Design of a Novel Trabecular Acetabular Cup and Selective Laser Melting Fabrication
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
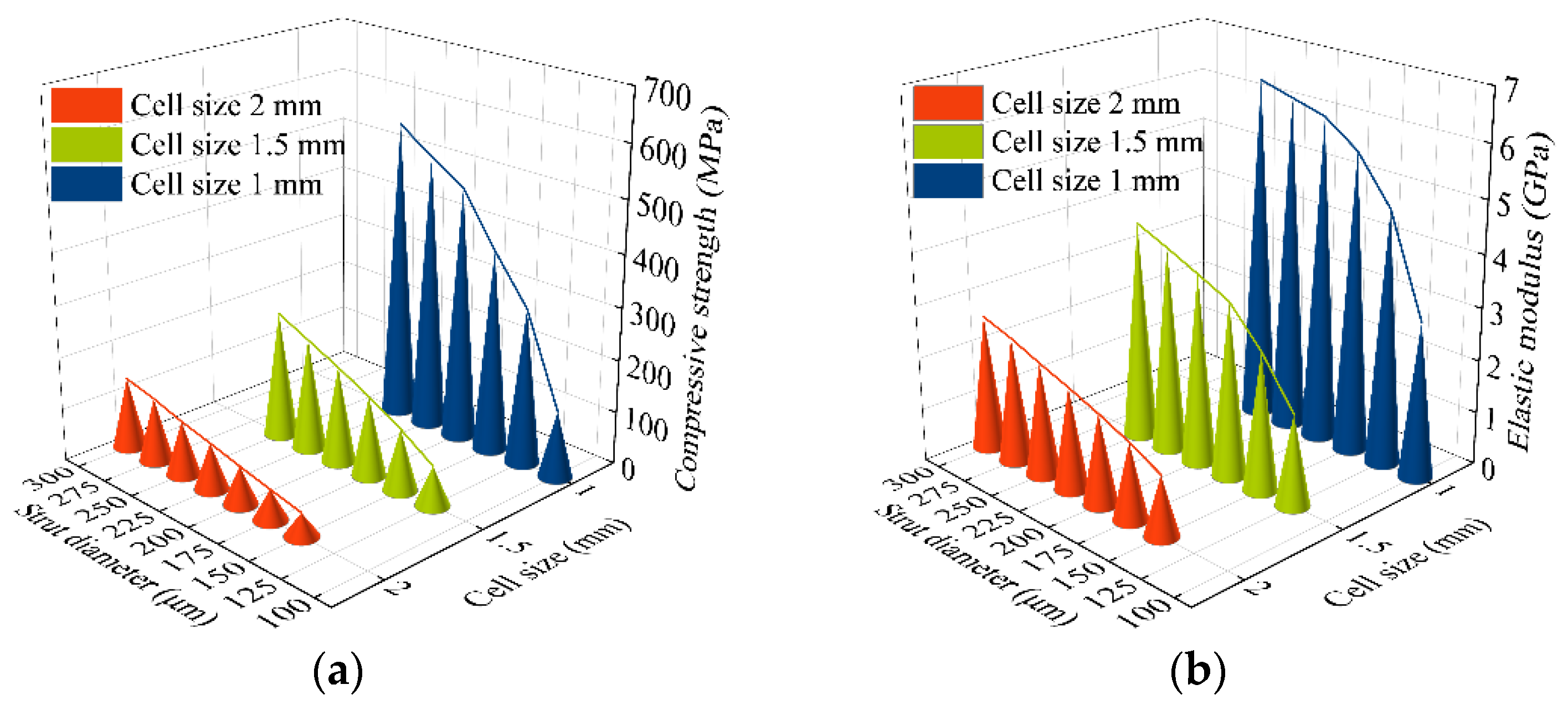
3.1. Compression Properties of Porous Ti6Al4V Alloy
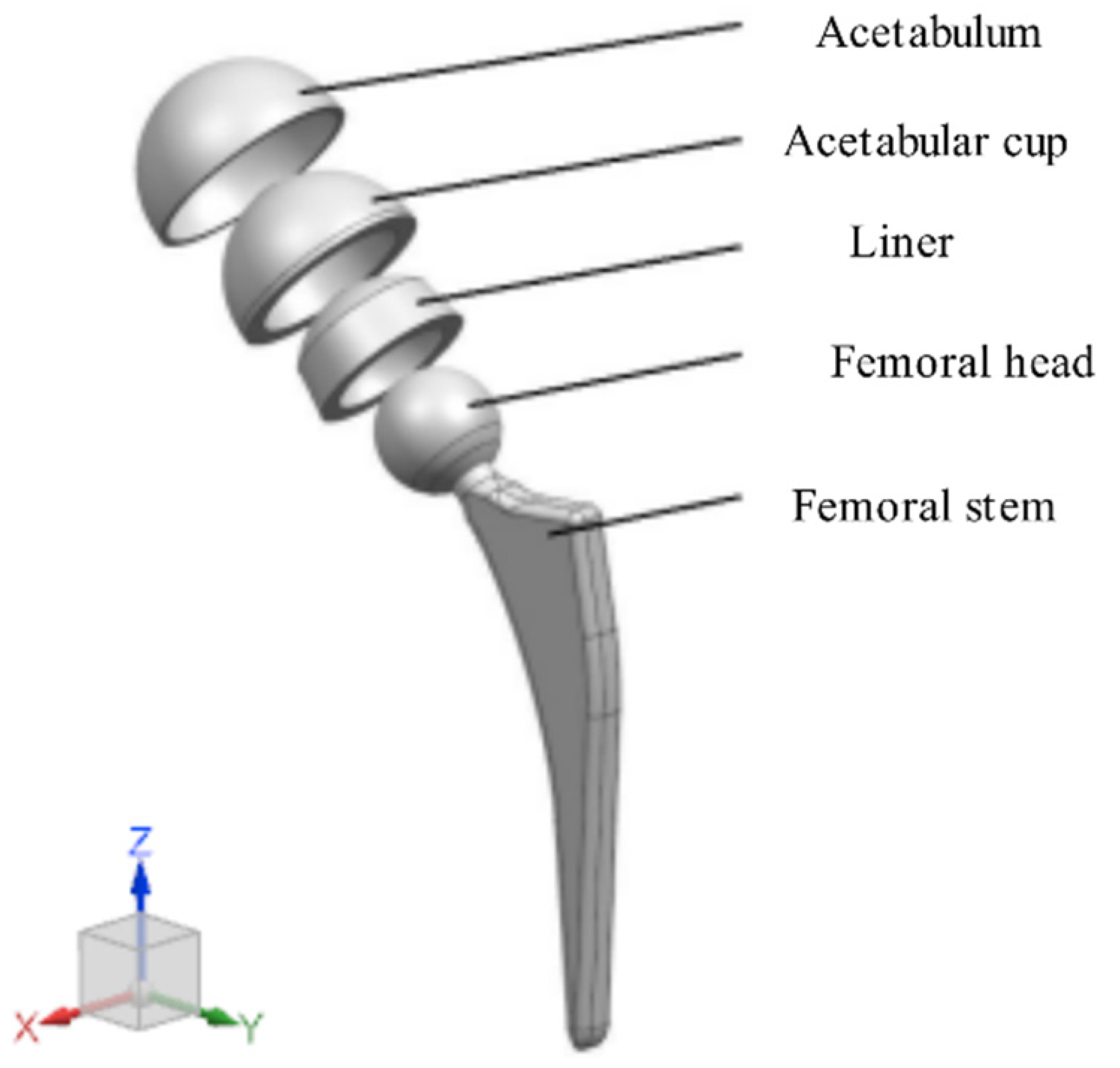
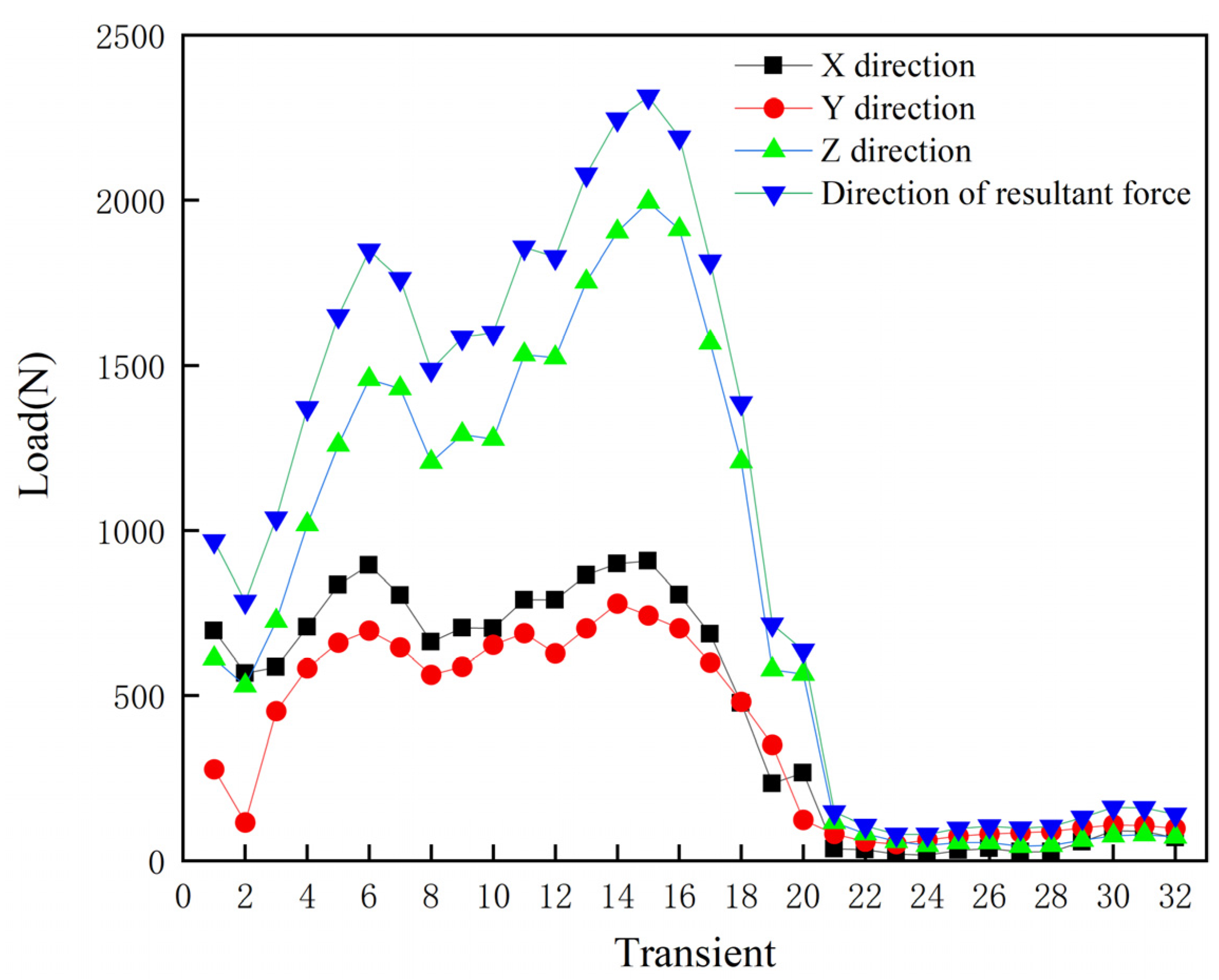
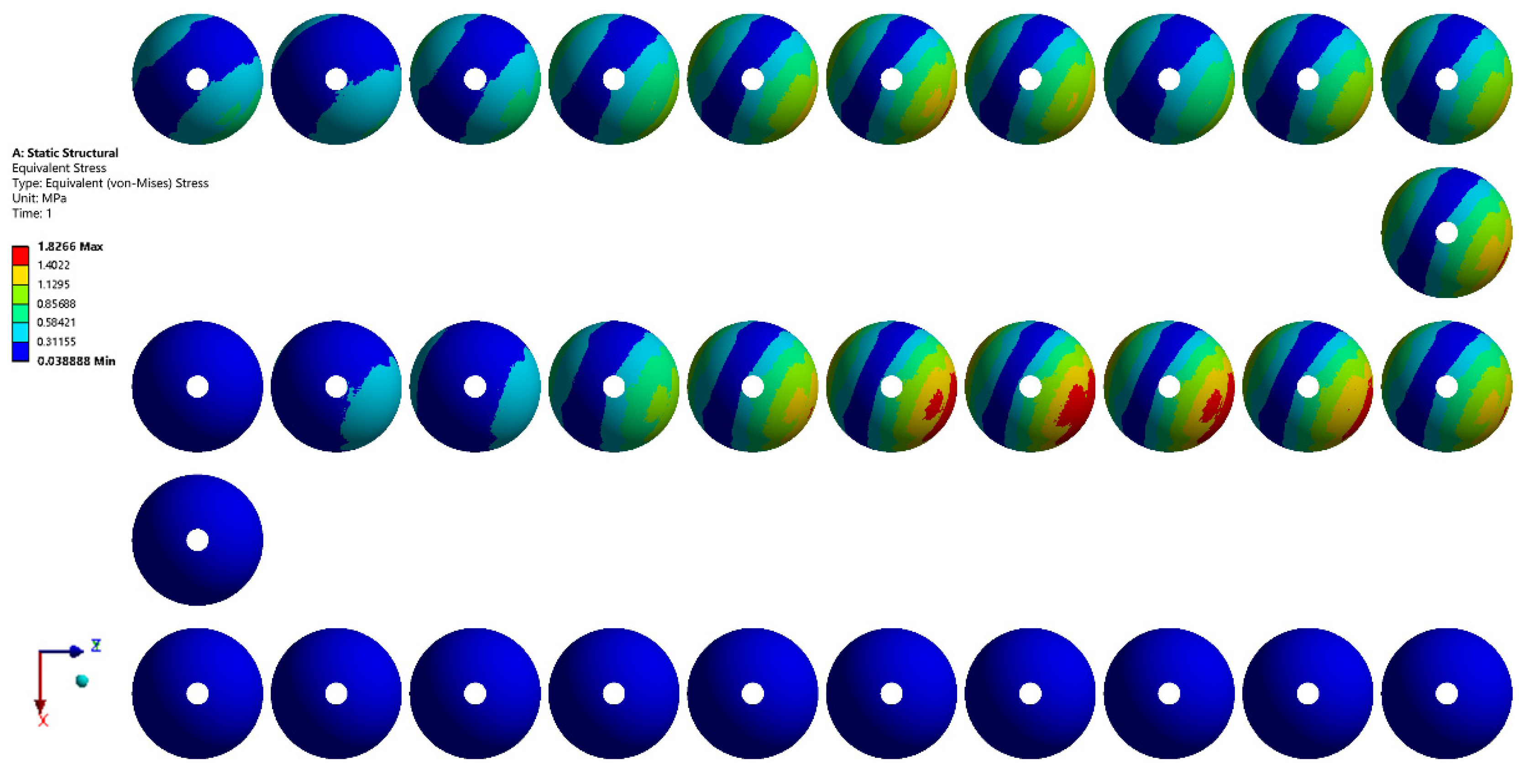
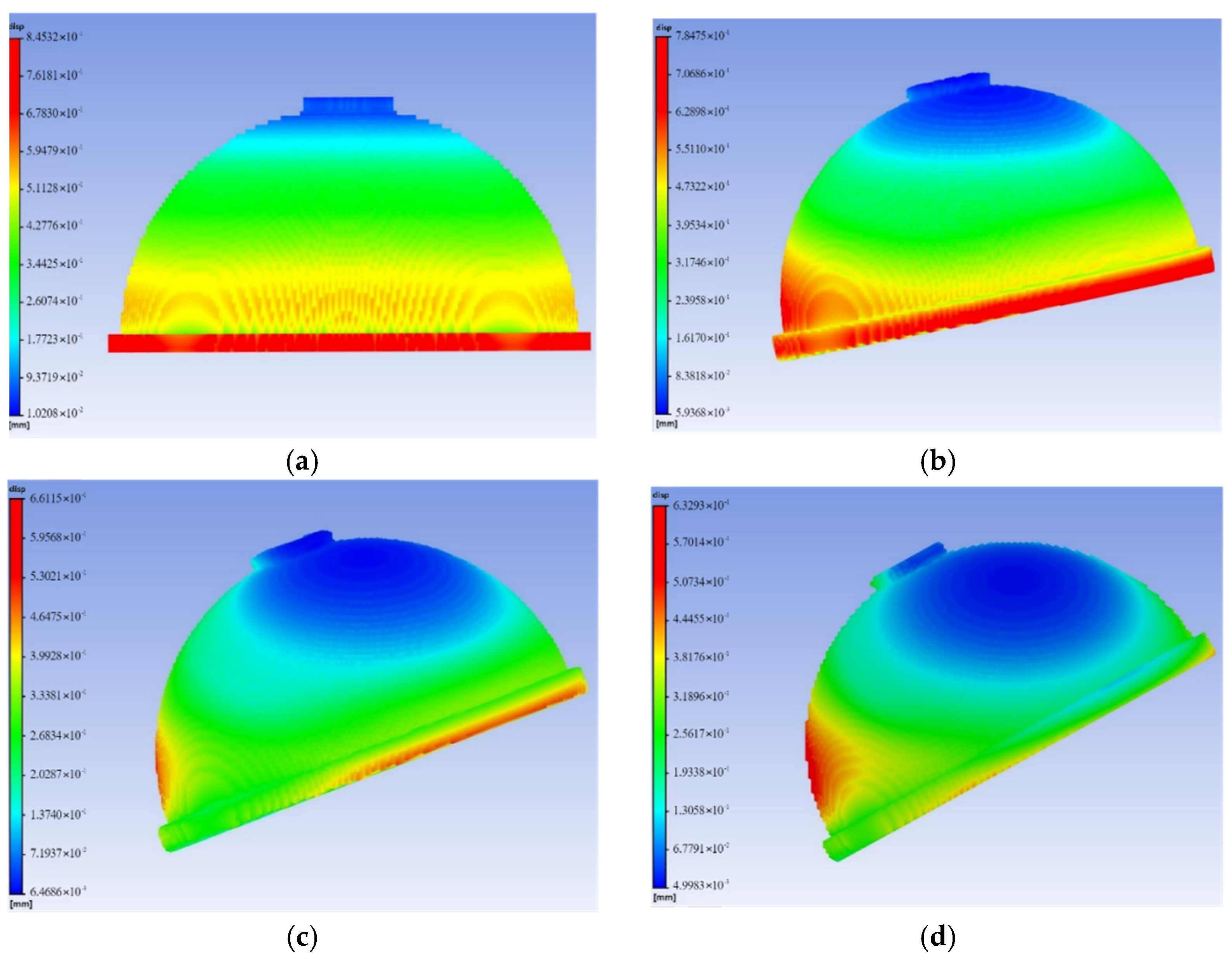
3.2. Finite Element Simulation of the Acetabular Cup under Gait Conditions
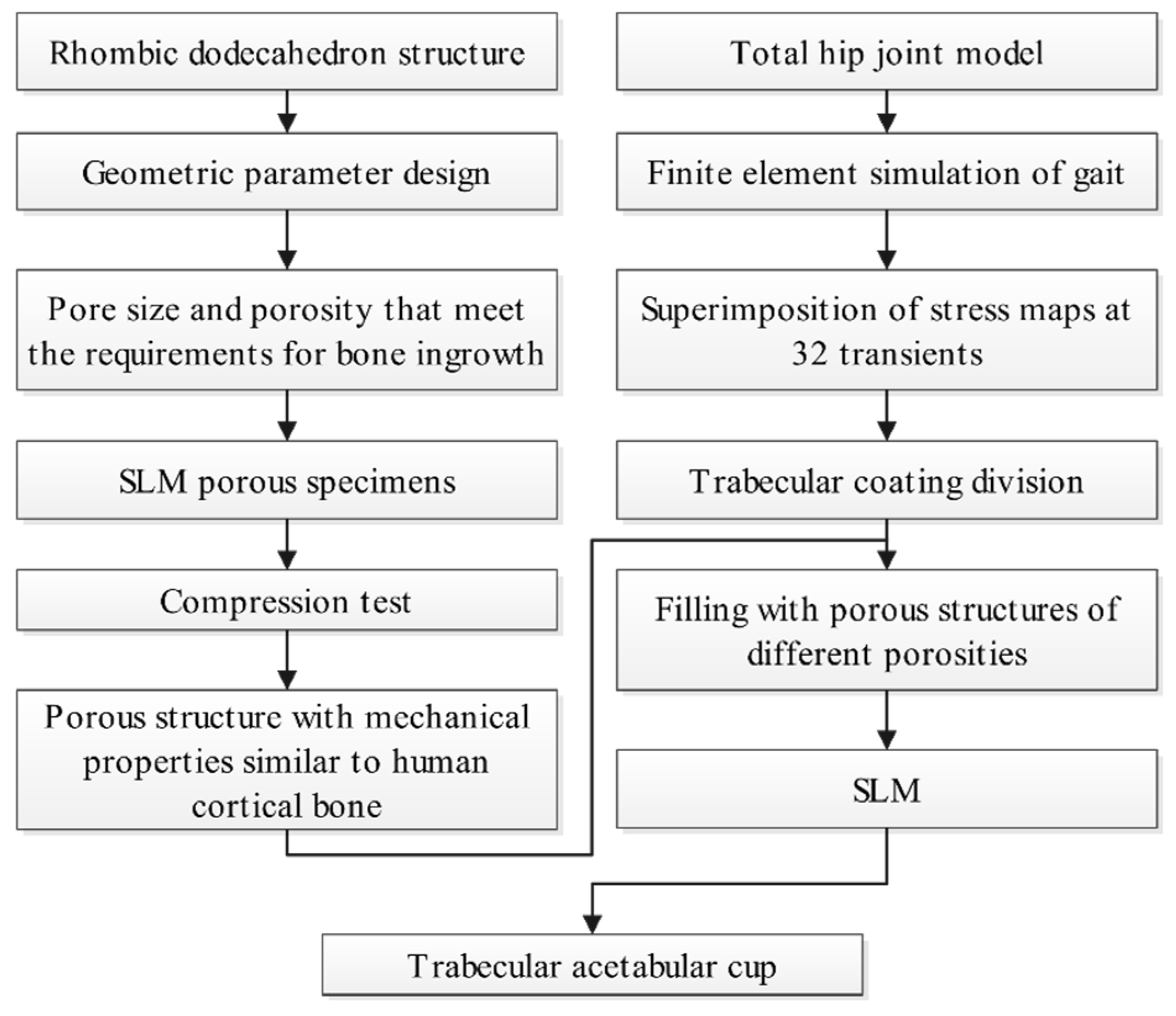
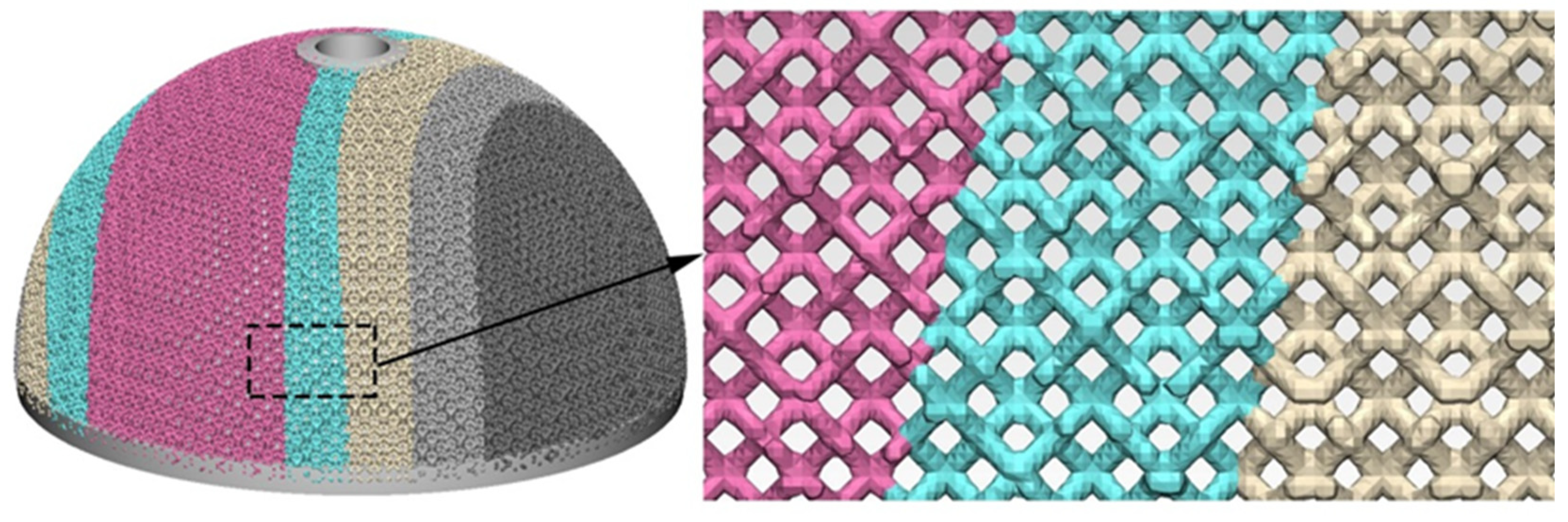
3.3. Design of Trabecular Acetabular Cup and Selective Laser Melting Fabrication
4. Conclusions
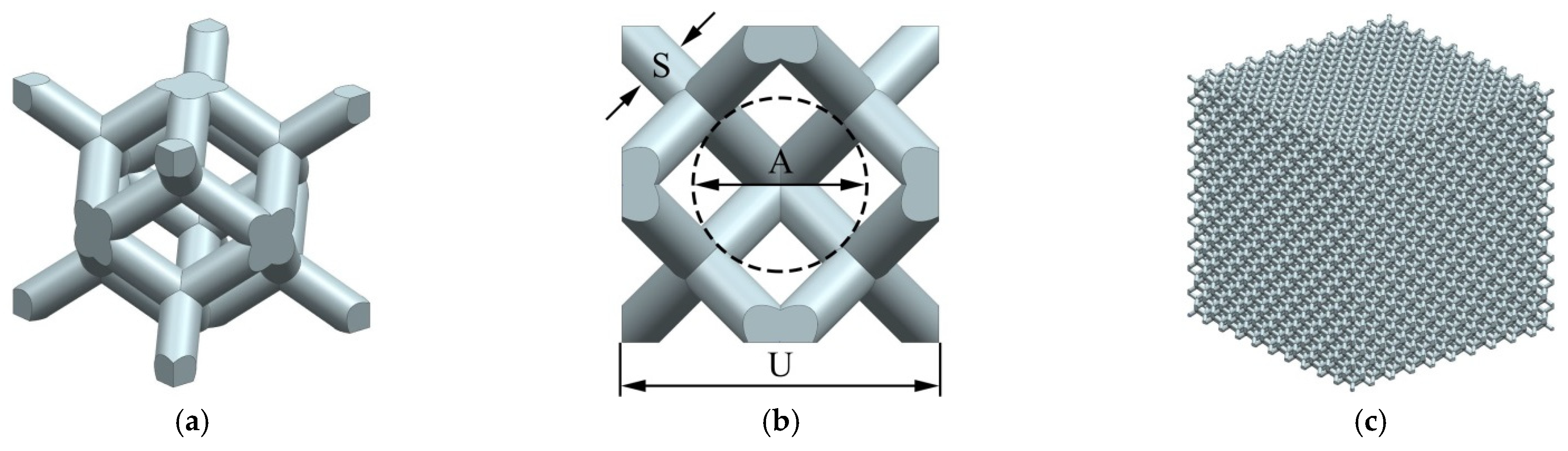
- A rhombic dodecahedron porous structure was designed, and the porous structure cells with sizes of 1, 1.5, and 2 mm were compared. By changing the diameter of the struts in the range of 100–300 μm, the porosity was controlled within 60–90%, and the pore size was between 500–1200 μm, which met the requirements for bone ingrowth.
- Porous Ti6Al4V specimens with cell sizes of 1, 1.5, and 2 mm, and different strut diameters, were printed using SLM technology and tested. Compression test results showed that the specimens with a cell size of 1.5 mm had a compressive strength of 78.16–242.94 MPa and an elastic modulus of 1.74–4.17 GPa, which were close to the values of human cortical bone.
- Finite element analysis of the hip prosthesis model under different gait conditions was carried out, and the stress map of the trabecular coating on the surface of the acetabular cup was obtained. According to the stress map, the trabecular coating was divided into six stress regions (A–F), and the stress values of each region were determined. The porous Ti6Al4V alloy with the corresponding compressive strength was filled in each area of the trabecular coating, and a trabecular acetabular cup with a porosity of 70.71–89.94% was obtained. This design optimized the porous layer on the outer surface of the traditional titanium alloy acetabular cup and could alleviate the “stress shielding” effect to the greatest extent.
- The thermal deformation and overhang problems were simulated before printing and the forming angles of 0°, 15°, 30°, and 45° were simulated. The simulation results showed that the forming deformation was the smallest when the forming angle was 45°. Lastly, the acetabular cup was printed by SLM technology, and heat treatment was performed to remove residual stress.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alonso-Rasgado, T.; Del-Valle-Mojica, J.F.; Jimenez-Cruz, D.; Bailey, C.G.; Board, T.N. Cement interface and bone stress in total hip arthroplasty: Relationship to head size. J. Orthop. Res. 2018, 36, 2966–2977. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.; Ahangarani, S.; Esmailian, M.; Shanaghi, A. Investigation on the corrosion behavior and biocompatibility of Ti-6Al-4V implant coated with HA/TiN dual layer for medical applications. Surf. Coat. Technol. 2020, 397, 126044. [Google Scholar] [CrossRef]
- Tan, X.P.; Tan, Y.J.; Chow, C.S.L.; Tor, S.B.; Yeong, W.Y. Metallic powder-bed based 3D printing of cellular scaffolds for orthopaedic implants: A state-of-the-art review on manufacturing, topological design, mechanical properties and biocompatibility. Mater. Sci. Eng. C 2017, 76, 1328–1343. [Google Scholar] [CrossRef]
- Teichtahl, A.J.; Wluka, A.E.; Wijethilake, P.; Wang, Y.; Ghasem-Zadeh, A.; Cicuttini, F.M. Wolff’s law in action: A mechanism for early knee osteoarthritis. Arthrit. Res. Ther. 2015, 17, 207. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, M.F. Cellular Solids Structures and Properties, 2nd ed.; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Kapfer, S.C.; Hyde, S.T.; Mecke, K.; Arns, C.H.; Schröder-Turk, G.E. Minimal surface scaffold designs for tissue engineering. Biomaterials 2011, 32, 6875–6882. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.S.; Yang, C.Y.; Cheng, W. Study on property model for porous materials 3 mathematical deduction. J. Mater. Eng. 2019, 47, 59–81. [Google Scholar] [CrossRef]
- Lai, Y.X.; Cao, H.J.; Wang, X.L.; Chen, S.K.; Zhang, M.; Wang, N.; Yao, Z.H.; Dai, Y.; Xie, X.; Zhang, P. Porous composite scaffold incorporating osteogenic phytomolecule icariin for promoting skeletal regeneration in challenging osteonecrotic bone in rabbits. Biomaterials 2018, 153, 1–13. [Google Scholar] [CrossRef]
- Nune, K.C.; Misra, R.D.K.; Gaytan, S.M.; Murr, L.E. Interplay between cellular activity and three-dimensional scaffold-cell constructs with different foam structure processed by electron beam melting. J. Biomed. Mater. Res. A 2015, 103, 1677–1692. [Google Scholar] [CrossRef]
- Mihalcea, E.; Vergara-HernÁNdez, H.J.; Jimenez, O.; Olmos, L.; ChÁVez, J.; Arteaga, D. Design and characterization of Ti6Al4V/20CoCrMo−highly porous Ti6Al4V biomedical bilayer processed by powder metallurgy. Trans. Nonferr. Metal. Soc. 2021, 31, 178–192. [Google Scholar] [CrossRef]
- Kapat, K.; Srivas, P.K.; Dhara, S. Coagulant assisted foaming—A method for cellular Ti6Al4V: Influence of microstructure on mechanical properties. Mater. Sci. Eng. A 2017, 689, 63–71. [Google Scholar] [CrossRef]
- Marin, E.; Fedrizzi, L.; Zagra, L. Porous metallic structures for orthopaedic applications: A short review of materials and technologies. Eur. Orthop. Traumatol. 2010, 1, 103–109. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Li, J.X.; Zhang, C.G.; Xie, J.J.; Zhou, X.Y.; Anmin, W. Design Optimization and Manufacturing of Bio-fixed tibial implants using 3D printing technology. J. Mech. Behav. Biomed. Mater. 2021, 117, 104415. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, Z.Y.; Tse, Y.; Huang, C.S.; Zhang, W.W. Optimization of processing parameters and establishment of a relationship between microstructure and mechanical properties of SLM titanium alloy. Opt. Laser Technol. 2019, 112, 159–167. [Google Scholar]
- Pal, S.; Lojen, G.; Hudak, R.; Rajtukova, V.; Brajlih, T.; Kokol, V.; Drstvenšek, I. As-fabricated surface morphologies of Ti-6Al-4V samples fabricated by different laser processing parameters in selective laser melting. Addit. Manuf. 2020, 33, 101147. [Google Scholar] [CrossRef]
- Ge, J.G.; Huang, J.; Lei, Y.P.; O’Reilly, P.; Ahmed, M.; Zhang, C.; Yan, X.C.; Yin, S. Microstructural features and compressive properties of SLM Ti6Al4V lattice structures. Surf. Coat. Technol. 2020, 403, 126419. [Google Scholar] [CrossRef]
- Elsayed, M.; Ghazy, M.; Youssef, Y.; Essa, K. Optimization of SLM process parameters for Ti6Al4V medical implants. Rapid Prototyp. J. 2019, 25, 433–447. [Google Scholar] [CrossRef]
- Liu, J.W.; Sun, Q.D.; Zhou, C.G.; Wang, Z.; Li, H.; Guo, K.; Sun, J. Achieving Ti6Al4V alloys with both high strength and ductility via selective laser melting. Mater. Sci. Eng. A 2019, 766, 138319. [Google Scholar] [CrossRef]
- Xu, Y.L.; Li, T.T.; Cao, X.Y.; Tan, Y.Q.; Luo, P.H. Compressive Properties of 316L Stainless Steel Topology-Optimized Lattice Structures Fabricated by Selective Laser Melting. Adv. Eng. Mater. 2021, 23, 2000957. [Google Scholar] [CrossRef]
- Sala, R.; Regondi, S.; Pugliese, R. Design Data and Finite Element Analysis of 3D Printed Poly(ε-Caprolactone)-Based Lattice Scaffolds: Influence of Type of Unit Cell, Porosity, and Nozzle Diameter on the Mechanical Behavior. Eng 2022, 3, 2. [Google Scholar] [CrossRef]
- Tao, P.; Zhong, J.W.; Li, H.X.; Hu, Q.D.; Gong, S.L.; Xu, Q.Y. Microstructure, Mechanical Properties, and Constitutive Models for Ti–6Al–4V Alloy Fabricated by Selective Laser Melting (SLM). Metals 2019, 9, 447. [Google Scholar] [CrossRef]
- Sidambe, A.T.; Todd, I.; Hatton, P.V. Effects of build orientation induced surface modifications on the in vitro biocompatibility of electron beam melted Ti6Al4V. Powder Metall. 2016, 59, 57–65. [Google Scholar] [CrossRef]
- Zhao, B.J.; Wang, H.; Qiao, N.; Wang, C.; Hu, M. Corrosion resistance characteristics of a Ti-6Al-4V alloy scaffold that is fabricated by electron beam melting and selective laser melting for implantation in vivo. Mater. Sci. Eng. C 2017, 70, 832–841. [Google Scholar] [CrossRef]
- Baltatu, I.; Sandu, A.V.; Vlad, M.D.; Spataru, M.C.; Vizureanu, P.; Baltatu, M.S. Mechanical Characterization and In Vitro Assay of Biocompatible Titanium Alloys. Micromachines 2022, 13, 430. [Google Scholar] [CrossRef] [PubMed]
- Baltatu, M.S.; Vizureanu, P.; Sandu, A.V.; Florido-Suarez, N.; Saceleanu, M.V.; Mirza-Rosca, J.C. New Titanium Alloys, Promising Materials for Medical Devices. Materials 2021, 14, 5934. [Google Scholar] [CrossRef] [PubMed]
- Spataru, M.C.; Butnaru, M.; Sandu, A.V.; Vulpe, V.; Vlad, M.D.; Baltatu, M.S.; Geanta, V.; Voiculescu, I.; Vizureanu, P.; Solcan, C. In-depth assessment of new Ti-based biocompatible materials. Mater. Chem. Phys. 2021, 258, 123959. [Google Scholar] [CrossRef]
- Deng, Z.B.; Zhou, C.C.; Fan, Y.J.; Peng, J.P.; Zhu, X.D.; Pei, X.; Yin, G.F.; Zhang, X.D. Design and characterization of porous titanium scaffold for bone tissue engineering. Rare Met. Mater. Eng. 2016, 45, 2287–2292. [Google Scholar]
- Stamboulis, A.G.; Boccaccini, A.R.; Hench, L.L. Novel Biodegradable Polymer/Bioactive Glass Composites for Tissue Engineering Applications. Adv. Eng. Mater. 2002, 4, 105–109. [Google Scholar] [CrossRef]
- Mircheski, I.; Gradišar, M. 3D finite element analysis of porous Ti-based alloy prostheses. Comput. Method Biomech. 2016, 19, 1531–1540. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.Q.; Cao, W.; Chen, F. Research on prediction of contact stress of acetabular lining based on principal component analysis and support vector regression. Biotechnol. Biotechnol. Equip. 2021, 35, 462–468. [Google Scholar] [CrossRef]
- Xu, Y.L.; Zhang, D.Y.; Hu, S.T.; Chen, R.P.; Gu, Y.L.; Kong, X.S.; Tao, J.M.; Jiang, Y.J. Mechanical properties tailoring of topology optimized and selective laser melting fabricated Ti6Al4V lattice structure. J. Mech. Behav. Biomed. Mater. 2019, 99, 225–239. [Google Scholar] [CrossRef]
- Pedersen, D.R.; Brand, R.A.; Davy, D.T. Pelvic muscle and acetabular contact forces during gait. J. Biomech. 1997, 30, 959–965. [Google Scholar] [CrossRef]
- Koza, E.; Leonowicz, M.; Wojciechowski, S.; Simancik, F. Compressive strength of aluminium foams. Mater. Lett. 2004, 58, 132–135. [Google Scholar] [CrossRef]
- Alkhatib, S.E.; Mehboob, H.; Tarlochan, F. Finite Element Analysis of Porous Titanium Alloy Hip Stem to Evaluate the Biomechanical Performance During Walking and Stair Climbing. J. Bionic. Eng. 2019, 16, 1103–1115. [Google Scholar] [CrossRef]
- Mehboob, H.; Tarlochan, F.; Mehboob, A.; Chang, S.-H. Finite element modelling and characterization of 3D cellular microstructures for the design of a cementless biomimetic porous hip stem. Mater. Des. 2018, 149, 101–112. [Google Scholar] [CrossRef]
- Gharbi, A.; Ayadi, S.; Jouini, N.; Schoenstein, F.; Oudadess, H.; Feki, H.E.; Cheikhrouhou-Koubaa, W. Original implementation of low-temperature SPS for bioactive glass used as a bone biomaterial. J. Mech. Behav. Biomed. Mater. 2022, 126, 104988. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.P.; Chen, L.J.; Chen, D.Y.; Shao, C.S.; Yi, M.F.; Zhang, B. Effects of pore size and porosity of surface-modified porous titanium implants on bone tissue ingrowth. Trans. Nonferr. Metal. Soc. 2019, 29, 2534–2545. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Wang, C.Y.; Wang, C.W.; Yang, G. Study on Porous Ti6Al4V Alloy Formed by Selective Laser Melting and Its Heat Treatment. Rare Met. Mater. Eng. 2022, 51, 1690–1696. [Google Scholar]


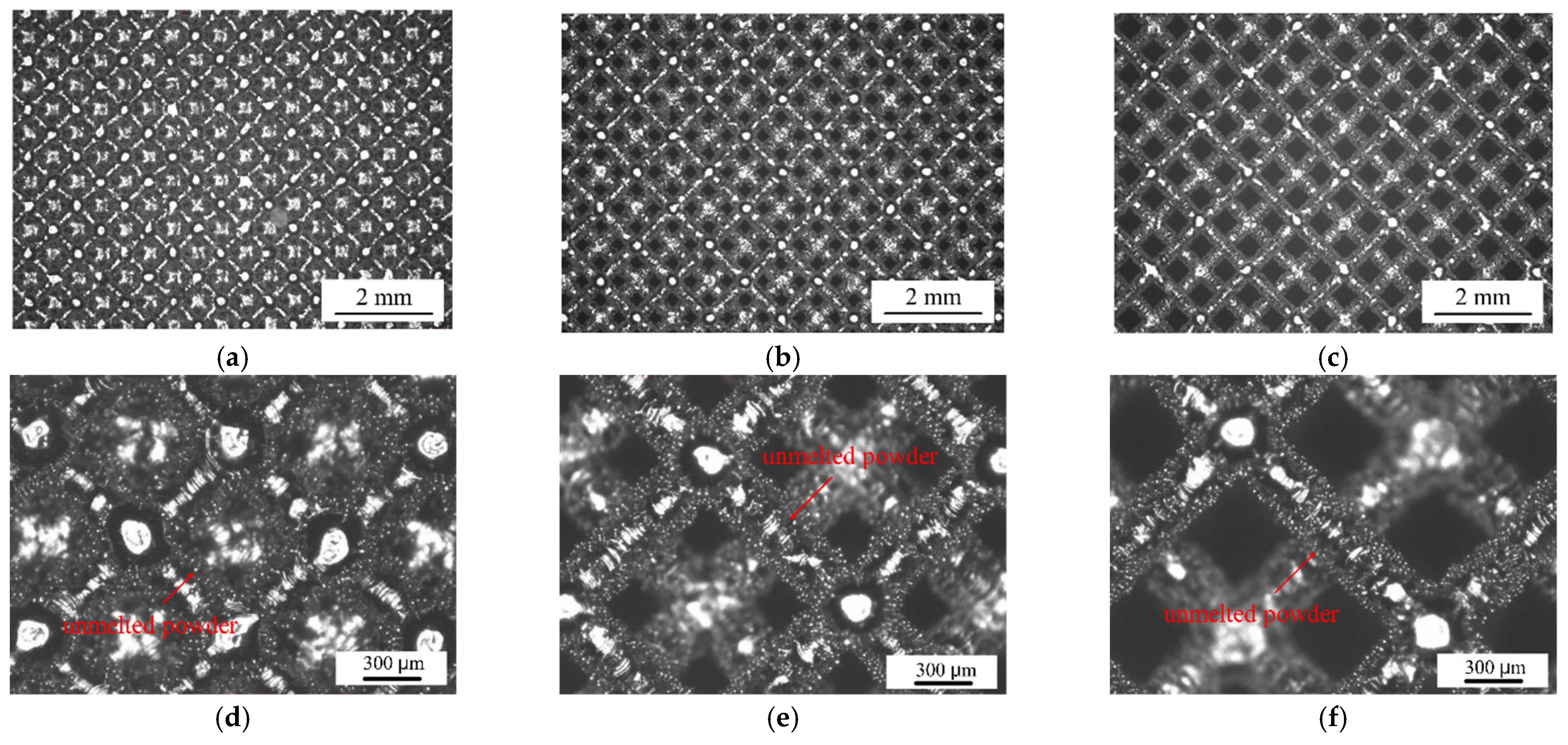




| Powder | N | C | H | Fe | O | Al | V | Ti |
|---|---|---|---|---|---|---|---|---|
| Ti6Al4V ELI | 0.012 | 0.012 | 0.0052 | 0.17 | 0.12 | 6.48 | 3.96 | Bal |
| ASTM F136 Standard | 0.05 | 0.08 | 0.012 | 0.25 | 0.13 | 5.5–6.5 | 3.5–4.5 | Bal |
| Cell Size, U (mm) | Strut Diameter, S (μm) | Porosity, P (%) | Pore size, A (μm) |
|---|---|---|---|
| 1 | 100 | 90.59 | 607.1 |
| 125 | 85.87 | 582.1 | |
| 150 | 80.47 | 557.1 | |
| 175 | 74.55 | 532.1 | |
| 200 | 68.23 | 507.1 | |
| 225 | 61.65 | 482.1 | |
| 1.5 | 125 | 89.94 | 935.7 |
| 150 | 85.88 | 910.7 | |
| 175 | 81.28 | 885.7 | |
| 200 | 76.21 | 860.7 | |
| 225 | 70.71 | 835.7 | |
| 250 | 64.86 | 810.7 | |
| 2 | 150 | 88.99 | 1264.2 |
| 175 | 85.31 | 1239.2 | |
| 200 | 81.17 | 1214.2 | |
| 225 | 76.64 | 1189.2 | |
| 250 | 71.73 | 1164.2 | |
| 275 | 66.49 | 1139.2 | |
| 300 | 60.95 | 1114.2 |
| Model | Material | Elastic Modulus (GPa) | Poisson’s Ratio |
|---|---|---|---|
| Acetabulum | Cortical bone | 20 | 0.3 |
| Liner | UHMWPE | 1 | 0.45 |
| Acetabular cup, femoral head, and stem | Ti6Al4V | 113 | 0.342 |
| Laser Power (W) | Scan Speed (mm/s) | Scan Spacing (μm) | Powder Layer Thickness (μm) |
|---|---|---|---|
| 200 | 1200 | 140 | 30 |
| Region | Stress (MPa) | Porous Ti6Al4V Alloy | ||
|---|---|---|---|---|
| Simulated Value | Scaled Value | Compressive Strength (MPa) | Porosity (%) | |
| A | 1.827 | 219.24 | 221.08 | 70.71 |
| B | 1.402 | 168.24 | 185.42 | 76.21 |
| C | 1.130 | 135.60 | 154.32 | 81.28 |
| D | 0.857 | 102.84 | 118.65 | 85.88 |
| E | 0.584 | 70.08 | 78.16 | 89.94 |
| F | 0.312 | 37.44 | 78.16 | 89.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Sun, B.; Zhang, Y.; Wang, C.; Yang, G. Design of a Novel Trabecular Acetabular Cup and Selective Laser Melting Fabrication. Materials 2022, 15, 6142. https://doi.org/10.3390/ma15176142
Wang C, Sun B, Zhang Y, Wang C, Yang G. Design of a Novel Trabecular Acetabular Cup and Selective Laser Melting Fabrication. Materials. 2022; 15(17):6142. https://doi.org/10.3390/ma15176142
Chicago/Turabian StyleWang, Congyu, Baoyu Sun, Yongdi Zhang, Congwei Wang, and Guang Yang. 2022. "Design of a Novel Trabecular Acetabular Cup and Selective Laser Melting Fabrication" Materials 15, no. 17: 6142. https://doi.org/10.3390/ma15176142
APA StyleWang, C., Sun, B., Zhang, Y., Wang, C., & Yang, G. (2022). Design of a Novel Trabecular Acetabular Cup and Selective Laser Melting Fabrication. Materials, 15(17), 6142. https://doi.org/10.3390/ma15176142






