Properties and Durability of Cement Mortar Using Calcium Stearate and Natural Pozzolan for Concrete Surface Treatment
Abstract
1. Introduction
2. Materials
2.1. Diatomite
2.2. Yellow Clay
2.3. Calcium Stearate
2.4. Other Materials
3. Methods
3.1. Experimental Plan
- After slowly mixing the cement and sand, water was added and slowly mixed for 30 s at a speed of 140 ± 5 r/min.
- The mixer was stopped and restarted at a medium speed of 285 ± 10 r/min for 30 s.
- The mixer was stopped for 90 s before mixing was performed at 285 ± 10 r/min for 60 s.
3.2. Experimental Method
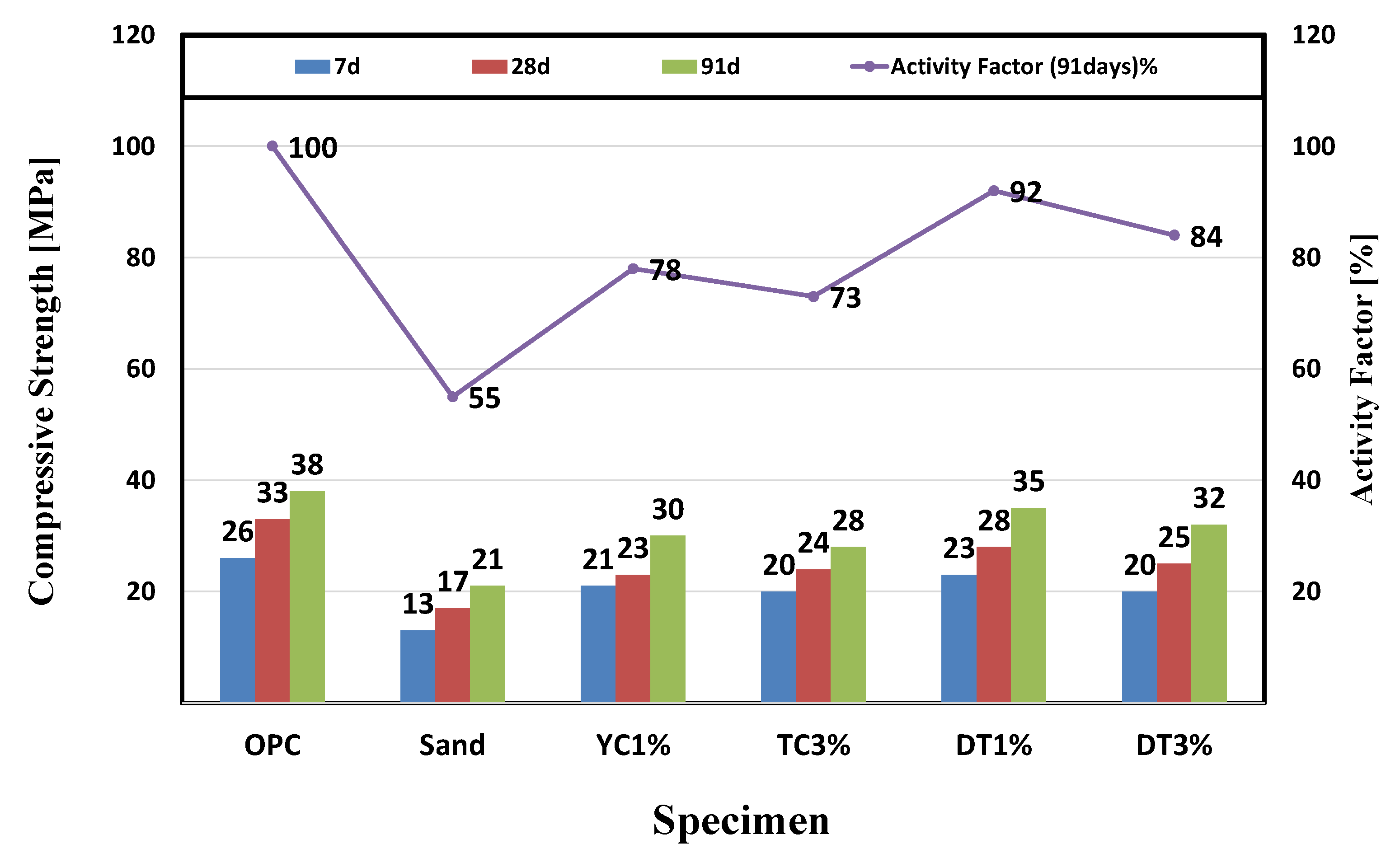
3.2.1. Evaluation of Flow, Air Content, Compressive Strength, and Activity Factor
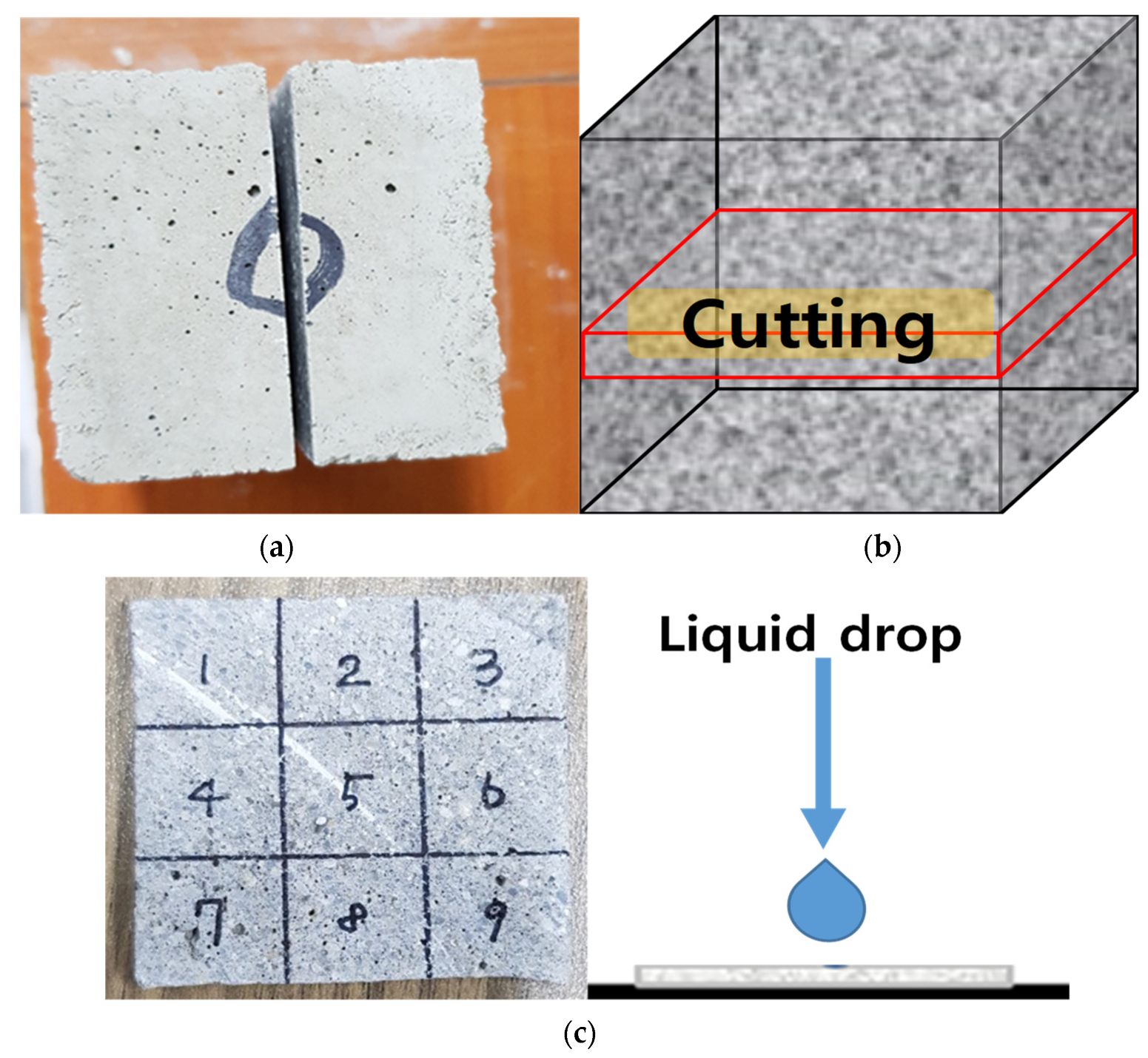
3.2.2. Contact Angle Measurement
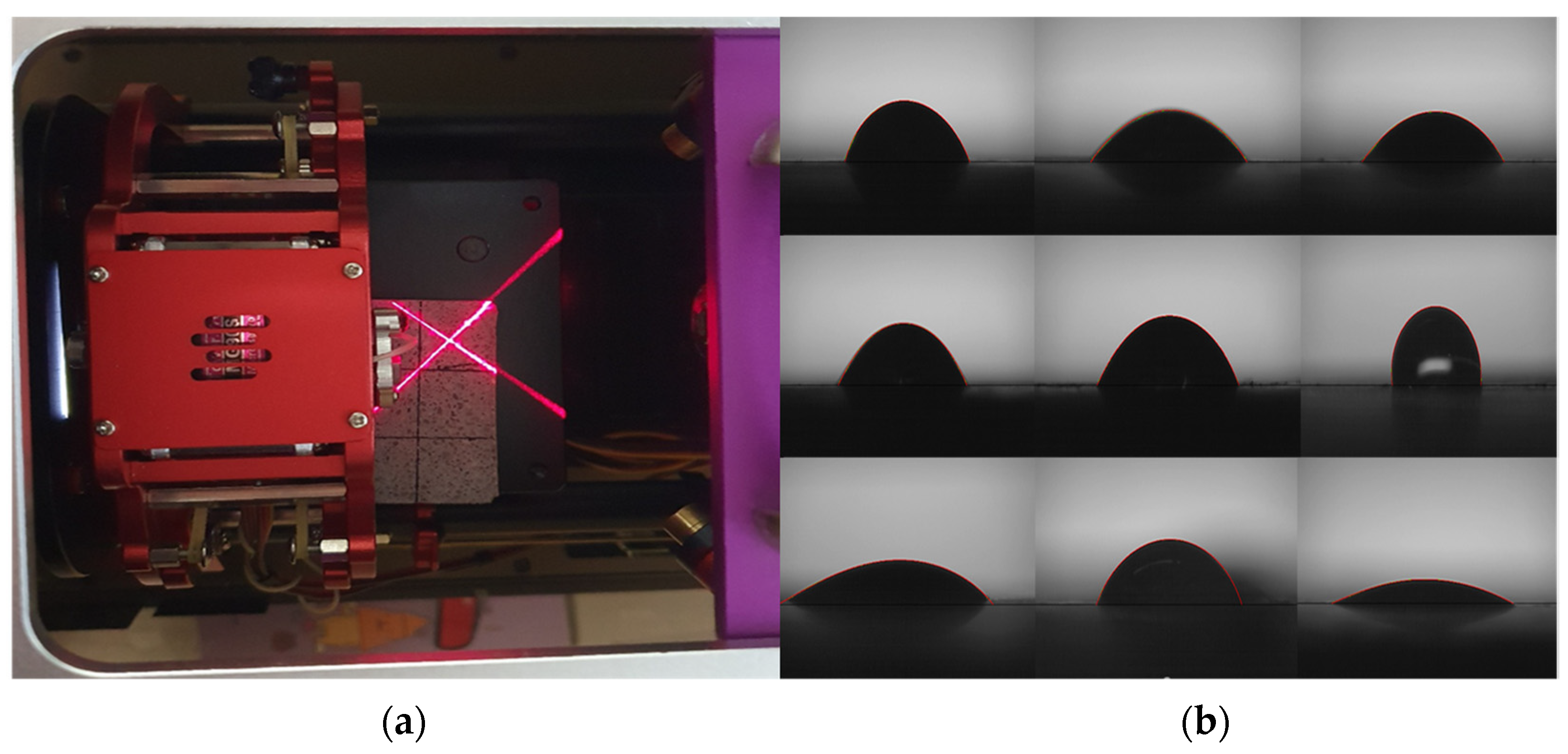
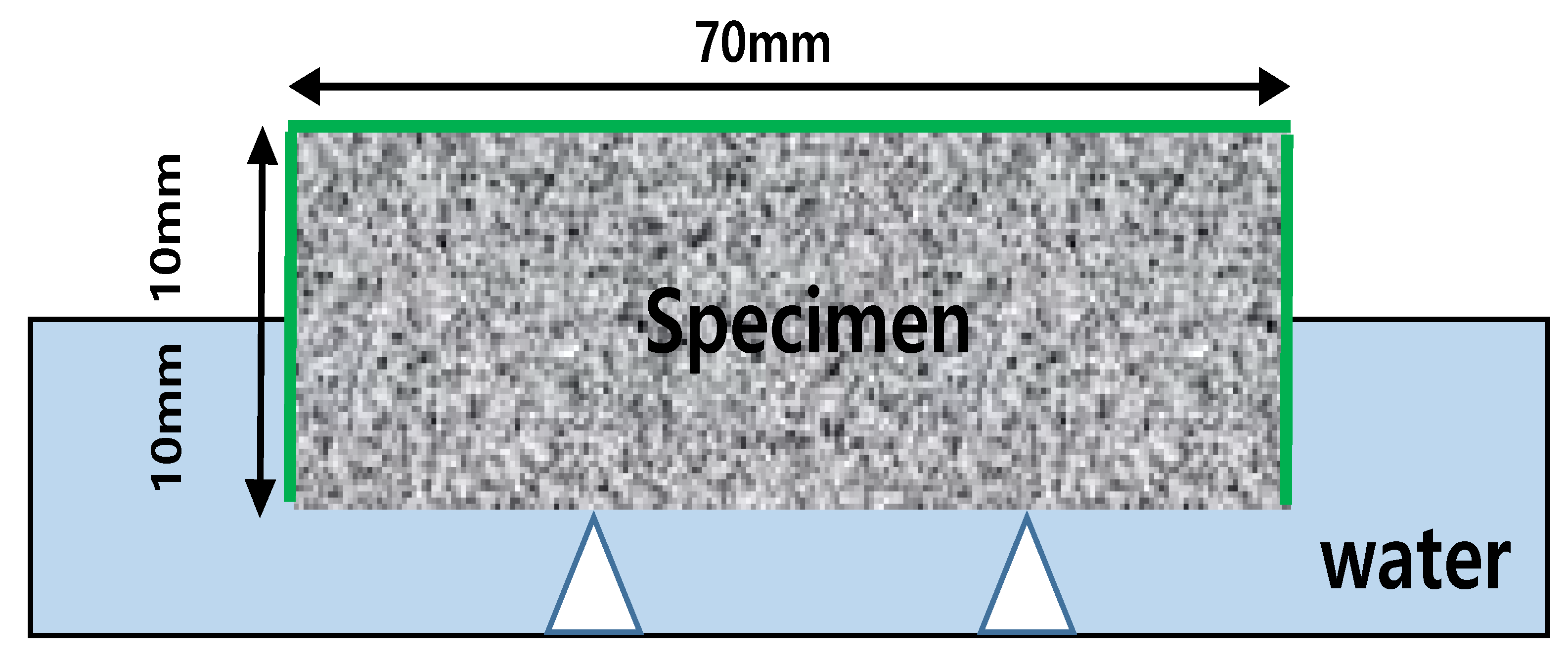
3.2.3. Chloride Ion Diffusion Coefficient Measurement
3.2.4. Water Absorption
3.2.5. MIP
4. Experimental Results
4.1. Evaluation of Physical Properties
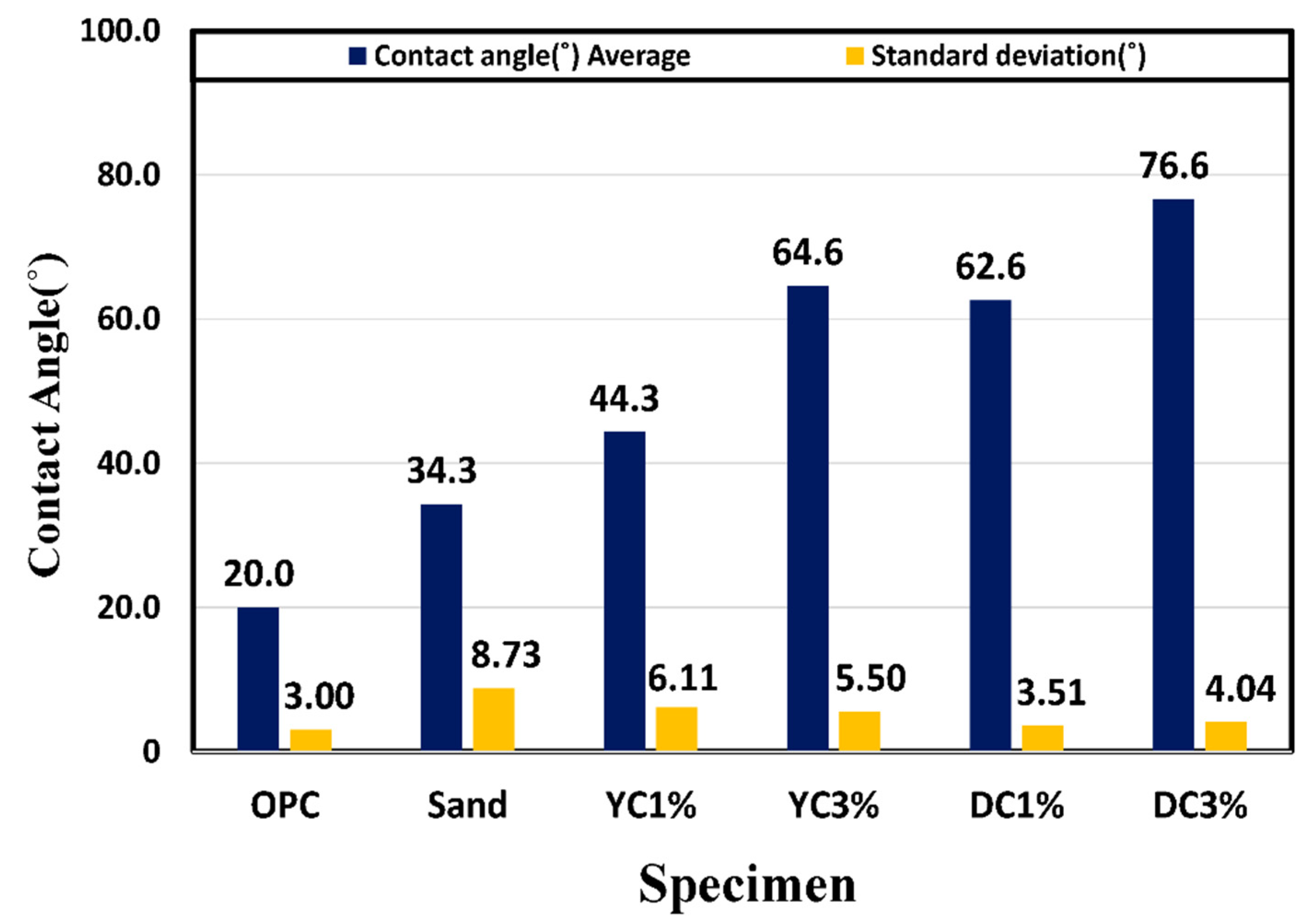
4.2. Contact Angle
4.3. Chloride Ion Diffusion Coefficient
4.4. Water Absorption Test
4.5. MIP
5. Conclusions
- (1)
- It was confirmed that the compressive strength decreased as the mixing amount of calcium stearate increased. Compared with the reference mortar OPC, the lowest compressive strength was 21 MPa for the sand test specimen, and the highest compressive strength was 35 MPa for the DT 1% test specimen. This indicates that calcium stearate tends to delay the hydration of cement and reduce the amount of hydrate generated.
- (2)
- From the contact angle measurement result it was confirmed that the size of the contact angle increased as the mixing amount of calcium stearate increased. Among the experimental specimen excluding the reference mortar, the experimental sand specimen was measured at the lowest value at 34 and the highest measurement at DC 3% to 76. Considering that the angle of OPC is 20, it was found that the resistance to moisture is about 3.5 times or more.
- (3)
- Chloride ion diffusion coefficient diffusivity was measured to be highest at 12.5 × 10−12 m2/s for the sand specimens and the lowest at DT 1% to 8.8 × 10−12 m2/s. The specimens with pozzolanic admixtures and calcium stearate exhibited resistance to water. They also showed a decrease in the extent of penetration of migrating chlorides, resulting in a lower chloride ion diffusion coefficient than that of the reference mortar.
- (4)
- As a result of the water penetration test, the sand test piece lost resistance to water and showed the largest amount of water absorption (11.8 g). The DT 3% specimens were found to have the highest resistance to moisture with a moisture absorption of 2.4 g. In the diatomite and yellow clay specimens, calcium stearate adhering to pores on the surface of the particles did not separate and was located inside the specimen and was judged to have high moisture resistance. From this, it can be inferred that calcium stearate is resistant to water in the non-hydraulic state (natural state).
- (5)
- The MIP test results, which are similar results to the compressive strength, were shown. It was shown that OPC, YC3%, and DT3% had a large void distribution in the 10–100 nm fine section, and conversely, the sand specimen had a large void distribution of 103–106. A large amount of SiO2 in diatomite and yellow clay and ocher produces calcium silicate (CSH) hydrate is due to the pozzolan reaction with C3S and C2S hydration products of cement.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, S.Y.; Nam, G.Y.; Kim, J.H. Effects of water-repellent on the physical properties of water paint. J. Korea Inst. Build. Constr. 2014, 14, 259–265. [Google Scholar] [CrossRef][Green Version]
- Lee, B.J.; Lee, J.; Kim, Y.Y. Durability performance of concrete penetrated and coated by polydimethylsiloxane for penetrating water repellency. J. Korea Concr. Inst. 2017, 29, 607–613. [Google Scholar]
- Dhir, R.K.; Hewlett, P.C.; Chan, Y.N. Near-surface characteristics of concrete: Assessment and development of in situ test methods. Mag. Concr. Res. 1987, 39, 183–195. [Google Scholar] [CrossRef]
- Song, H.W.; Lee, C.H.; Ann, K.Y. Factors influencing chloride transport in concrete structures exposed to marine environments. Cem. Concr. Compos. 2008, 30, 113–121. [Google Scholar] [CrossRef]
- Meyer, A. Importance of the surface layer for the durability of concrete structures. Spec. Publ. 1987, 100, 49–62. [Google Scholar]
- Basheer, P.A.M.; Basheer, L.; Cleland, D.J.; Long, A.E. Surface treatments for concrete: Assessment methods and reported performance. Constr. Build. Mater. 1997, 11, 413–429. [Google Scholar] [CrossRef]
- Pan, X.; Shi, Z.; Shi, C.; Ling, T.C.; Li, N. A review on surface treatment for concrete—Part 2: Performance. Constr. Build. Mater. 2017, 133, 81–90. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Jalali, S. Sulphuric acid resistance of plain, polymer modified, and fly ash cement concretes. Constr. Build. Mater. 2009, 23, 3485–3491. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Brenna, A.; Bolzoni, F.; Berra, M.; Pastore, T.; Ormellese, M. Effect of polymer modified cementitious coatings on water and chloride permeability in concrete. Constr. Build. Mater. 2013, 49, 720–728. [Google Scholar] [CrossRef]
- Medeiros, M.; Helene, P. Efficacy of surface hydrophobic agents in reducing water and chloride ion penetration in concrete. Mater. Struct. 2008, 41, 59–71. [Google Scholar] [CrossRef]
- Maravelaki-Kalaitzaki, P. Hydraulic Lime Mortars with Siloxane for Waterproofing Historic Masonry. Cem. Concr. Res. 2007, 37, 283–290. [Google Scholar] [CrossRef]
- Moradllo, M.K.; Sudbrink, B.; TylerLey, M. Determining the Effective Service Life of Silane Treatments in Concrete Bridge Decks. Constr. Build. Mater. 2016, 116, 121–127. [Google Scholar] [CrossRef]
- Park, M.J.; Lee, B.J.; Kim, J.S.; Kim, W.Y. Effect of Concrete Strength on Chloride Ion Penetration Resistance and Chemical Resistance of Concrete Coated by Siloxane-based Water Repellent. J. Korea Concr. Inst. 2018, 30, 583–590. [Google Scholar] [CrossRef]
- Yoon, C.B.; Kim, W.S.; Lee, H.S. An experimental study on the effect of the mixing of water-repellent impregnated natural zeolite on the resistance chloride penetration and microstructure of cement mortar. J. Arch. Inst Korea 2020, 36, 207–213. [Google Scholar]
- Shim, H.B.; Lee, M.S. An Experimental Study on Water Resistance of Penetrating Water Repellency of Emulsified Silicon Type Exposed to The Outdoor Environment. J. Korea Concr. Inst. 2004, 16, 477–484. [Google Scholar] [CrossRef]
- Maryoto, A.; Sthenly Gan, B.; Intang Setyo Hermanto, N.; Setijadi, R. Effect of calcium stearate in the mechanical and physical properties of concrete with PCC and fly ash as binders. Materials 2020, 13, 1394. [Google Scholar] [CrossRef] [PubMed]
- Maryoto, A. Resistance of concrete with calcium stearate due to chloride attack tested by accelerated corrosion. Procedia Eng. 2017, 171, 511–516. [Google Scholar] [CrossRef]
- Maryoto, A.; Gan, B.S.; Hermanto, N.I.S.; Setijadi, R. Corrosion resistance of self-compacting concrete containing calcium stearate. J. Eng. Sci. Tech. 2018, 13, 3263–3276. [Google Scholar]
- Jeong, G.Y. Quantitative determination of cristobalite content in diatomite and filtered food. J. Miner. Soc. Korea 2019, 32, 313–321. [Google Scholar] [CrossRef]
- Janotka, I.; Krajči, Ľ.; Uhlík, P.; Bačuvčík, M. Natural and Calcined Clayey Diatomite as Cement Replacement Materials: Microsture and Pore Structure Study. Int. J. Res. Eng. Technol. 2014, 3, 20–26. [Google Scholar]
- Degimenci, N.; Yilmaz, A. Use of Diatomite as Partial Replacement for Portland Cement in Cement Mortars. Constr. Build. Mater. 2009, 23, 284–288. [Google Scholar] [CrossRef]
- Ergun, A. Effects of the Usage of Diatomite and Water Marble Powder as Partial Replacement of Cement on the Mechanical Properties of Concrete. Constr. Build. Mater. 2011, 25, 806–812. [Google Scholar] [CrossRef]
- Yilmaz, B.; Hocaoglu, E. Fly Ash and Limestone in Diatomite-Blended Portland Cement. Adv. Cem. Res. 2011, 23, 151–159. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Meng, G. Sintering kinetics of porous ceramics from natural diatomite. J. Am. Ceram. Soc. 2005, 88, 1826–1830. [Google Scholar] [CrossRef]
- Wang, H.T.; Liu, X.Q.; Chen, F.L.; Meng, G.Y.; Sørensen, O.T. Kinetics and mechanism of sinter process for macro-porous alumina ceramic by extrusion. J. Am. Ceram. Soc. 1998, 81, 781–784. [Google Scholar] [CrossRef]
- Jung, S.G.; Bae, K.N.; Jeong, J.Y.; Kim, S.D. Characteristics on the adsorption and photocatalytic degradation by an air filter coated with TiO2 for hazardous air pollutants. J. Korean Soc. Indoor Environ. 2005, 2, 138–150. [Google Scholar]
- Kwon, S.J.; Kim, H.J.; Yoon, Y.S. The evaluation of durability performance in mortar curbs containing activated hwangtoh. J. Korean Recycl. Constr. Resour. Inst. 2020, 8, 520–527. [Google Scholar]
- Go, S.S.; Lee, H.C.; Lee, J.Y.; Kim, J.H.; Chung, C.W. Experimental investigation of mortars using activated hwangtoh. Constr. Build. Mater. 2009, 23, 1438–1445. [Google Scholar] [CrossRef]
- Choi, H.Y.; Hwang, H.Z.; Kim, M.H.; Kim, M.H. A study on the development of hwangtoh admixture for the application of cement mortar. J. Archit. Inst. Korea 2000, 16, 95–102. [Google Scholar]
- Kang, S.S.; Lee, S.L.; Hwang, H.Z.; Cho, M.C. Hydration heat and shrinkage of concrete using hwangtoh binder. J. Korea Concr. Inst. 2008, 20, 549–555. [Google Scholar]
- Hwang, H.Z.; Roh, T.H.; Kim, J.I. Characteristics of strength and durability of hwangto-concrete according to its mixing condition. J. Korea Inst. Ecol. Archit. Environ. 2008, 8, 55–60. [Google Scholar]
- Naseroleslami, R.; Chari, M.N. The effects of calcium stearate on mechanical and durability aspects of self-consolidating concretes incorporating silica fume/natural zeolite. Constr. Build. Mater. 2019, 225, 384–400. [Google Scholar] [CrossRef]
- Langley, W.D.; Rosenbaum, M.G.; Rosenbaum, M.M. The solubility of calcium stearate in solutions containing bile and in water. J. Biol. Chem. 1932, 99, 271–278. [Google Scholar] [CrossRef]
- KSL5201; Potland Cement. Korean Industrial Standards: Seoul, Korea, 2017.
- KSL5105; Testing Method for Compressive Strength of Hydraulic Cement Mortar. Korean Industrial Standards: Seoul, Korea, 2017.
- KSL5111; Flow Table for Use in Tests of Hydraulic Cement. Korean Industrial Standards: Seoul, Korea, 2017.
- C39; Standard Test Method for Compressive Strength of Concrete Cylinders. ASTM International: West Conshohocken, PA, USA, 2012.
- KSF2421; Standard Test Method for Air of Fresh Concrete by the Pressure Method (Air Receiver Method). Korean Industrial Standards: Seoul, Korea, 2016.
- KSL5405; Fly Ash. Korean Industrial Standards: Seoul, Korea, 2018.
- Takayoshi, I. A numerical method for determining surface tension of sessile drop. J. Korean Ceram. Soc. 1996, 33, 1325–1330. [Google Scholar]
- Flores-Vivian, I.; Hejazi, V.; Kozhukhova, M.I.; Nosonovsky, M.; Sobolev, K. Self-assembling particle-siloxane coatings for superhydrophobic concrete. ACS Appl. Mater. Interfaces 2013, 5, 13284–13294. [Google Scholar] [CrossRef]
- She, W.; Wang, X.; Miao, C.; Zhang, Q.; Zhang, Y.; Yang, J.; Hong, J. Biomimetic superhydrophobic surface of concrete: Topographic and chemical modification assembly by direct spray. Constr. Build. Mater. 2018, 181, 347–357. [Google Scholar] [CrossRef]
- Li, G.; Yue, J.; Guo, C.; Ji, Y. Influences of modified nanoparticles on hydrophobicity of concrete with organic film coating. Constr. Build. Mater. 2018, 169, 1–7. [Google Scholar] [CrossRef]
- Lange, A.; Hirata, T.; Plank, J. Influence of the HLB value of polycarboxylate superplasticizers on the flow behavior of mortar and concrete. Cem. Concr. Res. 2014, 60, 45–50. [Google Scholar] [CrossRef]
- Nie, S.; Zhang, W.; Hu, S.; Liu, Z.; Wang, F. Improving the fluid transport properties of heat-cured concrete by internal curing. Constr. Build. Mater. 2018, 168, 522–531. [Google Scholar] [CrossRef]
- Yoon, C.B.; Lee, H.S. Experimental study on the evaluation of physical performance and durability of cement mortar mixed with water repellent impregnated natural zeolite. Materials 2020, 13, 3288. [Google Scholar] [CrossRef]
- NT Build 492; Concrete, Mortar and Cement-Based Repair Materials: Chloride Migration Coefficient from Non-Steady-State Migration Experiments. Nordtest: Espoo, Finland, 1999.
- Tang, L. Electrically accelerated methods for determining chloride diffusivity in concrete—Current development. Mag. Concr. Res. 1996, 48, 173–179. [Google Scholar] [CrossRef]
- Park, J.H.; Park, C.; Joh, S.H.; Lee, H.S. Effect of curing condition on resistance to chloride ingress in concrete using ground granulated blast furnace slag. Materials 2019, 12, 3233. [Google Scholar] [CrossRef] [PubMed]
- KSF4919; Cement-Polymer Modified Waterproof Coatings. Korean Industrial Standards: Seoul, Korea, 2008.
- Lagazzo, A.; Vicini, S.; Cattaneo, C.; Botter, R. Effect of fatty acid soap on microstructure of lime-cement mortar. Constr. Build. Mater. 2016, 116, 384–390. [Google Scholar] [CrossRef]
- Lanzón, M.; Martínez, E.; Mestre, M.; Madrid, J.A. Use of zinc stearate to produce highly-hydrophobic adobe materials with extended durability to water and acid-rain. Constr. Build. Mater. 2017, 139, 114–122. [Google Scholar] [CrossRef]
- Nemati Chari, M.; Naseroleslami, R.; Shekarchi, M. The impact of calcium stearate on characteristics of concrete. Asian J. Civ. Eng. 2019, 20, 1007–1020. [Google Scholar] [CrossRef]
- Chen, R.; Liu, J.; Mu, S. Chloride ion penetration resistance and microstructural modification of concrete with the addition of calcium stearate. Constr. Build. Mater. 2022, 321, 126188. [Google Scholar] [CrossRef]
- Klein, N.S.; Bachmann, J.; Aguado, A.; Toralles-Carbonari, B. Evaluation of the wettability of mortar component granular materials through contact angle measurements. Cem. Concr. Res. 2012, 42, 1611–1620. [Google Scholar] [CrossRef]
- Bachmann, J.; Horton, R.; van der Ploeg, R.R.; Woche, S. Modified sessile drop method for assessing initial soil–water contact angle of sandy soil. Soil Sci. Soc. Am. J. 2000, 64, 564–567. [Google Scholar] [CrossRef]
- Adamson, A.W.; Gast, A.P. Physical chemistry of surfaces. Intersci. Publ. 1967, 150, 180. [Google Scholar] [CrossRef]
- Maekawa, K.; Ishida, T. Modeling of structural performances under coupled environmental and weather actions. Mater. Struct. 2002, 35, 591–602. [Google Scholar] [CrossRef]
- ACI 212.3R-10; Report on Chemical Admixtures for Concrete, Chapter 15: Permeability reducing admixtures. Reported by ACI Committee 212. ACI: Farmington, MI, USA, 2017.
- Jang, H.S.; Kang, H.J.; Song, J.Y.; Oh, S.K. An experimental study about the water leakage structure of waterproofing layer performance demobilization method using of stick expansion type complex of flexible material. Proc. Korean Inst. Build. Constr. Conf. 2005, 79–83. [Google Scholar]
- Feng, Z.; Wang, F.; Xie, T.; Ou, J.; Xue, M.; Li, W. Integral hydrophobic concrete without using silane. Constr. Build. Mater. 2019, 227, 116678. [Google Scholar] [CrossRef]
- Li, Q.; Yang, K.; Yang, C. An alternative admixture to reduce sorptivity of alkali-activated slag cement by optimising pore structure and introducing hydrophobic film. Cem. Concr. Compos. 2019, 95, 183–192. [Google Scholar] [CrossRef]
- Chen, H.; Feng, P.; Du, Y.; Jiang, J.; Sun, W. The effect of superhydrophobic nano-silica particles on the transport and mechanical properties of hardened cement pastes. Constr. Build. Mater. 2018, 182, 620–628. [Google Scholar] [CrossRef]









| Name | Chemical Composition (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | K2O | Etc. /Lg. loss | LOI | |
| OPC | 19.29 | 5.16 | 2.87 | 61.68 | 4.17 | 2.53 | 0.89 | 3.41 | 2.3 |
| Name | Chemical Composition (%) | ||||||||
| SiO2 | Al2O3 | Fe2O3 | MgO | CaO | K2O | Etc. /Lg. Loss | |||
| Yellow clay | 42.9 | 37.0 | 6.4 | 1.1 | 1.3 | 0.8 | 10.5 | ||
| Diatomite | 78.5 | 10.1 | 2.8 | 2.1 | 1.9 | 1.8 | 2.8 | ||
| Name | Chemical Composition (%) | ||||||||
| Chemical Formula | Density (g/cm3) | pH | Melting Point | Molecular Weight (g/mol) | |||||
| Calcium stearate | C36H70CaO4 | 1.08 | 7–9 | 147–149 | 607 | ||||
| Name | W/B (%) * | Unit Weight (kg/m3) | |||||
|---|---|---|---|---|---|---|---|
| Cement | Water | Sand | CS * | YC * | DT * | ||
| OPC * | 50% | 510 | 255 | 1530 | - | - | - |
| Sand | 50% | 510 | 257.5 | 1530 | 5.1 | - | - |
| YC1% * | 50% | 510 | 260.1 | 1530 | 5.1 | 5.1 | - |
| YC3% * | 50% | 510 | 270.3 | 1530 | 15.3 | 15.3 | - |
| DT1% * | 50% | 510 | 260.1 | 1530 | 5.1 | - | 5.1 |
| DT3% * | 50% | 510 | 270.3 | 1530 | 15.3 | - | 15.3 |
| Surface Contact Angle | Penetrability |
|---|---|
| >130° | High repellency |
| 110–130° | repellency |
| 90–110° | Slight wetting |
| 30–90° | Pronounced wetting |
| <30° | Complete surface wetting |
| Initial Current I30 V (with 30 V) (mA) | Applied Voltage U (after Adjustment) (V) | Possible New Initial Current I0 (mA) | Test Duration (h) |
|---|---|---|---|
| I0 < 5 | 60 | I0 < 10 | 96 |
| 5 ≤ I0 < 10 | 60 | 10 ≤ I0 < 20 | 48 |
| 10 ≤ I0 < 15 | 60 | 20 ≤ I0 < 30 | 24 |
| 15 ≤ I0 < 20 | 50 | 25 ≤ I0 < 35 | 24 |
| 20 ≤ I0 < 30 | 40 | 25 ≤ I0 < 40 | 24 |
| 30 ≤ I0 < 40 | 35 | 35 ≤ I0 < 50 | 24 |
| 40 ≤ I0 < 60 | 30 | 40 ≤ I0 < 60 | 24 |
| 60 ≤ I0 < 90 | 25 | 50 ≤ I0 < 75 | 24 |
| 90 ≤ I0 < 120 | 20 | 60 ≤ I0 < 80 | 24 |
| 120 ≤ I0 < 180 | 15 | 60 ≤ I0 < 90 | 24 |
| 180 ≤ I0 < 360 | 10 | 60 ≤ I0 < 120 | 24 |
| I0 ≥ 360 | 10 | I0 ≥ 120 | 6 |
| Name | Air Content (%) | Flow (mm) |
|---|---|---|
| OPC * | 5.6 | 165 |
| Sand | 9.2 | 190 |
| YC1% * | 5.9 | 170 |
| YC3% * | 5.5 | 160 |
| DT1% * | 6.0 | 175 |
| DT3% * | 5.2 | 165 |
| Name | Compressive Strength (MPa) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7 d | 28 d | 91 d | |||||||||||||
| 1st | 2nd | 3rd | Average | * SD | 1st | 2nd | 3rd | Average | * SD | 1st | 2nd | 3rd | Average | * SD | |
| OPC | 25 | 25 | 28 | 26 | 1.7 | 35 | 31 | 33 | 33 | 2.0 | 38 | 39 | 36 | 38 | 1.5 |
| Sand | 16 | 10 | 13 | 13 | 3.0 | 15 | 20 | 16 | 17 | 2.6 | 16 | 25 | 22 | 21 | 4.5 |
| YC1% | 23 | 18 | 22 | 21 | 2.6 | 25 | 22 | 22 | 23 | 1.7 | 32 | 29 | 29 | 30 | 1.7 |
| YC3% | 18 | 20 | 23 | 20 | 2.5 | 21 | 24 | 25 | 24 | 2.0 | 32 | 29 | 29 | 28 | 1.7 |
| DT1% | 24 | 24 | 22 | 23 | 1.1 | 28 | 29 | 27 | 28 | 1.0 | 34 | 34 | 36 | 35 | 1.1 |
| DT3% | 22 | 19 | 20 | 20 | 1.5 | 23 | 25 | 27 | 25 | 2.0 | 32 | 34 | 30 | 32 | 2.0 |
| Name | Dry (g) | Wet (g) | Absorption (g) | Normalized ValueOPC (%) |
|---|---|---|---|---|
| OPC | 118.9 | 125.4 | 6.5 | 100 |
| Sand | 117.3 | 129.1 | 11.8 | 181 |
| YC1% | 119.5 | 122.9 | 3.4 | 52.3 |
| YC3% | 118.4 | 121.4 | 3.0 | 46.1 |
| DT1% | 119.1 | 122.4 | 3.3 | 50.8 |
| DT3% | 120.8 | 123.2 | 2.4 | 37.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-H.; Yoon, C.-B. Properties and Durability of Cement Mortar Using Calcium Stearate and Natural Pozzolan for Concrete Surface Treatment. Materials 2022, 15, 5762. https://doi.org/10.3390/ma15165762
Park J-H, Yoon C-B. Properties and Durability of Cement Mortar Using Calcium Stearate and Natural Pozzolan for Concrete Surface Treatment. Materials. 2022; 15(16):5762. https://doi.org/10.3390/ma15165762
Chicago/Turabian StylePark, Jang-Hyun, and Chang-Bok Yoon. 2022. "Properties and Durability of Cement Mortar Using Calcium Stearate and Natural Pozzolan for Concrete Surface Treatment" Materials 15, no. 16: 5762. https://doi.org/10.3390/ma15165762
APA StylePark, J.-H., & Yoon, C.-B. (2022). Properties and Durability of Cement Mortar Using Calcium Stearate and Natural Pozzolan for Concrete Surface Treatment. Materials, 15(16), 5762. https://doi.org/10.3390/ma15165762






