Multicomponent Low Initial Molar Ratio of SiO2/Al2O3 Geopolymer Mortars: Pilot Research
Abstract
:1. Introduction
2. Methods and Materials
2.1. Methods
2.1.1. XRF: X-ray Fluorescence Method
2.1.2. Grain Size Distribution
2.1.3. Thermal Analysis (DTA/DTG/TA)
2.1.4. Strength Test
2.1.5. Leaching Test
2.1.6. Total Carbon and Sulphur Examination
2.1.7. SEM (Scanning Electron Microscopy) Examination
2.1.8. XRD: X-ray Diffraction
2.2. Characterisation of Components
2.2.1. PVG: PV Glass
2.2.2. KC: Kaolinite Clay
2.2.3. ALC: Alumina-Lime Cement
2.2.4. GGBFS: Ground Granulated Blast Furnace Slag
2.2.5. ACC: Autoclaved Cellular Concrete
2.2.6. FAB: Fly Ash from Biomass Combustion
2.2.7. FAC: Fly Ash from Coal Combustion
2.2.8. Standard Sand
2.2.9. Alkaline-Activating Solution
2.3. Selection of Ingredients Proportions, Preparing Mixtures
3. Results with Analysis
3.1. Composition Analysis
3.2. Strength Tests
3.3. Leaching Test
3.4. SEM Examination
3.5. XRD Examination—Crystalline Phases
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weckend, S.; Wade, A.; Heath, G. End-of-Life Management Solar Photovoltaic Panels. IRENA and IEA-PVPS. Available online: https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2016/IRENA_IEAPVPS_End-of-Life_Solar_PV_Panels_2016.pdf (accessed on 5 July 2016).
- IRENA. Future of Solar Photovoltaic: Deployment, Investment, Technology, Grid Integration and Socio-Economic Aspects (A Global Energy Transformation: Paper); International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2019; Available online: https://irena.org/-/media/Files/IRENA/Agency/Publication/2019/Nov/IRENA_Future_of_Solar_PV_2019.pdf (accessed on 6 January 2020).
- The International Renewable Energy Agency (IRENA). Future of Solar Photovoltaic. Deployment, Investment, Technology, Grid Integration and Socio-Economic Aspects. Available online: https://irena.org/Search?keywords=pv%20end%20of%20life (accessed on 6 January 2020).
- European Commission. Set of Documents. Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en (accessed on 11 December 2019).
- Industrial Decarbonisation Strategy. Available online: https://www.gov.uk/government/publications/industrial-decarbonisation-strategy (accessed on 17 March 2021).
- The Long-Term Strategy of the United States: Pathways to Net-Zero Greenhouse Gas Emissions by 2050; United States Department of State and the United States Executive Office of the President: Washington, DC, USA, 2021. Available online: https://www.whitehouse.gov/wp-content/uploads/2021/10/US-Long-Term-Strategy.pdf (accessed on 27 September 2021).
- Onyango, S. African Countries Are Joining the World in Decarbonising, but Is this Fair? Available online: https://www.theafricareport.com/131724/african-countries-are-joining-the-world-in-decarbonising-but-is-this-fair/ (accessed on 30 September 2021).
- Japan 2021 Energy Policy Review, International Energy Agency. Available online: https://www.iea.org/reports/japan-2021 (accessed on 4 August 2021).
- EU-Japan Centre for Industrial Cooperation. Green Technology. Set of Documents. Available online: https://www.eu-japan.eu/tags/green-technology (accessed on 21 July 2021).
- Lee, H.; Schrag, D. The Reforms Needed for ‘Deep Decarbonisation’ in China. Available online: https://chinadialogue.net/en/climate/the-reforms-needed-for-deep-decarbonisation-in-china/ (accessed on 7 April 2022).
- Yum, W.S.; Yu, J.; Jeon, D.; Song, H.; Sim, S.; Kim, D.H.; Oh, J.E. Mechanical and Durability Properties of Cementless Concretes Made Using Three Types of CaO-Activated GGBFS Binders. Materials 2022, 15, 271. [Google Scholar] [CrossRef] [PubMed]
- Parcesepe, E.; De Masi, R.F.; Lima, C.; Mauro, G.M.; Maddaloni, G.; Pecce, M.R. Experimental Evaluation of the Mechanical Strengths and the Thermal Conductivity of GGBFS and Silica Fume Based Alkali-Activated Concrete. Materials 2021, 14, 7717. [Google Scholar] [CrossRef] [PubMed]
- Słomka-Słupik, B. Self-Immobilizing Metals Binder for Construction Made of Activated Metallurgical Slag, Slag from Lignite Coal Combustion and Ash from Biomass Combustion. Materials 2021, 14, 3101. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, T.; Lee, S.; Choi, H. Performance Evaluation of Cementless Composites with Alkali-Sulfate Activator for Field Application. Materials 2020, 13, 5410. [Google Scholar] [CrossRef]
- Kwon, Y.-H.; Kang, S.-H.; Hong, S.-G.; Moon, J. Acceleration of Intended Pozzolanic Reaction under Initial Thermal Treatment for Developing Cementless Fly Ash Based Mortar. Materials 2017, 10, 225. [Google Scholar] [CrossRef]
- Park, H.; Jeong, Y.; Jeong, J.-H.; Oh, J.E. Strength Development and Hydration Behavior of Self-Activation of Commercial Ground Granulated Blast-Furnace Slag Mixed with Purified Water. Materials 2016, 9, 185. [Google Scholar] [CrossRef]
- Siddika, A.; Hajimohammadi, A.; Mamun, M.A.A.; Alyousef, R.; Ferdous, W. Waste Glass in Cement and Geopolymer Concretes: A Review on Durability and Challenges. Polymers 2021, 13, 2071. [Google Scholar] [CrossRef]
- Provis, J.L.; Deventer, J.S.J. Geopolymers. Structures, Processing, Properties and Industrial Applications; A Volume in Woodhead Publishing Series in Civil and Structural Engineering; Woodhead Publishing: Sawston, UK, 2009. [Google Scholar] [CrossRef]
- Singh, N.B. 19-Fly Ash in the Construction Industry. In Handbook of Fly Ash; Kamal, K.K., Ed.; Butterworth-Heinemann: Oxford, UK, 2022; pp. 565–610. [Google Scholar] [CrossRef]
- Gao, X.; Yao, X.; Xie, R.; Li, X.; Cheng, J.; Yang, T. Performance of fly ash-based geopolymer mortars with waste cathode ray tubes glass fine aggregate: A comparative study with cement mortars. Constr. Build. Mater. 2022, 344, 128243. [Google Scholar] [CrossRef]
- Long, W.-J.; Zhang, X.; Xie, J.; Kou, S.; Luo, Q.; Wei, J.; Lin, C.; Feng, G.-L. Recycling of waste cathode ray tube glass through fly ash-slag geopolymer mortar. Constr. Build. Mater. 2022, 322, 126454. [Google Scholar] [CrossRef]
- Yoo, D.-Y.; Lee, S.K.; You, I.; Oh, T.; Lee, Y.; Zi, G. Development of strain-hardening geopolymer mortar based on liquid-crystal display (LCD) glass and blast furnace slag. Constr. Build. Mater. 2022, 331, 127334. [Google Scholar] [CrossRef]
- Derinpinar, A.N.; Karakoç, M.B.; Özcan, A. Performance of glass powder substituted slag based geopolymer concretes under high temperature. Constr. Build. Mater. 2022, 331, 127318. [Google Scholar] [CrossRef]
- Si, R.; Guo, S.; Dai, Q.; Wang, J. Atomic-structure, microstructure and mechanical properties of glass powder modified metakaolin-based geopolymer. Constr. Build. Mater. 2020, 254, 119303. [Google Scholar] [CrossRef]
- Dadsetan, S.; Siad, H.; Lachemi, M.; Sahmaran, M. Extensive evaluation on the effect of glass powder on the rheology, strength, and microstructure of metakaolin-based geopolymer binders. Constr. Build. Mater. 2021, 268, 121168. [Google Scholar] [CrossRef]
- Toniolo, N.; Rincón, A.; Roether, J.A.; Ercole, P.; Bernardo, E.; Boccaccini, A.R. Extensive reuse of soda-lime waste glass in fly ash-based geopolymers. Constr. Build. Mater. 2018, 188, 1077–1084. [Google Scholar] [CrossRef]
- Redden, R.; Neithalath, N. Microstructure, strength, and moisture stability of alkali activated glass powder-based binders. Cem. Concr. Compos. 2014, 45, 46–56. [Google Scholar] [CrossRef]
- Toniolo, N.; Boccaccini, A.R. Fly ash-based geopolymers containing added silicate waste. A review. Ceram. Int. 2017, 43, 14545–14551. [Google Scholar] [CrossRef]
- Zhao, J.; Tong, L.; Li, B.; Chen, T.; Wang, C.; Yang, G.; Zheng, Y. Eco-friendly geopolymer materials: A review of performance improvement, potential application and sustainability assessment. J. Clean. Prod. 2021, 307, 127085. [Google Scholar] [CrossRef]
- Nikolov, A.; Rostovsky, I.; Nugteren, H. Geopolymer materials based on natural zeolite. Case Stud. Constr. Mater. 2017, 6, 198–205. [Google Scholar] [CrossRef]
- Skripkiūnas, G.; Vasarevičius, S.; Danila, V. Immobilization of copper indium selenide solar module waste in concrete constructions. Cem. Concr. Compos. 2018, 85, 174–182. [Google Scholar] [CrossRef]
- Máčalová, K.; Václavík, V.; Dvorský, T.; Figmig, R.; Charvát, J.; Lupták, M. The Use of Glass from Photovoltaic Panels at the End of Their Life Cycle in Cement Composites. Materials 2021, 14, 6655. [Google Scholar] [CrossRef]
- Mariaková, D.; Jirkalová, Z.; Řepka, J.; Vlach, T.; Hájek, P. Utilization of photovoltaic panels waste glass in high-performance concrete. AIP Conf. Proc. 2021, 2322, 020020. [Google Scholar] [CrossRef]
- Stehlík, M.; Knapová, J.; Kostka, V. Possibilities of Use of Glass Recyclate from Photovoltaic Panels for Concrete Masonry Units. In IOP Conference Series: Materials Science and Engineering, Proceedings of the XXV International Conference and Meeting of Departments, CONSTRUMAT 2019, Zuberec, Slovakia, 29–31 May 2019; Volume 549, p. 012006. Available online: https://iopscience.iop.org/article/10.1088/1757-899X/549/1/012006 (accessed on 10 May 2019).
- Hao, H.; Lin, K.-L.; Wang, D.Y.; Chao, S.-J.; Shiu, H.-S.; Cheng, Y.-W.; Hwang, C.-L. Elucidating characteristics of geopolymer with solar panel waste glass. Environ. Eng. Manag. J. 2015, 14, 79–87. Available online: http://www.eemj.icpm.tuiasi.ro/pdfs/vol14/no1/10_49_Hao_12.pdf (accessed on 15 February 2015).
- Khale, D.; Chaudhary, R. Mechanism of geopolymerization and factors influencing its development: A review. J. Mater. Sci. 2007, 42, 729–746. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Bartzas, G. Effect of sulphate and nitrate anions on heavy metal immobilisation in ferronickel slag geopolymers. Appl. Clay Sci. 2013, 73, 103–109. [Google Scholar] [CrossRef]
- Xu, J.Z.; Zhou, Y.L.; Chang, Q.; Qu, H.Q. Study on the factors of affecting the immobilization of heavy metals in fly ash-based geopolymers. Mater. Lett. 2006, 60, 820–822. [Google Scholar] [CrossRef]
- Palomo, A.; Palacios, M. Alkali-activated cementitious materials: Alternative matrices for the immobilisation of hazardous wastes: Part II. Stabilisation of chromium and lead. Cem. Con. Res. 2003, 33, 289–295. [Google Scholar] [CrossRef]
- Medina, T.J.; Arredondo, S.P.; Corral, R.; Jacobo, A.; Zárraga, R.A.; Rosas, C.A.; Cabrera, F.G.; Bernal, J.M. Microstructure and Pb2+ Adsorption Properties of Blast Furnace Slag and Fly Ash based Geopolymers. Minerals 2020, 10, 808. [Google Scholar] [CrossRef]
- Ariffin, N.; Abdullah, M.M.; Zainol, R.R.; Murshed, M.F. Geopolymer as an Adsorbent of Heavy Metal: A Review. In Proceedings of the AIP Conference Proceedings, Krabi, Thailand, 29–30 April 2017; Volume 1885, p. 020030. [Google Scholar] [CrossRef]
- Deja, J. Immobilization of Cr6+, Cd2+, Zn2+ and Pb2+ in alkali-activated slag binders. Cem. Concr. Res. 2002, 32, 1971–1977. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.G.S.; Van Deventer, J.S.J.; Lorenzen, L. The potential use of geopolymeric materials to immobilise toxic metals: Part I. Theory and applications. Miner. Eng. 1997, 10, 659–669. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Davitovits, J.; Davitovits, M.; Davitovits, N. Process for Obtaining a Geopolymericalumno-Slicate and Products thus Obtained. US Patent No. 5,342,595, 30 August 1994. Available online: https://patentimages.storage.googleapis.com/1d/3e/be/2fa9a5ebece332/US5342595.pdf (accessed on 25 June 2017).
- Davidovits, J. Geopolymers of the first generation: SILIFACE-Process, Geopolymer 88. In Proceedings of the First European Conference on Soft Mineralurgy, Compiegne, France, 1–3 June 1988; Volume 1, pp. 49–67. [Google Scholar]
- Glukhovsky, V.D.; Rostovskaja, G.S.; Rumyna, G.V. High strength slag-alkaline cements. In Proceedings of the Seventh International Congress on the Chemistry of Cement, Paris, France, 30 June–5 July 1980; Volume 3, pp. 164–168. [Google Scholar]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the composition and application of biomass ash. Part 1. Phase–mineral and chemical composition and classification. Fuel 2013, 105, 40–76. [Google Scholar] [CrossRef]
- Demirbaş, A. Sustainable cofiring of biomass with coal. Energy Convers. Manag. 2003, 44, 1465–1479. [Google Scholar] [CrossRef]
- Palomo, A.; Grutzeck, M.W.; Blanco, M.T. Alkali-activated fly ashes: A cement for the future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Khalifa, A.Z.; Cizer, Ö.; Pontikes, Y.; Heath, A.; Patureau, P.; Bernal, S.A.; Marsh, A.T.M. Advances in alkali-activation of clay minerals. Cem. Concr. Res. 2020, 132, 106050. [Google Scholar] [CrossRef]
- Pytel, Z.; Małolepszy, J. Influence of Kaolin Clay Roasting Conditions on Its Pozzolanic Properties (In Polish, Wpływ Warunków Prażenia Gliny Kaolinowej Na Jej Własności Pucolanowe). In Proceedings of the II Scientific and Technical Conference on Materials Issues in Civil Engineering MATBUD ’98, Kraków, Poland, 17–19 June 1998; Available online: https://www.researchgate.net/publication/298784978_WPLYW_WARUNKOW_PRAZENIA_GLINY_KAOLINOWEJ_NA_JEJ_WLASNOSCI_PUCOLANOWE (accessed on 21 July 2022).
- Lemougna, P.N.; Wang, K.; Tang, Q.; Melo, U.C.; Cui, X. Recent developments on inorganic polymers synthesis and applications. Ceram. Int. 2016, 42, 15142–15159. [Google Scholar] [CrossRef]
- Medri, V.; Fabbri, S.; Dedecek, J.; Sobalik, Z.; Tvaruzkova, Z.; Vaccari, A. Role of the morphology and the dehydroxylation of metakaolins on geopolymerization. Appl. Clay Sci. 2010, 50, 538–545. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.; Li, L. A review: The comparison between alkali-activated slag (Si+Ca) and metakaolin (Si+Al) cements. Cem. Concr. Res. 2010, 40, 1341–1349. [Google Scholar] [CrossRef]
- Yip, C.K.; Lukey, G.C.; van Deventer, J.S.J. The coexistence of geopolymeric gel and calcium silicate hydrate at the early stage of alkaline activation. Cem. Concr. Res. 2005, 35, 1688–1697. [Google Scholar] [CrossRef]
- Mozgawa, W.; Deja, J. Spectroscopic studies of alkaline activated slag geopolymers. J. Mol. Struct. 2009, 924–926, 434–441. [Google Scholar] [CrossRef]
- Shilar, F.A.; Ganachari, S.V.; Patil, V.B.; Khan, M.Y.; Khadar, S.D.A. Molarity activity effect on mechanical and microstructure properties of geopolymer concrete: A review. Case Stud. Constr. Mater. 2022, 16, e01014. [Google Scholar] [CrossRef]
- Gołek, Ł.; Deja, J. The hydration conditions of aluminosilicate glasses and the usable properties and phase composition of hardened pates. In Proceedings of the conference Dni Betonu, Wisła, Poland, 13–15 September 2008; Available online: https://www.dnibetonu.com/wp-content/pdfs/2008/golek_deja.pdf (accessed on 24 September 2008).
- Chi, M.; Huang, R. Binding mechanism and properties of alkali-activated fly ash/slag mortars. Constr. Build. Mater. 2013, 40, 291–298. [Google Scholar] [CrossRef]
- Buchwald, A.; Hilbig, H.; Kaps, C. Alkali-activated metakaolin-slag blends—performance and structure in dependence of their composition. J. Mater. Sci. 2007, 42, 3024–3032. [Google Scholar] [CrossRef]
- Swanepoel, J.C.; Strydom, C.A. Utilisation of fly ash in a geopolymeric material. Appl. Geochem. 2002, 17, 1143–1148. [Google Scholar] [CrossRef]
- Lombardi, F.; Mangialardi, T.; Piga, L.; Sirini, P. Mechanical and leaching properties of cement solidified hospital solid waste incinerator fly ash. Waste Manag. 1998, 18, 99–106. [Google Scholar] [CrossRef]
- Juengsuwattananon, K.; Winnefeld, F.; Chindaprasirt, P.; Pimraksa, K. Correlation between initial SiO2/Al2O3, Na2O/Al2O3, Na2O/SiO2 and H2O/Na2O ratios on phase and microstructure of reaction products of metakaolin-rice husk ash geopolymer. Constr. Build. Mater. 2019, 226, 406–417. [Google Scholar] [CrossRef]
- PN-EN ISO 12677:2011; Chemical Analysis of Refractory Products By X-ray Fluorescence (XRF)-Fused Cast-Bead Method. PKN: Warsaw, Poland, 2011.
- ISO 13320:2009; Particle Size Analysis-Laser Diffraction Methods. Publication Date: 2009-10. Technical Committee: ISO/TC 24/SC 4 Particle Characterization. ICS: 19.120 Particle Size Analysis. Sieving. ISO: Geneva, Switzerland, 2009. Available online: https://www.iso.org/standard/44929.html (accessed on 6 January 2020).
- PN-EN 196-1:2016-07; Metody Badania Cementu-Część 1: Oznaczanie Wytrzymałości (Cement Test Methods-Part 1: Determination of Strength). PKN: Warsaw, Poland, 2018.
- PN-EN 12457-2:2006; Polish Version, Characterization of Waste-Leaching-Compliance Test for Leaching of Granular Waste Materials and Sludge-Part 2: One-Stage Batch Test with a Liquid/Solid Ratio of 10 L/kg for Materials with a Particle Size of less than 4 mm (no Reduction or with Size Reduction. PKN: Warsaw, Poland, 2006.
- PN-EN 12457-2:2006; Polish Version, Characterization of Waste-Leaching-Compliance Test for Leaching of Granular Waste Materials and Sludge-Part 4: One-Stage Batch Test with a Liquid/Solid Ratio of 10 L/kg for Materials with a Particle Size of less than 10 mm (no Reduction or with Size Reduction. PKN: Warsaw, Poland, 2006.
- PN-EN ISO 11885:2009; English Version, Water Quality-Determination of Selected Elements by Inductively Induced Plasma Optical Emission Spectrometry (ICP-OES). PKN: Warsaw, Poland, 2009.
- PN-EN ISO 10304-1:2009; English Version, Water Quality-Determination of Dissolved Anions by Ion Chromatography-Part 1: Determination of Bromides, Chlorides, Fluorides, Nitrates, Nitrites, Phosphates and Sulphates. PKN: Warsaw, Poland, 2009.
- PN-EN ISO 12846:2012; English Version, Water Quality-Determination of Mercury-Atomic Absorption Spectrometry (AAS) Method with or without Enrichment. PKN: Warsaw, Poland, 2012.
- PN-EN 15167-1:2007; Polish Version, Ground Granular Blast Furnace Slag for Use in Concrete, Mortar and Paste-Part 1: Definitions, Specifications and Compliance Criteria. PKN: Warsaw, Poland, 2007.
- Bolewski, A.; Manecki, A. Mineralogia Szczegółowa (Detailed Mineralogy); Publishing house PAE: Warsaw, Poland, 1993. [Google Scholar]
- Rackley, S.A. 7-Adsorption capture systems. In Rackley, Carbon Capture and Storage, 2nd ed.; Stephen, A., Ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 151–185. [Google Scholar] [CrossRef]
- Samarakoon, M.H.; Ranjith, P.G.; De Silva, V.R.S. Effect of soda-lime glass powder on alkali-activated binders: Rheology, strength and microstructure characterization. Constr. Build. Mater. 2020, 241, 118013. [Google Scholar] [CrossRef]
- Provis, J.L. Geopolymers and other alkali activated materials: Why, how, and what? Mater. Struct. 2014, 47, 11–25. [Google Scholar] [CrossRef]
- Palomo, A.; Krivenko, P.; Garcia-Lodeiro, I.; Kavalerova, E.; Maltseva, O.; Fernández-Jiménez, A. A review on alkaline activation: New analytical perspectives. Mater. Construcción 2014, 64, 315. [Google Scholar] [CrossRef]
- Palomo, A.; Maltseva, O.; Garcia-Lodeiro, I.; Fernández-Jiménez, A. Portland Versus Alkaline Cement: Continuity or Clean Break: “A Key Decision for Global Sustainability”. Front. Chem. 2021, 9, 705475. [Google Scholar] [CrossRef]





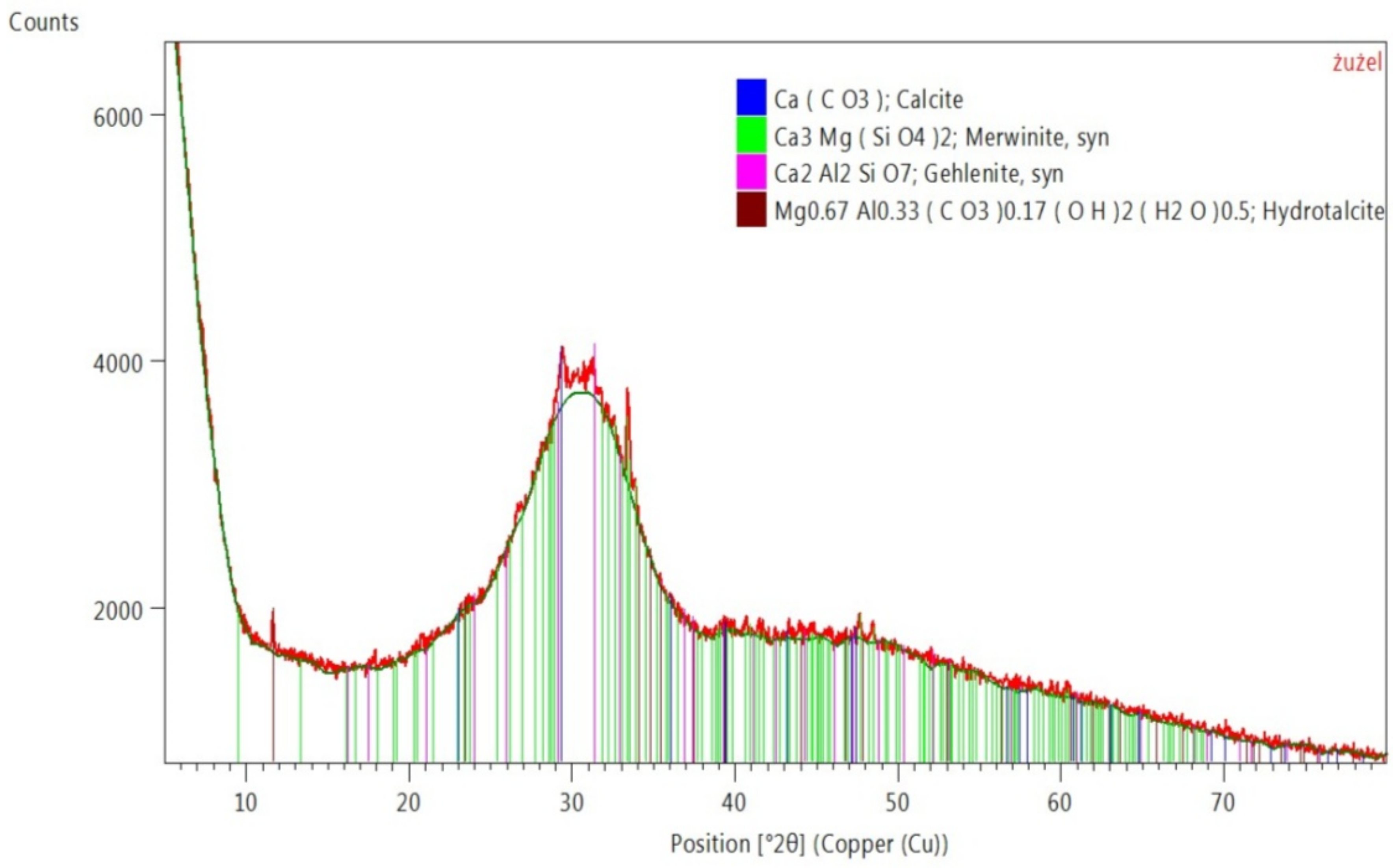













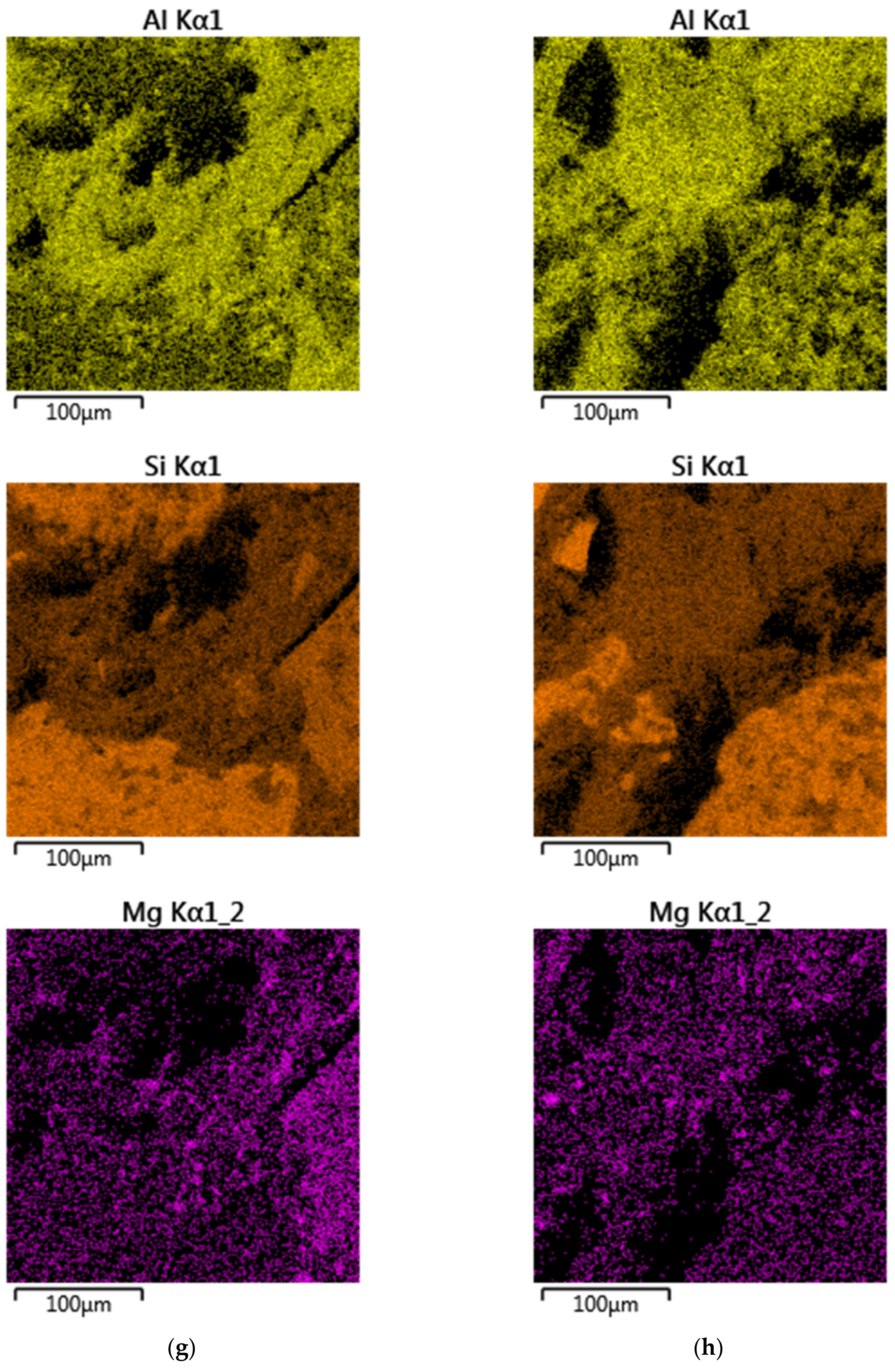



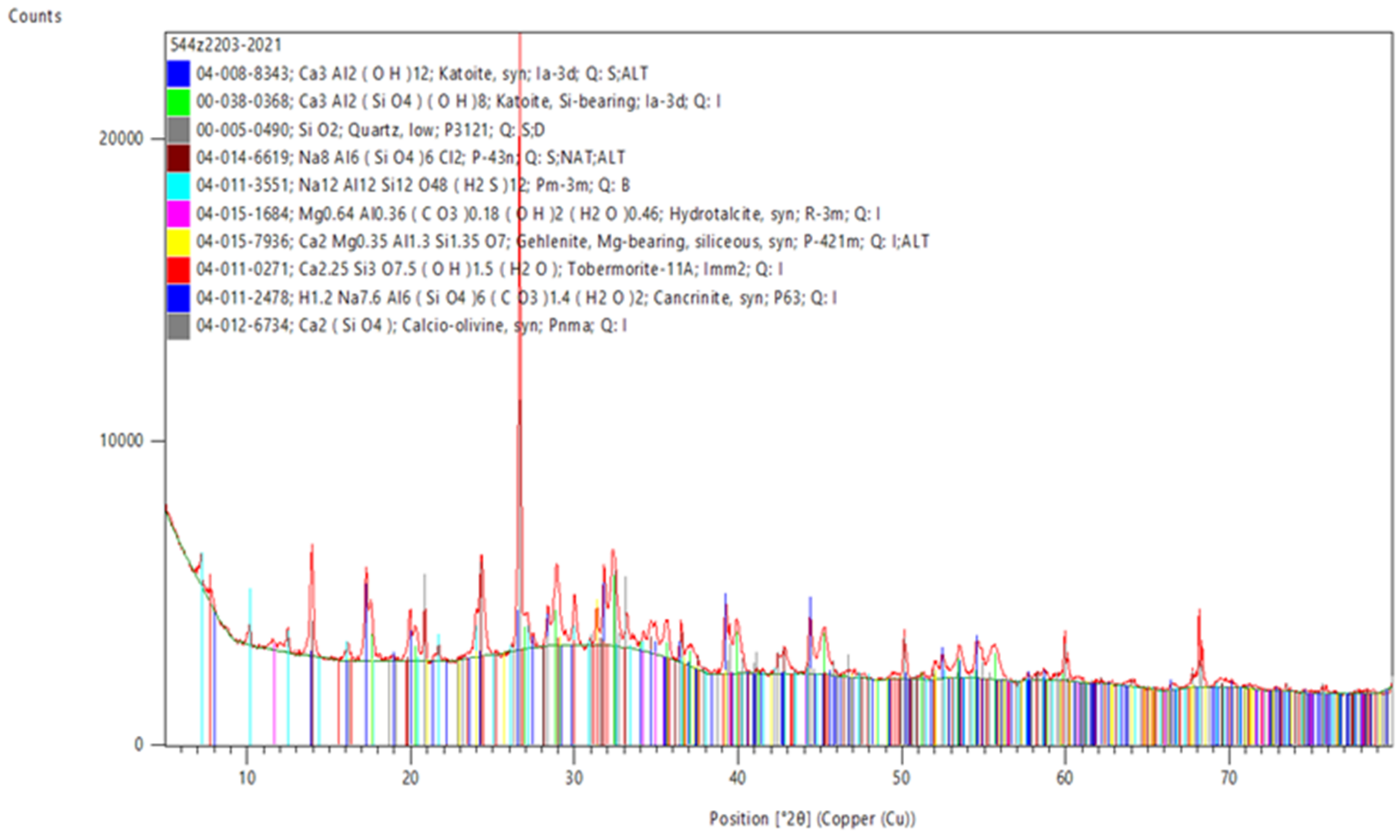
| Component (PL Abbreviation) | PV Glass(S1) | Kaolin Clay | Ground Granulated Blast Furnace Slag | Alumina-Lime Cement (CG-40) | Autoclaved Cellular Concrete (SOL.1) | Fly Ash from Coal Combustion (WR25.1) | Fly Ash from Biomass Combustion (PLB_T1) |
|---|---|---|---|---|---|---|---|
| Abbreviation used in this article | PVG | KC | GGBFS | ALC | ACC | FAC | FAB |
| Content, wt.% | |||||||
| Silica as SiO2 | 72.39 | 47.46 | 40.43 | 3.05 | 67.45 | 43.53 | 60.25 |
| Aluminium as Al2O3 | 1.10 | 36.75 | 7.88 | 42.52 | 2.32 | 25.30 | 5.87 |
| Sodium as Na2O | 13.41 | 0.02 | 0.46 | 0.08 | 0.26 | 2.22 | 0.72 |
| Calcium as CaO | 9.01 | 0.23 | 43.27 | 35.87 | 17.26 | 4.42 | 11.47 |
| Iron as Fe2O3 | 0.05 | 0.92 | 0.81 | 15.31 | 1.00 | 6.80 | 2.86 |
| Magnesium as MgO | 3.09 | 0.24 | 6.97 | 0.50 | 0.29 | 2.61 | 3.51 |
| Potassium as K2O | 0.02 | 0.88 | 0.29 | 0.09 | 0.60 | 4.67 | 6.91 |
| Titanium as TiO2 | 0.02 | 0.24 | 0.28 | 2.01 | 0.06 | 1.39 | 0.39 |
| Manganese as MnO | <0.01 | 0.02 | 0.16 | 0.07 | 0.02 | 0.07 | 0.37 |
| Phosphorus as P2O5 | <0.01 | 0.08 | 0.02 | 0.06 | 0.04 | 3.41 | 1.63 |
| Chromium as Cr2O3 | 0.01 | 0.01 | 0.01 | 0.08 | 0.01 | - | 0.02 |
| Zirconium as ZrO2 | <0.01 | <0.01 | <0.01 | 0.05 | <0.01 | - | 0.04 |
| LOI (550 °C) | 0.31 | ||||||
| LOI (1025 °C) | 13.35 | 0.46 | 0.29 | 10.44 | 26.31 | 5.88 | |
| Phase | Amount, wt.% |
|---|---|
| Amorphous phase | 26.6 |
| Quartz | 38.4 |
| Tobermorite | 24.1 |
| Calcite | 4.0 |
| Anorthite | 1.5 |
| Microcline | 2.1 |
| Anhydrite | 2.0 |
| Gypsum | 1.5 |
| Phase | Amount, wt.% |
|---|---|
| Amorphous phase | 51.3 |
| Quartz | 26.6 |
| Potassium chloride (KCl) | 0.5 |
| Potassium chloride (K0.9Na0.1Cl) | 0.4 |
| Calcite | 2.9 |
| Anhydrite | 1.8 |
| Plagioclase (labradorite) | 6.6 |
| Feldspare (microcline) | 6.7 |
| Mica | 0.7 |
| Lime | 0.1 |
| Akermanite | 1.3 |
| Cordierite | 0.3 |
| Leucite | 0.6 |
| Mixture | Oxide Molar Ratio | Atomic Ratio | ||||
|---|---|---|---|---|---|---|
| SiO2/Al2O3 | Na2O/SiO2 | Na2O/Al2O3 | Si/Al | Na/Si | Na/Al | |
| Mixture 5 | 2.28 | 0.33 | 0.75 | 1.14 | 0.66 | 0.75 |
| Mixture 6 | 2.33 | 0.33 | 0.76 | 1.17 | 0.65 | 0.76 |
| Mixture 7 | 2.12 | 0.34 | 0.73 | 1.06 | 0.69 | 0.73 |
| Component | Type of Mixture with Components Amount. % | % Mass of Binder Component | ||||||
|---|---|---|---|---|---|---|---|---|
| M.5.0 | M.5.1 | M.6.0 | M.6.1 | M.7.0 | M.7.1 | % | ||
| PVG | PV glass | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 | 5 |
| FAB | Fly ash from biomass combustion | 1.1 | 1.1 | 5 | ||||
| ACC | Autoclaved cellular concrete | 1.1 | 1.1 | |||||
| FAC | Fly ash from coal combustion | 1.1 | 1.1 | |||||
| KC-0 | Kaolin clay | 3.2 | 3.2 | 3.2 | 15 | |||
| KC-1 | Sintered kaolin clay | 3.2 | 3.2 | 3.2 | ||||
| GGBFS | Slag | 6.5 | 6.5 | 6.5 | 6.5 | 6.5 | 6.5 | 30 |
| ALC | Alumina-lime cement | 6.5 | 6.5 | 6.5 | 6.5 | 6.5 | 6.5 | 30 |
| A.1 | Na2SiO3·5H2O | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 | 5 |
| A.2 | NaOH | 2.2 | 2.2 | 2.2 | 2.2 | 2.2 | 2.2 | 10 |
| SS | Standard sand | 67.2 | 67.2 | 67.2 | 67.2 | 67.2 | 67.2 | - |
| DW | Distilled water | 11.2 | 11.2 | 11.2 | 11.2 | 11.2 | 11.2 | - |
| Component | PV Glass (PVG) | Specimen M.5.1 | Specimen M.5.1.1 |
|---|---|---|---|
| Chlorides as Cl− | <1.00 | <1.00 | <1.00 |
| Sulphates as SO42− | <1.00 | 16.6 | 11.2 |
| Zinc as Zn | <0.001 | 0.005 | 0.003 |
| Cadmium as Cd | <0.001 | <0.001 | <0.001 |
| Copper as Cu | 0.005 | <0.001 | <0.001 |
| Lead as Pb | 0.006 | <0.001 | <0.001 |
| Nickel as Ni | <0.001 | <0.001 | <0.001 |
| Barium as Ba | 0.020 | <0.001 | <0.001 |
| Chromium as Cr | <0.001 | 0.029 | 0.025 |
| Mercury as Hg | 0.00020 | 0.06 | 0.11 |
| Arsenic as As | <0.01 | 0.029 | 0.035 |
| Molybdenum as Mo | <0.02 | <0.02 | <0.02 |
| Cobalt as Co | <0.01 | <0.01 | <0.01 |
| Tin as Sn | <0.02 | <0.02 | <0.02 |
| Spectrum Label | Spectrum 19 | Spectrum 20 | Spectrum 21 | Spectrum 22 | Spectrum 23 | Spectrum 24 |
|---|---|---|---|---|---|---|
| C | 2.94 | 3.84 | 4.03 | 1.58 | 2.89 | |
| O | 52.09 | 55.74 | 55.36 | 37.83 | 56.36 | 32.86 |
| Na | 3.29 | 4.60 | 3.60 | 4.98 | 0.82 | 1.62 |
| Mg | 0.22 | 1.01 | 0.49 | 2.99 | ||
| Al | 12.50 | 13.66 | 15.00 | 15.97 | 1.16 | 12.27 |
| Si | 10.58 | 12.65 | 14.99 | 13.09 | 37.62 | 4.84 |
| S | 0.12 | 0.22 | 0.20 | 0.44 | 0.03 | 0.58 |
| K | 0.17 | 0.12 | 0.13 | 0.31 | 0.13 | |
| Ca | 14.22 | 6.88 | 5.56 | 18.81 | 0.78 | 36.68 |
| Ti | 0.53 | 0.15 | 0.72 | 0.38 | ||
| Fe | 3.33 | 1.13 | 0.64 | 3.28 | 0.33 | 10.64 |
| Spectrum Label | Spectrum 25 | Spectrum 26 | Spectrum 27 | Spectrum 28 | Spectrum 29 | Spectrum 30 | Spectrum 31 |
|---|---|---|---|---|---|---|---|
| C | 3.21 | 4.87 | 4.12 | 3.49 | 2.72 | 1.24 | 5.59 |
| O | 51.93 | 53.71 | 50.47 | 54.80 | 54.12 | 33.45 | 51.94 |
| Na | 4.65 | 5.71 | 7.90 | 4.01 | 2.04 | 4.80 | 8.66 |
| Mg | 0.10 | 0.07 | 4.12 | 0.61 | 0.42 | 0.28 | 2.84 |
| Al | 6.48 | 5.39 | 11.16 | 9.18 | 8.01 | 6.78 | 11.96 |
| Si | 6.49 | 27.49 | 9.81 | 6.67 | 5.17 | 7.53 | 11.18 |
| S | 0.08 | 0.12 | 0.23 | 0.22 | 0.21 | 0.18 | 0.20 |
| K | 0.07 | 0.14 | 0.14 | 0.18 | 0.26 | ||
| Ca | 14.80 | 2.28 | 10.06 | 16.87 | 20.28 | 11.53 | 6.36 |
| Ti | 6.12 | 0.19 | 0.32 | 1.62 | 17.40 | ||
| Fe | 6.07 | 0.23 | 1.83 | 3.84 | 5.40 | 16.63 | 1.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Słomka-Słupik, B.; Wiśniewska, P.; Bargieł, W. Multicomponent Low Initial Molar Ratio of SiO2/Al2O3 Geopolymer Mortars: Pilot Research. Materials 2022, 15, 5943. https://doi.org/10.3390/ma15175943
Słomka-Słupik B, Wiśniewska P, Bargieł W. Multicomponent Low Initial Molar Ratio of SiO2/Al2O3 Geopolymer Mortars: Pilot Research. Materials. 2022; 15(17):5943. https://doi.org/10.3390/ma15175943
Chicago/Turabian StyleSłomka-Słupik, Barbara, Paulina Wiśniewska, and Wiktor Bargieł. 2022. "Multicomponent Low Initial Molar Ratio of SiO2/Al2O3 Geopolymer Mortars: Pilot Research" Materials 15, no. 17: 5943. https://doi.org/10.3390/ma15175943






