The Influence of Heat Treatment on Corrosion Resistance and Microhardness of Hot-Dip Zinc Coating Deposited on Steel Bolts
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
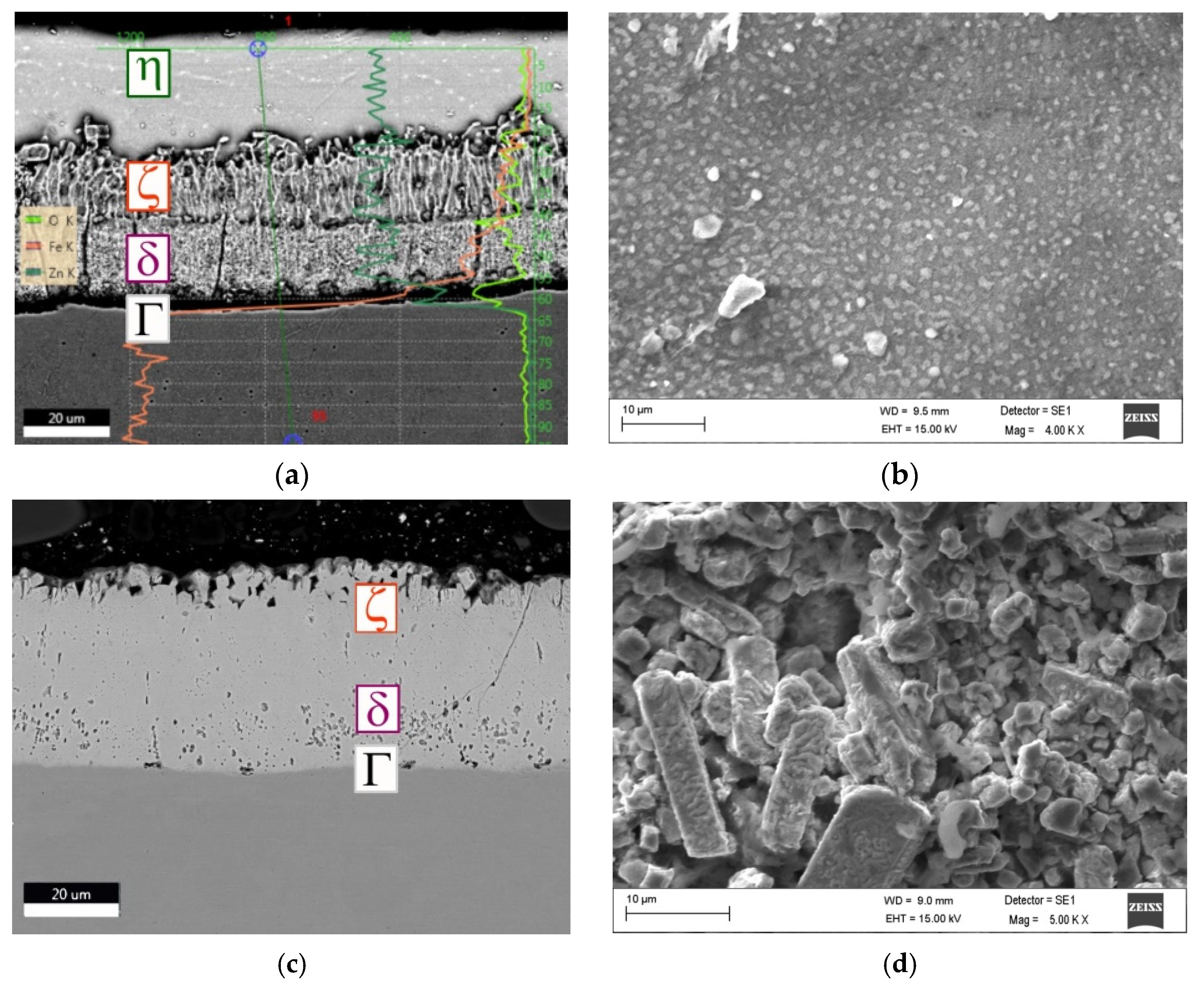
3.1. Coating Thickness and Microhardness Measurements
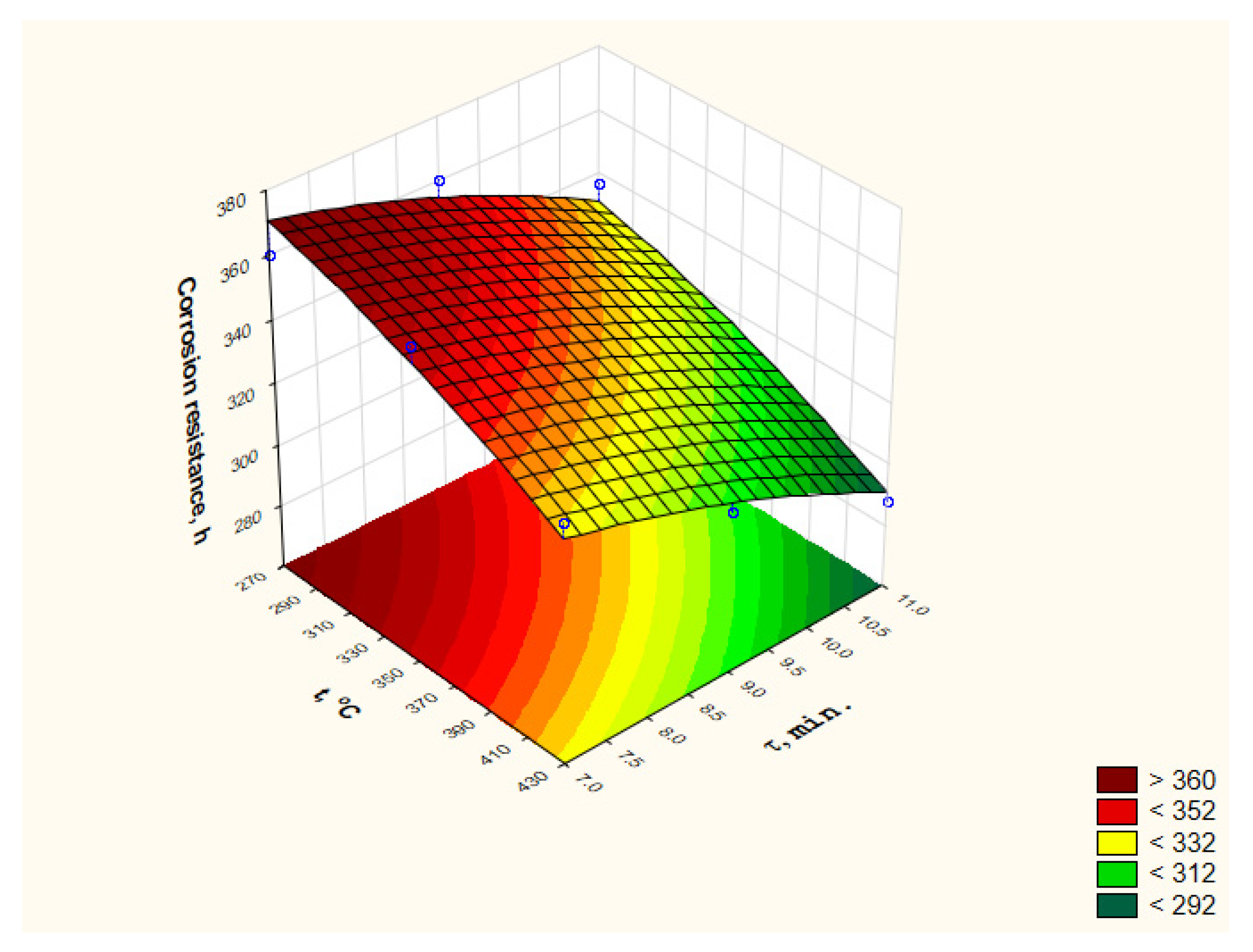
3.2. Corrosion Resistance Measurements
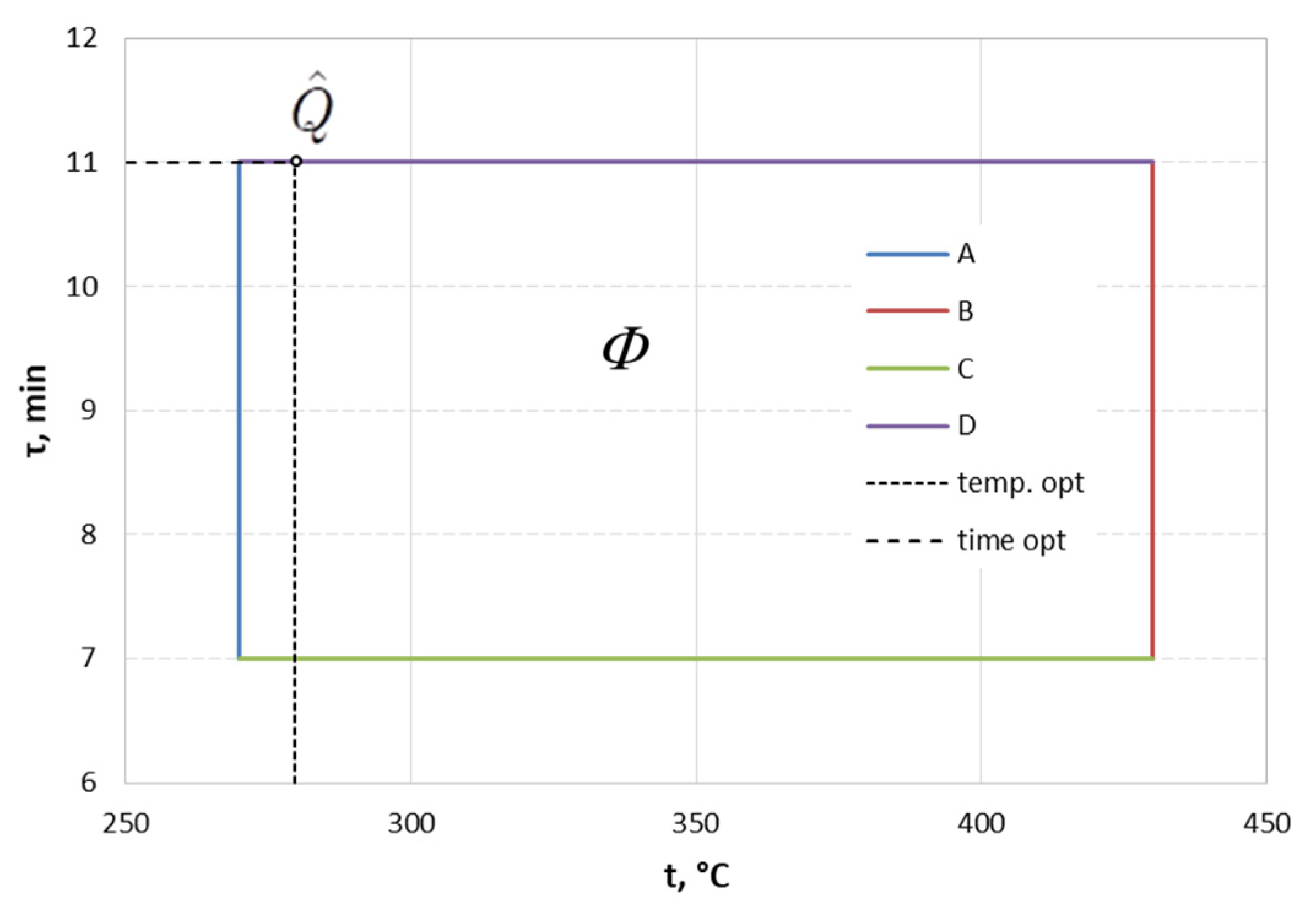
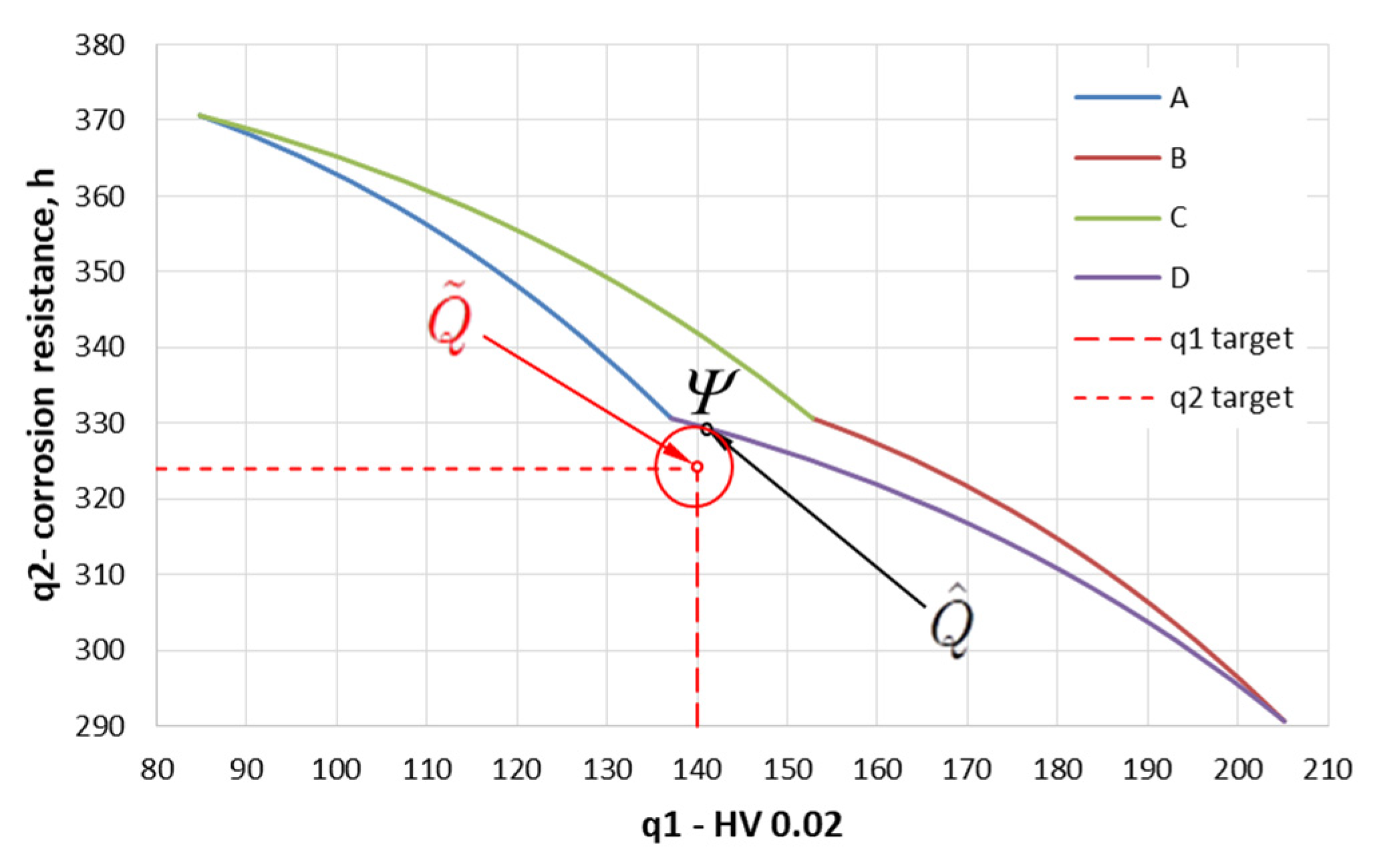
3.3. Solving the Optimization Problem
4. Conclusions
- (1)
- Microhardness measured on the heads of bolts subjected to the HT in the tested temperature range showed max. 4 times increase in the HV in the outer surface layer (85–204 HV 0.02) in relation to the reference samples (52 HV).
- (2)
- The observed changes in the hardness and corrosion resistance of the zinc coating are a consequence of changes in its structure. As the treatment time and temperature increase, the range of occurrence of the harder phase ζ increases too, at the expense of the η phase range (the total thickness of the coating does not change).
- (3)
- Only after a heat treatment conducted at 270 °C and a duration of 7 and 9 min and a heat treatment at 350 °C and a duration of 7 min, the corrosion resistance of the tested coatings did not decrease concerning coatings without heat treatment.
- (4)
- The obtained results confirm that it is possible to increase the hardness of the tested zinc coatings deposited on the bolts to a value of approx. 120 HV without reducing their corrosion resistance.
- (5)
- The obtained solution of the two-criteria optimization task allows for determining the most favorable/optimal parameters of heat treatment of hot-dip zinc coating (temperature and time) with reference to the postulated values of microhardness and corrosion resistance.
- (6)
- The determined optimal parameters of for the heat treatment of bolts (fulfilled the assumptions formulated in the work, i.e., HV = 140; corrosion resistance reduction ≤10%) in the tested range of variability of parameters are as follows: temperature 280 °C and time 11 min. The presented values of heat treatment parameters allow obtaining a coating hardness of 141 HV and coating corrosion resistance of 329 h.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Metals Price List—London Metal Exchange. Available online: www.lme.com (accessed on 27 July 2022).
- Maaß, P.; Peißker, P. Handbook of Hot-Dip Galvanization; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011. [Google Scholar]
- Report of the International Lead and Zinc Study Group. Available online: www.ilzsg.org/static/statistics.aspx (accessed on 26 August 2021).
- Kania, H.; Sipa, J. Microstructure Characterization and Corrosion Resistance of Zinc Coating Obtained on High-Strength Grade 10.9 Bolts Using a New Thermal Diffusion Process. Materials 2019, 12, 1400. [Google Scholar] [CrossRef]
- Votava, J. Anticorrosion protection of strength bolts. Acta Univ. Agric. et Silvic. Mendel. Brun. 2012, 60, 181–188. [Google Scholar] [CrossRef]
- Chung, P.P.; Wang, J.; Durandet, Y. Deposition processes and properties of coatings on steel fasteners—A review. Friction 2019, 7, 389–416. [Google Scholar] [CrossRef]
- Zhmurkin, D. Corrosion Resistance of Bolt Coatings; Tyco Electronics Corporation: Kawasaki, Japan, 2009; pp. 1–7. [Google Scholar]
- Evans, D.W. Next Generation Technology for Corrosion Protection in Ground Support. In Proceedings of the 14th Coal Operators’ Conference, Wollongong, Australia, 12–14 February 2014; The University of Wollongong: Wollongong, Australia, 2014; pp. 177–185. [Google Scholar]
- Meikle, T.; Tadolini, S.C.; Sainsbury, B.-A.; Bolton, J. Laboratory and field testing of bolting systems subjected to highly corrosive environments. Int. J. Min. Sci. Technol. 2017, 27, 101–106. [Google Scholar] [CrossRef]
- Królikowska, A.; Zubielewicz, M. Różnorodność cynkowych powłok zanurzeniowych i jej wpływ na właściwości antykorozyjne. Ochr. Przed Korozją 2013, 56, 430–435. [Google Scholar]
- Georges, C.; Colinet, B.; Sturel, T.; Allely, C.; Lhoist, V.; Cornette, D. Development of electro galvanized AHSS with tensile strength ranging from 1200 to 1500 MPa for automotive application with no risk of delayed fracture. In Proceedings of the Conference Galvatech 2017, Tokyo, Japan, 11 December 2017. [Google Scholar]
- Kwiatkowski, L. Cynk i ochrona przed korozją. Ochr. Przed Korozją 2004, 47, 256–257. [Google Scholar]
- Kania, H. Cynkowanie wysokotemperaturowe stali z zakresu Sandelina. Ochr. Przed Korozją 2011, 54, 587–590. [Google Scholar]
- Jiang, J.H.; Bin Ma, A.; Du Fan, X.; Gong, M.Z.; Zhang, L.Y. Sherardizing and Characteristic of Zinc Protective Coating on High-Strength Steel Bridge Cable Wires. Adv. Mater. Res. 2010, 97–101, 1368–1372. [Google Scholar] [CrossRef]
- Natrup, F.; Graf, W. Sherardizing: Corrosion protection of steels by zinc diffusion coatings. Thermochem. Surf. Eng. Steels 2015, 62, 737–750. [Google Scholar] [CrossRef]
- Shibli, S.; Manu, R.; Beegum, S. Studies on the influence of metal oxides on the galvanic characteristics of hot-dip zinc coating. Surf. Coatings Technol. 2008, 202, 1733–1737. [Google Scholar] [CrossRef]
- Marder, A. The metallurgy of zinc-coated steel. Prog. Mater. Sci. 2000, 45, 191–271. [Google Scholar] [CrossRef]
- Jordan, C.E.; Marder, A.R. Fe-Zn phase formation in interstitial-free steels hot-dip galvanized at 450°C: Part I 0.00 wt% Al-Zn baths. J. Mater. Sci. 1997, 32, 5593–5602. [Google Scholar] [CrossRef]
- Pu, D.; Pan, Y. First-principles investigation of oxidation mechanism of Al-doped Mo5Si3 silicide. Ceram. Int. 2022, 48, 11518–11526. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, S. Insight into the oxidation mechanism of MoSi2: Ab-initio calculations. Ceram. Int. 2018, 44, 19583–19589. [Google Scholar] [CrossRef]
- Pan, Y.; Pu, D. First-principles investigation of oxidation behavior of Mo5SiB2. Ceram. Int. 2020, 46, 6698–6702. [Google Scholar] [CrossRef]
- Pan, Y.; Wen, M. Insight into the oxidation mechanism of Nb3Si(111) surface: First-principles calculations. Mater. Res. Bull. 2018, 107, 484–491. [Google Scholar] [CrossRef]
- Pan, Y.; Pu, D.; Li, Y.; Zheng, Q. Origin of the antioxidation mechanism of RuAl(1 1 0) surface from first-principles calculations. Mater. Sci. Eng. B 2020, 259, 114580. [Google Scholar] [CrossRef]
- Wang, S.; Pan, Y.; Lin, Y. First-principles study of the effect of Cr and Al on the oxidation resistance of WSi2. Chem. Phys. Lett. 2018, 698, 211–217. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, J.; Wang, D.; Deng, H. Influence of vacancy on the elastic properties, ductility and electronic properties of hexagonal C40 MoSi2 from first-principles calculations. Vacuum 2020, 179, 109438. [Google Scholar] [CrossRef]
- Popczyk, M.; Łosiewicz, B. Wpływ obróbki cieplnej na odporność korozyjną powłok stopowych Zn-Ni-P-W. Ochr. Przed Korozją 2012, 55, 132–134. [Google Scholar]
- Łosiewicz, B.; Popczyk, M. Wpływ obróbki cieplnej na odporność korozyjną elektrolitycznych powłok Ni-W. Ochr. Przed Korozją 2012, 55, 128–131. [Google Scholar]
- Staszewska-Samson, K.; Scendo, M. Wpływ podwyższonej i wysokiej temperatury na właściwości antykorozyjne powłoki diamentopodobnej na stali S355. Ochr. Przed Korozją 2019, 62, 197–203. [Google Scholar]
- Li, W.; Li, C.-J.; Liao, H.; Coddet, C. Effect of heat treatment on the microstructure and microhardness of cold-sprayed tin bronze coating. Appl. Surf. Sci. 2007, 253, 5967–5971. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Barman, T.K.; Sahoo, P. Effects of heat treatment on tribological behavior of electroless ni–b coating at elevated temperatures. Surf. Rev. Lett. 2017, 24, 1850014. [Google Scholar] [CrossRef]
- Azadeh, M.; Toroghinejad, M.R. Effect of Heat Treatment on Formability of Hot-dip Galvanized Low Carbon Steel Sheet. ISIJ Int. 2009, 49, 1945–1951. [Google Scholar] [CrossRef]
- Szabadi, L.; Kalácska, G.; Pék, L.; Pálinkás, I. Abrasive wear of different hot-dip galvanized multilayers. Sustain. Constr. Des. 2011, 2, 82–91. [Google Scholar] [CrossRef]
- Chung, P.; Esfahani, M.; Wang, J.; Cook, P.; Durandet, Y. Effects of heat treatment on microstructure evolution and corrosion performance of mechanically plated zinc coatings. Surf. Coatings Technol. 2019, 377, 124916. [Google Scholar] [CrossRef]
- Tatarek, P.; Liberski, H.; Kania, H.; Formanek, B.; Podolski, P. Porównawcze badania odporności korozyjnej powłok cynkowych otrzymanych różnymi metodami. Ochr. Przed Korozją 2008, 4–5, 131–134. [Google Scholar]
- Kuklík, V.; Kudláček, J. Hot-Dip Galvanizing of Steel Structures; Elsevier Science: Oxford, UK, 2016. [Google Scholar] [CrossRef]
- Kania, H. Wpływ dodatku Pb do kąpieli cynkowej na odporność korozyjną powłok. Ochr. Przed Korozją 2016, 10, 368–371. [Google Scholar] [CrossRef]
- Kania, H.; Liberski, P.; Kwiatkowski, L. Wpływ dodatku niklu w kąpieli do cynkowania na odporność korozyjną powłok zanurzeniowych. Ochr. Przed Korozją 2010, 10, 471–474. [Google Scholar]
- Kania, H.; Liberski, P. Wpływ dodatku Sn na strukturę i odporność korozyjną powłok otrzymywanych na stalach z zakresu Sandelina. Ochr. Przed Korozją 2009, 10, 388–391. [Google Scholar]
- Liberski, P.; Kania, H. Wpływ dodatków stopowych w kąpieli cynkowej na jakość otrzymywanych powłok. Inżynieria Mater. 2009, 30, 182–188. [Google Scholar]
- Pistofidis, N.; Vourlias, G.; Konidaris, S.; Pavlidou, E.; Stergiou, A.; Stergioudis, G. The effect of bismuth on the structure of zinc hot-dip galvanized coatings. Mater. Lett. 2007, 61, 994–997. [Google Scholar] [CrossRef]
- Liberski, P.; Podolski, P.; Kama, H.; Gierek, A.; Mendala, J. Corrosion Resistance of Zinc Coatings Obtained in High-Temperature Baths. Mater. Sci. 2003, 39, 652–657. [Google Scholar] [CrossRef]
- Pokorný, P. Influence of Fe-Zn intermetallic layer on corrosion behaviour of galvanized concrete reinforcement. Koroze a Ochr. Mater. 2016, 60, 91–100. [Google Scholar] [CrossRef]
- Yadav, A.; Katayama, H.; Noda, K.; Masuda, H.; Nishikata, A.; Tsuru, T. Effect of Fe–Zn alloy layer on the corrosion resistance of galvanized steel in chloride containing environments. Corros. Sci. 2007, 49, 3716–3731. [Google Scholar] [CrossRef]
- Ramanauskas, R.; Juškėnas, R.; Kaliničenko, A.; Garfias-Mesias, L.F. Microstructure and corrosion resistance of electrodeposited zinc alloy coatings. J. Solid State Electrochem. 2004, 8, 416–421. [Google Scholar] [CrossRef]
- Jędrzejczyk, D.; Szatkowska, E. Changes in Properties of Hot-Dip Zinc Coating Resulting from Heat Treatment. Arch. Metall. Mater. 2019, 64, 201–206. [Google Scholar] [CrossRef]
- Jędrzejczyk, D.; Skotnicki, W. Comparison of the Tribological Properties of the Thermal Diffusion Zinc Coating to the Classic and Heat Treated Hot-Dip Zinc Coatings. Materials 2021, 14, 1655. [Google Scholar] [CrossRef]
- Jędrzejczyk, D.; Szatkowska, E. The Impact of Heat Treatment on the Behavior of a Hot-Dip Zinc Coating Applied to Steel During Dry Friction. Materials 2021, 14, 660. [Google Scholar] [CrossRef]
- PN-EN ISO 10684:2006; Fasteners, Zinc Coatings Applied by Hot-Dip Method. Polish Committee for Standardization: Warsaw, Poland, 2006.
- Abbas, M.M.; Rasheed, M. Solid State Reaction Synthesis and Characterization of Cu doped TiO2 Nanomaterials. J. Physics: Conf. Ser. 2021, 1795, 012059. [Google Scholar] [CrossRef]
- Aukštuolis, A.; Girtan, M.; Mousdis, G.A.; Mallet, R.; Socol, M.; Rasheed, M.; Stanculescu, A. Measurement of charge carrier mobility in perovskite nanowire films by photo-celiv method. Rom. Acad. Ser. A—Math. Phys. Tech. Sci. Inf. Sci. 2017, 18, 34–41. [Google Scholar]
- Hestenes, M.R.; Stiefel, E. Methods of Conjugate Gradients for Solving Linear Systems. J. Res. Natl. Bur. Stand. 1952, 49, 409–436. [Google Scholar] [CrossRef]
- PN-EN ISO 2178:2016-06; Non-Magnetic Coatings on Magnetic Substrate. Coating Thickness Measurement. Magnetic Method. Polish Committee for Standardization: Warsaw, Poland, 2016.
- Massalski, T.B.; Okamoto, H.; Subramanian, P.R.; Kacprzak, L. Binary Alloy Phase Diagrams; ASM International: Novelty, OH, USA, 1990. [Google Scholar]
- Culcasi, J.; Seré, P.; Elsner, C.; Di Sarli, A. Control of the growth of zinc–iron phases in the hot-dip galvanizing process. Surf. Coatings Technol. 1999, 122, 21–23. [Google Scholar] [CrossRef]
- Pokorný, P.; Cinert, J.; Pala, Z. Fe-Zn intermetallic phases prepared by diffusion annealing and spark-plasma sintering. Mater. Teh. 2016, 50, 253–256. [Google Scholar] [CrossRef]
- Kopyciński, D.; Siekaniec, D.; Szczęsny, A.; Guzik, E. Oddziaływanie warstwy wierzchniej odlewu z żeliwa sferoidalnego na wzrost powłoki cynkowej podczas metalizacji zanurzeniowej. Inżynieria Mater. 2015, 2, 65–68. [Google Scholar] [CrossRef][Green Version]
- Kania, H.; Mendala, J.; Kozuba, J.; Saternus, M. Development of Bath Chemical Composition for Batch Hot-Dip Galvanizing—A Review. Materials 2020, 13, 4168. [Google Scholar] [CrossRef]
- Chatterjee, B. Sherardizing. Met. Finish. 2004, 102, 40–46. [Google Scholar] [CrossRef]
- Pokorny, P.; Kolisko, J.; Balik, L.; Novak, P. Reaction kinetics of the formation of intermetallic Fe-Zn during hot-dip galvanizing of steel. Metalurgija 2016, 55, 111–114. [Google Scholar]
- Wołczyński, W.; Kucharska, B.; Garzeł, G.; Sypień, A.; Pogoda, Z.; Okane, T. Part III. Kinetics of the (Zn)—Coating Deposition During Stable and Meta-Stable Solidifications. Arch. Met. Mater. 2015, 60, 199–207. [Google Scholar] [CrossRef]
- PN-EN ISO 9227:2017-06; Corrosion Tests in Artificial Atmospheres—Salt Spray Tests. Polish Committee for Standardization: Warsaw, Poland, 2017.
- Stadnicki, J. Teoria I Praktyka Rozwiązywania Zadań Optymalizacj; Wydawnictwo Naukowo-Techniczne: Warszawa, Poland, 2006. [Google Scholar]
- Lisowski, J. Metoda Gradientu Sprzężonego Hestenesa-Stiefela; Akademia Morska w Gdyni: Gdynia, Poland, 2017. [Google Scholar]




| Parameter of Heat Treatment | No. of Fractional Plan Variant | |
|---|---|---|
| Temperature t, °C | Time τ, min. | |
| 270 | 7 | 1 |
| 9 | 2 | |
| 11 | 3 | |
| 350 | 7 | 4 |
| 9 | 5 | |
| 11 | 6 | |
| 430 | 7 | 7 |
| 9 | 8 | |
| 11 | 9 | |
| Phase Kind | Phase Hardness | ||
|---|---|---|---|
| (a) | (b) | ||
| Г | Г, Fe3Zn10 | 326 HB | 326 |
| Г1, Fe5Zn21 | - | 505 HV | |
| δ | δ, FeZn10 | - | 358 HV |
| δ, FeZn7 | 270 HB | - | |
| ζ | ζ, FeZn13 | 220 HB | 208 HV |
| η | η, Zn(Fe) | 70 HB | 52 HV |
| Phase Kind | Content Fe,% | Phase Kind | Content Fe, % | Phase Kind | Content Fe, % |
|---|---|---|---|---|---|
| (a) | (b) | (c) | |||
| Г, Fe3Zn10 | 18–31 | Г1, Fe3Zn10 | 21–28 | Г, Fe3Zn10 | 23.7–31.5 |
| Г1, Fe5Zn21 | 19–24 | ||||
| δ, FeZn10δ, FeZn7 | 8–137–10 [58] | δ, FeZn10δ, FeZn7 [59] | 7–11.510.87 | δ1, FeZn10 | 8.1–13.4 |
| ζ, FeZn13 | 6–7 | ζ, FeZn13 | 5–6 | ζ, FeZn13 | 6.5–7.5 |
| η, Zn(Fe) | 0.04 | η, Zn(Fe) | 0 | η, Zn(Fe) | 0.03 |
| Parameter of Heat Treatment | Time to Red Corrosion Appearance, Hours/Days | |
|---|---|---|
| Temperature t, °C | Time τ, min. | |
| 0 | 0 | 360/15 |
| 270 | 7 | 360/15 |
| 9 | 360/15 | |
| 11 | 336/14 | |
| 350 | 7 | 360/15 |
| 9 | 336/14 | |
| 11 | 312/13 | |
| 430 | 7 | 336/147 |
| 9 | 312/13 | |
| 11 | 288/12 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jędrzejczyk, D.; Szatkowska, E. The Influence of Heat Treatment on Corrosion Resistance and Microhardness of Hot-Dip Zinc Coating Deposited on Steel Bolts. Materials 2022, 15, 5887. https://doi.org/10.3390/ma15175887
Jędrzejczyk D, Szatkowska E. The Influence of Heat Treatment on Corrosion Resistance and Microhardness of Hot-Dip Zinc Coating Deposited on Steel Bolts. Materials. 2022; 15(17):5887. https://doi.org/10.3390/ma15175887
Chicago/Turabian StyleJędrzejczyk, Dariusz, and Elżbieta Szatkowska. 2022. "The Influence of Heat Treatment on Corrosion Resistance and Microhardness of Hot-Dip Zinc Coating Deposited on Steel Bolts" Materials 15, no. 17: 5887. https://doi.org/10.3390/ma15175887
APA StyleJędrzejczyk, D., & Szatkowska, E. (2022). The Influence of Heat Treatment on Corrosion Resistance and Microhardness of Hot-Dip Zinc Coating Deposited on Steel Bolts. Materials, 15(17), 5887. https://doi.org/10.3390/ma15175887







