1. Introduction
In metals and alloys, the hardness normally increases with the decreasing grain size. In steels, particularly carbon steels, a well-known phase transition (martensite transformation) occurs when the steel is cooled down quickly from the austenitization temperature. This transformation process commonly has a pronounced effect on the mechanical properties, particularly hardness, since a fine microstructure forms in the steel after this transformation. As the mechanical properties are significantly affected by this transformation, the martensitic transformation mechanism has been an attractive topic in the fundamental research field of steels. Nevertheless, the ultimate fine α-Fe produced via this transformation process remains unclear to date. In order to observe the fine α-Fe precisely, it is necessary to conduct an in-depth characterization of the substructure in the freshly formed martensite right after martensitic transformation.
In Fe-C binary alloys, the martensitic transformation begins at the martensite start temperature (Ms), which decreases as the carbon content increases. In general, the microstructural investigations on the as-quenched martensite are carried out at room temperature. Thus, the observed martensitic structure at room temperature has unavoidably experienced an auto-tempering process at the temperature interval between Ms and room temperature. In the Fe-C binary alloy system, the martensite, which begins to transform at a temperature close to room temperature, can then be treated as a freshly formed martensite since its substructure experiences little auto-tempering. Even if it is kept at room temperature, the as-quenched martensite unavoidably suffers the auto-tempering treatment or the natural aging upon exposure to air. Thus, it is better to investigate the as-quenched martensite as soon as quenching treatment is completed. The martensite structure was initially investigated by using optical microscopy and X-ray diffraction. The initial X-ray studies on the crystal structure of iron and steel has suggested that the martensite is composed of a 2-nm fine structure [
1]. Later, the as-quenched high carbon martensite was regarded as a single crystal [
2,
3,
4,
5,
6,
7,
8,
9,
10,
11]. Nevertheless, unlike large carbide particles, large martensite plates have never been successfully extracted from the as-quenched samples for X-ray characterization. It is also impossible to characterize the martensite substructure by an optical microscope since the resolution is too low. Later in 1960s, a high density of body-centered cubic (bcc) {112}<111>-type twins were observed in the as-quenched high carbon martensite [
12,
13,
14,
15,
16,
17,
18,
19,
20]. Obviously, the existence of the twins has indicated that the high carbon martensite is no longer a single crystal due to the presence of twinning boundaries.
Recent results carried by TEM observations on the as-quenched martensite have revealed that nanoclusters of α-Fe formed in the as-quenched high-carbon martensite [
21,
22]. However, contrast comparison with retained austenite has not been studied, and the formation mechanism of such ultrafine α-Fe grains has not been explained yet. The results about the observations of the ultrafine α-Fe crystals in as-quenched martensite are quite surprising since it is contradictory to the traditional description of the Fe-C martensite structure. Thus, the martensite substructure of cast-iron alloys will be investigated in detail by means of TEM in this study. The cast-iron alloy is a high-carbon alloy with a comparative volume fraction of retained austenite and quenched martensite structure in the as-quenched state. Thus, it is easier to use the TEM technique to observe both phases in a small observation field.
3. Results
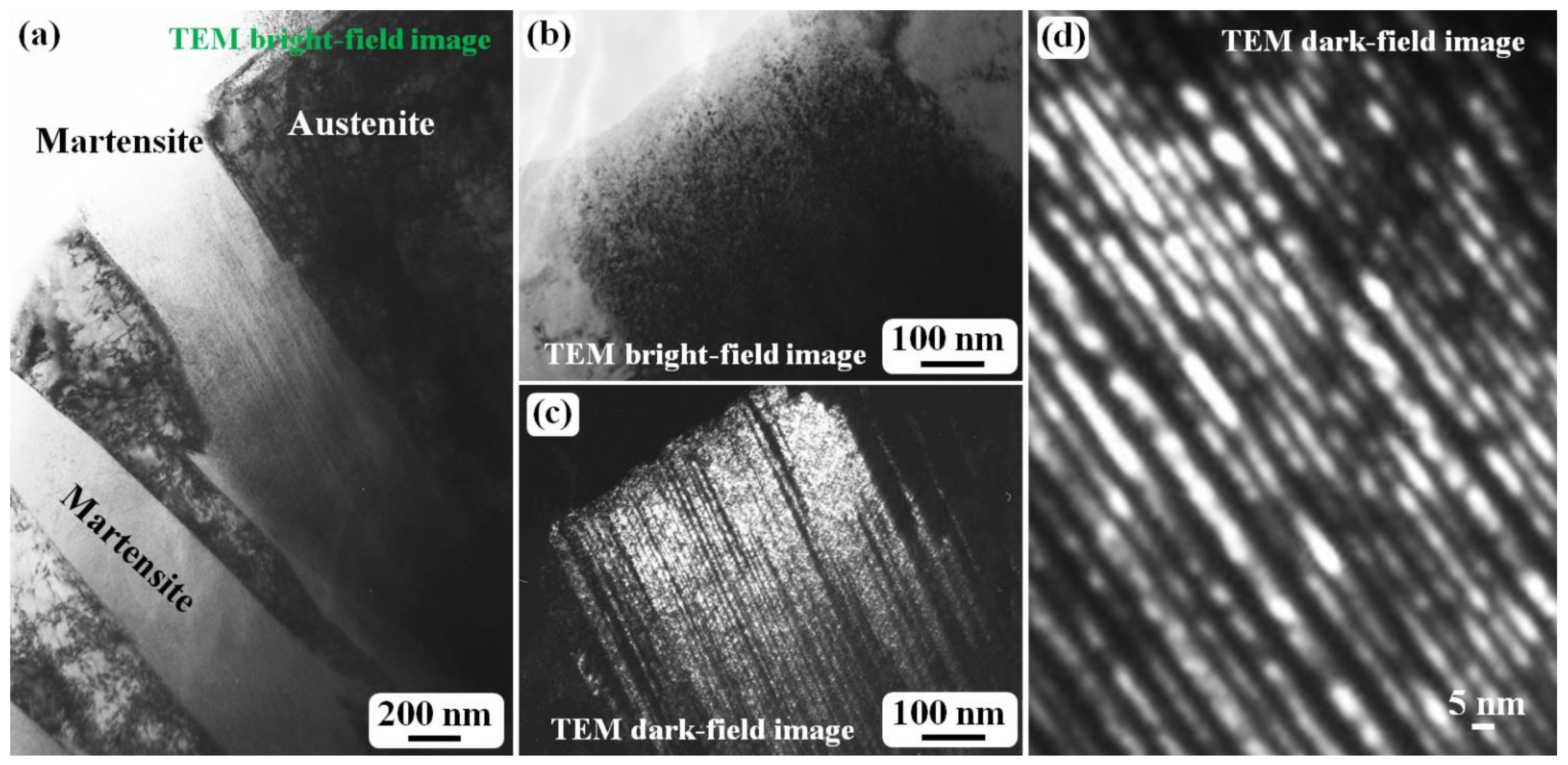
Figure 1 shows the morphology and twin density of the martensite structure in as-quenched cast iron samples. A plate-like or lenticular martensite morphology is observed, as shown in
Figure 1a. The two martensite plates in
Figure 1a are both off zone axes, which results in the bright contrast. After one of the martensite plates was tilted to align with the zone axis, which means the zone axis is parallel to the incident electron beam, the martensite shows black contrast under a bright field mode, as shown in
Figure 1b, and it is difficult to observe a detailed substructure inside the martensite. However, the corresponding dark field image (
Figure 1c) clearly reveals a high density of twins. In an enlarged dark field image (
Figure 1d), it can be clearly seen that the twin width is several nanometers, and the thinner ones are just 1–2 nm. In the twinned part (bright-contrast region), the smallest α-Fe crystal is also 1–2 nm. In
Figure 1d, if the bright contrast is treated as the twinned crystal part, then the dark contrast region corresponds to the matrix, and the ω-Fe phase particles are distributed in the twinning boundary region [
24]. The ultrafine α-Fe grains in the matrix region and those in the twinned region have a BCC {112}<111>-type twinning relationship rather than a twinned crystal with a sharp twinning boundary to split one crystal into two parts (matrix and twinned parts). This is due to the existence of the ω-Fe phase distributed in the twinning boundary.
Figure 2 shows a general TEM observation for an as-quenched cast iron sample. The images in
Figure 2a,b were obtained in a TEM bright field and dark field modes, respectively. In general, compared to the bright field image, the TEM dark field image can show a better or higher contrast for observing a much finer structure. In
Figure 2a, a region containing fringes, which are caused by the sample thickness variation, is outlined by the red dashed circle. Except for the contrast of the thickness of the fringe, the whole martensite in both the bright and dark field images shows a uniform contrast. Such a uniform contrast can be easily found in
Figure 3, which is another dark field image showing a very fine structure. The whole martensite structure shows a uniform contrast no matter how large it is. However, the contrast is not uniform across the austenite, and the dislocation-like contrast can be clearly observed.
The TEM image contrast can be caused by several reasons, such as the second phase contrast, grain orientations, thickness contrast, defects (dislocations, twin boundaries, etc.), element segregation contrast or chemical composition variation contrast, and so on. It is quite difficult to explain the origin of the image contrast in a TEM sample. However, the present TEM sample consists mainly of iron, with the light element carbon more or less uniformly distributed in the austenite. The contrast variation inside one austenite grain can be caused by the difference in the local thickness in the specimen and/or defects (sub-grain boundaries or dislocations).
Figure 3 shows a dark field TEM image containing both an austenite and martensite structure. The austenite region can be simply recognized from the contrast difference between the two structures (martensite and austenite). In the enlarged image on which a local martensite region has been inserted, neither a dislocation contrast nor an obvious contrast caused by the thickness variation can be seen. From the enlarged image, it is easy to see the contrast from ultra-fine grains with almost the same crystal orientation and the grain size of 1–2 nm. Such fine grains are the only reason for the formation of the uniform contrast in martensite. Thus, in an as-quenched high-carbon martensite, the fresh martensite is composed of ultra-fine grains and sub-grain boundaries between them. Since Fe is the main element in the cast iron alloy, and selected area electron diffraction (SAED) has confirmed that the nanocrystals have a body-centered cubic (bcc) crystal structure, these nanocrystals can be deemed as α-Fe grains with almost the same orientation. It can be estimated from the dark field images that the size of these nanocrystals is about 1–2 nm. Some regions in dark field images show a larger particle size, which is likely to be the aggregation of several ultrafine α-Fe grains due to very close orientation.
The martensitic substructure always shows a uniform contrast regardless of its orientation.
Figure 4 shows the TEM observation with the electron beam parallel to the [
13] axis of α-Fe. Both the bright field image (
Figure 4a) and the corresponding dark field image (
Figure 4c) show a clear contrast between the ultrafine particles and the surrounding matrix. The SAED pattern in
Figure 4b has confirmed that two crystalline phases (α-Fe, ω-Fe(C)) coexist [
24,
25,
26,
27,
28,
29]. The dark field image was taken by using α-Fe (110) diffraction spot. Thus, both phases are nanocrystals with almost the same size.
The absence of the twinning contrast in the martensite is ascribed to the fact that the twinning planes are not parallel or close to the electron beam direction. When the twinning planes form a certain angle with the incident electron beam direction (the observation direction), the twin boundary ω-Fe
3C ultrafine particles completely overlap with the α-Fe grains [
24,
25,
26,
27,
28,
29]. Thus, it is difficult to recognize the ω-Fe
3C particles from the high-resolution TEM lattice image (
Figure 4d) due to the lattice coherence between the two phases. From both the SAED pattern and the high-resolution lattice image, the α-Fe (110) plane completely overlaps with the ω-Fe
3C (0
1
) plane, as indicated by the red dashed line in
Figure 4d. The white dashed line corresponds to the ω-Fe
3C (10
0) plane and the interplanar spacing is exactly three times that of the α-Fe {112} planes, although it is unable to observe in the high-resolution lattice image due to the resolution limitation.
The above experimental observations from different observation directions have revealed that the martensite substructure is composed of ultrafine α-Fe and ω-Fe3C grains in the as-quenched state regardless of the observation directions, and the grain size is about 1–2 nm. Observations from all directions show almost the same grain size, and 1–2 nm is the smallest size for crystalline phases in both metals and alloys. The formation mechanism about such a small size grain will be discussed later.
Figure 5 shows TEM observation from location on the as-quenched martensite sample. The bright field image and its corresponding SAED pattern are shown in
Figure 5a,b, respectively. The SAED pattern is similar to that shown in
Figure 4b so that the index is not shown in
Figure 5b in order to exhibit the diffraction spots more clearly. The dark field images shown in
Figure 5c–f were obtained by using different diffraction spots, as indicated by the yellow arrows. In all of these dark field images, nanocrystals with sizes of 1–2 nm were observed inside the martensite. As can be seen from one of the enlarged images in
Figure 6, the fundamental structure inside the martensite is composed of nanocrystals alone, and the size of all these nanocrystals is about 1–2 nm.
The TEM dark field images have shown that the martensite substructure is composed of nanocrystals no matter what observation directions and diffraction spots were used. Since the grain size is about 1–2 nm, which is the smallest crystalline size for pure Fe crystal, the martensite in this condition can be treated as freshly formed martensite suffering few auto-tempering effects. In fact, all twinned martensite consists of nanocrystals as its substructure [
21,
22].
4. Discussion
The martensite structure is formed in the austenite (γ-Fe) matrix with an fcc crystal lattice structure via a well-known γ → α transformation in pure iron. In addition, the martensite consists of two crystalline phases, namely α-Fe and ω-Fe
3C, in Fe-C binary alloys [
24,
25,
27,
28]. The detailed γ → α transformation pathway has been explained in the previous publication [
30]. Here, emphasis will be placed on the formation mechanism of the α-Fe grains with a size of about 1–2 nm. The present observation result actually matches well with the suggestion based on X-ray studies carried out one hundred years ago [
1]. At that time, by comparing the X-ray diffraction line width obtained from an extremely fine-grained gold colloid, the author (A. Westgren) has given a clear suggestion that the martensite is composed of a 2-nm-particle structure.
The schematic in
Figure 7 shows the γ → α transformation pathway.
Figure 7a is the model presented in ref. [
30].
Figure 7b is the Bain model, which has been known and widely accepted for a long period [
7,
8,
9,
10].
Figure 7c is the projection of
Figure 7a along the
cfcc-Fe direction. The red dashed lines correspond to the projection of α-Fe along its <110> direction, and the blue dashed line is parallel to both the (1
0)
γ-Fe plane and one of the {112}
α-Fe planes. Those atoms on the blue dashed line are kept unchanged during the transformation, while the atoms in different layers in the shadow region, which are parallel to the blue dashed line, will experience a shift of either √5/20)
afcc-Fe (~0.4014Å for
afcc-Fe = 3.59 Å) or 2 × 0.4014 Å along the arrow direction, as shown in
Figure 7c.
As explained in
Figure 7d, the formation of a large three-dimensional α-Fe crystal requires a continuous shift of the atoms in other layers outside the shadow region. For example, the atoms at the “B” or “B′” positions in
Figure 7d need to shift three times as long as 0.4014 Å (~1.204 Å). One more layer-shifting process results in an atom movement of about 4 × 0.4014 Å = 1.6056 Å, which is longer than half (~1.25 Å) of the distance of two neighboring atoms in that layer. Thus, one α-Fe grain can have a size of about 2 × a
fcc-Fe ×
(~10.15 Å and ~1 nm). This means that the α-Fe crystal cannot grow in size during the transformation process. This is believed to be the fundamental reason for the formation of 1–2 nm-size ultrafine α-Fe grains in the initially formed martensite structure. At the same time, the formation of α-Fe grains or the γ → α transformation can occur anywhere as long as there are no carbon atoms. In
Figure 7d, the distance from “B” to “B′” is about 0.75 nm, which is still smaller than 1–2 nm estimated from the grain morphology in the TEM dark field images. Thus, a practical recrystallization among the nanocrystals must have occurred during the transformation.
Based on the Bain model shown in
Figure 7b, a large α-Fe grain could not form either. Actually, there is no suitable modeling to explain the formation of a large α-Fe crystal during the γ → α transformation process to date. Although, the α-Fe structure shown in
Figure 7d can be formed without a size limit on the (1
0)
γ-Fe plane based on the present atomic shift. In fact, numerous α-Fe unit cells will form at the same time everywhere. These homogeneously formed α-Fe unit cells on various (1
0)
γ-Fe planes will possess almost the same <111>
α-Fe orientation on the (1
0)
γ-Fe plane or one of the {112}
α-Fe planes, as experimentally revealed in
Figure 4. A small mis-orientation can result in the formation of sub-grain boundaries between ultrafine α-Fe grains, and therefore, the nanocrystals form the initial martensitic substructure. Such a nanocrystal has been commonly observed in quenched twinned martensite regardless of the carbon content [
21,
22].
It is well-known that the martensitic transformation results in the formation of fine-grain α-Fe in steels. However, how small the fine α-Fe grain could be remains unclear. Based on the present experimental results, it is quite evident that the smallest size of the α-Fe grains formed after martensite transformation is just 1–2 nm. Upon tempering, α-Fe grains with different grain sizes can form via recrystallization [
21]. Obviously, such tiny α-Fe grains cannot be formed by a long-range atomic shearing or displacive movement mechanism, which was proposed based on the single-crystal martensite with a relatively large crystal size. Such a nanocrystal in the martensite generates an enormous amount of sub-grain boundaries naturally, which results in a volume expansion of the martensite and thus the crack formation in the martensite.
Figure 8 shows a SEM image revealing cracks in as-quenched martensite. These cracks, which were normally formed during the quenching process, pass through the whole martensite.