The Mechanical and Energy Release Performance of THV-Based Reactive Materials
Abstract
:1. Introduction
2. Sample Preparation
3. Experiment
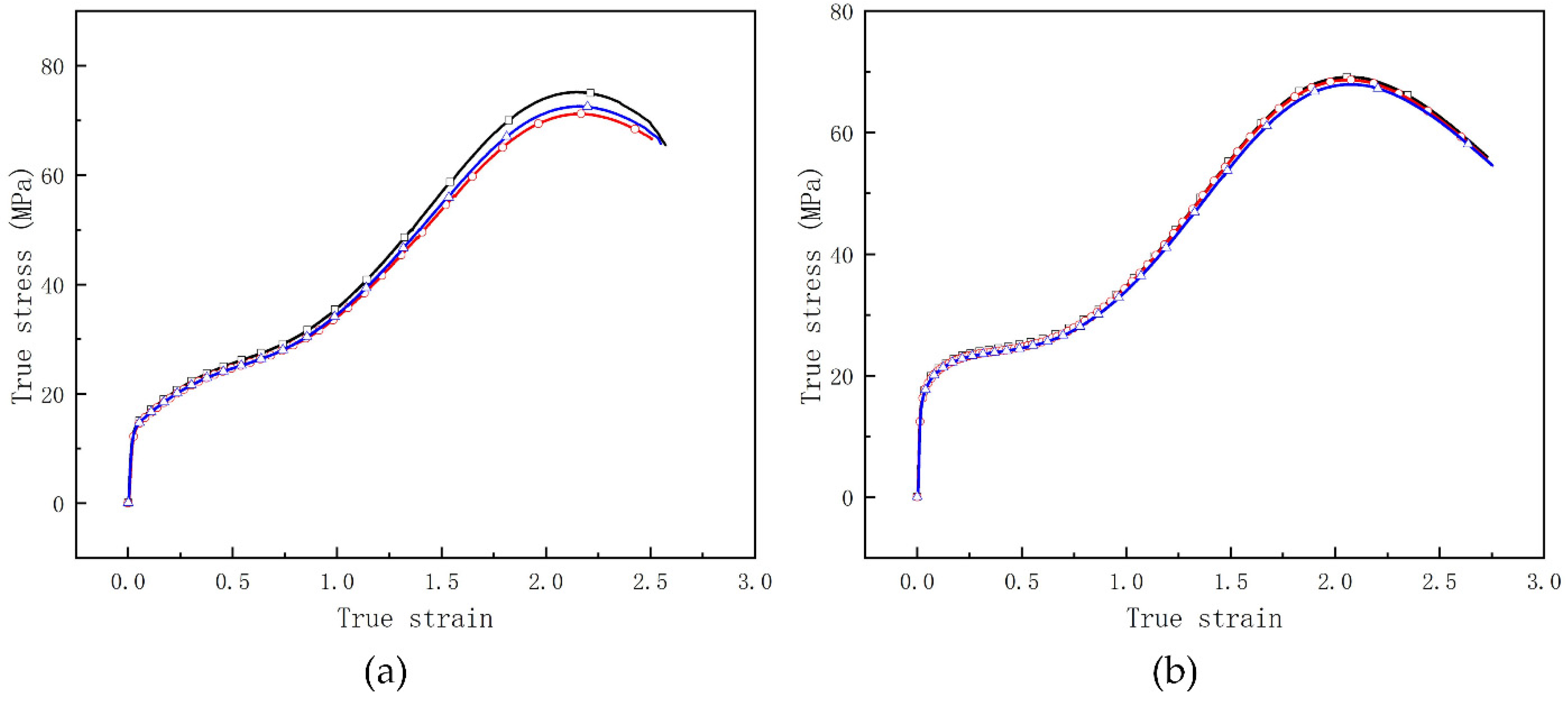
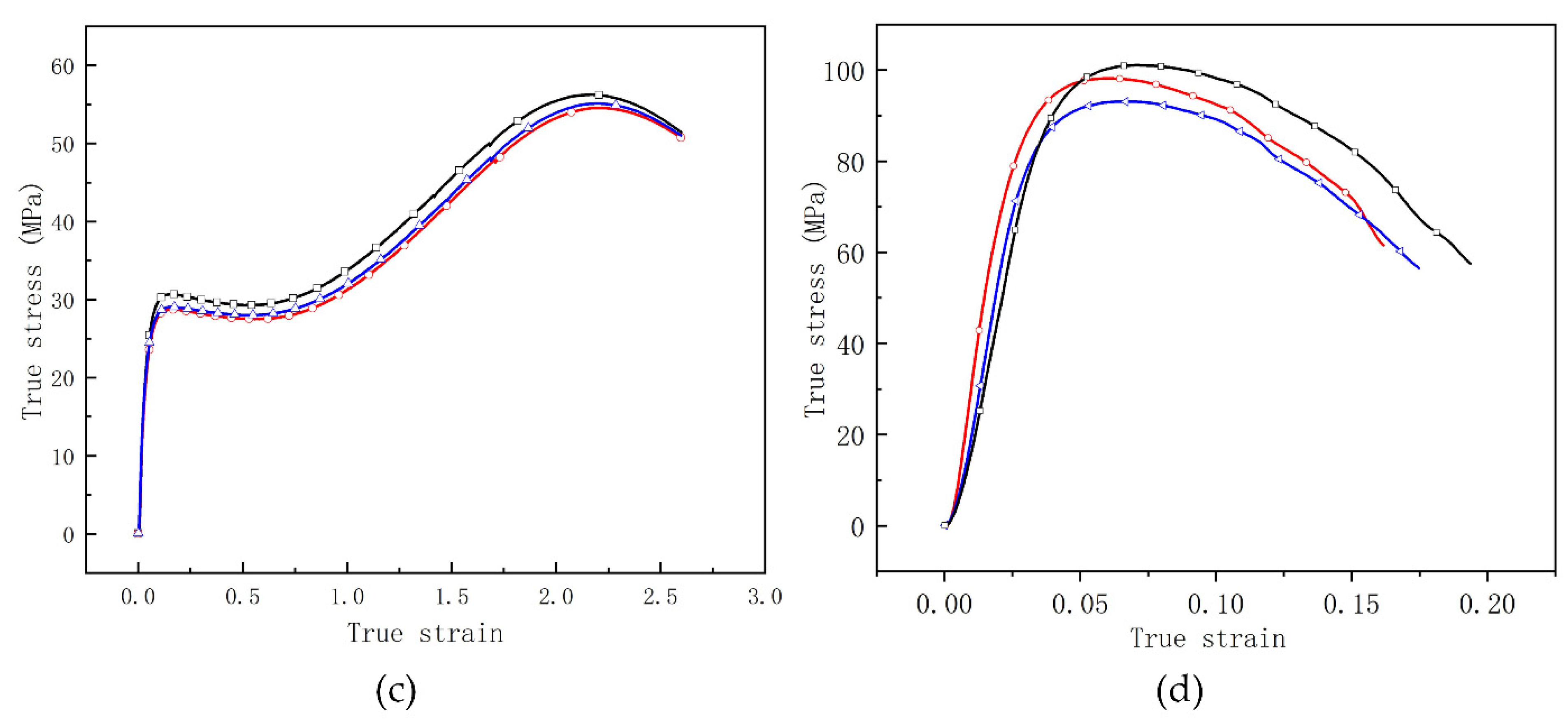
3.1. Quasi-Static Compressive Test
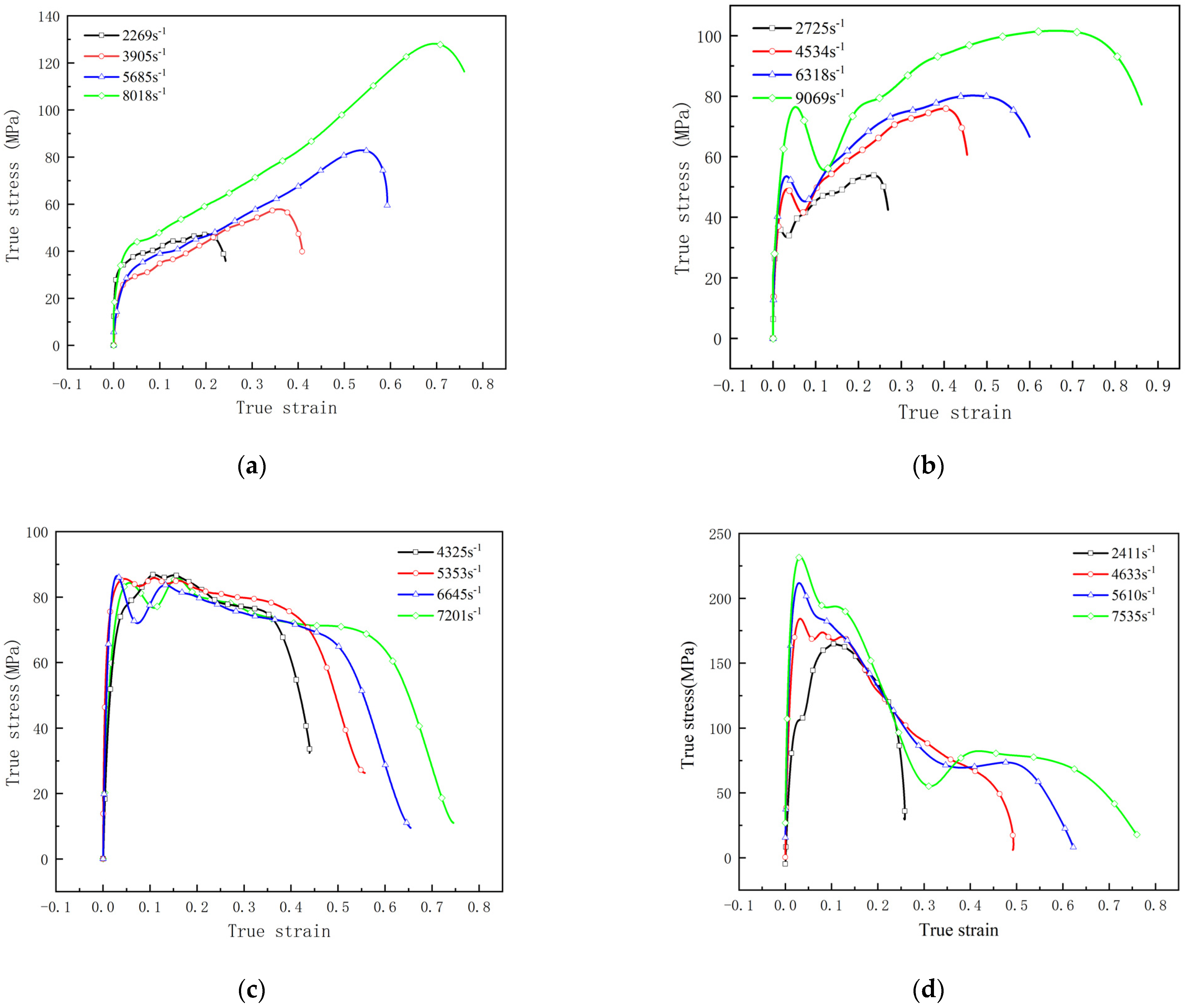
3.2. SHPB Test
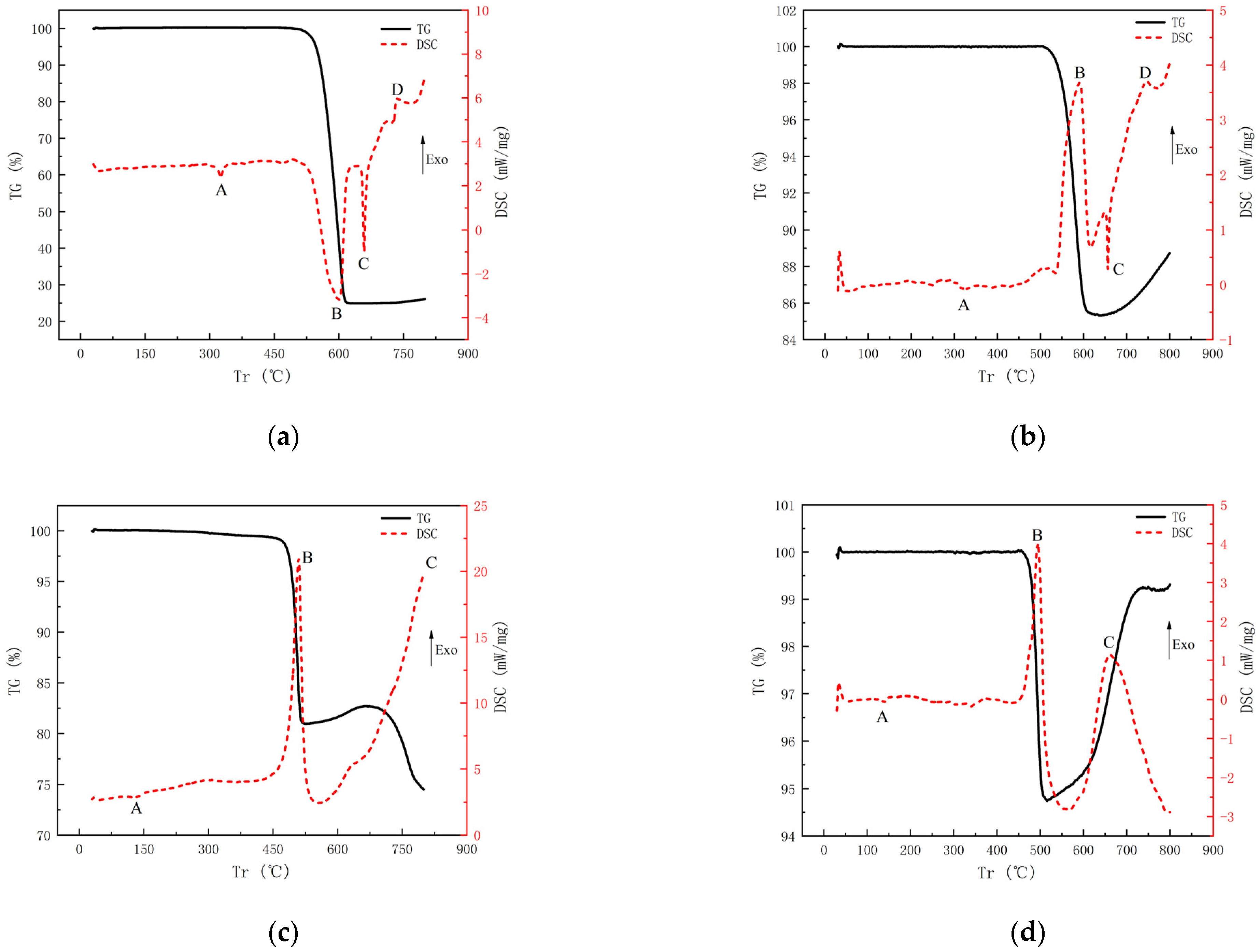
3.3. DSC/TG Test
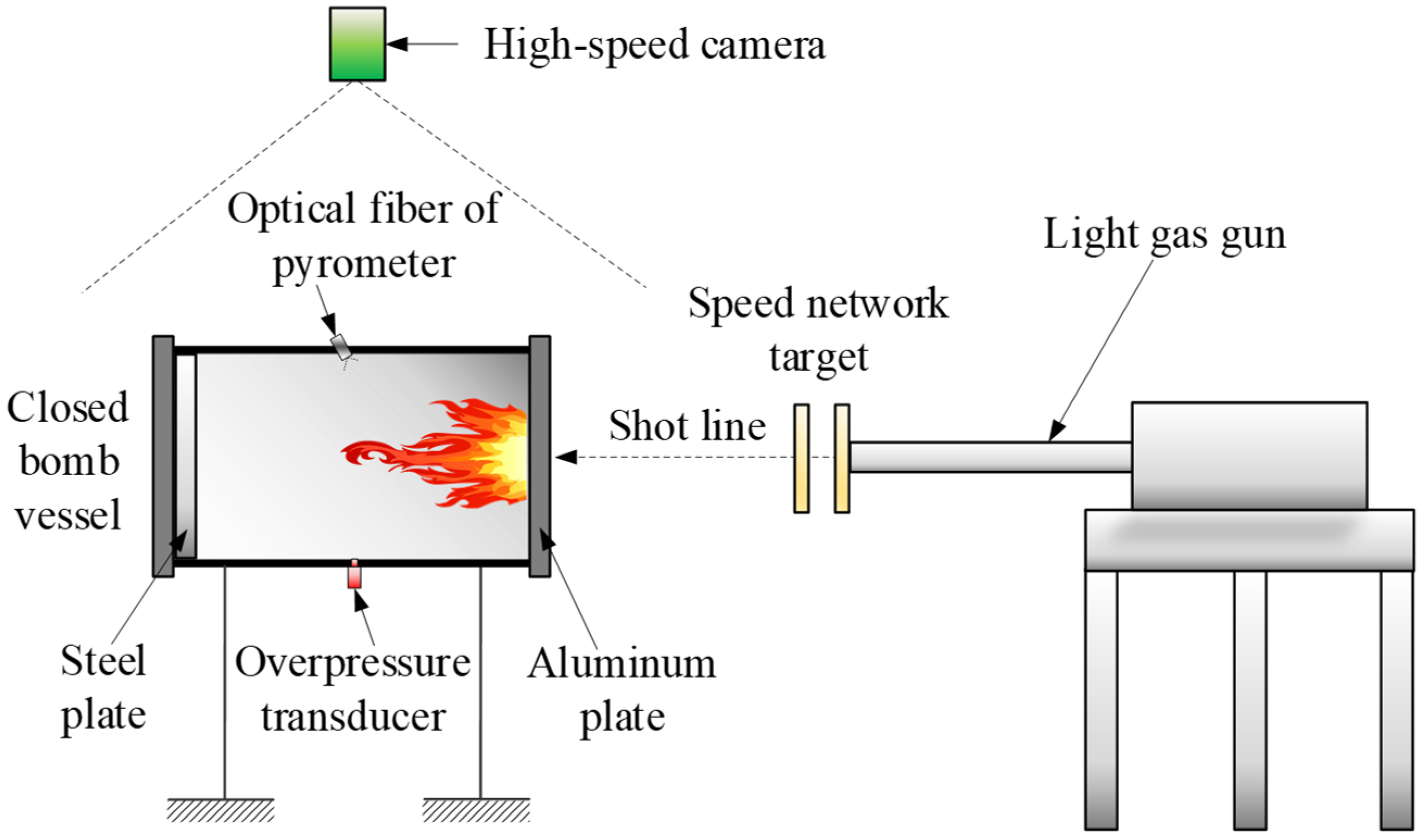
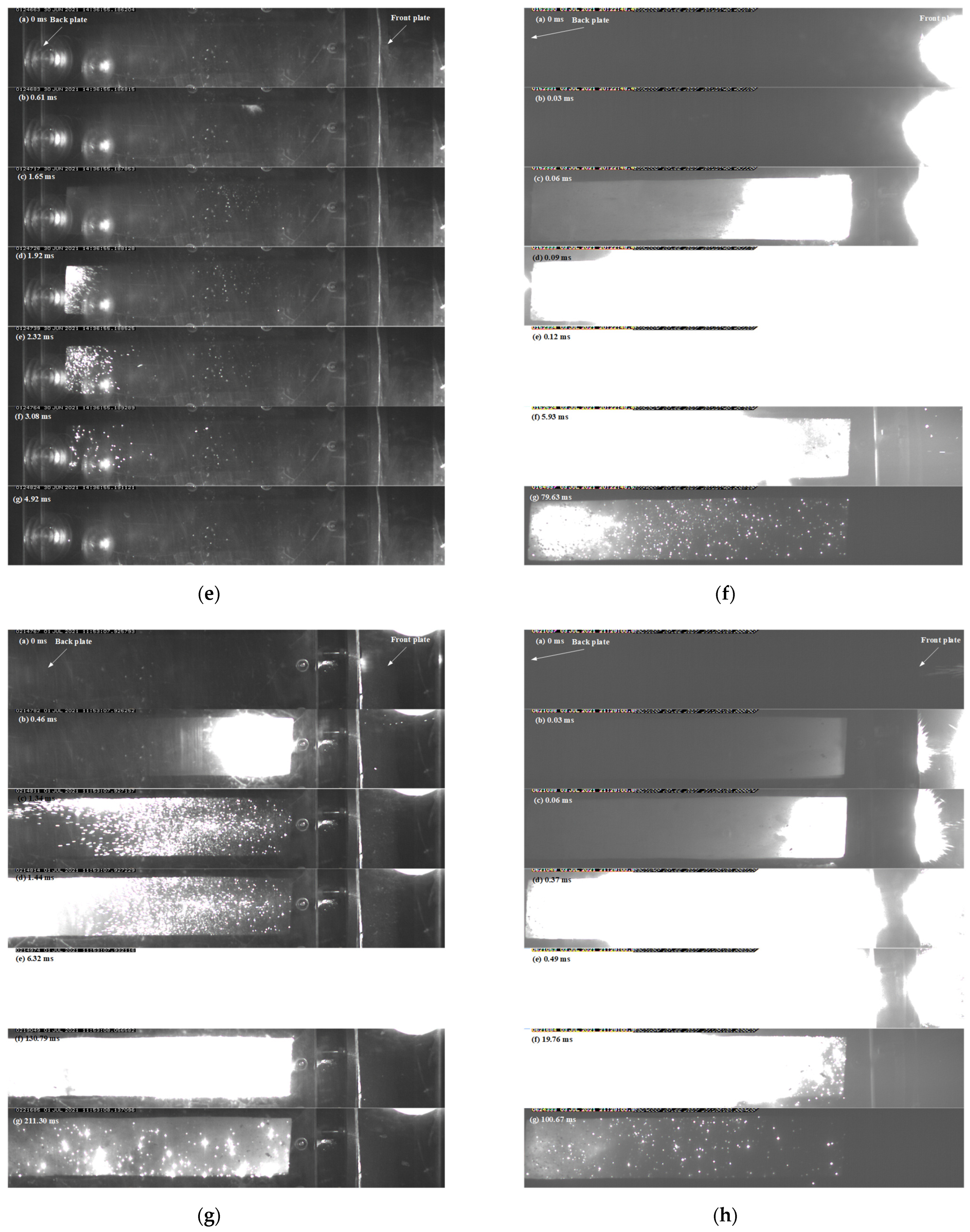
3.4. Ballistic Experiment
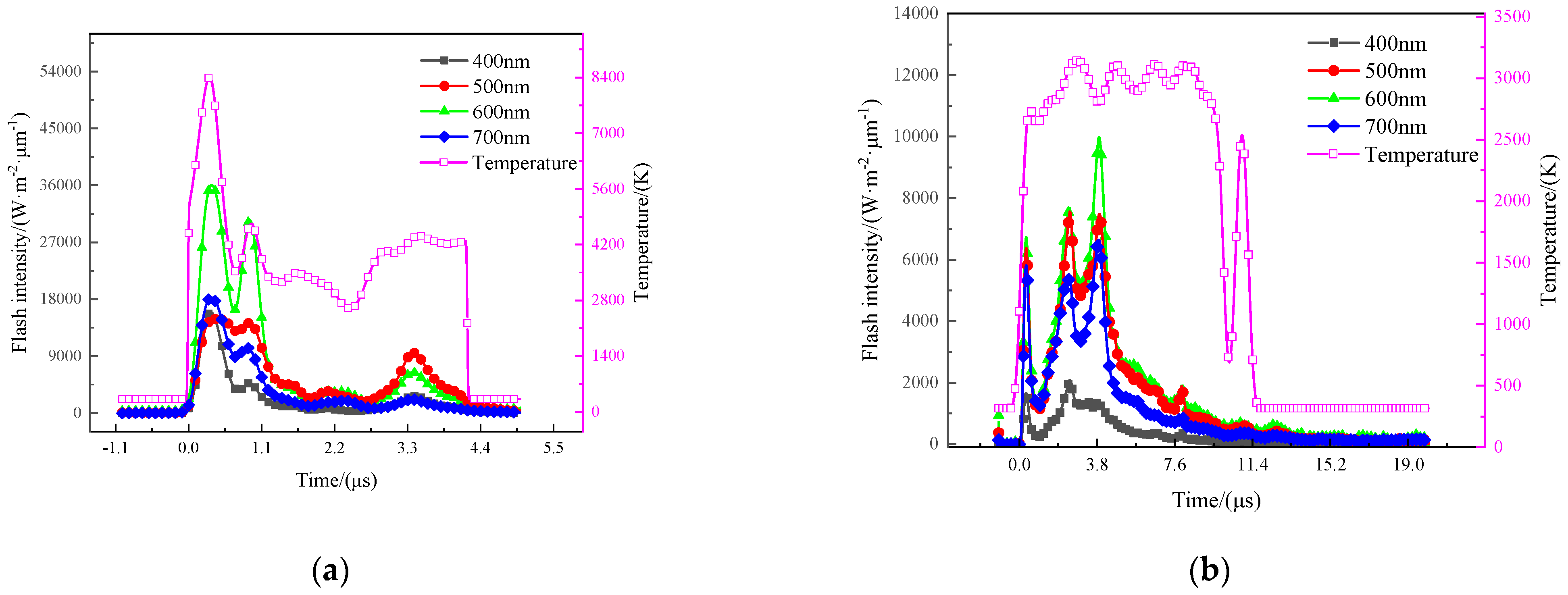
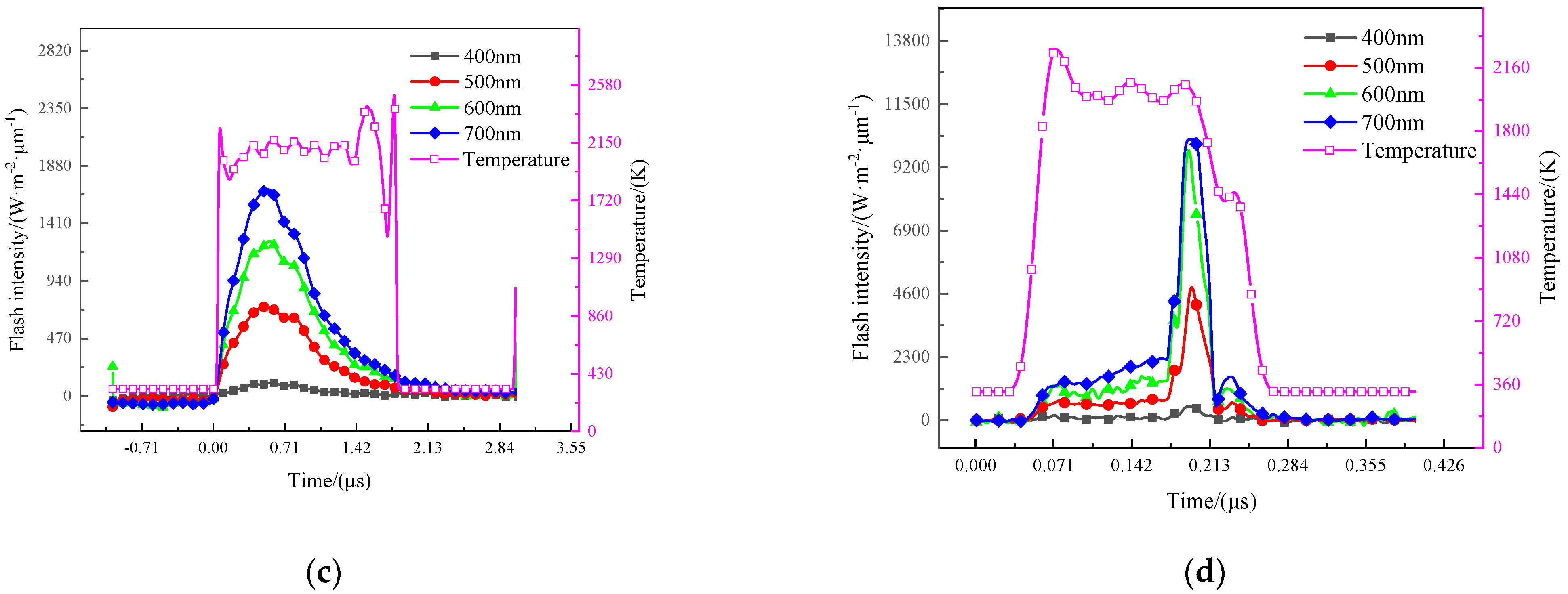
3.4.1. Temperature of the Gas-Phase Product
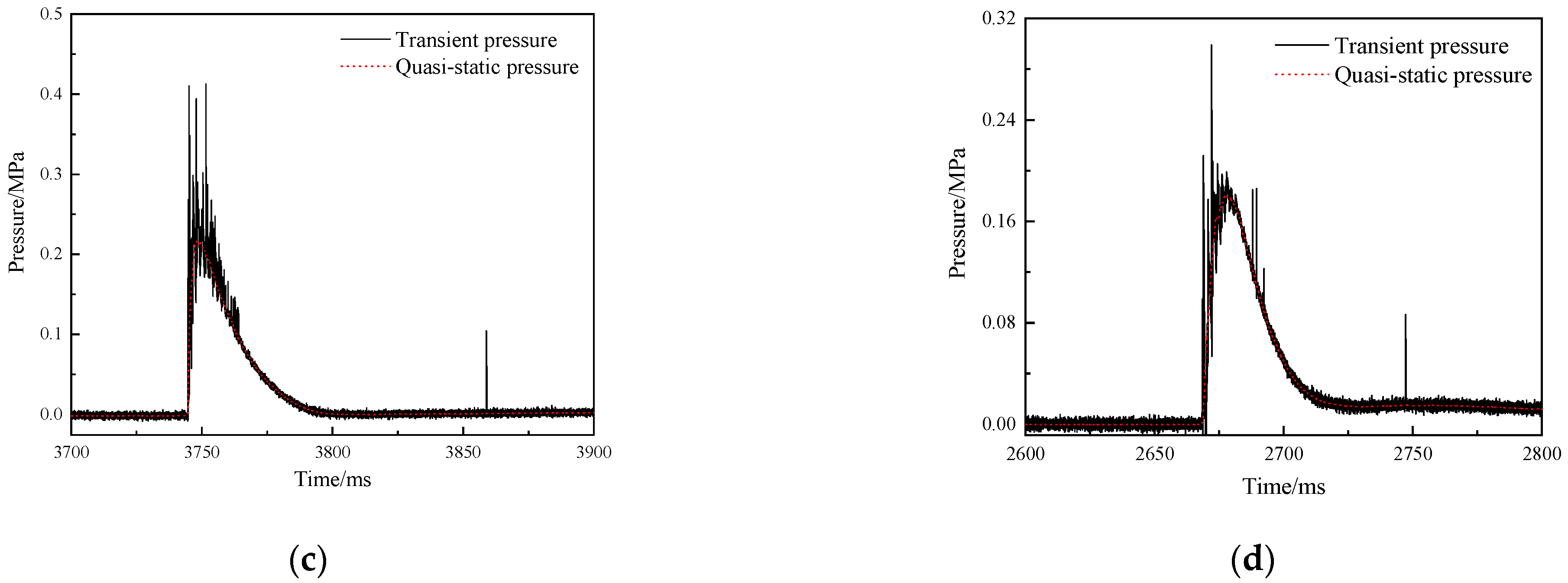
3.4.2. Overpressure in the Closed Bomb Vessel
4. Result and Discussion
4.1. Mechanical Performance of RMs
4.2. The Fracture Mechanism
4.3. Thermal Behavior under DSC/TG Tests
4.4. The Energy Release Behavior of RMs
4.5. The Total Energy Release of THV-Based RMs
5. Conclusions
- (1)
- For the compression tests with quasi-static strain rate, the THV-based RMs have a unique strain softening effect whereas the PTFE-based RMs have a remarkable strain strengthening effect, that is, the stress decreases with the increase in strain after the materials yield. This phenomenon is mainly caused by the different glass transition temperatures of them. The glass transition temperature of THV is 5 °C, which is below the test temperature. In this case, the disordered chains of molecules in RMs will be “unfrozen” so that the relative motion between them is promoted to a great extent under loading, leading to their unique strain softening effect.
- (2)
- Thermal analysis indicates that the THV-based RMs have more than one exothermic peak because of the complex component in THV. The first exothermic peak leads to a 17.9% drop in mass, which is consistent with the content of TFE (17.40%) in THV. The subsequent exothermic peak may be associated with exothermic reaction caused by the HFP and VDF. In addition, the rupture of the alumina layer outside the Al core plays an important part in the onset of the whole chemical reaction of Al/PTFE RMs. If the alumina layer is ruptured under the impact load, a chemical reaction will bring forward the decomposition temperature of PTFE. Additionally, the increase in the TG curve is caused by the reaction product’s desublimation in the crucible lid.
- (3)
- The reaction threshold is closely related to the mechanical characteristics of RMs. The introduction of tungsten (W) particles to PTFE RMs could not only enhance the density but also elevate the reaction threshold of RMs, whereas the reaction threshold of THV-based RMs is decreased when increasing Hf particles content to achieve an equivalent density of RMs projectile. This is because the high content of Hf powder makes it easier for the RMs to be fragmented and it increases the energy released near the crack after the fracture of the material to generate a hot spot at a higher temperature, thus making the RMs more prone to ignition reaction. However, the introduction of tungsten (W) particles to PTFE RMs make RMs not easy to be fragmented. As such, under current conditions, the THV-based RMs (88% Hf/12% THV) with a high density of 7.83 g/cm3 are adapted to release a lot of energy in thin, confined spaces.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eryong, H. Investigation of Mechanism and Performance of Spaced Ceramic Target under Impact of 12.7 mm Armor Piercing Projectile; National University of Defense Technology: Changsha, China, 2008. [Google Scholar]
- Shengcai, Z. Research on Damage Mechanism of Light Armored Target by PELE; Nanjing University of Science and Technology: Nanjing, China, 2010. [Google Scholar]
- Nielson, D.; Ashcroft, B.; Doll, D. Reactive Material Enhanced Munition Compositions and Projectiles Containing Same. U.S. Patent 10/801,948, 15 September 2005. [Google Scholar]
- Nielson, D.B.; Truitt, R.M.; Ashcroft, B.N. Reactive Material Enhanced Projectiles and Related Methods. U.S. Patent 9,103,641, 11 August 2015. [Google Scholar]
- Wu, J.X.; Liu, Q.; Feng, B.; Yin, Q.; Li, Y.C.; Wu, S.Z.; Huang, J.I.; Ren, X.X. Improving the energy release characteristics of PTFE/Al by doping magnesium hydride. Def. Technol. 2022, 18, 219–228. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, Y.F.; Yu, Q.B.; Ge, C.; Su, C.H.; Wang, H.F. Penetration and internal blast behavior of reactive liner enhanced shaped charge against concrete space. Def. Technol. 2022, 18, 952–962. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, Y.F.; Yu, Q.B.; Ge, C.; Su, C.H.; Wang, H.F. Energetic Materials Based on W/PTFE/Al: Thermal and Shock-Wave Initiation of Exothermic Reactions. Metals 2021, 11, 1355. [Google Scholar]
- Saikov, I.; Seropyan, S.; Malakhov, A.; Saikova, G.; Denisov, I.; Petrov, E. An effective way to enhance energy output and combustion characteristics of Al/PTFE. Combust. Flame 2020, 214, 419–425. [Google Scholar]
- Wang, J.; Zhang, L.; Mao, Y.; Gong, F. Sensitivity of Al-PTFE upon Low-Speed Impact. Propellants Explos. Pyrotech. 2019, 44, 630–636. [Google Scholar]
- Feng, B.; Qiu, C.L.; Zhang, T.H.; Hu, Y.F.; Li, H.G.; Xu, B.C. Investigation on mechanical properties and reaction characteristics of Al-PTFE composites with different Al particle size. Adv. Mater. Sci. Eng. 2019, 44, 630–636. [Google Scholar]
- Wu, J.X.; Fang, X.; Gao, Z.R.; Wang, H.X.; Huang, J.Y.; Wu, S.Z.; Li, Y.C. Study on initiation mechanism of reactive fragment to covered explosive. Trans. Beijing Inst. Technol. 2012, 32, 786–789. [Google Scholar]
- Wang, H.F.; Zheng, Y.F.; Yu, Q.B.; Liu, Z.W.; Yu, W. Damage effect of energetic fragment warhead. Chin. J. Energ. Mater. 2012, 19, 450–453. [Google Scholar]
- Ge, C.; Dong, Y.; Maimaitituersun, W. Microscale simulation on mechanical properties of Al/PTFE composite based on real microstructures. Materials 2016, 9, 590. [Google Scholar] [CrossRef]
- Baker, E.L.; Daniels, A.S.; Ng, K.W.; Martin, V.O.; Orosz, J.P. Barnie: A unitary demolition warhead. In Proceedings of the 19th International Symposium of Ballistics, Interlaken, Switzerland, 7–11 May 2001. [Google Scholar]
- Daniels, A.S.; Baker, E.L.; DeFisher, S.E.; Ng, K.W.; Pham, J. BAM BAM: Large Scale Unitary Demolition Warheads. In Proceedings of the 23rd International Symposium on Ballistics, Tarragona, Spain, 16–20 April 2007. [Google Scholar]
- Zhao, H.; Yu, Q.; Deng, B.; Cun, H. Experimental Study on Terminal Demolition Lethality of Reactive Fragments. Trans. Beijing Inst. Technol. 2020, 40, 375–381. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhang, J.; Qiao, L.; Shi, A.S.; Zhang, Y.G.; H, Y.; Guan, Z.W. Experimental study of the compression properties of Al/W/PTFE granular composites under elevated strain rates. Mater. Sci. Eng. A 2013, 581, 48–55. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhang, J.; Qiao, L.; Shi, A.S.; Zhang, Y.G.; He, Y.; Guan, Z.W. Influence of particle size grading on strength of Al/W/PTFE composite. Ordnance Mater. Sci. Eng. 2014, 6, 17–21. [Google Scholar]
- Ren, K.; Chen, J.; Qing, H.; Chen, R.; Chen, P.; Lin, Y.; Guo, B. Study on Shock-Induced Chemical Energy Release Behavior of Al/W/PTFE Reactive Material with Mechanical-Thermal-Chemical Coupling SPH Approach. Propellants Explos. Pyrotech. 2020, 45, 1937–1948. [Google Scholar] [CrossRef]
- Ren, K.; Chen, J.; Qing, H.; Chen, R.; Chen, P.; Lin, Y.; Guo, B. Investigation on the thermal behavior, mechanical properties and reaction characteristics of Al-PTFE composites enhanced by Ni particle. Materials 2018, 11, 1741. [Google Scholar]
- Wang, R.; Huang, J.; Liu, Q.; Wu, S.; Wu, J.; Ren, X.; Li, Y. Impact energy release characteristics of PTFE/Al/CuO reactive materials measured by a new energy release testing device. Polymers 2019, 11, 149. [Google Scholar]
- Huang, J.; Fang, X.; Wu, S.; Yang, L.; Yu, Z.; Li, Y. Mechanical Response and Shear-Induced Initiation Properties of PTFE/Al/MoO3 Reactive Composites. Materials 2018, 11, 1200. [Google Scholar] [CrossRef]
- Aboud, N.; Ferraro, D.; Taverna, M.; Descroix, S.; Smadja, C.; Tran, N.T. Dyneon THV, a fluorinated thermoplastic as a novel material for microchip capillary electrophoresis. Analyst 2016, 141, 5776–5783. [Google Scholar] [CrossRef]
- Feng, B.; Li, Y.C.; Hao, H.; Wang, H.X.; Hao, Y.F.; Fang, X. A Mechanism of Hot-spots Formation at the Crack Tip of Al-PTFE under Quasi-static Compression. Propellants Explos. Pyrotech. 2017, 42, 1366–1372. [Google Scholar] [CrossRef]
- Ge, C.; Yu, Q.; Zhang, H.; Qu, Z.; Wang, H.; Zheng, Y. On dynamic response and fracture-induced initiation characteristics of aluminum particle filled PTFE reactive material using hat-shaped specimens. Mater. Des. 2020, 188, 108472. [Google Scholar] [CrossRef]
- Xiao, J.; Nie, Z.; Wang, Z.; Du, Y.; Tang, E. Energy release behavior of Al/PTFE reactive materials powder in a closed chamber. J. Appl. Phys. 2020, 127, 165106. [Google Scholar] [CrossRef]
- Xiao, J. Experimenl Study on the Physical Characteristics of Flash Produced by Hypervelocity Impact with LY12 Aluminum Plate; Shenyang Ligong University: Shenyang, China, 2012. [Google Scholar]
- Yafei, H.; Enling, T.; Liping, H.; Meng, W.; Kai, G.; Jin, X.; Ruizhi, W.; Zhenbo, L. Evolutionary characteristics of thermal radiation induced by 2A12 aluminum plate under hypervelocity impact loading. Int. J. Impact Eng. 2019, 125, 173–179. [Google Scholar] [CrossRef]
- Thornhill, T.F.; Reinhart, W.D.; Chhabildas, L.C. Characterization of prompt flash sig-natures using high-speed broadband diode detectors. Int. J. Impact Eng. 2008, 35, 827–835. [Google Scholar] [CrossRef]
- Osborne, D.T.; Pantoya, M.L. Effect of Al particle size on the thermal degradation of Al/Teflon mixtures. Combust. Sci. Technol. 2007, 179, 1467–1480. [Google Scholar] [CrossRef]
- Dreizin, E.L.; Schoenitz, M. Correlating ignition mechanisms of aluminum-based reactive materials with thermoanalytical measurements. Prog. Energy Combust. Sci. 2015, 50, 81–105. [Google Scholar] [CrossRef]







| NO. | Specimen Formula | Theoretical Density (g/cm3) | Specimen Density (g/cm3) | Compactness |
|---|---|---|---|---|
| 1 | 26.5% Al/73.5% PTFE | 2.31 | 2.31 | 100% |
| 2 | 5.29% Al/80% W/14.71% PTFE | 7.73 | 7.73 | 100% |
| 3 | 62% Hf/38% THV | 4.14 | 3.93 | 94.93% |
| 4 | 88% Hf/12% THV | 7.83 | 7.28 | 92.98% |
| No. | Elasticity Modulus (MPa) | Yield Stress (MPa) | Yield Strain | Compressive Strength (MPa) | Failure Strain |
|---|---|---|---|---|---|
| 1 | 728 | 13.30 | 0.0322 | 72.57 | 2.14 |
| 2 | 661 | 16.89 | 0.0308 | 68.60 | 2.05 |
| 3 | 665 | 28.85 | 0.1205 | 55.12 | 2.18 |
| 4 | 3550 | 79.39 | 0.0257 | 98.17 | 0.06 |
| Formula | Strain Rate (s−1) | Yield Stress (MPa) | Yield Strain | Compressive Strength (MPa) | Failure Strain |
|---|---|---|---|---|---|
| 26.5% Al/73.5% PTFE | 2269 | 30.14 | 0.0173 | 47.21 | 0.20 |
| 3905 | 21.44 | 0.0122 | 57.88 | 0.36 | |
| 5685 | 23.04 | 0.0165 | 82.91 | 0.54 | |
| 8081 | 34.65 | 0.0161 | 128.15 | 0.69 | |
| 5.29% Al/80% W/14.71% PTFE | 2725 | 36.05 | 0.0154 | 53.84 | 0.23 |
| 4534 | 49.30 | 0.0337 | 75.90 | 0.40 | |
| 6318 | 53.52 | 0.0322 | 80.22 | 0.47 | |
| 9069 | 76.12 | 0.0479 | 101.65 | 0.66 | |
| 62% Hf/38% THV220 | 4325 | 74.03 | 0.0374 | 86.85 | 0.35 |
| 5353 | 85.56 | 0.0486 | 85.56 | 0.39 | |
| 6645 | 86.49 | 0.0302 | 86.49 | 0.49 | |
| 7201 | 84.32 | 0.0561 | 85.79 | 0.56 | |
| 88% Hf/12% THV220 | 2411 | 103.49 | 0.0241 | 164.71 | 0.11 |
| 4633 | 184.20 | 0.0321 | 184.20 | 0.03 | |
| 5610 | 211.79 | 0.0301 | 211.79 | 0.03 | |
| 7535 | 231.94 | 0.0318 | 231.94 | 0.03 |
| Formula | Density (g/cm3) | Velocity (m/s) | Over-Pressure (Mpa) | Temper-ature (K) | Pressure Potential Energy (kJ/g) | Internal Energy (kJ/g) | Energy Content (kJ/g) | Efficiency (%) | Duration (ms) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.42 | 635 | 0.128 | 2514 | 0.50 | 3.41 | 14.64 | 26.69 | 89.28 |
| 1 | 2.27 | 1880 | 0.0289 | 2169 | 0.50 | 3.15 | 14.64 | 24.90 | 9.44 |
| 2 | 7.92 | 464 | 0.089 | 2929 | 0.11 | 0.71 | 4.34 | 18.86 | 172.21 |
| 2 | 7.87 | 1150 | 0.106 | 2261 | 0.53 | 5.09 | 4.34 | 129.33 | 92.06 |
| 3 | 3.94 | 450 | 0.031 | - | 0.08 | - | 10.03 | - | 4.92 |
| 3 | 3.97 | 1500 | 0.216 | 4721 | 1.77 | 11.80 | 10.03 | 135.32 | 79.63 |
| 4 | 7.45 | 480 | 0.108 | 4664 | 0.13 | 1.99 | 6.97 | 30.42 | 211.30 |
| 4 | 7.24 | 1100 | 0.179 | 3140 | 0.88 | 7.57 | 6.97 | 121.15 | 100.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, M.; Wang, Y.; Wang, H.; Xiao, J. The Mechanical and Energy Release Performance of THV-Based Reactive Materials. Materials 2022, 15, 5975. https://doi.org/10.3390/ma15175975
Guo M, Wang Y, Wang H, Xiao J. The Mechanical and Energy Release Performance of THV-Based Reactive Materials. Materials. 2022; 15(17):5975. https://doi.org/10.3390/ma15175975
Chicago/Turabian StyleGuo, Mengmeng, Yanxin Wang, Haifu Wang, and Jianguang Xiao. 2022. "The Mechanical and Energy Release Performance of THV-Based Reactive Materials" Materials 15, no. 17: 5975. https://doi.org/10.3390/ma15175975
APA StyleGuo, M., Wang, Y., Wang, H., & Xiao, J. (2022). The Mechanical and Energy Release Performance of THV-Based Reactive Materials. Materials, 15(17), 5975. https://doi.org/10.3390/ma15175975






