Inclusion of Natural Antioxidants of Mango Leaves in Porous Ceramic Matrices by Supercritical CO2 Impregnation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Obtaining of Ethanolic Extracts from Mango Leaves
2.3. Supercritical Solvent Impregnation (SSI)
2.4. Determination of Antioxidant Capacity Assay with DPPH and FRAP
2.4.1. DPPH Method
2.4.2. FRAP Method
2.5. Phenolic Compounds Determination in the Impregnated Silica
2.5.1. Total Phenolic Content
2.5.2. HPLC Analysis
2.6. Physical Characterization of Impregnated Silica
2.7. Statistic Analysis
3. Results and Discussion
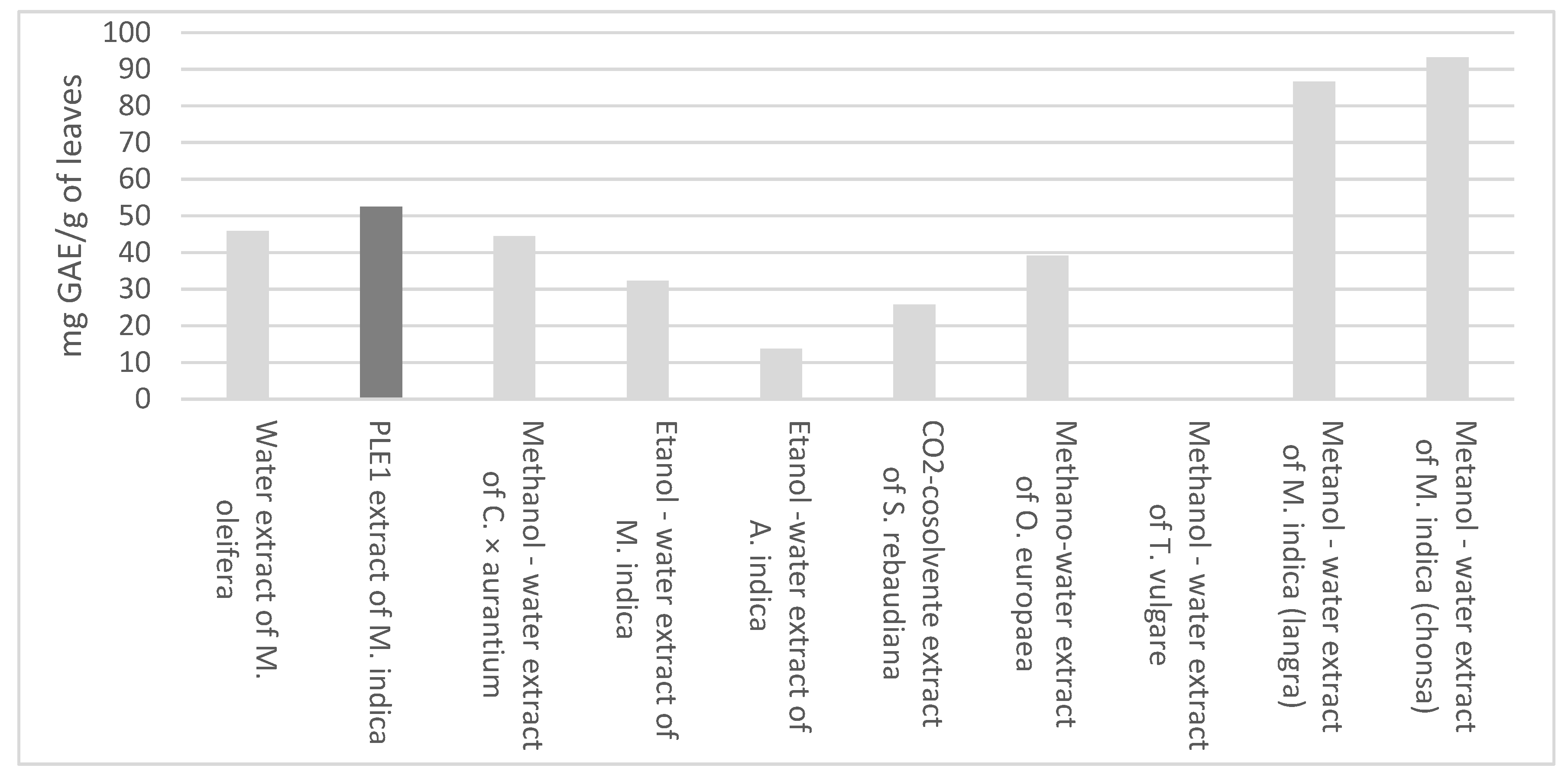
3.1. Chemical and Functional Characterization of Extract
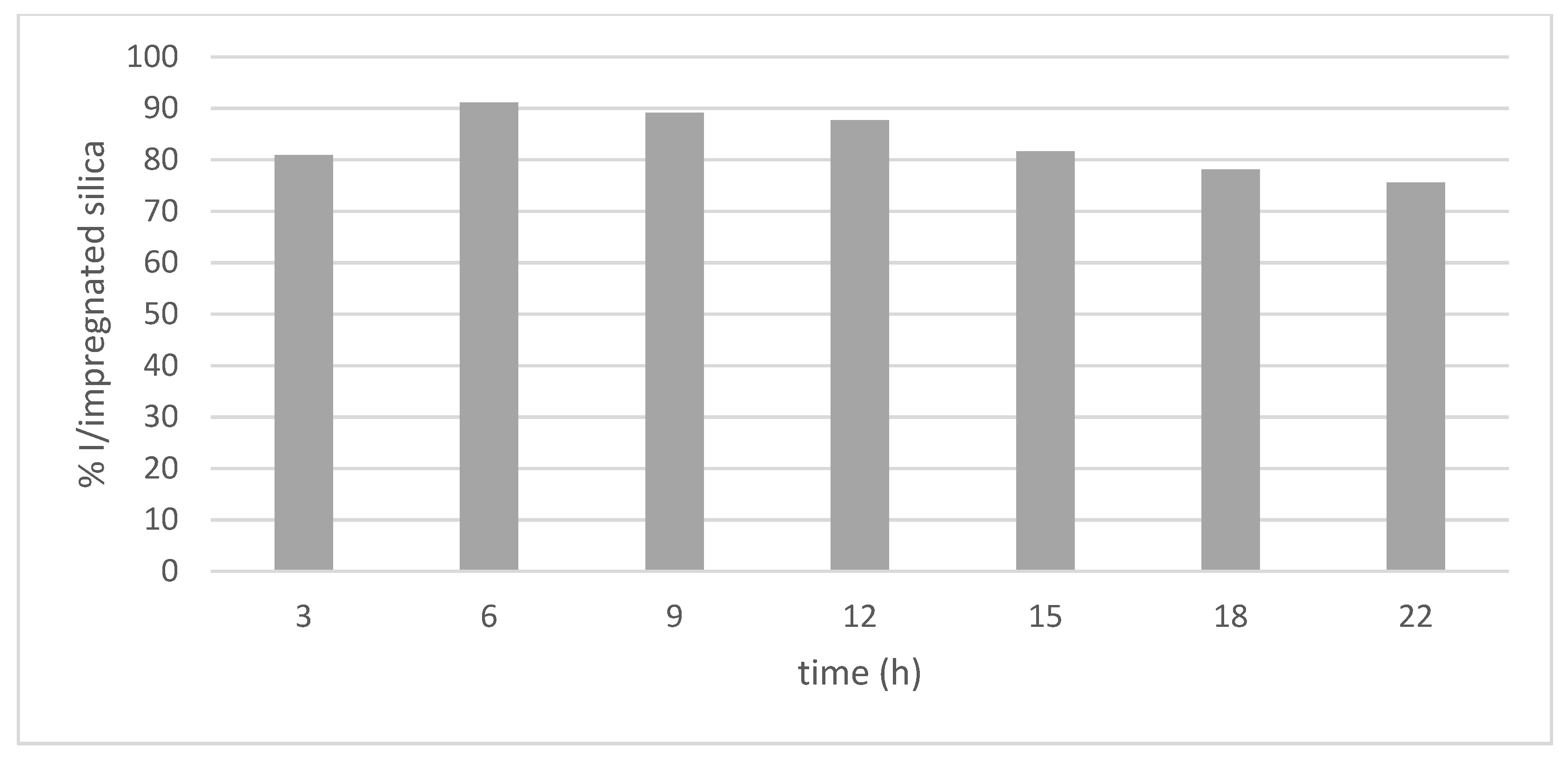
3.2. Impregnation Process—Time Influence
3.3. Impregnation Process—Pressure Influence
3.4. Impregnation Process—Concentration of the Extract and Temperature Influence
3.5. Impregnation Process—Type of Silica
3.6. Morphology of Particles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexander, E.M.; Aguiyi, J.C.; Ogwu, O.S.; Simeon, O.; Imoleayo, O.O.; Debola, O.O.; Pam, D. The in vivo Antioxidant Protective Activity of Mangifera indica Cold Aqueous Leaf Extract in Drosophila Melanogaster. J. Adv. Biol. Biotechnol. 2019, 22, 1–7. [Google Scholar] [CrossRef]
- Singh, U.P.; Singh, D.P.; Singh, M.; Maurya, S.; Srivastava, J.S.; Singh, R.B.; Singh, S.P. Characterization of phenolic compounds in some Indian mango cultivars. Int. J. Food Sci. Nutr. 2004, 55, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ponce, T.; Casas, L.; Mantell, C.; Rodríguez, M.; Martínez De La Ossa, E. Extraction of antioxidant compounds from different varieties of Mangifera indica leaves using green technologies. J. Supercrit. Fluids 2012, 72, 168–175. [Google Scholar] [CrossRef]
- Fernández-Ponce, T.; Casas, L.; Mantell, C.; Martínez De La Ossa, E. Use of high pressure techniques to produce Mangifera indica L. leaf extracts enriched in potent antioxidant phenolic compounds. Innov. Food Sci. Emerg. Technol. 2015, 29, 94–106. [Google Scholar] [CrossRef]
- Martínez, R.; Torres, P.; Meneses, M.A.; Figueroa, J.G.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, technological and in vitro antioxidant properties of mango, guava, pineapple and passion fruit dietary fibre concentrate. Food Chem. 2012, 135, 1520–1526. [Google Scholar] [CrossRef]
- Meneses, M.A.; Caputo, G.; Scognamiglio, M.; Reverchon, E.; Adami, R. Antioxidant phenolic compounds recovery from Mangifera indica L. by-products by supercritical antisolvent extraction. J. Food Eng. 2015, 163, 45–53. [Google Scholar] [CrossRef]
- Fernández-Ponce, M.T.; López-Biedma, A.; Sánchez-Quesada, C.; Casas, L.; Mantell, C.; Gaforio, J.J.; Martínez de la Ossa, E.J. Selective antitumoural action of pressurized mango leaf extracts against minimally and highly invasive breast cancer. Food Funct. 2017, 8, 3610–3620. [Google Scholar] [CrossRef]
- Abdullah, A.-S.H.; Mohammed, A.S.; Rasedee, A.; Mirghani, M.E.S. Oxidative stress-mediated apoptosis induced by ethanolic mango seed extract in cultured estrogen receptor positive breast cancer MCF-7 cells. Int. J. Mol. Sci. 2015, 16, 3528–3536. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, J.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; Martínez de la Ossa, E.J. Impregnation of mango leaf extract into a polyester textile using supercritical carbon dioxide. J. Supercrit. Fluids 2017, 128, 208–217. [Google Scholar] [CrossRef]
- Kikic, I.; Vecchione, F. Supercritical impregnation of polymers. Curr. Opin. Solid State Mater. Sci. 2003, 7, 399–405. [Google Scholar] [CrossRef]
- Belizón, M.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; Martínez De La Ossa-Fernández, E.J. Supercritical impregnation of antioxidant mango polyphenols into a multilayer PET/PP food-grade film. J. CO2 Util. 2018, 25, 56–67. [Google Scholar] [CrossRef]
- García-Casas, I.; Montes, A.; Valor, D.; Pereyra, C.; Martínez de la Ossa, E.J. Impregnation of mesoporous silica with mangiferin using supercritical CO2. J. Supercrit. Fluids 2018, 140, 129–136. [Google Scholar] [CrossRef]
- Cejudo Bastante, C.; Casas Cardoso, L.; Mantell Serrano, C.; Martínez de la Ossa, E.J. Supercritical impregnation of food packaging films to provide antioxidant properties. J. Supercrit. Fluids 2017, 128, 200–207. [Google Scholar] [CrossRef]
- García-Casas, I.; Crampon, C.; Montes, A.; Pereyra, C.; Martínez de la Ossa, E.J.; Badens, E. Supercritical CO2 impregnation of silica microparticles with quercetin. J. Supercrit. Fluids 2019, 143, 157–161. [Google Scholar] [CrossRef]
- Banchero, M.; Mohamed, S.S.Y.; Leone, F.; Lopez, F.; Ronchetti, S.; Manna, L.; Onida, B. Supercritical solvent impregnation of different drugs in mesoporous nanostructured ZnO. Pharmaceutics 2019, 11, 340. [Google Scholar] [CrossRef] [PubMed]
- Bouledjouidja, A.; Masmoudi, Y.; Van Speybroeck, M.; Schueller, L.; Badens, E. Impregnation of Fenofibrate on mesoporous silica using supercritical carbon dioxide. Int. J. Pharm. 2016, 499, 1–9. [Google Scholar] [CrossRef]
- Liu, X.; Jia, J.; Duan, S.; Zhou, X.; Xiang, A.; Lian, Z.; Ge, F. Zein/MCM-41 nanocomposite film incorporated with cinnamon essential oil loaded by modified supercritical CO2 impregnation for long-term antibacterial packaging. Pharmaceutics 2020, 12, 169. [Google Scholar] [CrossRef]
- Ushiki, I.; Takahashi, N.; Shimizu, T.; Sato, Y.; Ota, M.; Smith, R.L.; Inomata, H. Adsorption equilibria of rhodium acetylacetonate with MCM-41, MSU-H, and HMS silica substrates in supercritical carbon dioxide for preparing catalytic mesoporous materials. J. Supercrit. Fluids 2017, 120, 240–248. [Google Scholar] [CrossRef]
- Belhadj-Ahmed, F.; Badens, E.; Llewellyn, P.; Denoyel, R.; Charbit, G. Impregnation of vitamin E acetate on silica mesoporous phases using supercritical carbon dioxide. J. Supercrit. Fluids 2009, 51, 278–286. [Google Scholar] [CrossRef]
- Hunt, A.J.; Budarin, V.L.; Comerford, J.W.; Parker, H.L.; Lazarov, V.K.; Breeden, S.W.; Macquarrie, D.J.; Clark, J.H. Deposition of palladium nanoparticles in SBA-15 templated silica using supercritical carbon dioxide. Mater. Lett. 2014, 116, 408–411. [Google Scholar] [CrossRef]
- Miyoshi Europe, S.A.S. France, Surface Treated Pigments SA Treatment. Available online: https://www.Miyoshikaseigroup.Com/En/Surfacetreatment/Surfacetreatment-Type/Silicone-Type-Treatment/Sa-Treatment/(accessed on 18 July 2022).
- Guamán-Balcázar, M.C.; Montes, A.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; Pereyra, C.; Martínez de la Ossa, E. Generation of potent antioxidant nanoparticles from mango leaves by supercritical antisolvent extraction. J. Supercrit. Fluids 2018, 138, 92–101. [Google Scholar] [CrossRef]
- Scherer, R.; Godoy, H.T. Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Swain, T.; Hillis, W.E. The phenolic constituents of Prunus domestica. I.—The quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Wu, L.; Li, L.Ç.; Chen, S.; Wang, L.; Lin, X. Deep eutectic solvent-based ultrasonic-assisted extraction of phenolic compounds from Moringa oleifera L. leaves: Optimization, comparison and antioxidant activity. Sep. Purif. Technol. 2020, 247, 117014. [Google Scholar] [CrossRef]
- Wu, L.; Wu, W.; Cai, Y.; Li, C.; Wang, L. HPLC fingerprinting-based multivariate analysis of phenolic compounds in mango leaves varieties: Correlation to their antioxidant activity and in silico α-glucoidase inhibitory ability. J. Pharm. Biomed. Anal. 2020, 191, 113616. [Google Scholar] [CrossRef]
- Sreelatha, S.; Padma, P.R. Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods Hum. Nutr. 2009, 64, 303–311. [Google Scholar] [CrossRef]
- Lagha-Benamrouche, S.; Madani, K. Phenolic contents and antioxidant activity of orange varieties (Citrus sinensis L. and Citrus aurantium L.) cultivated in Algeria: Peels and leaves. Ind. Crops Prod. 2013, 50, 723–730. [Google Scholar] [CrossRef]
- Shewale, S.; Rathod, V.K. Extraction of total phenolic content from Azadirachta indica or (neem) leaves: Kinetics study. Prep. Biochem. Biotechnol. 2018, 48, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Ameer, K.; Chun, B.S.; Kwon, J.H. Optimization of supercritical fluid extraction of steviol glycosides and total phenolic content from Stevia rebaudiana (Bertoni) leaves using response surface methodology and artificial neural network modeling. Ind. Crop. Prod. 2017, 109, 672–685. [Google Scholar] [CrossRef]
- Şahin, S.; Elhussein, E.; Bilgin, M.; Lorenzo, J.M.; Barba, F.J.; Roohinejad, S. Effect of drying method on oleuropein, total phenolic content, flavonoid content, and antioxidant activity of olive (Olea europaea) leaf. J. Food Process. Preserv. 2018, 42, e13604. [Google Scholar] [CrossRef]
- Sultana, B.; Hussain, Z.; Asif, M.; Munir, A. Investigation on the Antioxidant Activity of Leaves, Peels, Stems Bark, and Kernel of Mango (Mangifera indica L.). J. Food Sci. 2012, 77, 849–852. [Google Scholar] [CrossRef]
- Guamán-Balcázar, M.; Montes, A.; Pereyra, C.; Martínez de la Ossa, E. Precipitation of mango leaves antioxidants by supercritical antisolvent process. J. Supercrit. Fluids 2017, 128, 218–226. [Google Scholar] [CrossRef]
- Barrande, M.; Bouchet, R.; Denoyel, R. Tortuosity of Porous Particles. Anal. Chem. 2007, 79, 9115–9121. [Google Scholar] [CrossRef]
- Murga, R.; Sanz, M.T.; Beltrán, S.; Cabezas, J.L. Solubility of some phenolic compounds contained in grape seeds, in supercritical carbon dioxide. J. Supercrit. Fluids 2002, 23, 113–121. [Google Scholar] [CrossRef]
- Dao, D.S.; Yamada, H.; Yogo, K. Large-pore mesostructured silica impregnated with blended amines for CO2 Capture. Ind. Eng. Chem. Res. 2013, 52, 13810–13817. [Google Scholar] [CrossRef]
- Al-Oweini, R.; El-Rassy, H. Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si(OR)4 and R00Si(OR0)3 precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]
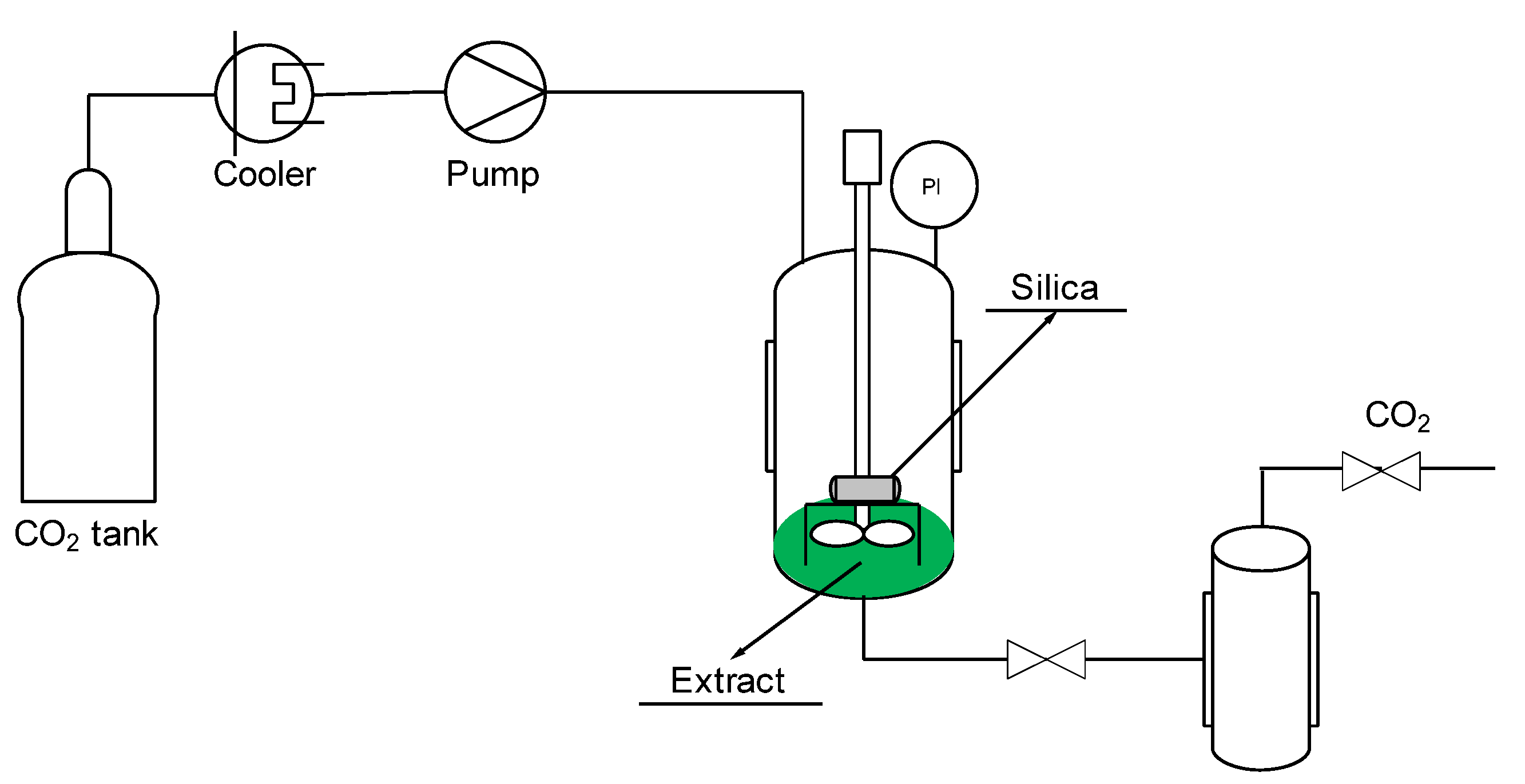
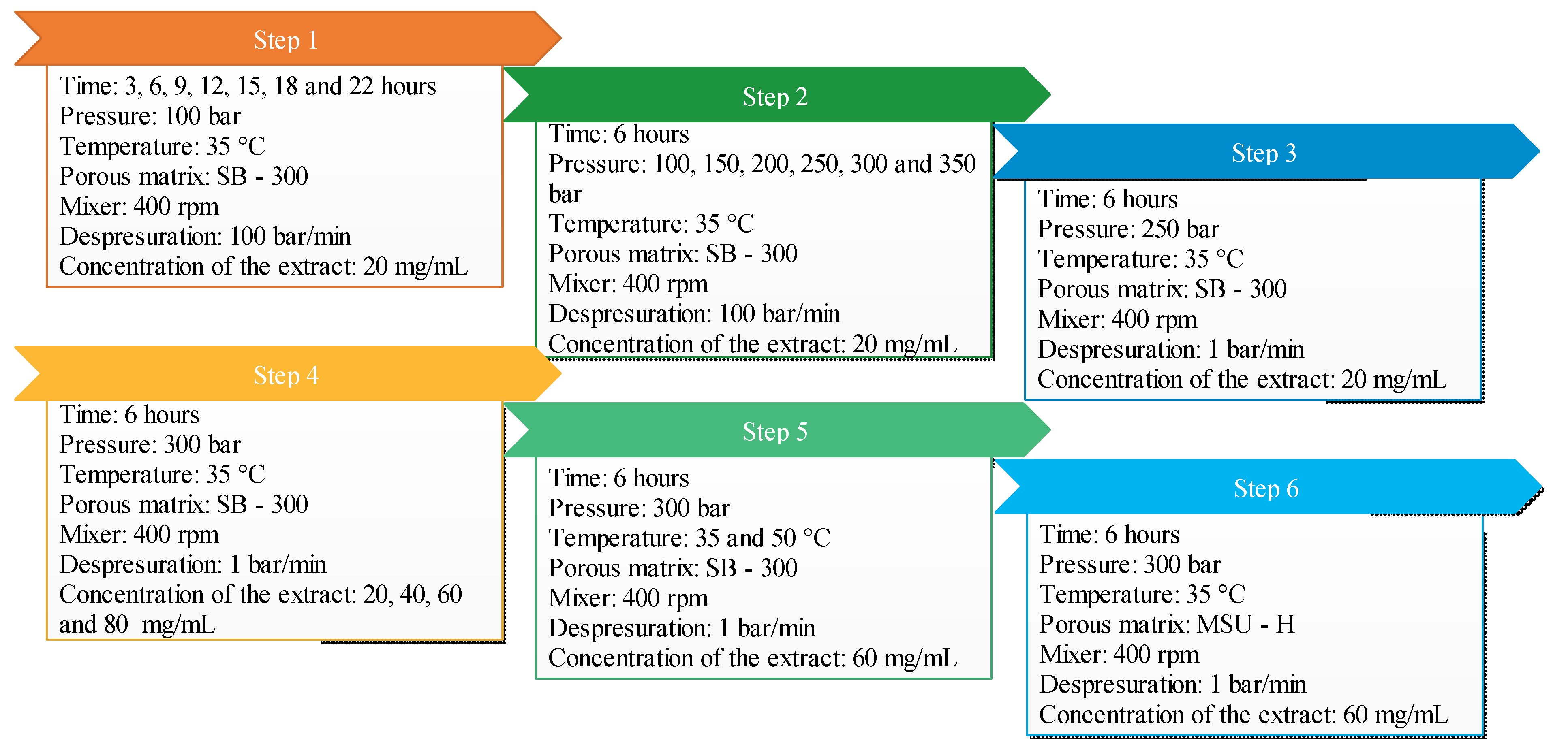

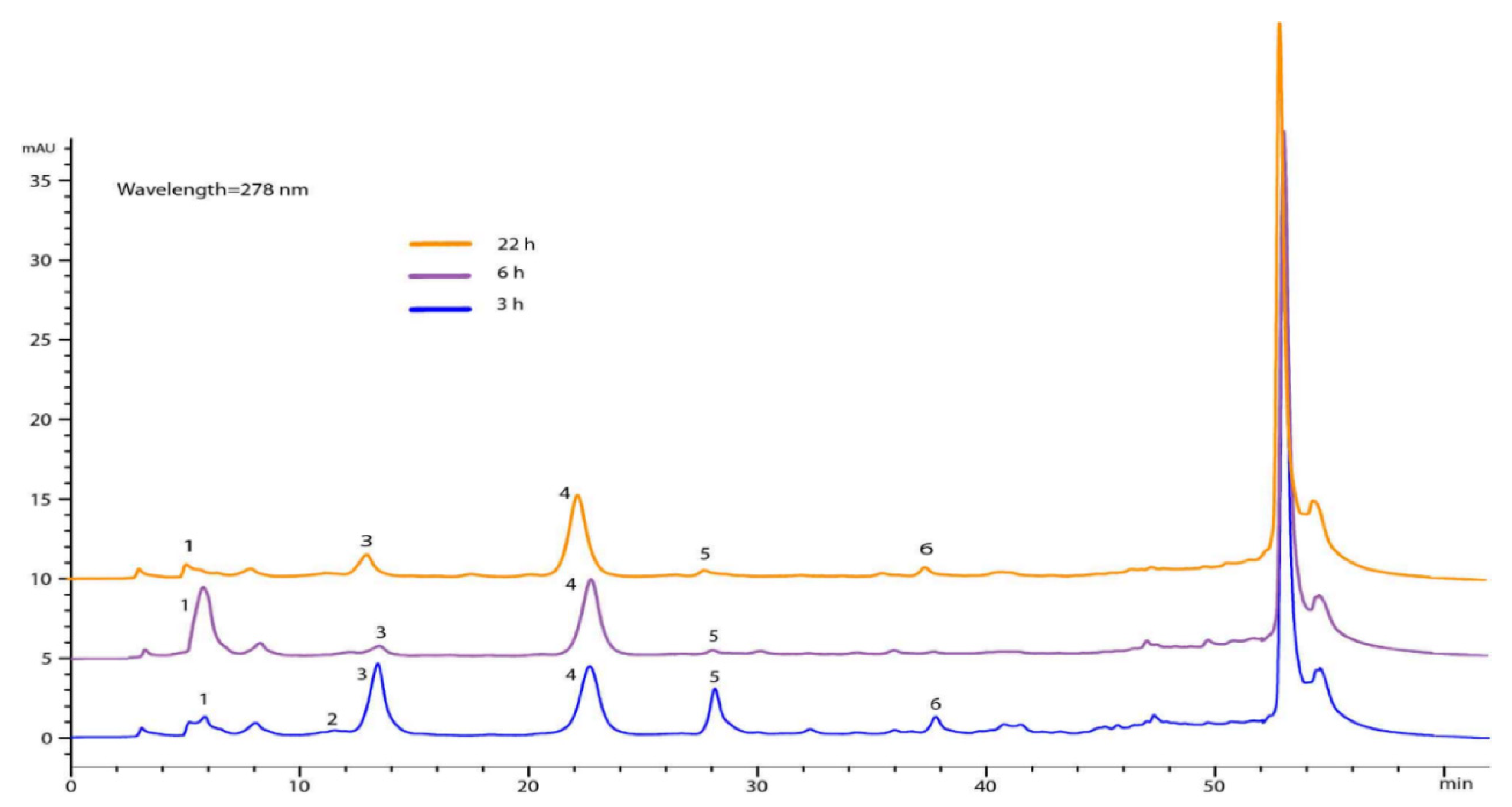


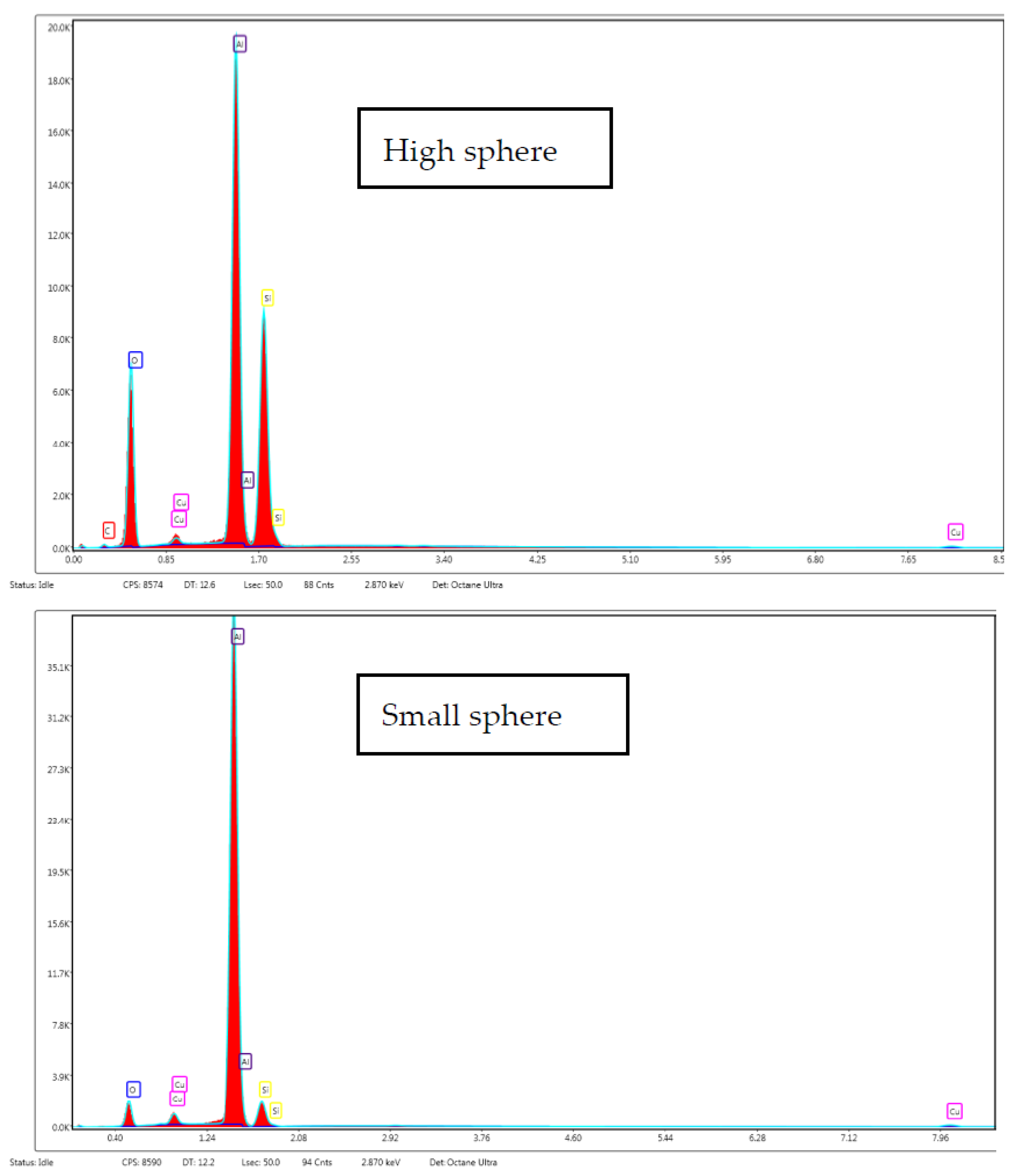
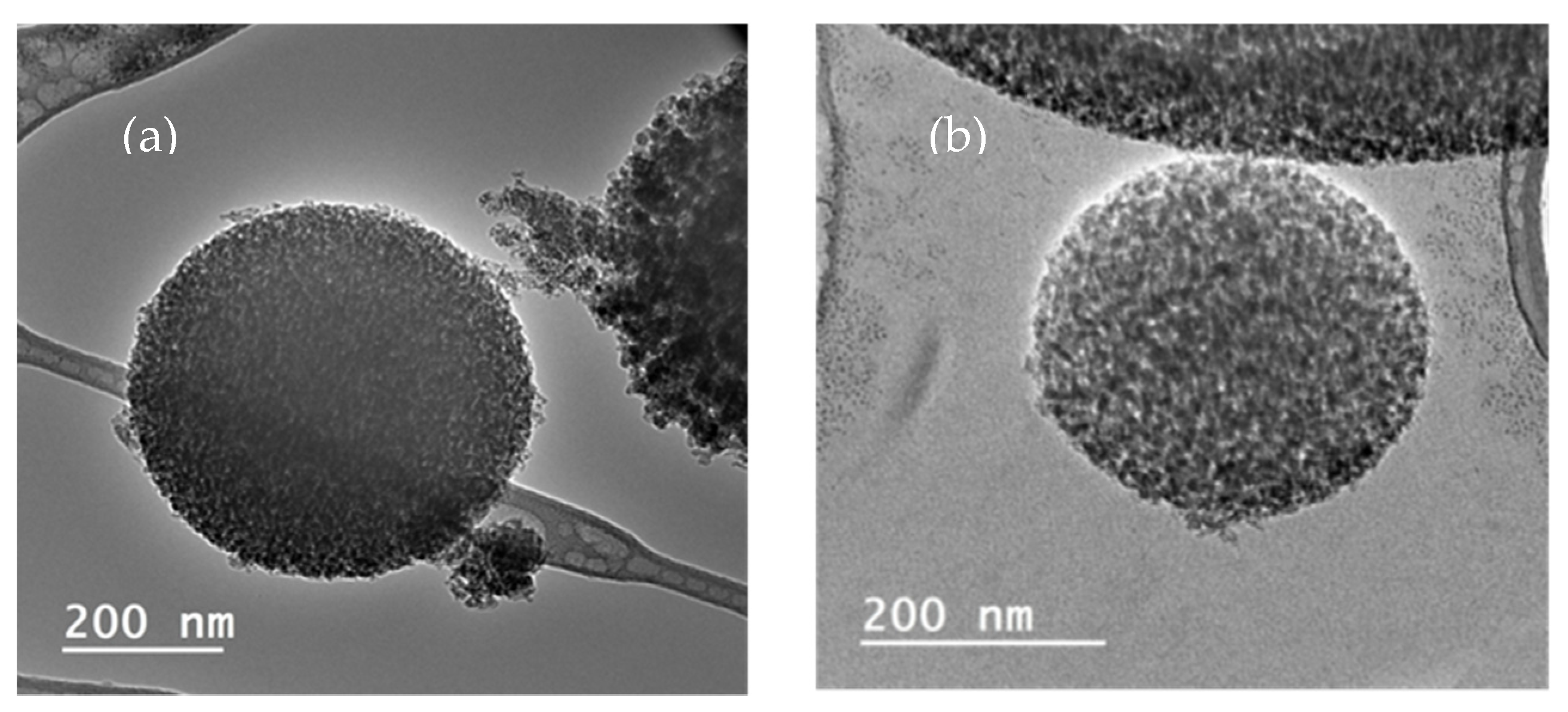
| Experiment | Time of Impregnation (h) | Pressure (bar) | Temperature (°C) | Concentration of Extract (mg/mL) | Porous Matrix |
|---|---|---|---|---|---|
| 1 | 3 | 100 | 35 | 20 | Silica SB-300 |
| 2 | 6 | 100 | 35 | 20 | Silica SB-300 |
| 3 | 9 | 100 | 35 | 20 | Silica SB-300 |
| 4 | 12 | 100 | 35 | 20 | Silica SB-300 |
| 5 | 15 | 100 | 35 | 20 | Silica SB-300 |
| 6 | 18 | 100 | 35 | 20 | Silica SB-300 |
| 7 | 22 | 100 | 35 | 20 | Silica SB-300 |
| 8 | 6 | 150 | 35 | 20 | Silica SB-300 |
| 9 | 6 | 200 | 35 | 20 | Silica SB-300 |
| 10 | 6 | 250 | 35 | 20 | Silica SB-300 |
| 11 * | 6 | 250 | 35 | 20 | Silica SB-300 |
| 11 | 6 | 300 | 35 | 20 | Silica SB-300 |
| 12 | 6 | 350 | 35 | 20 | Silica SB-300 |
| 13 | 6 | 300 | 35 | 40 | Silica SB-300 |
| 14 | 6 | 300 | 35 | 60 | Silica SB-300 |
| 15 | 6 | 300 | 35 | 80 | Silica SB-300 |
| 16 | 6 | 300 | 50 | 60 | MSU-H |
| 17 | 6 | 300 | 35 | 60 | MSU-H |
| Yield % | Total Phenol mg GAE/g Extract | AAI µg DPPH/µg Extract | FRAP µmol TE/g Extract | DPPH µmol TE/g Extract |
|---|---|---|---|---|
| 18.75 | 279.53 ± 3.27 | 2.58 ± 0.21 | 1898.78 ± 42.33 | 2637.46 ± 00.00 |
| Gallic Acid | Iriflophenone 3-C-β-D-Glucoside | Iriflophenone 3-C-(2-O-p-Hydroxybenzoyl)-β-D-Glucoside | Mangiferin |
|---|---|---|---|
| 5.84 ± 0.02 | 12.08 ± 0.01 | 5.53 ± 0.06 | 7.51 ± 0.09 |
| Iriflophenone-3-C-(2-O-galloryl)-β-D-glucoside | Quercetin 3-D-galactoside | Quercetin 3-β-D-glucoside | Quercetin-3-O-xyloside |
| 0.45 ± 0.02 | 0.69 ± 0.01 | NQ | 0.27 ± 0.02 |
| Quercetin-3-O-a-L arabinopyranoside | 1,2,3,4,6-penta-O-galloryl-β-D-glucose | Quercetin (Aglycone) | |
| 0.18 ± 0.07 | 0.14 ± 0.02 | 0.06 ± 0.03 | |
| mg Phenolic compounds/g of extract | |||
| N° | Matrix | P (Bar) | Ce (mg/mL) | T (°C) | Phenolic Compounds mg/L | |
|---|---|---|---|---|---|---|
| Gallic Acid | Iriflophenone 3-C-(2-O-P-Hydroxybenzoyl)-β-D-Glucoside | |||||
| 1 | SB-300 | 100 | 20 | 35 | 0.83 ± 0.01 ab | 1.68 ± 0.03 a |
| 2 | 150 | 20 | 35 | NQ | 4.68 ± 0.53 bd | |
| 3 | 200 | 20 | 35 | 0.74 ± 0.01 ab | 8.25 ± 0.10 c | |
| 5 | 250 * | 20 | 35 | 0.18 ± 0.04 c | 6.22 ± 0.10 bd | |
| 6 | 300 | 20 | 35 | 1.55 ± 0.33 d | 15.87 ± 1.21 e | |
| N° | Ce (mg/mL) | T (°C) | Silica | AAI µg (DPPH/µg Antioxidant) | IC50 (µg Antioxidant/mL) | DPPH (µmol TE/g Silica) | FRAP (µmol TE/g Silica) | TP (mg GAE/g of Silica) | Gallic Acid (mg/L) | Iriflophenone 3-C-(2-O-P-HyDroxybenzoyl)-β-D-Glucoside (mg/L) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 20 | 35 | SB-300 | 0.30 ± 0.01 ae | 77.21 ± 1.36 a | ND | ND | ND | 1.55 ± 0.33 a | 15.87 ± 1.21 a |
| 2 | 40 | 35 | SB-300 | 0.60 ± 0.03 b | 35.53 ± 1.66 b | ND | ND | ND | 0.45 ± 0.13 b | 15.79 ± 3.18 a |
| 3 | 60 | 35 | SB-300 | 0.74 ± 0.01 cd | 28.64 ± 0.39 c | ND | ND | ND | 2.52 ± 0.18 c | 35.62 ± 0.19 b |
| 4 | 80 | 35 | SB-300 | 0.44 ± 0.05 be | 54.16 ± 5.97 c | 4.63 ± 0.11 | 43.04 ± 0.07 | 2.51 ± 0.00 | 4.54 ± 0.02 d | 44.06 ± 0.28 c |
| 5 | 60 | 50 | SB-300 | 0.37 ± 0.00 ae | 65.67 ± 0.42 ac | ND | ND | ND | 2.73 ± 0.14 c | 30.85 ± 0.39 d |
| 6 | 60 | 35 | MSU-H | 1.05 ± 0.13 d | 17.28 ± 1.64 b | 1.80 ± 0.08 | 62.27 ± 1.75 | 4.32 ± 0.95 | 7.08 ± 0.25 e | 83.33 ± 0.46 e |
| 7 | 60 | 50 | MSU-H | 0.15 ± 0.02 e | 140.11 ± 15.28 d | ND | ND | ND | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guamán-Balcázar, M.d.C.; Montes, A.; Valor, D.; Coronel, Y.; De los Santos, D.M.; Pereyra, C.; Martínez de la Ossa, E.J. Inclusion of Natural Antioxidants of Mango Leaves in Porous Ceramic Matrices by Supercritical CO2 Impregnation. Materials 2022, 15, 5934. https://doi.org/10.3390/ma15175934
Guamán-Balcázar MdC, Montes A, Valor D, Coronel Y, De los Santos DM, Pereyra C, Martínez de la Ossa EJ. Inclusion of Natural Antioxidants of Mango Leaves in Porous Ceramic Matrices by Supercritical CO2 Impregnation. Materials. 2022; 15(17):5934. https://doi.org/10.3390/ma15175934
Chicago/Turabian StyleGuamán-Balcázar, María del Cisne, Antonio Montes, Diego Valor, Yorky Coronel, Desireé M. De los Santos, Clara Pereyra, and Enrique J. Martínez de la Ossa. 2022. "Inclusion of Natural Antioxidants of Mango Leaves in Porous Ceramic Matrices by Supercritical CO2 Impregnation" Materials 15, no. 17: 5934. https://doi.org/10.3390/ma15175934
APA StyleGuamán-Balcázar, M. d. C., Montes, A., Valor, D., Coronel, Y., De los Santos, D. M., Pereyra, C., & Martínez de la Ossa, E. J. (2022). Inclusion of Natural Antioxidants of Mango Leaves in Porous Ceramic Matrices by Supercritical CO2 Impregnation. Materials, 15(17), 5934. https://doi.org/10.3390/ma15175934








