Fe3O4/Diatomite-Decorated Cotton Evaporator for Continuous Solar Steam Generation and Water Treatment
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
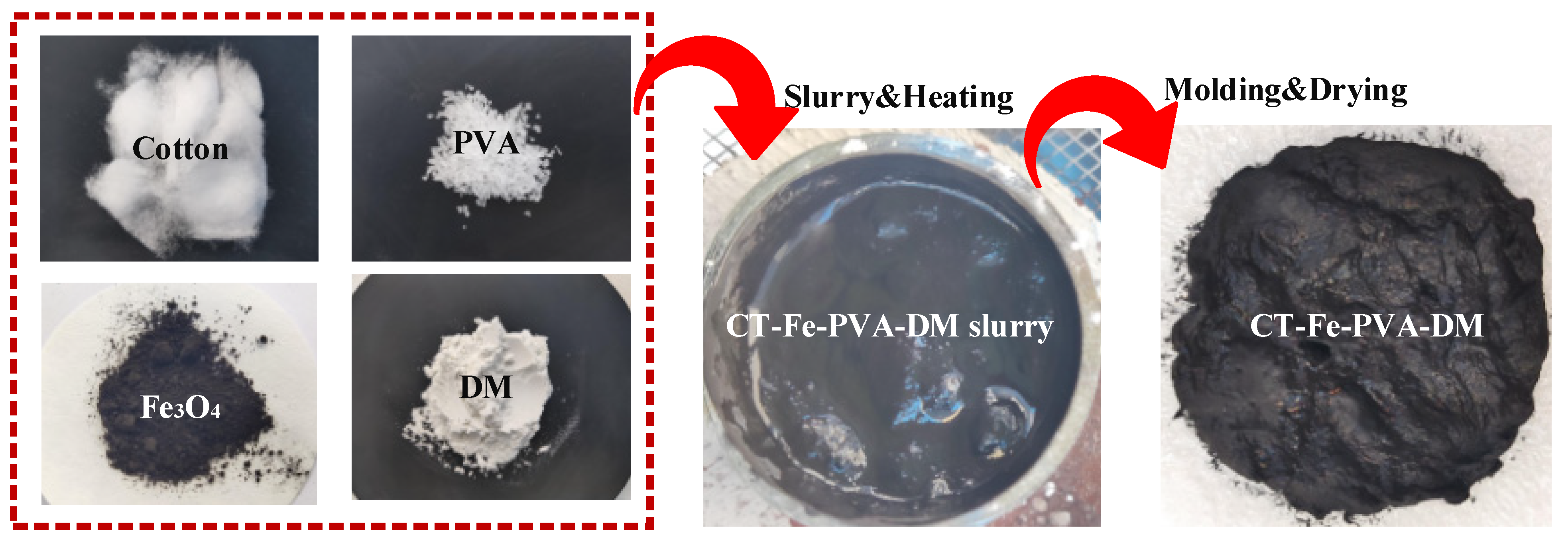
2.2. Preparation of Samples
2.3. Characterization of Samples
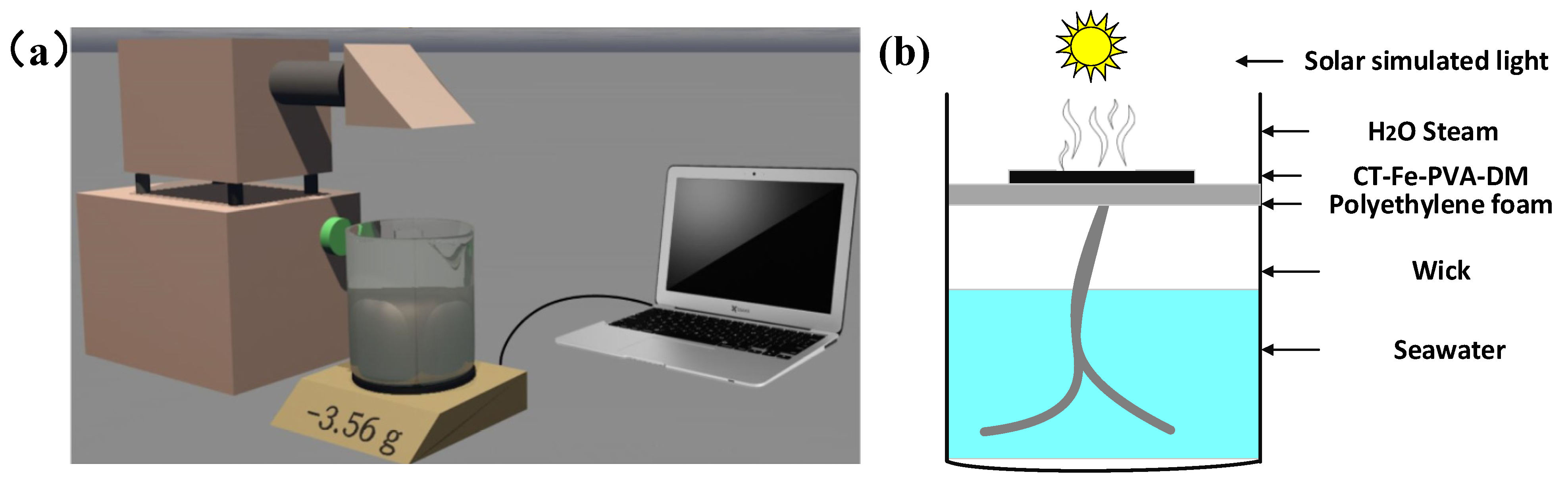
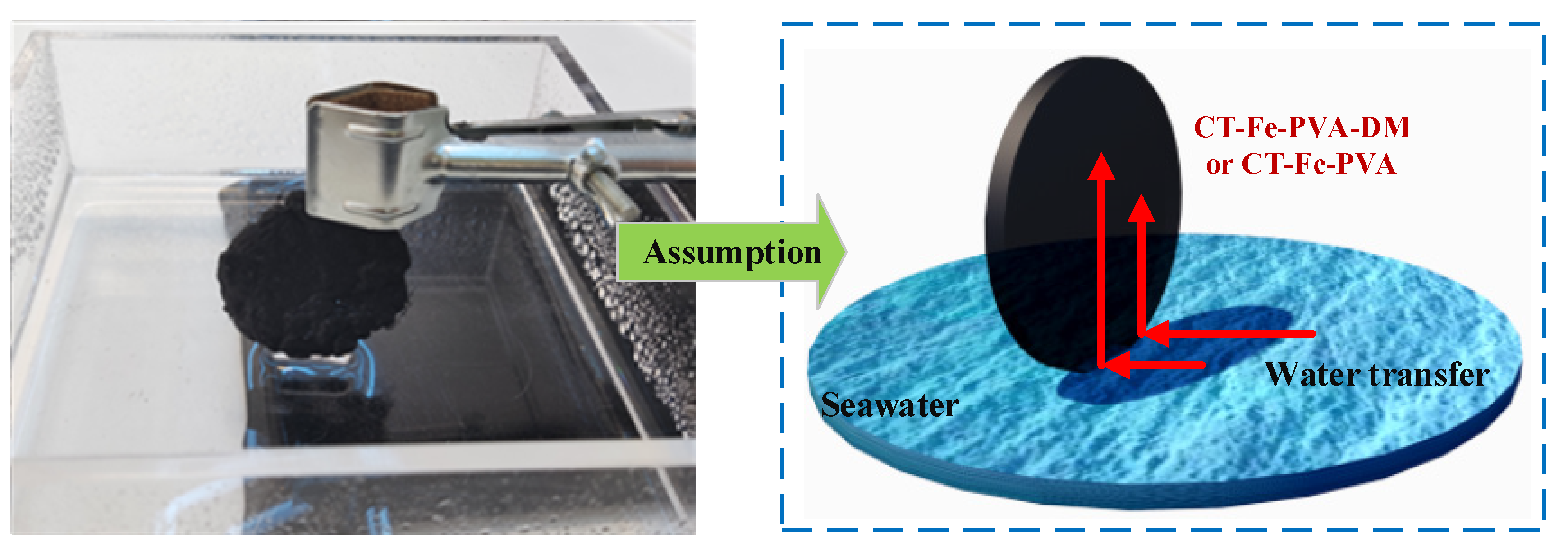
2.4. SSG Experiment
2.5. SSG Test
2.6. Solar Water Treatment
3. Results and Discussion
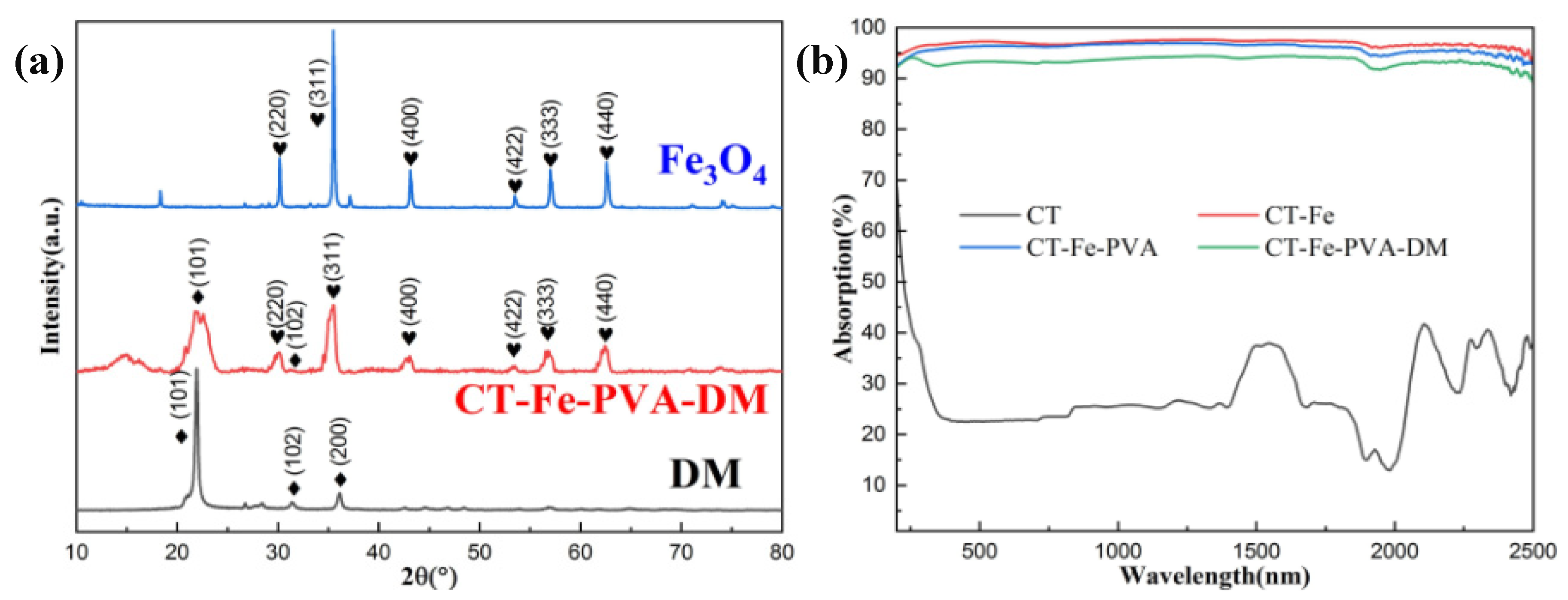
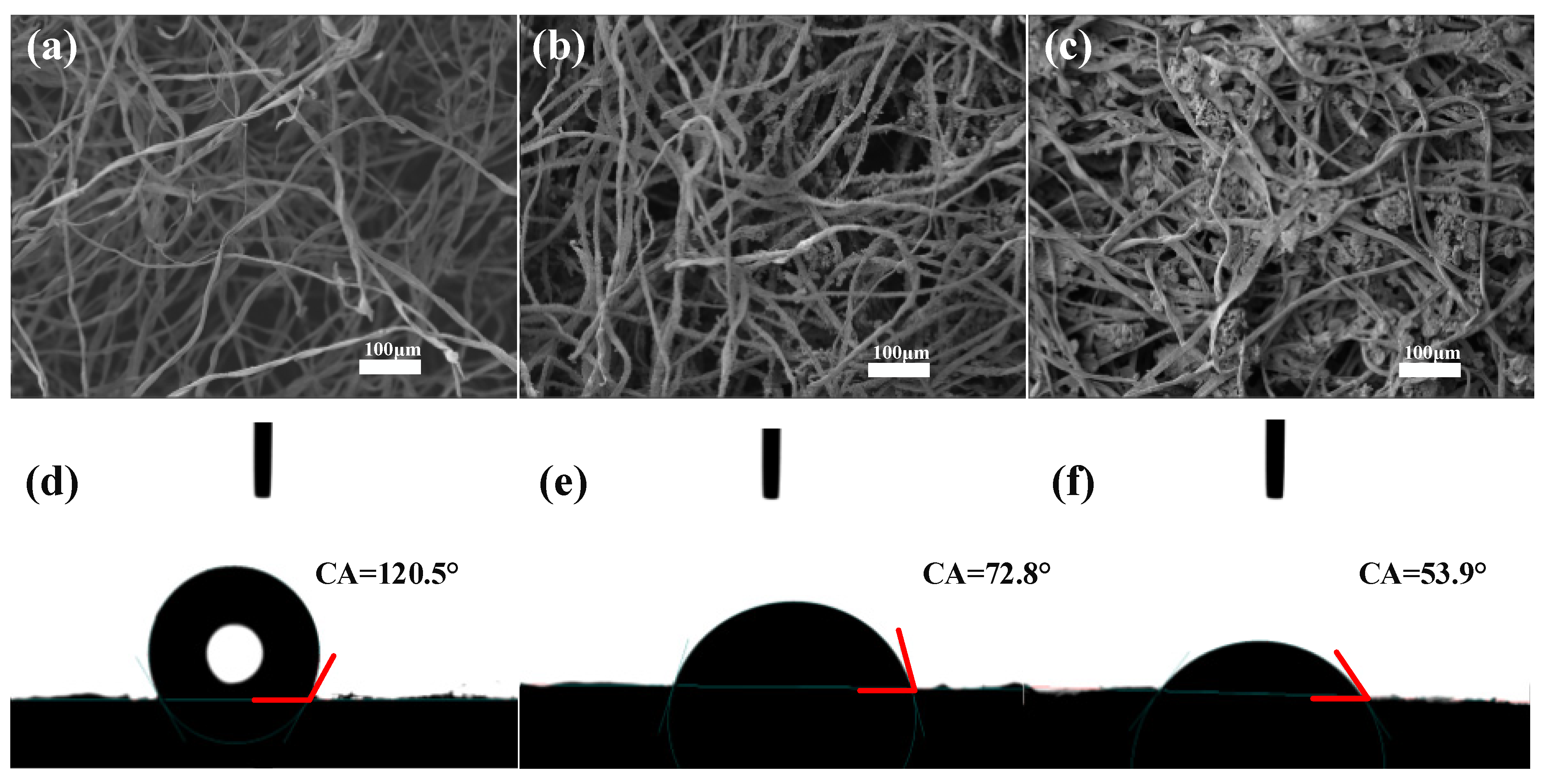
3.1. Characterization of Samples
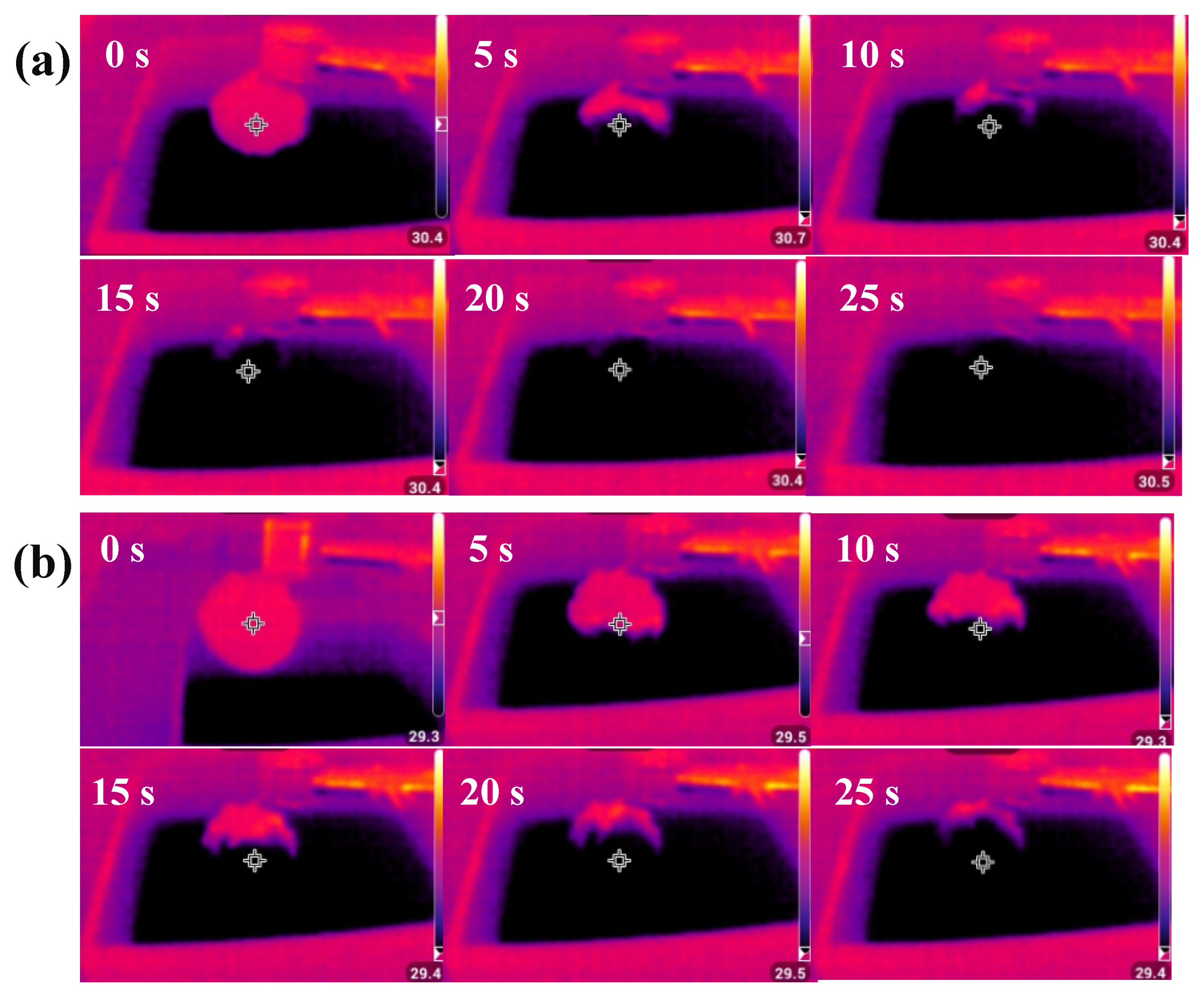
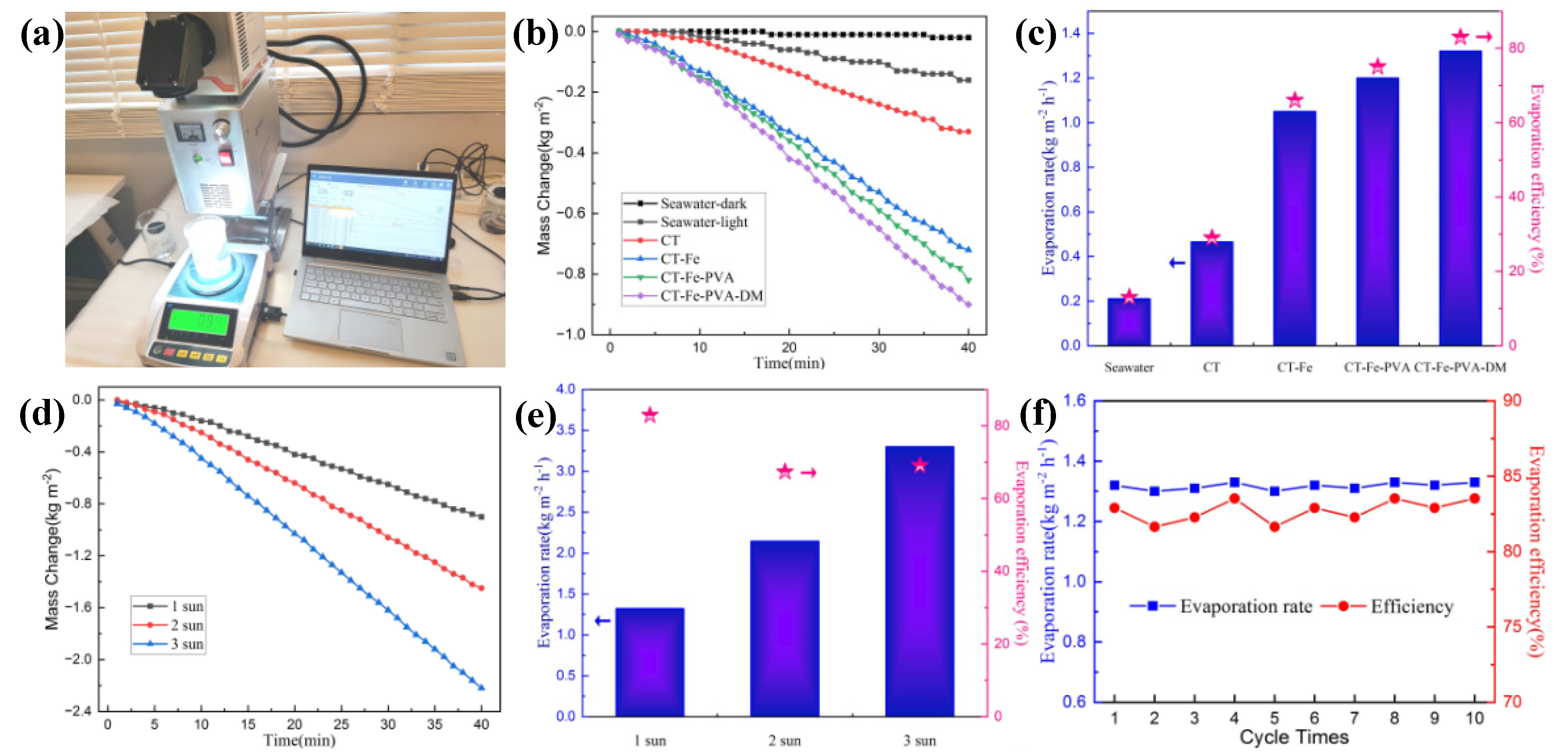
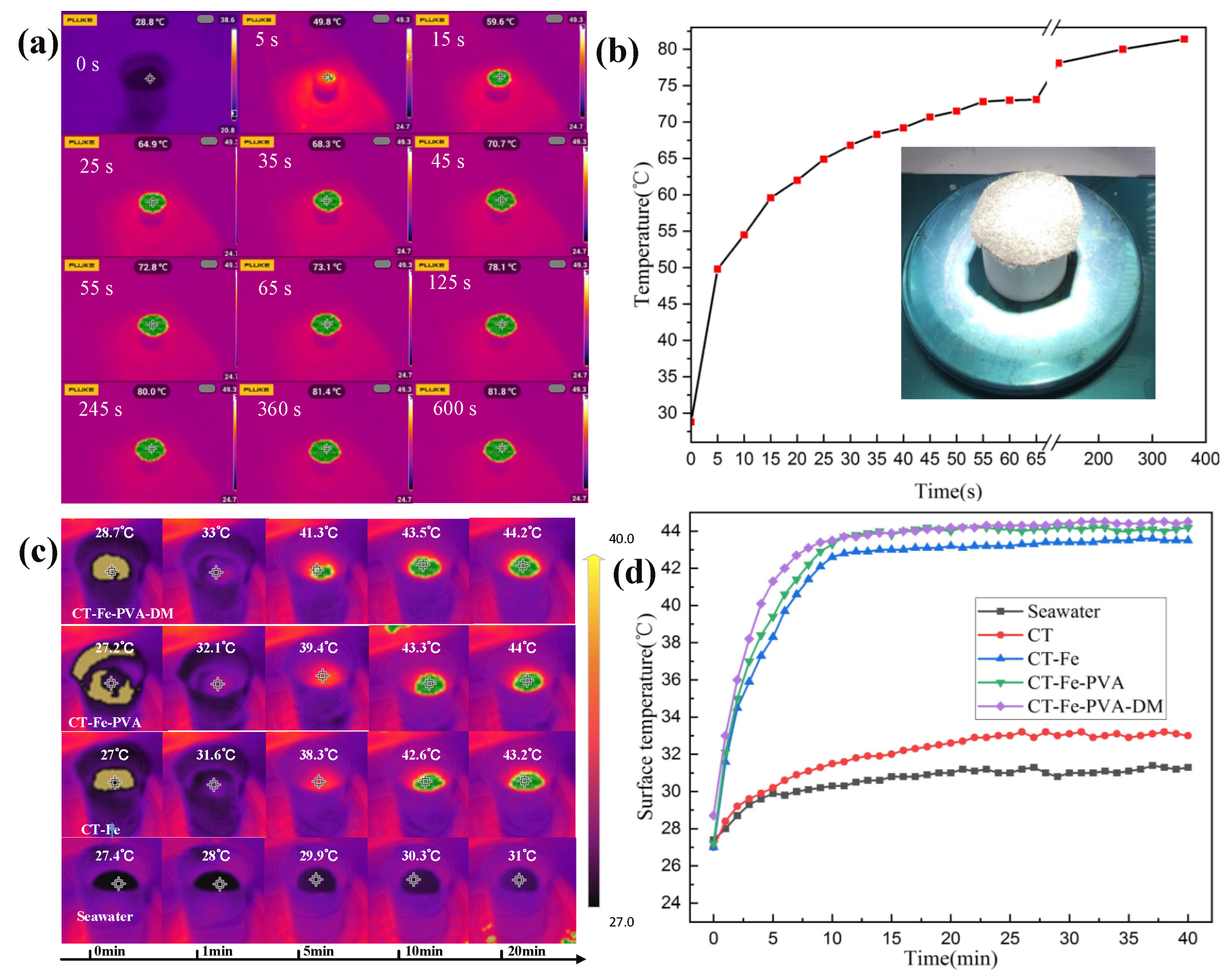
3.2. Photothermal Conversion and SSG Experiment
3.3. Water Treatment Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Montano-Medina, C.U.; Lopez-Martinez, L.M.; Ochoa-Teran, A.; Lopez-Maldonado, E.A.; Salazer-Gastelum, M.I.; Trujillo-Nvarrete, B.; Perez-Sicairos, S.; Cornejo-Bravo, J. New pyridyl and aniline-functionalized carbamoylcarboxylic acids for removal of metal ions from water by coagulation-flocculation process. Chem. Eng. J. 2022, 451, 138396. [Google Scholar] [CrossRef]
- Wu, Y.T.; Kong, R.; Ma, C.L.; Li, L.Z.; Zheng, Y.; Lu, Y.Z.; Liang, L.L.; Pang, Y.J.; Wu, Q.; Shen, Z.H.; et al. Simulation-Guided Design of Bamboo Leaf-Derived Carbon-Based High-Efficiency Evaporator for Sola-Driven Interface Water Evaporation. Energy Environ. Mater. 2022. [Google Scholar] [CrossRef]
- Wang, C.B.; Wang, J.L.; Li, Z.T.; Xu, K.Y.; Lei, T.; Wang, W.K. Superhydrophilic porous carbon foam as a self-desalting monolithic solar steam generation device with high energy efficiency. J. Mater. Chem. A 2020, 8, 9528–9535. [Google Scholar] [CrossRef]
- Yin, J.C.; You, X.; Zhang, Z.X.; Guo, Z.Z.; Wang, J.; Wang, X.B. Boron nanosheets loaded with MoS2 porous sponges for water purification. J. Water Process Eng. 2021, 41, 102048. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Luo, X.Y.; Song, X.Y.; Guo, W.; Yu, K.; Yang, C.Y.; Qu, F.Y. Turning waste into treasure: Carbonized walnut shell for solar-driven water evaporation. Mater. Lett. 2022, 307, 131057. [Google Scholar] [CrossRef]
- Peng, F.G.; Xu, J.; Bai, X.L.; Feng, G.P.; Zeng, X.H.; Ibn Raihan, M.R.; Bao, H.F. A Janus solar evaporator with 2D water path for highly efficient salt-resisting solar steam generation. Sol. Energy Mater. Sol. Cells 2021, 221, 110910. [Google Scholar] [CrossRef]
- Wang, Y.J.; Nie, J.L.; He, Z.; Zhi, Y.H.; Ma, X.H.; Zhong, P. Ti3C2Tx MXene Nanoflakes Embedded with Copper Indium Selenide Nanoparticles for Desalination and Water Purification through High-Efficiency Solar-Driven Membrane Evaporation. ACS Appl. Mater. Interfaces 2022, 14, 5876–5886. [Google Scholar] [CrossRef]
- Xiao, J.X.; Guo, Y.; Luo, W.Q.; Wang, D.; Zhong, S.K.; Yue, Y.R.; Han, C.N.; Lv, R.X.; Feng, J.B.; Wang, J.Q.; et al. A scalable, cost-effective and salt-rejecting MoS2/SA@melamine foam for continuous solar steam generation. Nano Energy 2021, 87, 106213. [Google Scholar] [CrossRef]
- Li, Z.T.; Wang, C.B.; Su, J.B.; Ling, S.; Wang, W.; An, M. Fast-Growing Field of Interfacial Solar Steam Generation: Evolutional Materials, Engineered Architectures, and Synergistic Applications. Solar RRL 2019, 3, 1800206. [Google Scholar] [CrossRef]
- Li, X.Q.; Lin, R.X.; Ni, G.; Xu, N.; Hu, X.Z.; Zhu, B.; Lv, G.X.; Li, J.L.; Zhu, S.N.; Zhu, J. Three-dimensional artificial transpiration for efficient solar waste-water treatment. Natl. Sci. Rev. 2018, 5, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Xu, N.; Hu, X.Z.; Xu, W.C.; Li, X.Q.; Zhou, L.; Zhu, S.N.; Zhu, J. Mushrooms as Efficient Solar Steam-Generation Devices. Adv. Mater. 2017, 29, 1606762. [Google Scholar] [CrossRef]
- Zhang, L.; Mu, L.; Zhou, Q.X.; Hu, X.G. Solar-assisted fabrication of dimpled 2H-MoS2 membrane for highly efficient water desalination. Water Res. 2020, 170, 115367. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Feng, M.; Zhang, F.; Lin, H.M.; Guo, W.; Yu, K.; Yang, C.Y.; Qu, F.Y. Three-dimensional water evaporator based on carbonized silkworm cocoon for highly effective solar-driven water evaporation and wastewater purification. Mater. Lett. 2022, 312, 131661. [Google Scholar] [CrossRef]
- Xu, Y.L.; Mu, H.C.; Han, X.S.; Sun, T.J.; Fan, X.F.; Lv, B.W.; Pan, Z.L.; Song, Y.X.; Song, C.W. A simple, flexible, and porous polypyrrole-wax gourd evaporator with excellent light absorption for efficient solar steam generation. Int. J. Energy Res. 2021, 45, 21476–21486. [Google Scholar] [CrossRef]
- Wang, S.; Fan, Y.K.; Wang, F.; Su, Y.N.; Zhou, X.; Zhu, Z.Q.; Sun, H.X.; Liang, W.D.; Li, A. Potentially scalable fabrication of salt-rejection evaporator based on electrogenerated polypyrrole-coated nickel foam for efficient solar steam generation. Desalination 2021, 505, 114982. [Google Scholar] [CrossRef]
- Mu, X.J.; Gu, Y.F.; Wang, P.F.; Wei, A.Y.; Tian, Y.Z.; Zhou, J.H.; Chen, Y.L.; Zhang, J.H.; Sun, Z.Q.; Liu, J.H.; et al. Strategies for breaking theoretical evaporation limitation in direct solar steam generation. Sol. Energy Mater. Sol. Cells 2021, 220, 110842. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Ren, L.P.; Xu, J.; Shang, B.; Liu, X.; Xu, W.L. Magnetically Driven Tunable 3D Structured Fe3O4 Vertical Array for High-Performance Solar Steam Generation. Small 2022, 18, e2105198. [Google Scholar] [CrossRef]
- Mnoyan, A.; Choi, M.; Kim, D.H.; Ku, B.J.; Kim, H.; Lee, K.J.; Yasin, A.S.; Nam, S.; Lee, K. Cheap, facile, and upscalable activated carbon-based photothermal layers for solar steam generation. RSC Adv. 2020, 10, 42432–42440. [Google Scholar] [CrossRef]
- Zhang, C.F.; Yuan, B.H.; Liang, Y.; Yang, L.X.; Bai, L.J.; Yang, H.W.; Wei, D.L.; Wang, F.; Wang, Q.; Wang, W.X.; et al. Carbon nanofibers enhanced solar steam generation device based on loofah biomass for water purification. Mater. Chem. Phys. 2021, 258, 123998. [Google Scholar] [CrossRef]
- Dong, X.L.; Gao, S.W.; Li, S.H.; Zhu, T.X.; Huang, J.Y.; Chen, Z.; Lai, Y.K. Bioinspired structural and functional designs towards interfacial solar steam generation for clean water production. Mater. Chem. Front. 2021, 5, 1510–1524. [Google Scholar] [CrossRef]
- Zhang, L.J.; Bai, B.; Hu, N.; Wang, H.L. Low-cost and facile fabrication of a candle soot/adsorbent cotton 3D-interfacial solar steam generation for effective water evaporation. Sol. Energy Mater. Sol. Cells 2021, 221, 110876. [Google Scholar] [CrossRef]
- Kiriarachchi, H.D.; Hassan, A.A.; Awad, F.S.; El-Shall, M.S. Metal-free functionalized carbonized cotton for efficient solar steam generation and wastewater treatment. RSC Adv. 2021, 12, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.L.; Ying, P.J.; Geng, Y.; Tian, Y.Q.; Li, M.; Sun, K. Narrow Band-Gap Ti2O3 Photo-thermal Conversion Based on CA/CF. Res. Explor. Lab. 2020, 39, 54–58. [Google Scholar]
- Yao, J.X.; Zhang, K.; Wang, W.; Zuo, X.Q.; Yang, Q.; Wu, M.Z.; Li, G. Great enhancement of electrochemical cyclic voltammetry stabilization of Fe3O4 microspheres by introducing 3DRGO. Electrochim. Acta 2018, 279, 168–176. [Google Scholar] [CrossRef]
- Xu, R.Q.; Wei, N.; Li, Z.K.; Song, X.J.; Li, Q.; Sun, K.Y.; Yang, E.Q.; Gong, L.K.; Sui, Y.L.; Tian, J.; et al. Construction of hierarchical 2D/2D Ti3C2/MoS2 nanocomposites for high-efficiency solar steam generation. J. Colloid Interface Sci. 2021, 584, 125–133. [Google Scholar] [CrossRef]
- Song, L.; Zhang, X.F.; Wang, Z.G.; Zheng, T.R.; Yao, J.F. Fe3O4/polyvinyl alcohol decorated delignified wood evaporator for continuous solar steam generation. Desalination 2021, 507, 115024. [Google Scholar] [CrossRef]
- Chen, R.; Zhu, K.H.; Gan, Q.M.; Yu, Y.Q.; Zhang, T.Q.; Liu, X.W.; Ye, M.M.; Yin, Y.D. Interfacial solar heating by self-assembled Fe3O4@C film for steam generation. Mater. Chem. Front. 2017, 1, 2620–2626. [Google Scholar] [CrossRef]
- Liu, H.J.; Alam, M.K.; He, M.T.; Liu, Y.; Wang, L.M.; Qin, X.H.; Yu, J.Y. Sustainable Cellulose Aerogel from Waste Cotton Fabric for High-Performance Solar Steam Generation. ACS Appl. Mater. Interfaces 2021, 13, 49860–49867. [Google Scholar] [CrossRef]
- Yan, M.; Weng, T.F.; Yu, X.; Li, M.H.; Qiao, Q.; Zhou, Y.T.; Li, Z.H.; Wei, J.; Yu, X.M. Light absorption enhancement by incorporating nanostructured-diatomite for improving performance of polymeric photodetector. Opt. Mater. 2021, 120, 111403. [Google Scholar] [CrossRef]
- He, L.H.; Li, L.; Zhou, C.; Li, W.H. Hydrophobic surface modification of diatomit with silane coupling agent KH-570. Mod. Chem. Ind. 2014, 34, 93–97. [Google Scholar]
- Ma, Y.H.; Cao, J.R. Preparation of mechanically robust Fe3O4/porous carbon/diatomite composite monolith for solar steam generation. Environ. Sci. Pollut. Res. Int. 2020, 27, 45775–45786. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.Z.; Wang, X.Y.; Gu, Y.F.; Mu, X.J.; Wang, P.F.; Wei, A.Y.; Zhang, J.H.; Chen, Y.L.; Sun, Z.Q.; Jia, L.F.; et al. Versatile PVA/CS/CuO aerogel with superior hydrophilic and mechanical properties towards efficient solar steam generation. Nano Select 2021, 2, 2380–2389. [Google Scholar] [CrossRef]
- Zou, Y.; Yang, P.; Yang, L.; Li, N.; Duan, G.G.; Liu, X.H.; Li, Y.W. Boosting solar steam generation by photothermal enhanced polydopamine/wood composites. Polymer 2021, 217, 123464. [Google Scholar] [CrossRef]
- Wang, Y.D.; Wu, X.; Gao, T.; Lu, Y.; Yang, X.F.; Chen, G.Y.; Owens, G.; Xu, H.L. Same materials, bigger output: A reversibly transformable 2D–3D photothermal evaporator for highly efficient solar steam generation. Nano Energy 2021, 79, 105477. [Google Scholar] [CrossRef]
- He, J.X.; Liu, F.; Xiao, C.H.; Sun, H.X.; Li, J.Y.; Zhu, Z.Q.; Liang, W.D.; Li, A. Fe3O4/PPy-Coated Superhydrophilic Polymer Porous Foam: A Double Layered Photothermal Material with a Synergistic Light-to-Thermal Conversion Effect toward Desalination. Langmuir 2021, 37, 12397–12408. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Huang, C.L.; Luo, X.; Rao, Z.H. High-performance solar steam generation of a paper-based carbon particle system. Appl. Therm. Eng. 2018, 142, 566–572. [Google Scholar] [CrossRef]
- He, Y.L.; Zhang, W.Q.; Rao, P.H.; Jin, P. Diatomite- and polyvinyl alcohol-modified nonwoven fabric in membrane bioreactor for wastewater reclamation. Desalination Water Treat. 2014, 57, 2952–2958. [Google Scholar] [CrossRef]
- Shan, X.L.; Lin, Y.W.; Zhao, A.Q.; Di, Y.S.; Hu, Y.J.; Guo, Y.J.; Gan, Z.X. Porous reduced graphene oxide/nickel foam for highly efficient solar steam generation. Nanotechnology 2019, 30, 425403. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Y.D.; Wu, P.; Zhao, J.Y.; Lu, Y.; Yang, X.F.; Xu, H.L. Dual-Zone Photothermal Evaporator for Antisalt Accumulation and Highly Efficient Solar Steam Generation. Adv. Funct. Mater. 2021, 31, 2102618. [Google Scholar] [CrossRef]
- Higgins, M.W.; Shakeel Rahmaan, A.R.; Devarapalli, R.R.; Shelke, M.V.; Jha, N. Carbon fabric based solar steam generation for wastewater treatment. Sol. Energy 2018, 159, 800–810. [Google Scholar] [CrossRef]
- Cao, P.; Zhao, L.M.; Zhang, J.; Zhang, L.W.; Yuan, P.; Zhang, Y.Y.; Li, Q.W. Gradient Heating Effect Modulated by Hydrophobic/Hydrophilic Carbon Nanotube Network Structures for Ultrafast Solar Steam Generation. ACS Appl. Mater. Interfaces 2021, 13, 19109–19116. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, X.F.; Liu, J.; Zeng, M.J.; Feng, X.Y.; Jia, X.Q.; Yu, Z.Z. Coating of Wood with Fe2O3-Decorated Carbon Nanotubes by One-Step Combustion for Efficient Solar Steam Generation. ACS Appl. Mater Interfaces 2021, 13, 22845–22854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.T.; Zhu, W.X.; Shi, S.; Hu, N.; Suo, Y.R.; Wang, J.L. Bioinspired foam with large 3D macropores for efficient solar steam generation. J. Mater. Chem. A 2018, 6, 16220–16227. [Google Scholar] [CrossRef]
- Ding, D.W.; Wu, H.; He, X.P.; Yang, F.; Gao, C.B.; Yin, Y.D.; Ding, S.J. A metal nanoparticle assembly with broadband absorption and suppressed thermal radiation for enhanced solar steam generation. J. Mater. Chem. A 2021, 9, 11241–11247. [Google Scholar] [CrossRef]
- Zhou, Q.X.; Li, H.; Li, D.D.; Wang, B.B.; Wang, H.; Bai, J.; Ma, S.H.; Wang, G. A graphene assembled porous fiber-based Janus membrane for highly effective solar steam generation. J. Colloid Interface Sci. 2021, 592, 77–86. [Google Scholar] [CrossRef]

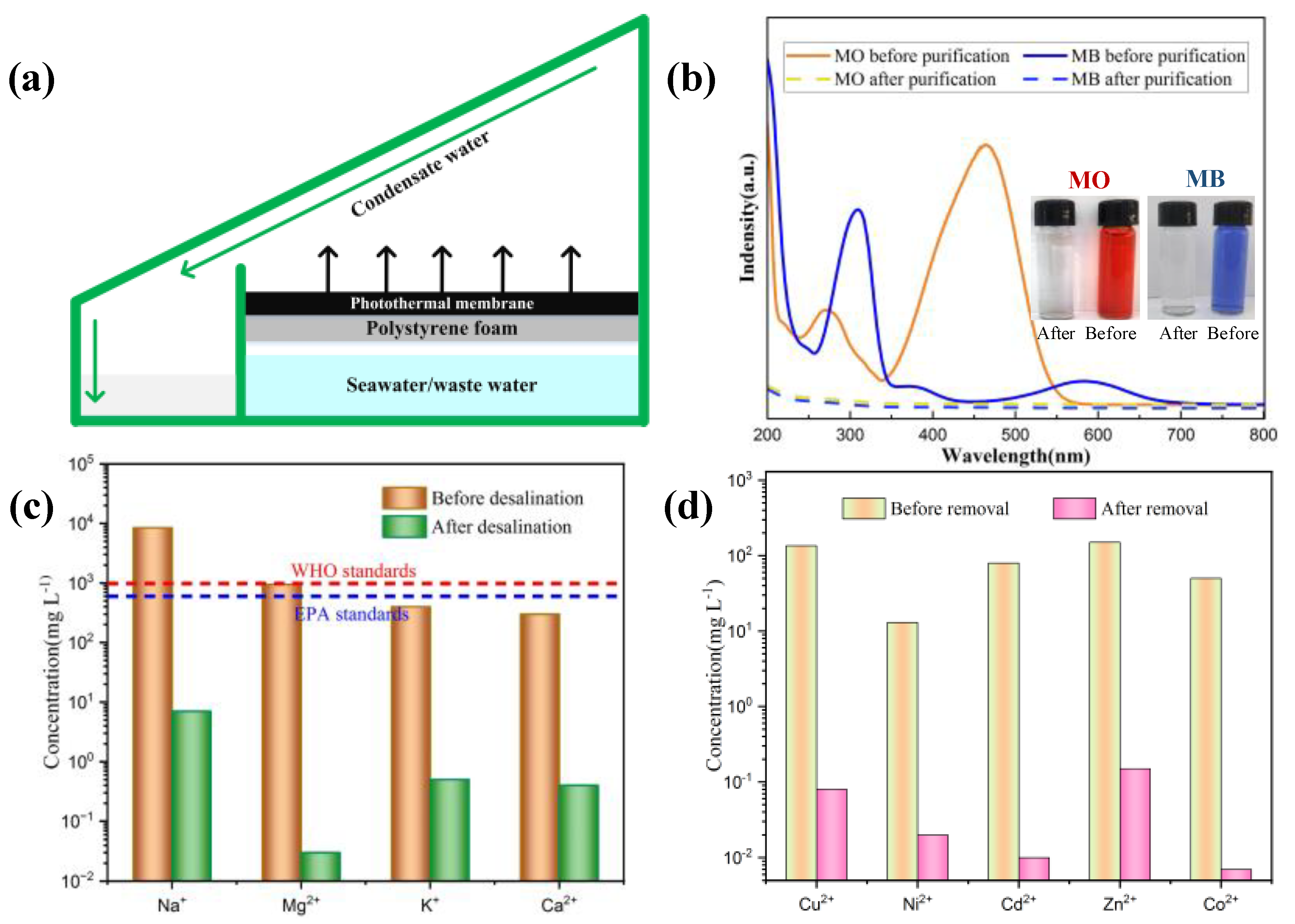
| Type | The Force Required to Break the Samples (Newton) |
|---|---|
| CT | 1.4 |
| CT-Fe | 1.6 |
| CT-Fe-PVA | 12 |
| CT-Fe-PVA-DM | 11 |
| Ion Type | Cu2+ | Ni2+ | Cd2+ | Zn2+ | Co2+ |
|---|---|---|---|---|---|
| Ion concentration before desalination (mg/L) | 135 | 13 | 80 | 150 | 50 |
| Ion concentration after desalination (mg/L) | 0.08 | 0.02 | 0.01 | 0.15 | 0.007 |
| Ion Type | Na+ | Mg2+ | K+ | Ca2+ |
|---|---|---|---|---|
| Ion concentration before desalination (mg/L) | 8520 | 950 | 400 | 310 |
| Ion concentration after desalination (mg/L) | 7.21 | 0.03 | 0.53 | 0.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Z.; Xu, H.; Yang, B.; Yao, J.; Li, G.; Guo, K.; Wang, N.; Liang, N. Fe3O4/Diatomite-Decorated Cotton Evaporator for Continuous Solar Steam Generation and Water Treatment. Materials 2022, 15, 6110. https://doi.org/10.3390/ma15176110
Bai Z, Xu H, Yang B, Yao J, Li G, Guo K, Wang N, Liang N. Fe3O4/Diatomite-Decorated Cotton Evaporator for Continuous Solar Steam Generation and Water Treatment. Materials. 2022; 15(17):6110. https://doi.org/10.3390/ma15176110
Chicago/Turabian StyleBai, Zhi, Haifeng Xu, Bo Yang, Jixin Yao, Guang Li, Kai Guo, Nan Wang, and Nannan Liang. 2022. "Fe3O4/Diatomite-Decorated Cotton Evaporator for Continuous Solar Steam Generation and Water Treatment" Materials 15, no. 17: 6110. https://doi.org/10.3390/ma15176110





