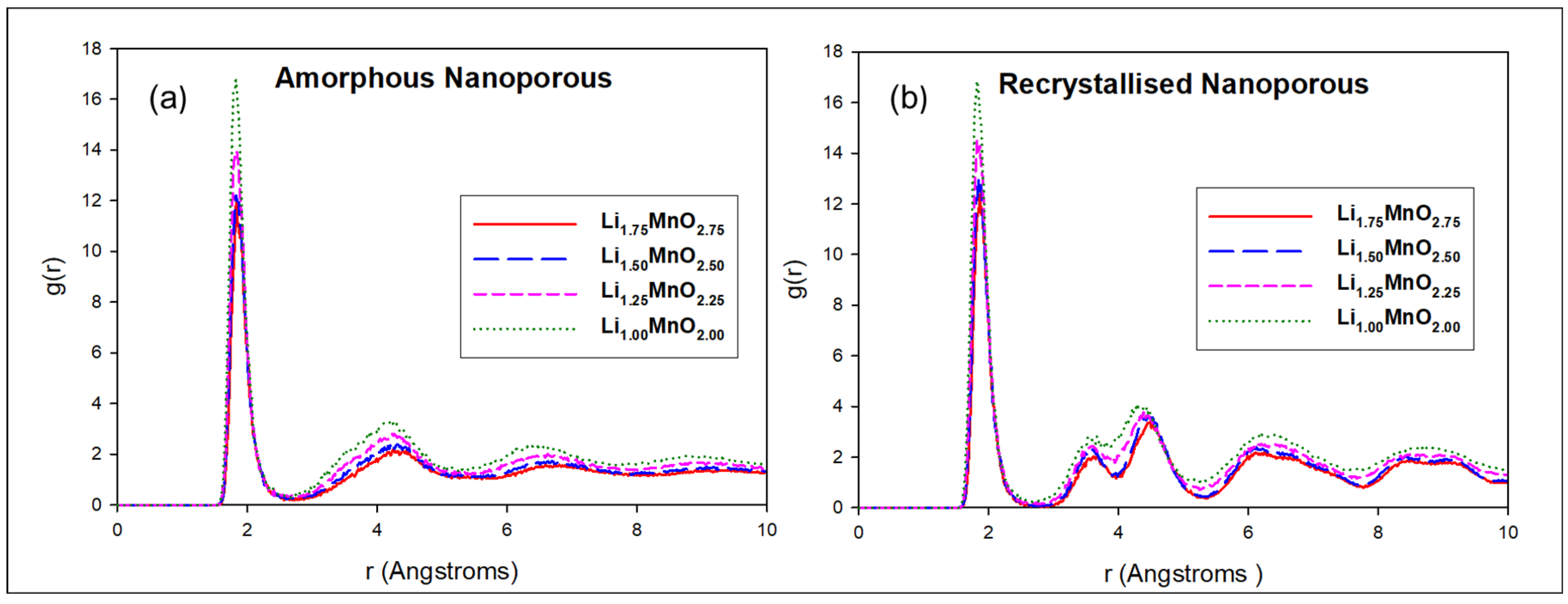
3.1. Radial Distribution Functions (RDFs)
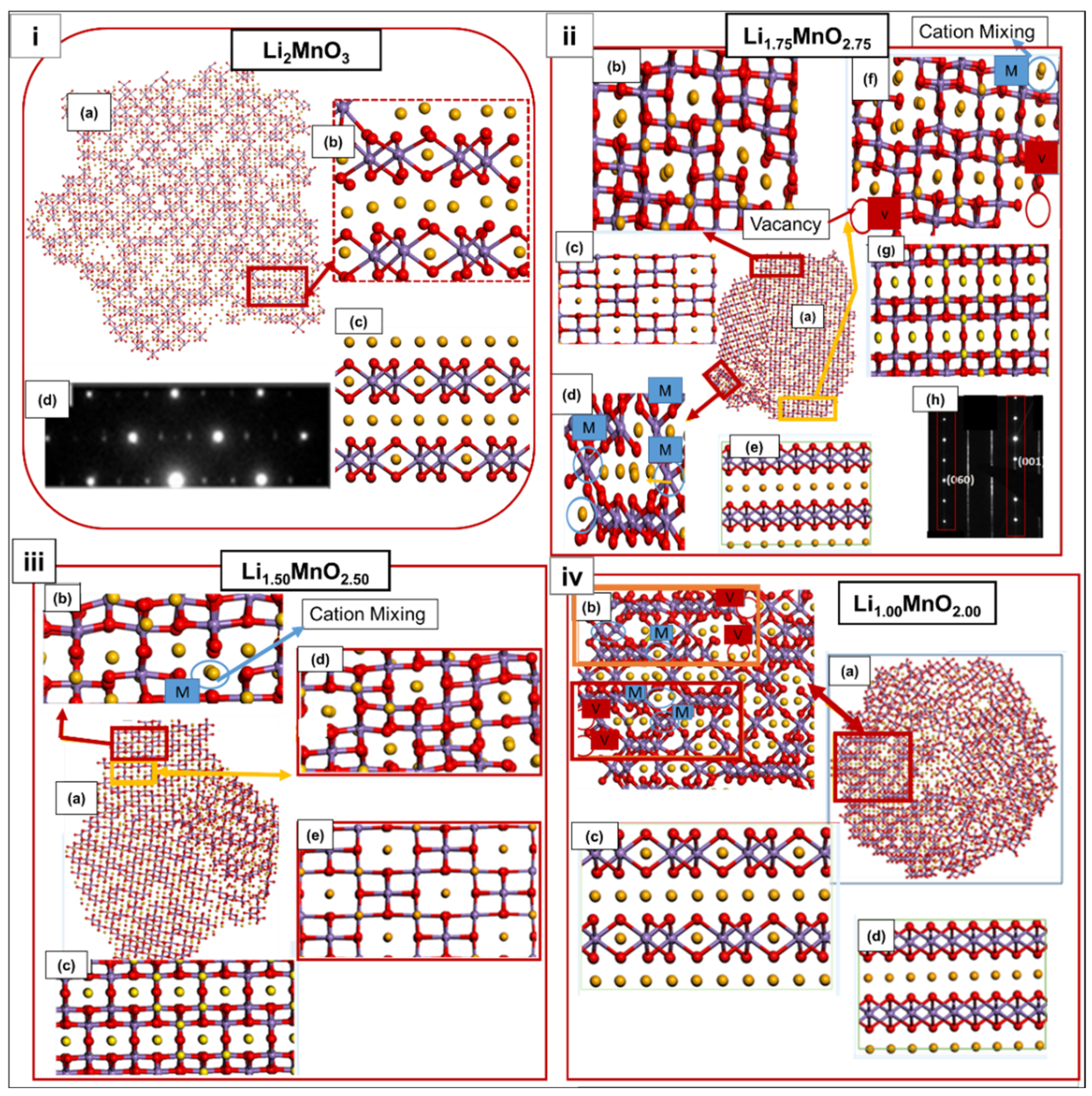
3.2. Structural Analysis
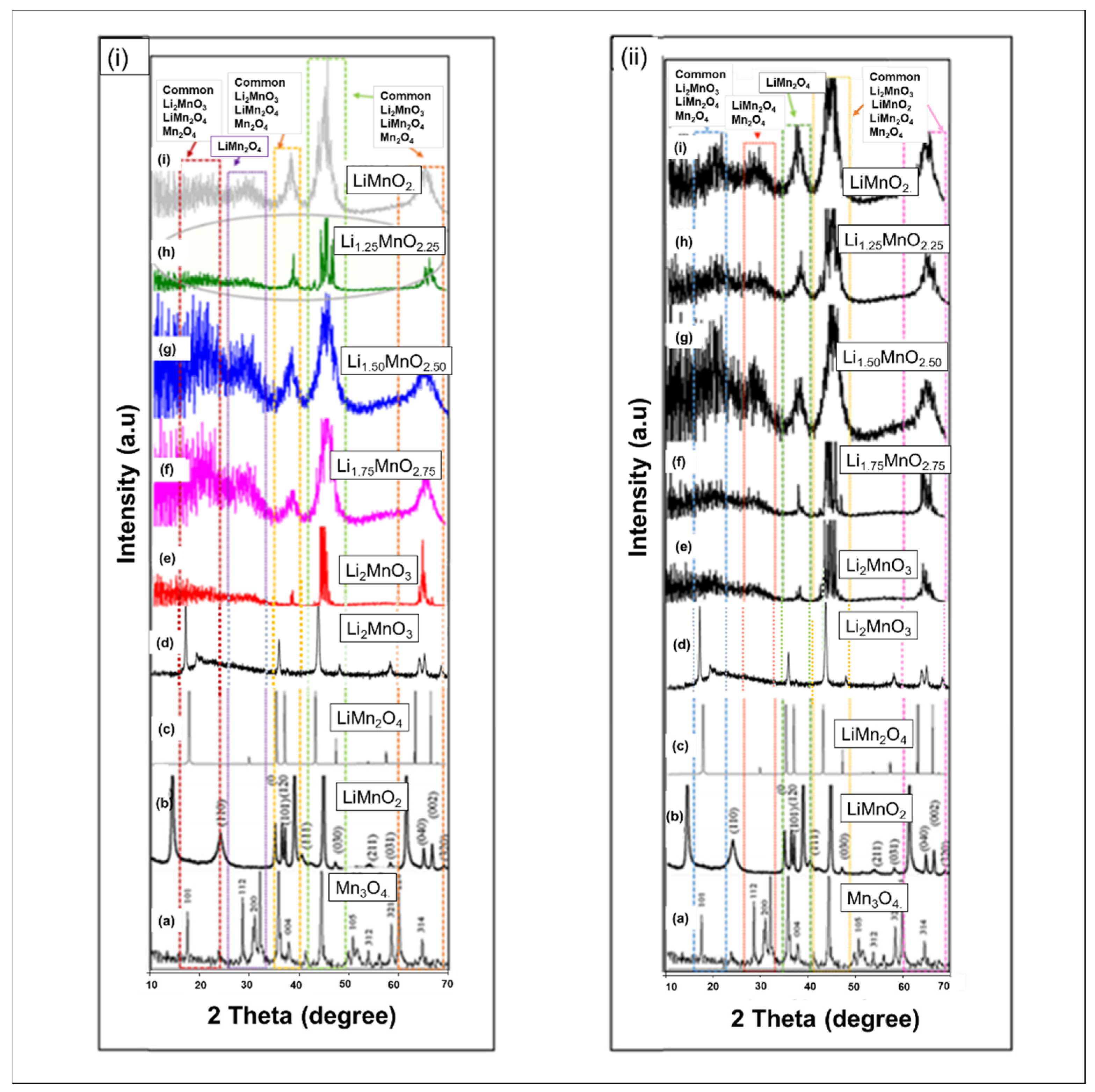
3.3. X-ray Diffraction Patterns (XRDs)
3.4. Diffusion Coefficient
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on the present, future and hybridized technologies. J. Mater. Chem. 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Marusczyk, A.; Albina, J.; Hammerschmidt, T.; Drautz, R.; Eckl, T.; Henkelman, G. Oxygen activity and peroxide formation as charge compensation mechanisms in Li2MnO3. J. Mater. Chem. 2017, 5, 15183–15190. [Google Scholar] [CrossRef]
- Li, F.; Zhang, X.; Lin, J.; Ma, J.; Zhang, S.; Yang, G. Unveiling the role of oxygen vacancy in Li2MnO3 upon delithiation. J. Phys. Chem. 2019, 123, 23403–23409. [Google Scholar] [CrossRef]
- Li, Q.; Yao, Z.; Lee, E.; Xu, Y.; Thackery, M.M.; Wolverton, C.; Dravid, V.P.; Wu, J. Dynamic imaging of crystalline defects in lithium-manganese oxide electrodes during electrochemical activation to high voltage. Nat. Commun. 2019, 10, 1692–1698. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cong, H.; Zan, L.; Zhang, Y. Oxygen vacancies and stacking faults introduced by low-temperature reduction improve the electrochemical properties of Li2MnO3 nanobelts as lithium-ion battery cathodes. ACS Appl. Mater. Interfaces 2017, 9, 38545–38555. [Google Scholar] [CrossRef]
- Sayle, T.X.T.; Caddeo, F.; Monama, N.O.; Kgatwane, K.M.; Ngoepe, P.E.; Sayle, D.C. Origin of electrochemical activity in nano-Li2MnO3; stabilization via a ‘point defect scaffold’. Nanoscale 2015, 7, 1167–1180. [Google Scholar] [CrossRef]
- Xie, H.; Cui, J.; Yao, Z.; Ding, X.; Zhang, Z.; Luo, D.; Lin, Z. Revealing the role of spinel phase on Li-rich layered oxides: A review. Chem. Eng. J. 2021, 427, 131978–131992. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, X.; Wang, C.; Bi, H.; Zhang, J.; Li, S.; Wang, M.; Che, R. Insight into the atomic structure of Li2MnO3 in Li-rich Mn-based cathode materials and the impact of its atomic arrangement on electrochemical performance. J. Mater. Chem. 2017, 5, 11214–11223. [Google Scholar] [CrossRef]
- Nakamura, T.N.; Gao, H.; Ohta, K.; Kimura, Y.; Tamenori, Y.; Nitta, K.; Ina, T.; Oishi, M.; Amezawa, K. Defect chemical studies on oxygen release from the Li-rich cathode material Li1.2Mn0.6Ni0.2O2-δ. J. Mater. Chem. A 2019, 7, 5009–5019. [Google Scholar] [CrossRef]
- Zhou, W. Effects of external mechanical loading on stress generation during lithiation in Li-ion battery electrodes. Electrochim. Acta 2015, 185, 28–33. [Google Scholar] [CrossRef]
- Ledwaba, R.S.; Kgatwane, K.M.; Sayle, D.C.; Ngoepe, P.E. Structural characterisation and mechanical properties of nanosized spinel LiMn2O4 cathode investigated using atomistic simulation. Mater. Res. Bull. 2022, 141, 111611. [Google Scholar] [CrossRef]
- Chen, R.; Zhao, T.; Zhang, X.; Liab, L.; Wu, F. Advanced cathode materials for lithium-ion batteries using nanoarchitectonics. Nanoscale Horiz. 2016, 1, 423–444. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, R.; Taguchi, N.; Kojima, T.; Takeichi, N.; Kiyobayashi, T. Improving the oxygen redox stability of NaCl-type cation disordered Li2MnO3 in a composite structure of Li2MnO3 and spinel-type LiMn2O4. J. Mater. Chem. 2019, 7, 5381–5390. [Google Scholar] [CrossRef]
- Long, B.R.; Croy, J.R.; Park, J.S.; Wen, J.; Miller, D.J.; Thackeray, M.M. Advances in Stabilizing ‘Layered-Layered’ xLi2MnO3·(1-x)LiMO2 (M=Mn, Ni, Co) Electrodes with a Spinel Component. J. Electrochem. Soc. 2014, 161, A2160–A2167. [Google Scholar] [CrossRef]
- Xiang, Y.; Wu, X. Enhanced electrochemical performances of Li2MnO3 cathode materials by Al doping. Ionics 2018, 24, 83–89. [Google Scholar] [CrossRef]
- Sun, Y.; Zan, L.; Zhang, Y. Enhanced electrochemical performances of Li2MnO3 cathode materials via adjusting oxygen vacancies content for lithium-ion batteries. Appl. Surf. Sci. 2019, 83, 270–277. [Google Scholar] [CrossRef]
- Lim, J.; Moon, J.; Gim, J.; Kim, S.; Kim, K.; Song, J.; Kang, J.; Im, W.B.; Kim, J. Fully activated Li2MnO3 nanoparticles by oxidation reaction. J. Mater. Chem. 2012, 22, 11772–11777. [Google Scholar] [CrossRef]
- Menon, A.S.; Ojwang, D.O.; Willhammar, T.; Peterson, V.K.; Edstrom, K.; Gomez, C.P.; Brant, W.R. The influence of synthesis routes on the crystallography, morphology and electrochemistry of Li2MnO3. ACS Appl. Mater. Interfaces 2020, 12, 5939–5950. [Google Scholar] [CrossRef]
- Maphanga, R.R.; Sayle, T.X.T.; Ngoepe, P.E.; Sayle, D.C. Amorphization and recrystallization study of lithium insertion into manganese dioxide. Phys. Chem. Chem. Phys. 2011, 13, 1307–1313. [Google Scholar] [CrossRef]
- Massel, F.; Hikima, K.; Rensmo, H.; Suzuki, K.; Hirayama, M.; Xu, C.; Younesi, R.; Liu, Y.-S.; Guo, J.; Kanno, R.; et al. Excess lithium in transition metal layers of epitaxially grown thin film cathodes of Li2MnO3 leads to rapid loss of covalency during first battery cycle. J. Phys. Chem. C 2019, 123, 28519–28526. [Google Scholar] [CrossRef]
- Guerrini, N.; Jin, L.; Lozano, J.G.; Luo, K.; Sobkowiak, A.; Tsuruta, K.; Massel, F.; Duda, L.-C.; Roberts, M.R.; Bruce, P.G. Charging mechanism of Li2MnO3. Chem. Mater. 2020, 32, 3733–3740. [Google Scholar] [CrossRef]
- Forester, T.R.; Smith, W. DL_POLY_2.0: A general-purpose parallel molecular dynamics simulation package. J. Mol. Dyn. 1996, 14, 136–141. [Google Scholar] [CrossRef]
- Watson, G.W.; Kelsey, E.T.; De Leeuw, N.H.; Harris, D.J.; Parker, S.C. Atomistic simulation of dislocation, surfaces and interfaces in MgO. J. Chem. Soc. Faraday Trans. 1996, 92, 433–438. [Google Scholar] [CrossRef]
- Sayle, T.X.T.; Catlow, C.R.A.; Maphanga, R.R.; Ngoepe, P.E.; Sayle, D.C. Generating MnO2 nanoparticles using simulated amorphization and recrystallization. J. Am. Chem. Soc. 2005, 127, 12828–12837. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Su, H.; Wua, T.; Jiang, Y.; Liu, D.; Yan, P.; Tian, H.; Yu, H. Atomic pair distribution function research on Li2MnO3 electrode structure evolution. Sci. Bull. 2019, 64, 553–561. [Google Scholar] [CrossRef]
- Xiao, L.; Xiao, J.A.; Yu, X.; Yan, P.; Zheng, J.; Engelhard, M.H.; Bhattacharya, P.; Wang, C.; Wang, C.; Yang, X.Q.; et al. Effects of structural defects on the electrochemical activation of Li2MnO3. Nano Energy 2015, 16, 143–151. [Google Scholar] [CrossRef]
- Matsunaga, T.; Komatsu, H.; Shimoda, K.; Minato, T.; Yonemura, M.; Kamiyama, T.; Kobayashi, S.; Kato, T.; Hirayama, T.; Ikuhara, Y.; et al. Dependence of structural defects in Li2MnO3 on synthesis temperature. Chem. Mater. 2016, 28, 4143–4150. [Google Scholar] [CrossRef]
- Ullah, A.K.M.A.; Kibria, A.K.M.F.; Akter, M.; Khan, M.N.I.; Shakhawat, A.R.M.T.; Firoz, H. Oxidative degradation of methylene blue using Mn3O4 nanoparticles. Water Conserv. Sci. Eng. 2017, 1, 249–256. [Google Scholar] [CrossRef]
- Tu, X.Y.; Shu, K.Y. X-ray diffraction study on phase transition of orthorhombic LiMnO2 in electrochemical conversions. J. Solid State Electrochem. 2008, 12, 245–249. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mogashoa, T.; Ledwaba, R.S.; Ngoepe, P.E. Analysing the Implications of Charging on Nanostructured Li2MnO3 Cathode Materials for Lithium-Ion Battery Performance. Materials 2022, 15, 5687. https://doi.org/10.3390/ma15165687
Mogashoa T, Ledwaba RS, Ngoepe PE. Analysing the Implications of Charging on Nanostructured Li2MnO3 Cathode Materials for Lithium-Ion Battery Performance. Materials. 2022; 15(16):5687. https://doi.org/10.3390/ma15165687
Chicago/Turabian StyleMogashoa, Tshidi, Raesibe Sylvia Ledwaba, and Phuti Esrom Ngoepe. 2022. "Analysing the Implications of Charging on Nanostructured Li2MnO3 Cathode Materials for Lithium-Ion Battery Performance" Materials 15, no. 16: 5687. https://doi.org/10.3390/ma15165687





