The Effect of a Chemical Foaming Agent and the Isocyanate Index on the Properties of Open-Cell Polyurethane Foams
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Samples
2.3. Methods and Testing
3. Results and Discussion
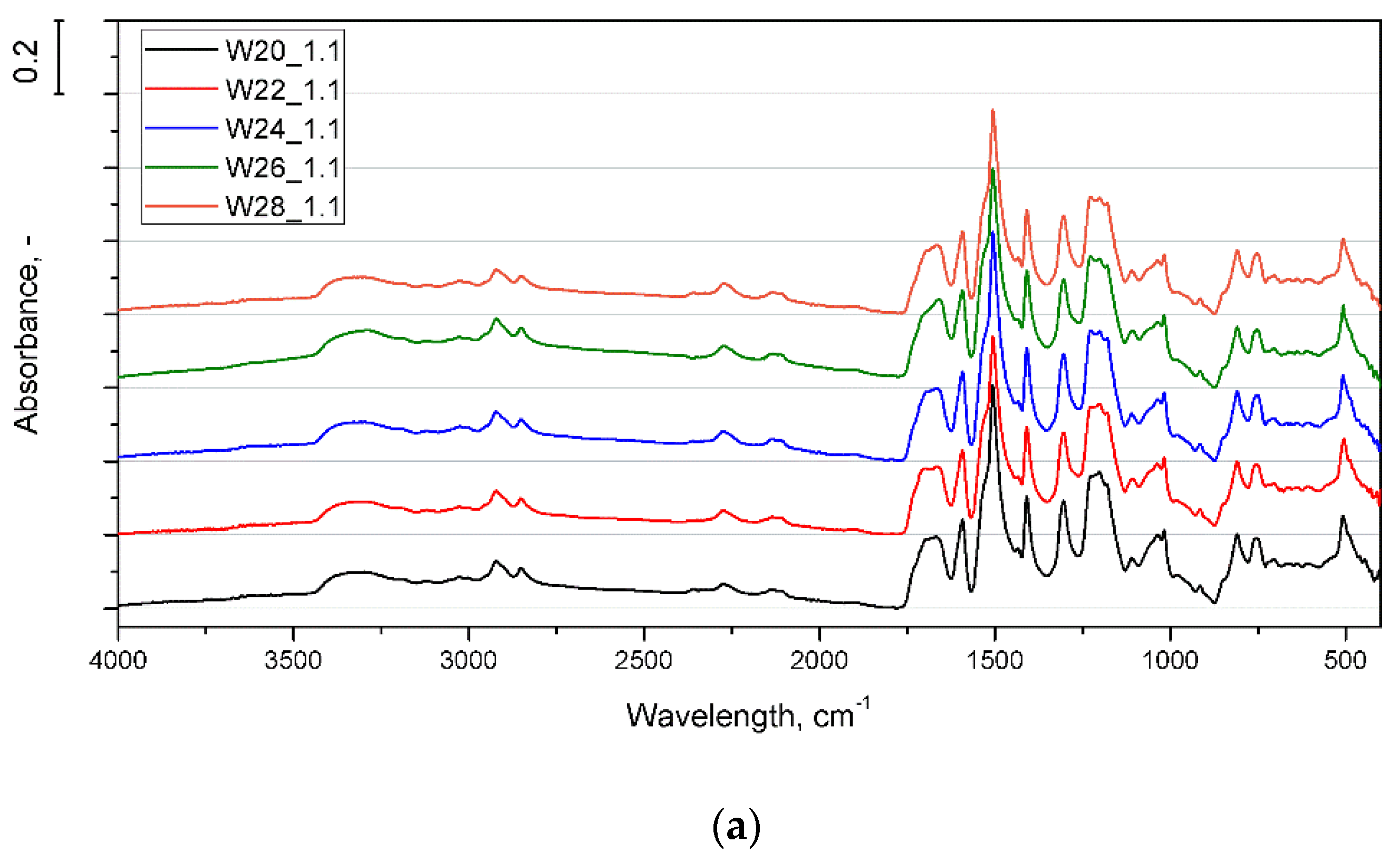
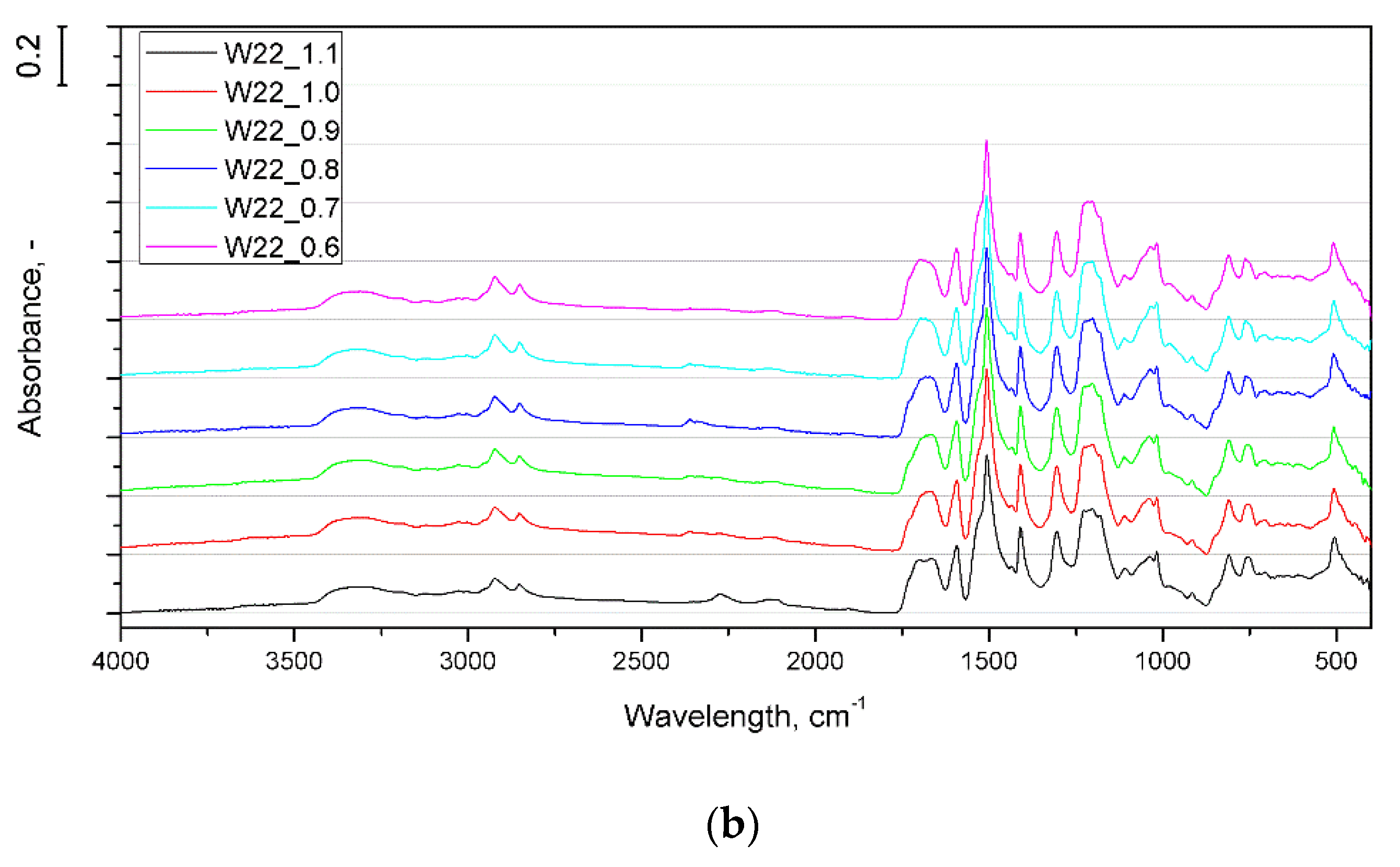
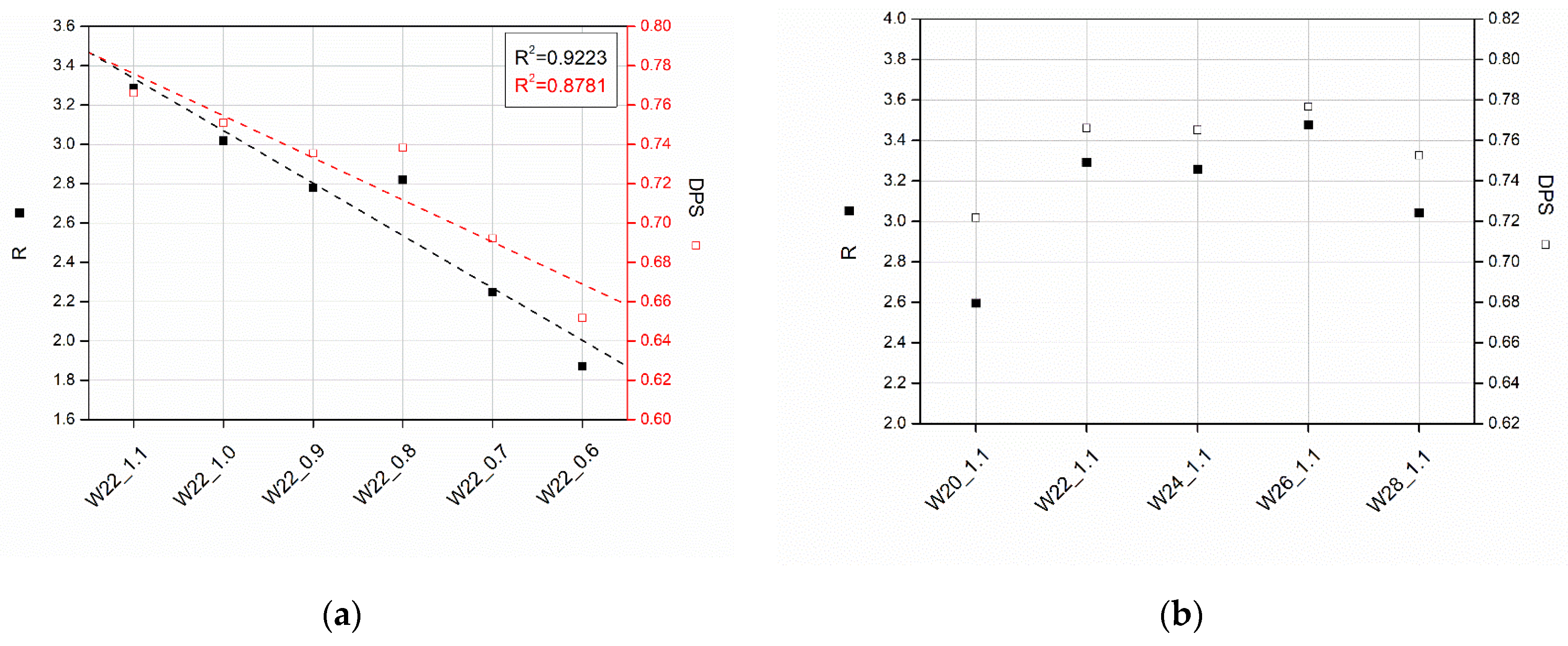
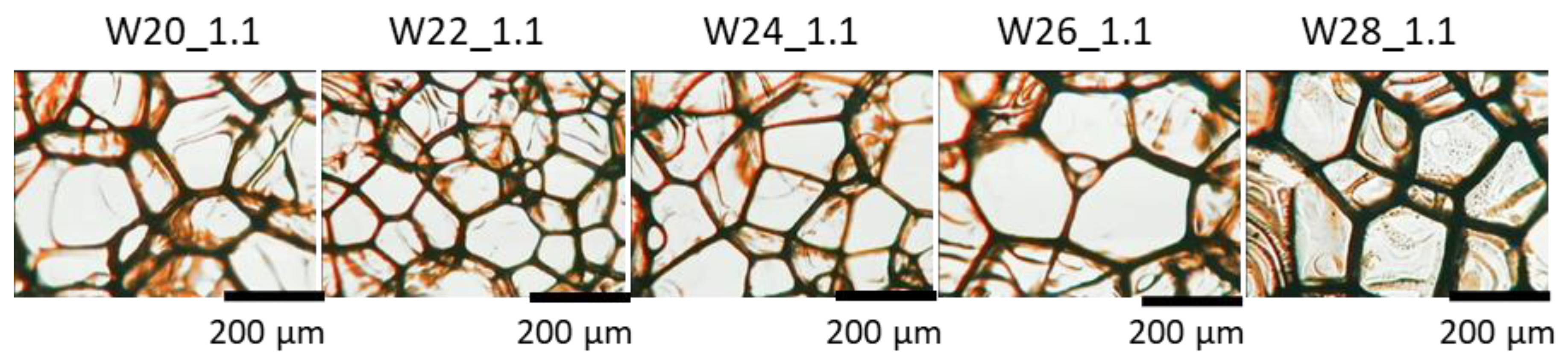
3.1. Effect of Water Content
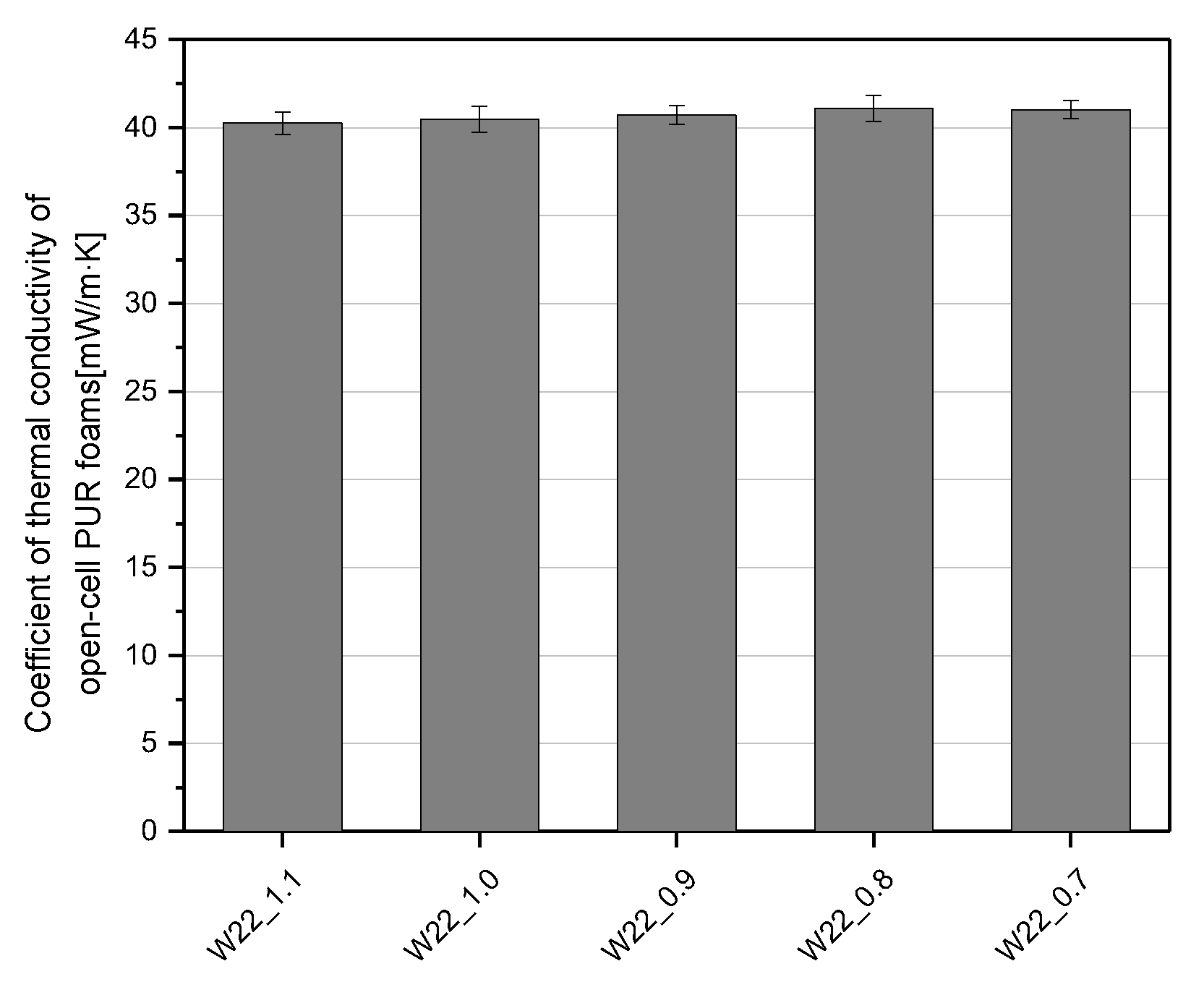
3.2. Effect of Isocyanate Index
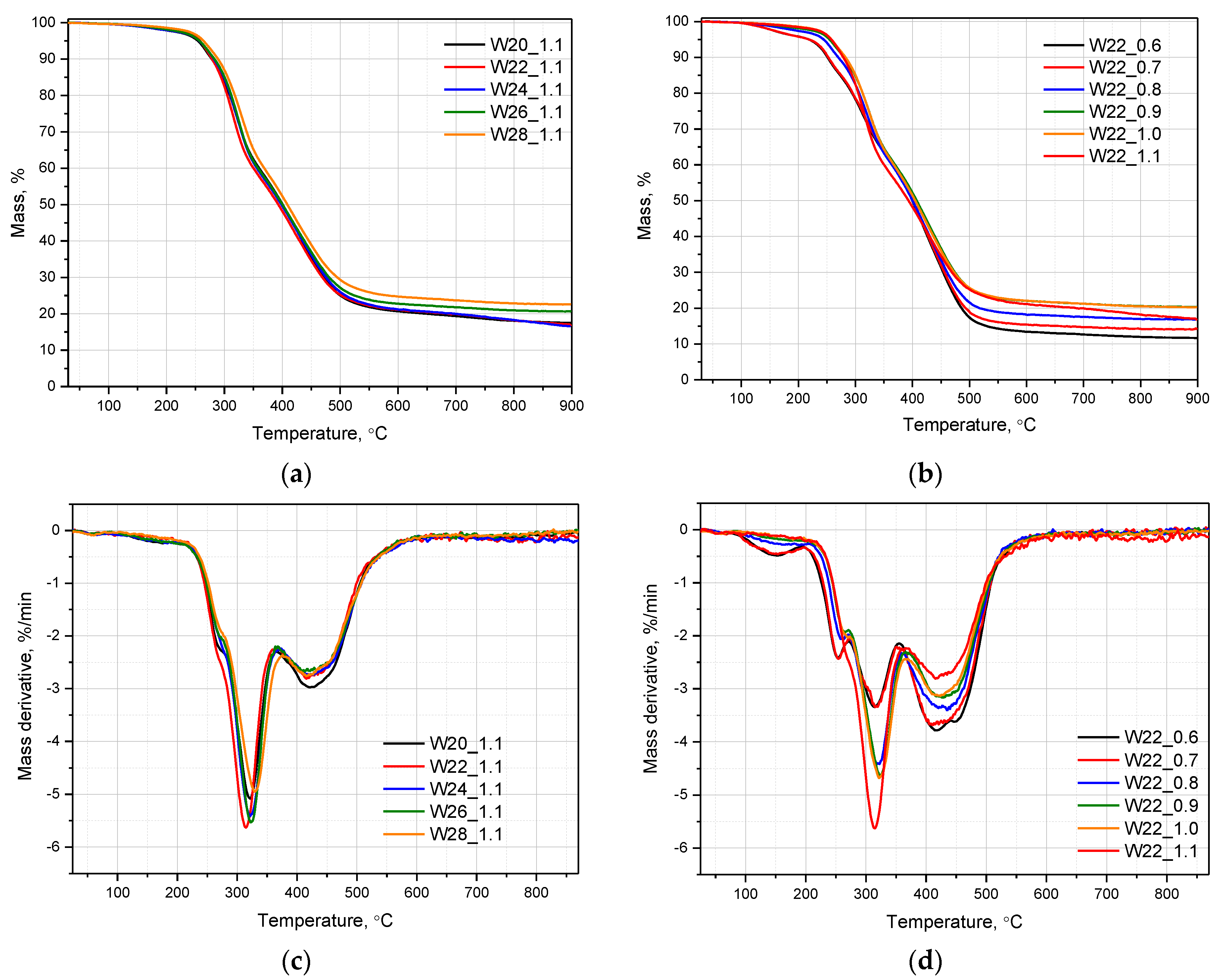
3.3. Thermal Stability of PU Foams
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Polyurethane Global Market Volume 2015–2026 | Statista, Online Resource. Available online: https://www.statista.com/statistics/720341/global-polyurethane-market-size-forecast/ (accessed on 20 April 2022).
- Tu, Y.C.; Fan, H.; Suppes, G.J.; Hsieh, F.H. Physical Properties of Water-Blown Rigid Polyurethane Foams Containing Epoxidized Soybean Oil in Different Isocyanate Indices. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Kairytė, A.; Kirpluks, M.; Ivdre, A.; Cabulis, U.; Vaitkus, S.; Pundienė, I. Cleaner production of polyurethane foam: Replacement of conventional raw materials, assessment of fire resistance and environmental impact. J. Clean. Prod. 2018, 183, 760–771. [Google Scholar] [CrossRef]
- Kurańska, M.; Polaczek, K.; Auguścik-Królikowska, M.; Prociak, A.; Ryszkowska, J. Open-cell polyurethane foams based on modified used cooking oil. Polimery 2020, 65, 216–225. [Google Scholar] [CrossRef]
- Kalmykova, Y.; Sadagopan, M.; Rosado, L. Circular Economy—From Review of Theories and Practices to Development of Implementation Tools. Resour. Conserv. Recycl. 2018, 135, 190–201. [Google Scholar] [CrossRef]
- Loizides, M.; Loizidou, X.; Orthodoxou, D.; Petsa, D. Circular Bioeconomy in Action: Collection and Recycling of Domestic Used Cooking Oil through a Social, Reverse Logistics System. Recycling 2019, 4, 16. [Google Scholar] [CrossRef]
- Geissdoerfer, M.; Savaget, P.; Bocken, N.M.P.; Jan, E. The Circular Economy e A new sustainability paradigm ? J. Clean. Prod. 2017, 143, 757–768. [Google Scholar] [CrossRef]
- Capros, P.; Zazias, G.; Evangelopoulou, S.; Kannavou, M.; Fotiou, T.; Siskos, P.; De Vita, A.; Sakellaris, K. Energy-system modelling of the EU strategy towards climate-neutrality. Energy Policy 2019, 134, 110960. [Google Scholar] [CrossRef]
- Prudhomme, R.; O’Donoghue, C.; Ryan, M.; Styles, D. Defining national biogenic methane targets: Implications for national food production & climate neutrality objectives. J. Environ. Manag. 2021, 295, 113058. [Google Scholar] [CrossRef]
- 2050 Long-Term Strategy. Online Resource. Available online: https://ec.europa.eu/clima/eu-action/climate-strategies-targets/2050-long-term-strategy_en (accessed on 31 March 2022).
- European Commission. Directorate-General for Climate Action, Going climate-neutral by 2050. In A Strategic Long-Term Vision for a Prosperous, Modern, Competitive and Climate-Neutral EU Economy; Publications Office: Brussels, Belgium, 2019. [Google Scholar] [CrossRef]
- Yong, J.Y.; Klemeš, J.J.; Varbanov, P.S.; Huisingh, D. Cleaner energy for cleaner production: Modelling, simulation, optimisation and waste management. J. Clean. Prod. 2016, 111, 1–16. [Google Scholar] [CrossRef]
- Kurańska, M.; Polaczek, K.; Auguścik-Królikowska, M.; Prociak, A.; Ryszkowska, J. Open-cell rigid polyurethane bio-foams based on modified used cooking oil. Polymer 2020, 190, 122164. [Google Scholar] [CrossRef]
- Ivdre, A.; Abolins, A.; Sevastyanova, I.; Kirpluks, M.; Cabulis, U.; Merijs-Meri, R. Rigid polyurethane foams with various isocyanate indices based on polyols from rapeseed oil and waste PET. Polymers 2020, 12, 738. [Google Scholar] [CrossRef] [PubMed]
- Kurańska, M.; Pinto, J.A.; Salach, K.; Barreiro, M.F.; Prociak, A. Synthesis of thermal insulating polyurethane foams from lignin and rapeseed based polyols: A comparative study. Sci. Direct 2020, 143, 111882. [Google Scholar] [CrossRef]
- Prociak, A. Poliuretanowe Materiały Termoizolacyjne Nowej Generacji; Wydawnictwo PK: Kraków, Poland, 2008. [Google Scholar]
- Prociak, A.; Ryszkowska, J.; Rokicki, G. Materiały Poliuretanowe; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2014; Volume 1, ISBN 9788301187842. [Google Scholar]
- Fridrihsone, A.; Romagnoli, F.; Kirsanovs, V.; Cabulis, U. Life Cycle Assessment of vegetable oil based polyols for polyurethane production. J. Clean. Prod. 2020, 266, 121403. [Google Scholar] [CrossRef]
- Kurańska, M.; Malewska, E.; Polaczek, K.; Prociak, A.; Kubacka, J. A pathway toward a new era of open-cell polyurethane foams—Influence of bio-polyols derived from used cooking oil on foams properties. Materials 2020, 13, 5161. [Google Scholar] [CrossRef] [PubMed]
- Park, D.H.; Park, G.P.; Kim, S.H.; Kim, W.N. Effects of isocyanate index and environmentally-friendly blowing agents on the morphological, mechanical, and thermal insulating properties of polyisocyanurate-polyurethane foams. Macromol. Res. 2013, 21, 852–859. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Singha, N.K.; Manjunath, B.S.; Naik, Y. Effect of Foam Density on the Properties of Water Blown Rigid Polyurethane Foam. J. Appl. Polym. Sci. 2008, 108, 1810–1817. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, J.W.; Choi, S.J.; Han, M.S.; Kim, J.M.; Kim, S.B.; Kim, W.N. Properties of Rigid Polyurethane Foams Blown by HFC-365mfc and Distilled Water. J. Ind. Eng. Chem. 2007, 13, 1076–1082. [Google Scholar]
- Prociak, A.; Malewska, E.; Bąk, S. Influence of isocyanate index on selected properties of flexible polyurethane foams modified with various bio-components. J. Renew. Mater. 2016, 4, 78–85. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, B.K.; Lim, H. Effect of isocyanate index on the properties of rigid polyurethane foams blown by HFC 365mfc. Macromol. Res. 2008, 16, 467–472. [Google Scholar] [CrossRef]
- Auguścik, M.; Kurańska, M.; Prociak, A.; Karalus, W.; Lipert, K.; Ryszkowska, J. Production and characterization of poly(urea-urethane) elastomers synthetized from rapeseed oil-based polyols Part I. Structure and properties. Polimery 2016, 61, 490–498. [Google Scholar] [CrossRef]
- Ribeiro da Silva, V.; Mosiewicki, M.A.; Yoshida, M.I.; Coelho da Silva, M.; Stefani, P.M.; Marcovich, N.E. Polyurethane Foams Based on Modified Tung Oil and Reinforced with Rice Husk Ash II: Mechanical Characterization. Polym. Test. 2013, 32, 665–672. [Google Scholar] [CrossRef]
- Reignier, J.; Méchin, F.; Sarbu, A. Chemical gradients in PIR foams as probed by ATR-FTIR analysis and consequences on fire resistance. Polym. Test. 2021, 93, 106972. [Google Scholar] [CrossRef]
- Zieleniewska, M.; Leszczyński, M.K.; Szczepkowski, L.; Bryśkiewicz, A.; Krzyżowska, M.; Bień, K.; Ryszkowska, J. Development and applicational evaluation of the rigid polyurethane foam composites with egg shell waste. Polym. Degrad. Stab. 2016, 132, 78–86. [Google Scholar] [CrossRef]
- Purinova Technical Data Sheet-Purios 500. Available online: https://purios.com/images/upload/product/purios-500/tds-purios-500.pdf (accessed on 11 February 2022).
- Kurańska, M.; Prociak, A. The influence of rapeseed oil-based polyols on the foaming process of rigid polyurethane foams. Ind. Crops Prod. 2016, 89, 182–187. [Google Scholar] [CrossRef]
- Fan, H.; Tekeei, A.; Suppes, G.J.; Hsieh, F.-H. Physical Properties of Soy-Phosphate Polyol-Based Rigid Polyurethane Foams. Int. J. Polym. Sci. 2012, 2012, 907049. [Google Scholar] [CrossRef]
- Cifarelli, A.; Boggioni, L.; Vignali, A.; Tritto, I.; Bertini, F.; Losio, S. Flexible Polyurethane Foams from Epoxidized Vegetable Oils and a Bio-Based Diisocyanate. Polymers 2021, 13, 612. [Google Scholar] [CrossRef]
- Singh, H.; Sharma, T.P.; Jain, A.K. Reactivity of the Raw Materials and Their Effects on the Structure and Properties of Rigid Polyurethane Foams. J. Appl. Polym. Sci. 2007, 106, 1014–1023. [Google Scholar] [CrossRef]
- Ugarte, L.; Gómez-Fernández, S.; Peña-Rodríuez, C.; Prociak, A.; Corcuera, M.A.; Eceiza, A. Tailoring Mechanical Properties of Rigid Polyurethane Foams by Sorbitol and Corn Derived Biopolyol Mixtures. ACS Sustain. Chem. Eng. 2015, 3, 3382–3387. [Google Scholar] [CrossRef]
- Mizera, K.; Ryszkowska, J. Polyurethane elastomers from polyols based on soybean oil with a different molar ratio. Polym. Degrad. Stab. 2016, 132, 21–31. [Google Scholar] [CrossRef]
- Gu, R.; Sain, M.M. Effects of Wood Fiber and Microclay on the Performance of Soy Based Polyurethane Foams. J. Polym. Environ. 2013, 21, 30–38. [Google Scholar] [CrossRef]
- Kirpluks, M.; Cabulis, U.; Avots, A. Flammability of Bio-Based Rigid Polyurethane Foam as Sustainable Thermal Insulation Material. In Insulation Materials in Context of Sustainability; InTech Open: London, UK, 2016; pp. 87–111. [Google Scholar] [CrossRef] [Green Version]

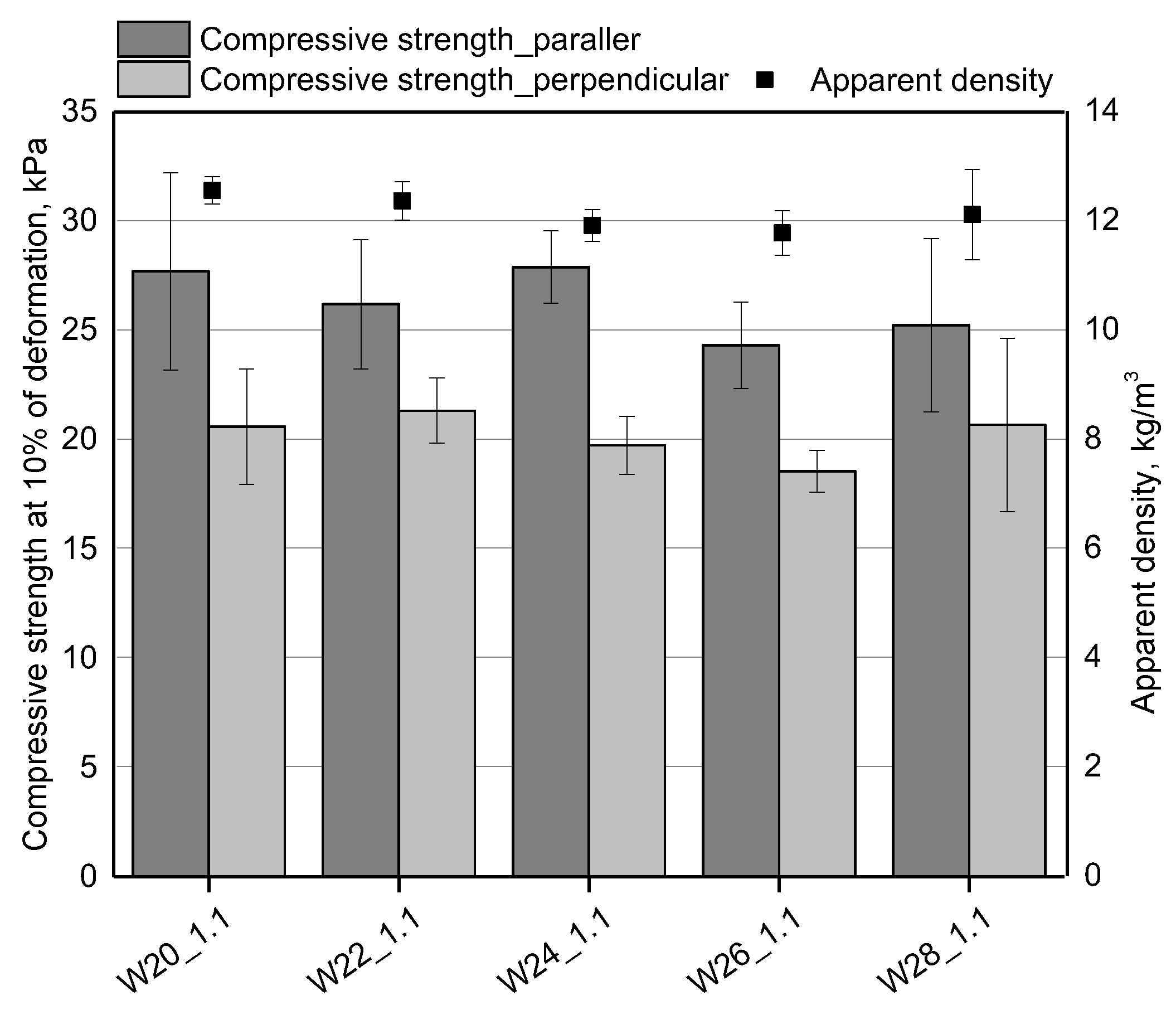
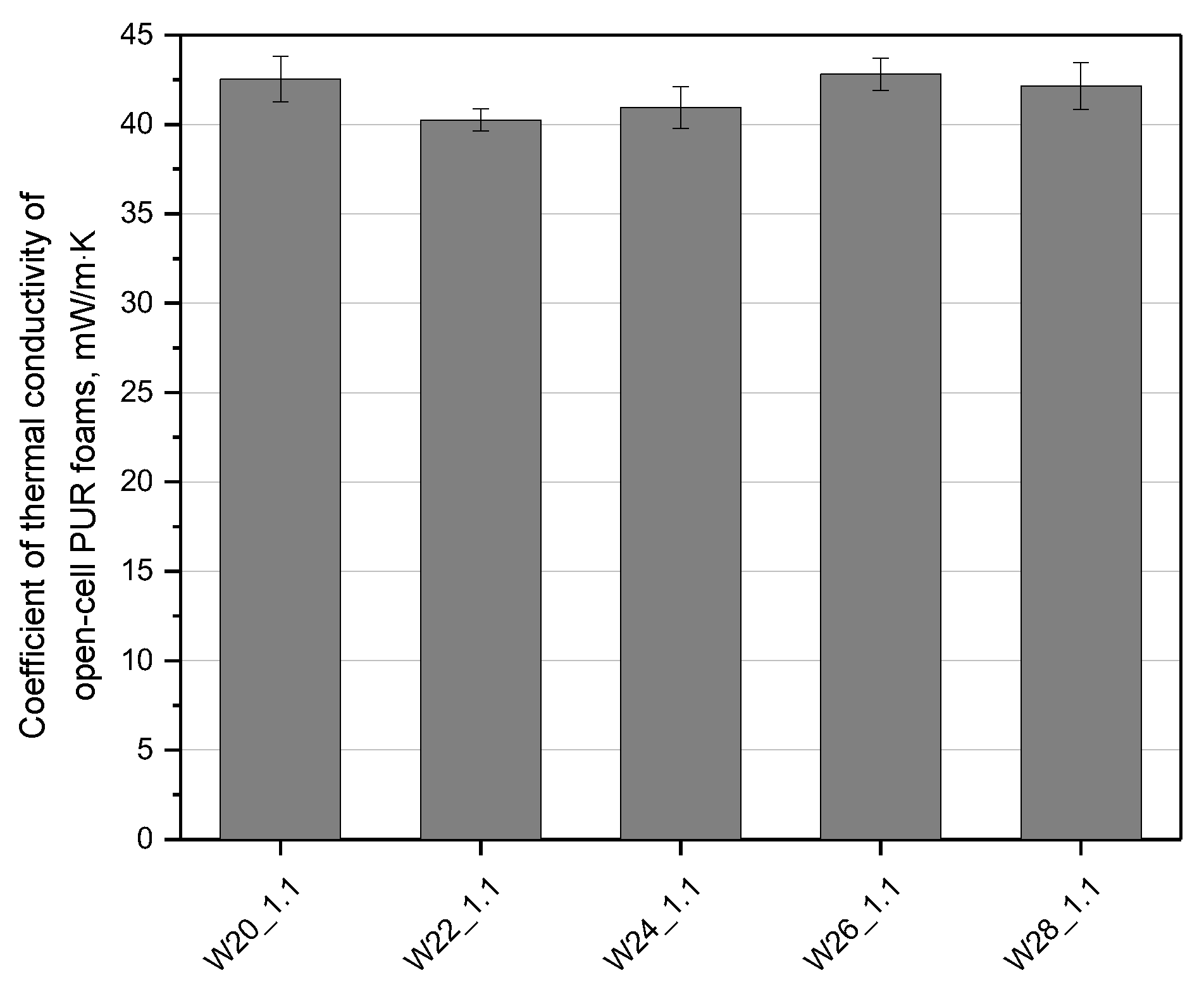
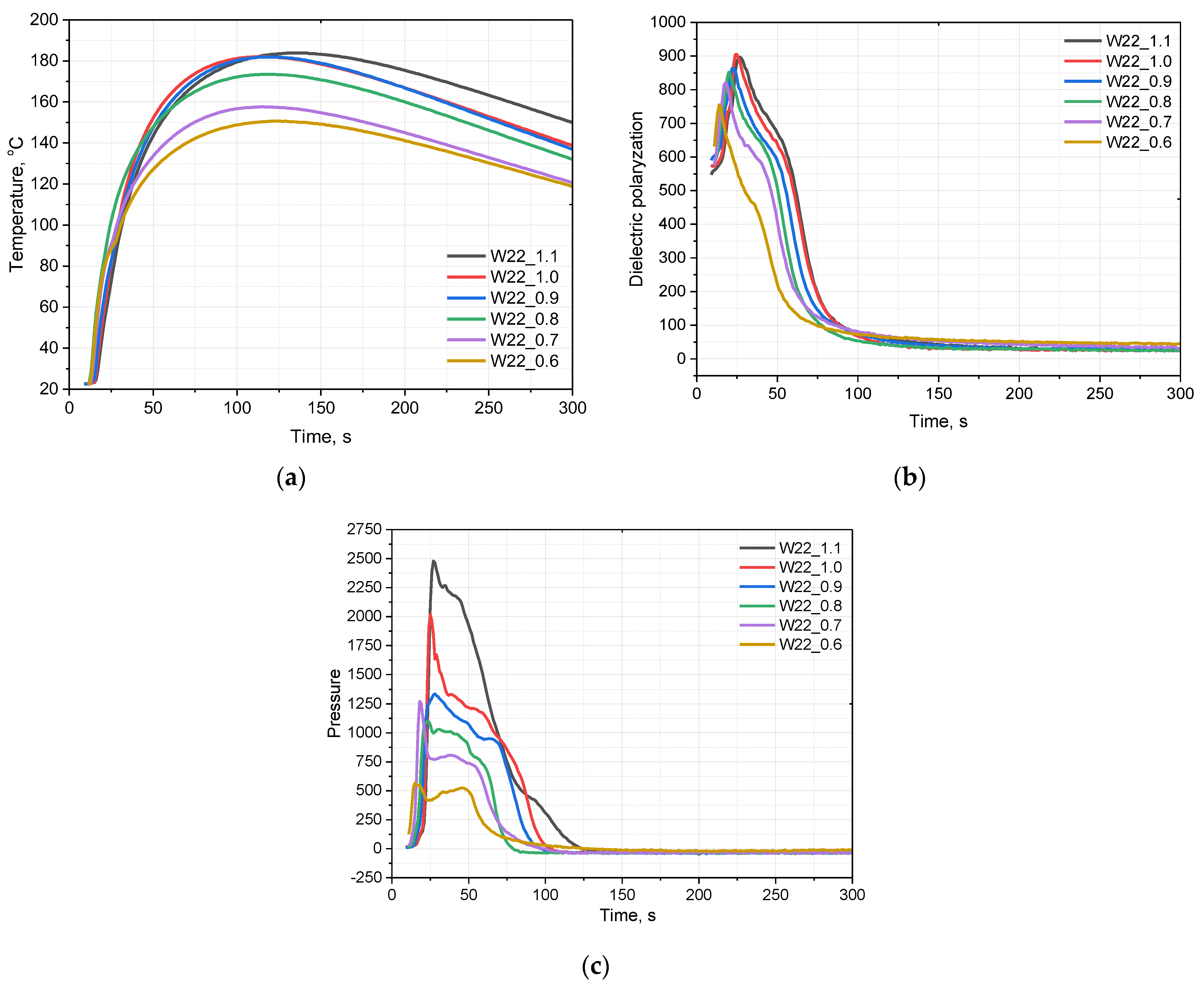
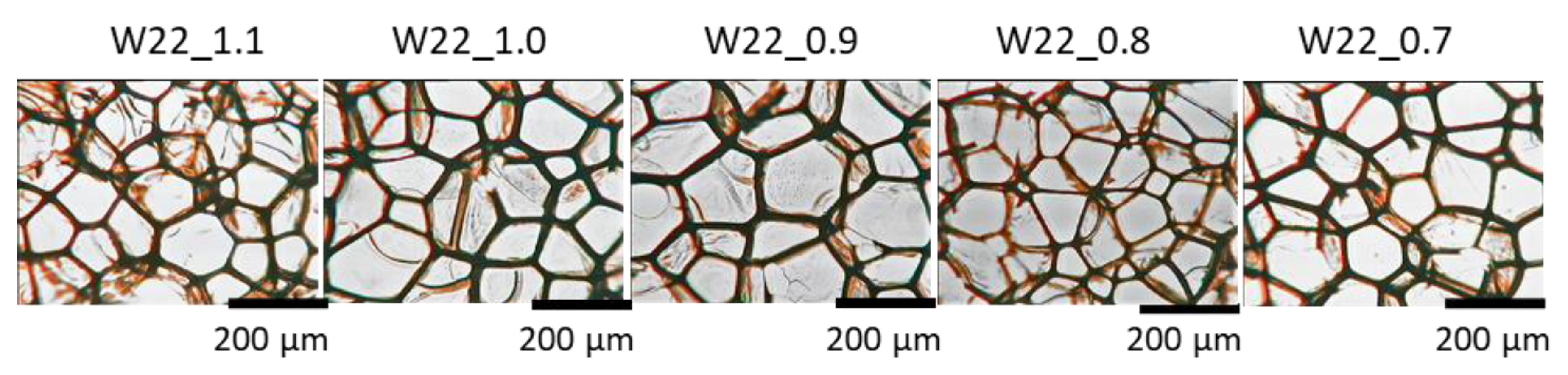

| Component, g | W20_1.1 | W22_1.1 | W24_1.1 | W26_1.1 | W28_1.1 |
|---|---|---|---|---|---|
| Bio-polyol TEA | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Polycat 142 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Tegostab 8870 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Tegostab 8523 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 |
| Ortegol 500 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| TEP | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Dabco EM400 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Water | 20.0 | 22.0 | 24.0 | 26.0 | 28.0 |
| PMDI | 431.8 | 464.9 | 498.1 | 531.2 | 564.3 |
| Isocyanate index | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 |
| Component, g | W22_1.1 | W22_1.0 | W22_0.9 | W22_0.8 | W22_0.7 | W22_0.6 |
|---|---|---|---|---|---|---|
| Bio-polyol TEA | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Polycat 142 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Tegostab 8870 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Tegostab 8523 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 |
| Ortegol 500 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| TEP | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Dabco EM400 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Water | 22.0 | 22.0 | 22.0 | 22.0 | 22.0 | 22.0 |
| PMDI | 464.9 | 422.7 | 380.4 | 338.1 | 295.2 | 253.6 |
| Isocyanate index | 1.1 | 1.0 | 0.9 | 0.8 | 0.7 | 0.6 |
| System Symbol | Number of Cells per cm2 | Cross-Section Average Cell Area [mm2] | Anisotropy Index | Cell Density, Number of Cells per cm3 × 105 | Content of Closed Cells, % |
|---|---|---|---|---|---|
| W20_1.1 | 1740 ± 255 | 0.0319 ± 0.0040 | 0.94 ± 0.05 | 0.73 ± 0.17 | <5 |
| W22_1.1 | 2430 ± 370 | 0.0253 ± 0.0047 | 0.95 ± 0.03 | 1.21 ± 0.28 | <5 |
| W24_1.1 | 1761 ± 438 | 0.0328 ± 0.0071 | 0.95 ± 0.11 | 0.75 ± 0.28 | <5 |
| W26_1.1 | 1625 ± 206 | 0.0334 ± 0.0033 | 0.94 ± 0.08 | 0.66 ± 0.12 | <5 |
| W28_1.1 | 2121 ± 194 | 0.0263 ± 0.0024 | 0.90 ± 0.11 | 0.98 ± 0.13 | <5 |
| System Symbol | ∆εl [%] | ∆εb [%] | ∆εd [%] |
|---|---|---|---|
| W20_1.1 | −0.08 ± 0.12 | −0.09 ± 0.24 | −0.50 ± 0.99 |
| W22_1.1 | −0.14 ± 0.12 | −0.17 ± 0.12 | −0.49 ± 0.37 |
| W24_1.1 | −0.13 ± 0.15 | −0.24 ± 0.17 | −0.58 ± 0.44 |
| W26_1.1 | −0.15 ± 0.11 | −0.16 ± 0.13 | −0.46 ± 0.47 |
| W28_1.1 | −0.08 ± 0.14 | −0.17 ± 0.18 | −0.50 ± 0.59 |
| System Symbol | Number of Cells per cm2 | Cross-Section Average Cell Area [mm2] | Anisotropy Index | Cell Density, Number of Cells per cm3 × 105 | Content of Closed Cells, % |
|---|---|---|---|---|---|
| W22_1.1 | 2430 ± 370 | 0.0181 ± 0.0047 | 0.95 ± 0.03 | 1.21 ± 0.28 | <5 |
| W22_1.0 | 2191 ± 148 | 0.0268 ± 0.0028 | 0.90 ± 0.04 | 1.03 ± 0.10 | <5 |
| W22_0.9 | 2260 ± 326 | 0.0252 ± 0.0044 | 0.86 ± 0.07 | 1.08 ± 0.23 | <5 |
| W22_0.8 | 2758 ± 343 | 0.0214 ± 0.0044 | 0.88 ± 0.04 | 1.46 ± 0.28 | <5 |
| W22_0.7 | 2306 ± 282 | 0.0245 ± 0.0031 | 0.90 ± 0.08 | 1.11 ± 0.21 | <5 |
| System Symbol | ∆εl [%] | ∆εb [%] | ∆εd [%] |
|---|---|---|---|
| W22_1.1 | −0.14 ± 0.12 | −0.17 ± 0.12 | −0.49 ± 0.37 |
| W22_1.0 | 0.00 ± 0.07 | 0.03 ± 0.10 | 0.08 ± 0.32 |
| W22_0.9 | 0.04 ± 0.10 | 0.02 ± 0.11 | 0.43 ± 0.37 |
| W22_0.8 | 0.07 ± 0.12 | 0.10 ± 0.09 | 0.08 ± 0.24 |
| W22_0.7 | −0.04 ± 0.12 | −0.03 ± 0.10 | 0.40 ± 0.51 |
| System Symbol | T5% [°C] | T10% [°C] | T20% [°C] | T50% [°C] | Residual Mass [%] |
|---|---|---|---|---|---|
| W20_1.1 | 254.2 | 278.1 | 309.6 | 400.7 | 17.5 |
| W22_1.1 | 257.6 | 278.7 | 305.0 | 393.1 | 16.63 |
| W24_1.1 | 256.8 | 281.8 | 310.6 | 398.4 | 16.69 |
| W26_1.1 | 257.1 | 282.7 | 312.0 | 400.5 | 20.54 |
| W28_1.1 | 263.8 | 289.0 | 318.0 | 408.7 | 22.56 |
| W22_1.0 | 257.7 | 282.6 | 312.7 | 404.7 | 20.28 |
| W22_0.9 | 254.4 | 280.7 | 312.6 | 407.4 | 20.33 |
| W22_0.8 | 244.1 | 269.9 | 306.5 | 400.0 | 17.03 |
| W22_0.7 | 218.4 | 252.4 | 295.3 | 400.4 | 14.03 |
| W22_0.6 | 218.1 | 251.1 | 294.2 | 399.7 | 11.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamińska, K.; Barczewski, M.; Kurańska, M.; Malewska, E.; Polaczek, K.; Prociak, A. The Effect of a Chemical Foaming Agent and the Isocyanate Index on the Properties of Open-Cell Polyurethane Foams. Materials 2022, 15, 6087. https://doi.org/10.3390/ma15176087
Kamińska K, Barczewski M, Kurańska M, Malewska E, Polaczek K, Prociak A. The Effect of a Chemical Foaming Agent and the Isocyanate Index on the Properties of Open-Cell Polyurethane Foams. Materials. 2022; 15(17):6087. https://doi.org/10.3390/ma15176087
Chicago/Turabian StyleKamińska, Klaudia, Mateusz Barczewski, Maria Kurańska, Elżbieta Malewska, Krzysztof Polaczek, and Aleksander Prociak. 2022. "The Effect of a Chemical Foaming Agent and the Isocyanate Index on the Properties of Open-Cell Polyurethane Foams" Materials 15, no. 17: 6087. https://doi.org/10.3390/ma15176087
APA StyleKamińska, K., Barczewski, M., Kurańska, M., Malewska, E., Polaczek, K., & Prociak, A. (2022). The Effect of a Chemical Foaming Agent and the Isocyanate Index on the Properties of Open-Cell Polyurethane Foams. Materials, 15(17), 6087. https://doi.org/10.3390/ma15176087








