High-Efficiency Iron Extraction from Low-Grade Siderite via a Conveyor Bed Magnetization Roasting–Magnetic Separation Process: Kinetics Research and Applications
Abstract
:1. Introduction
2. Materials and Methods
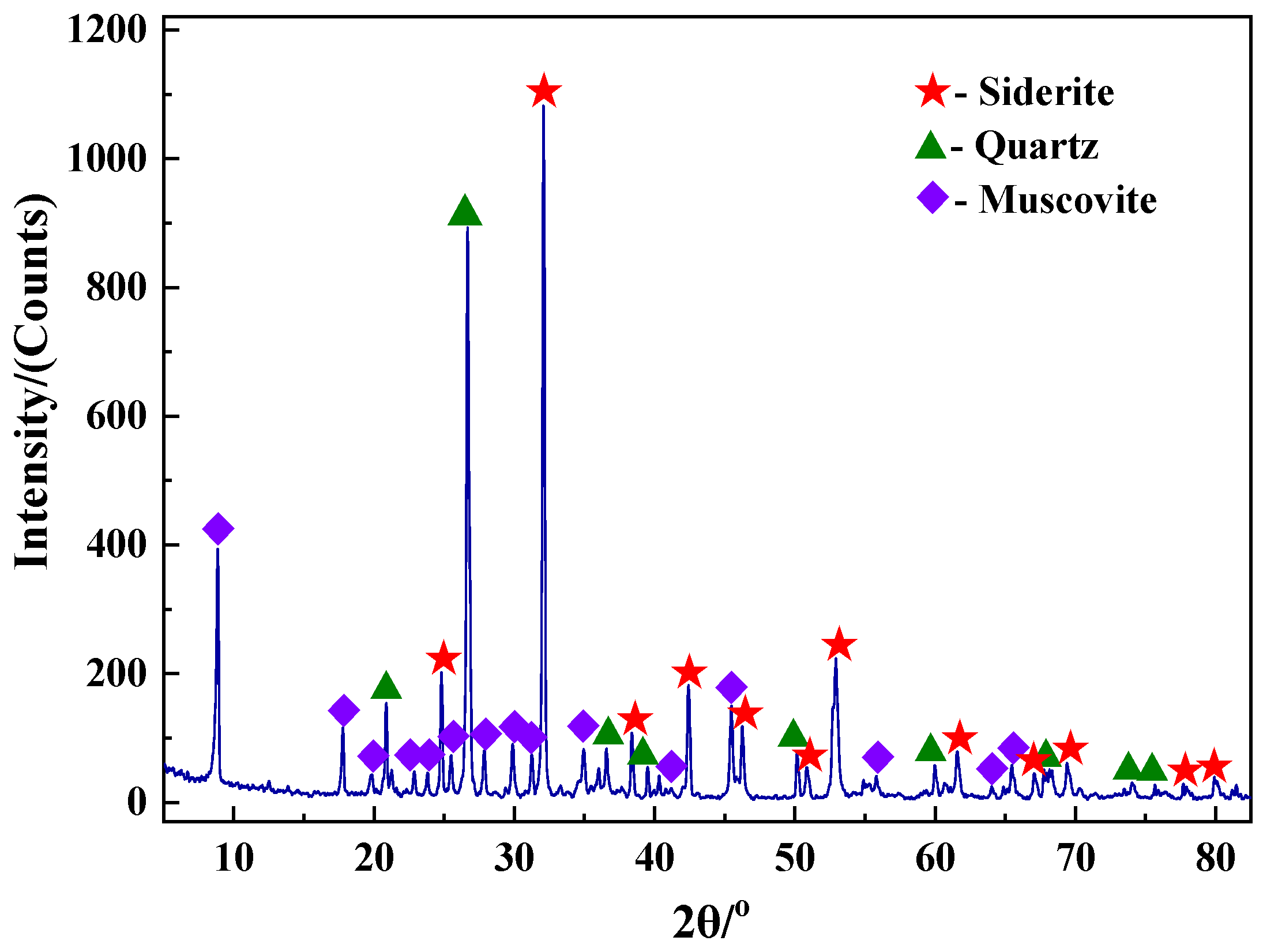
2.1. Raw Materials
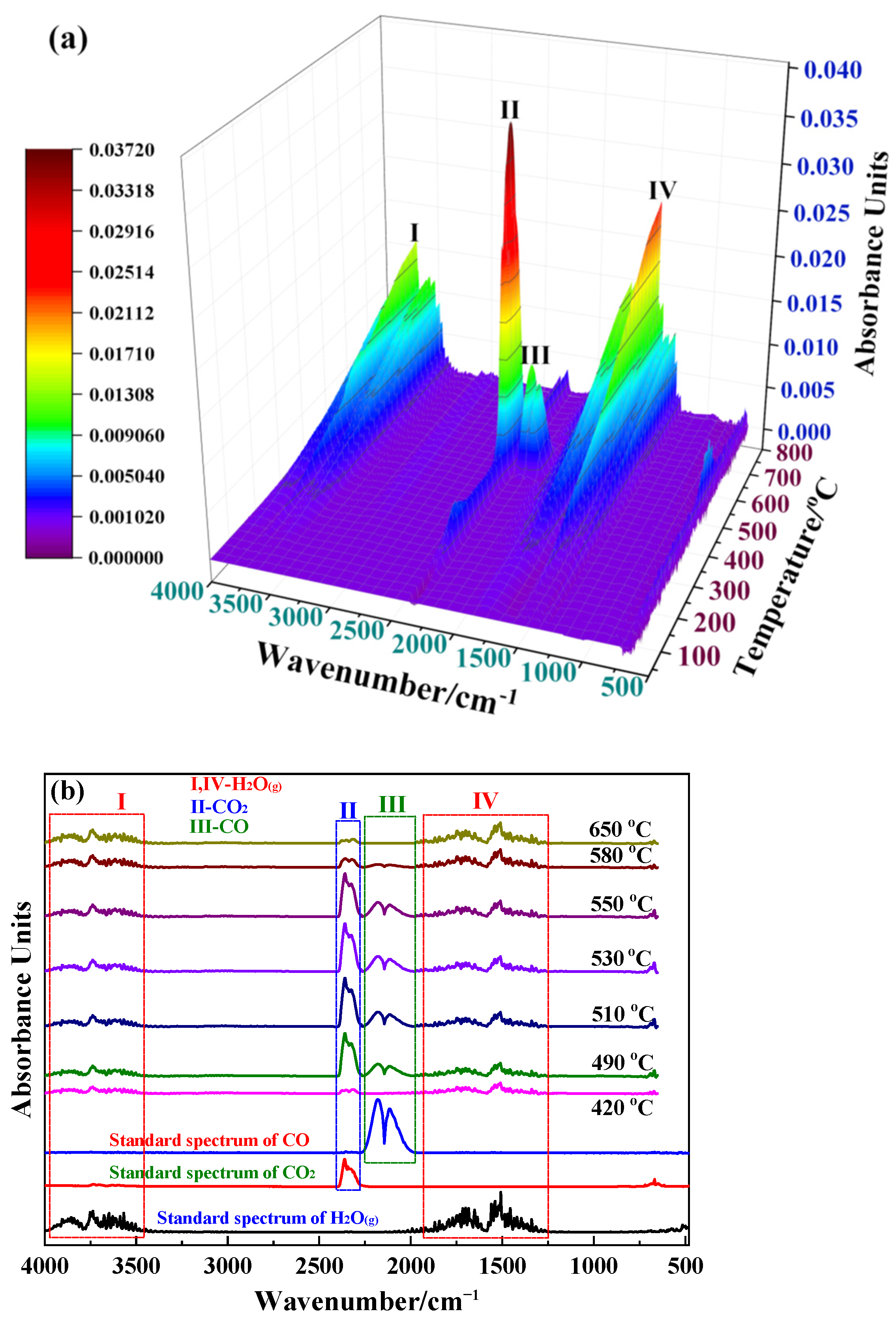
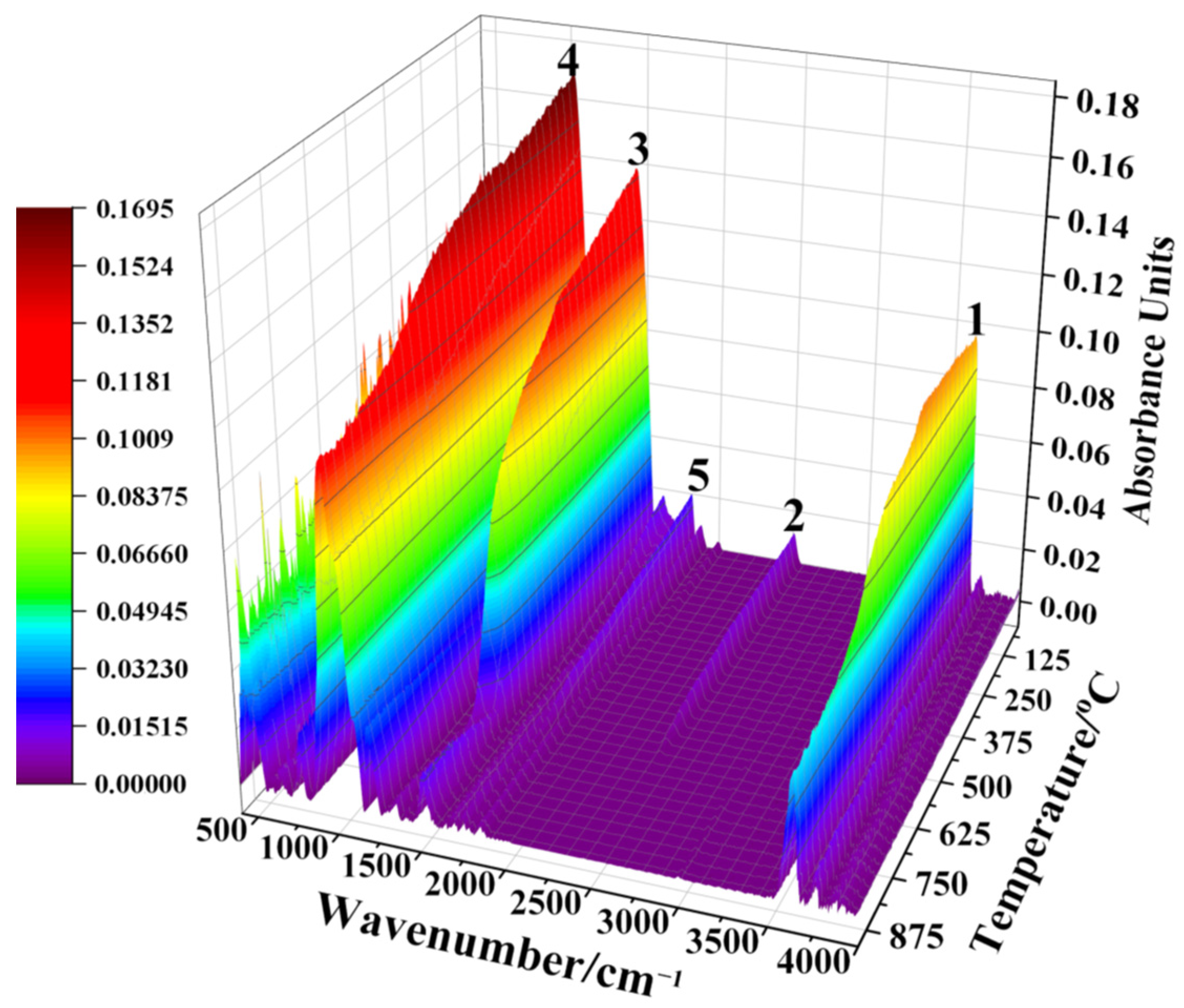
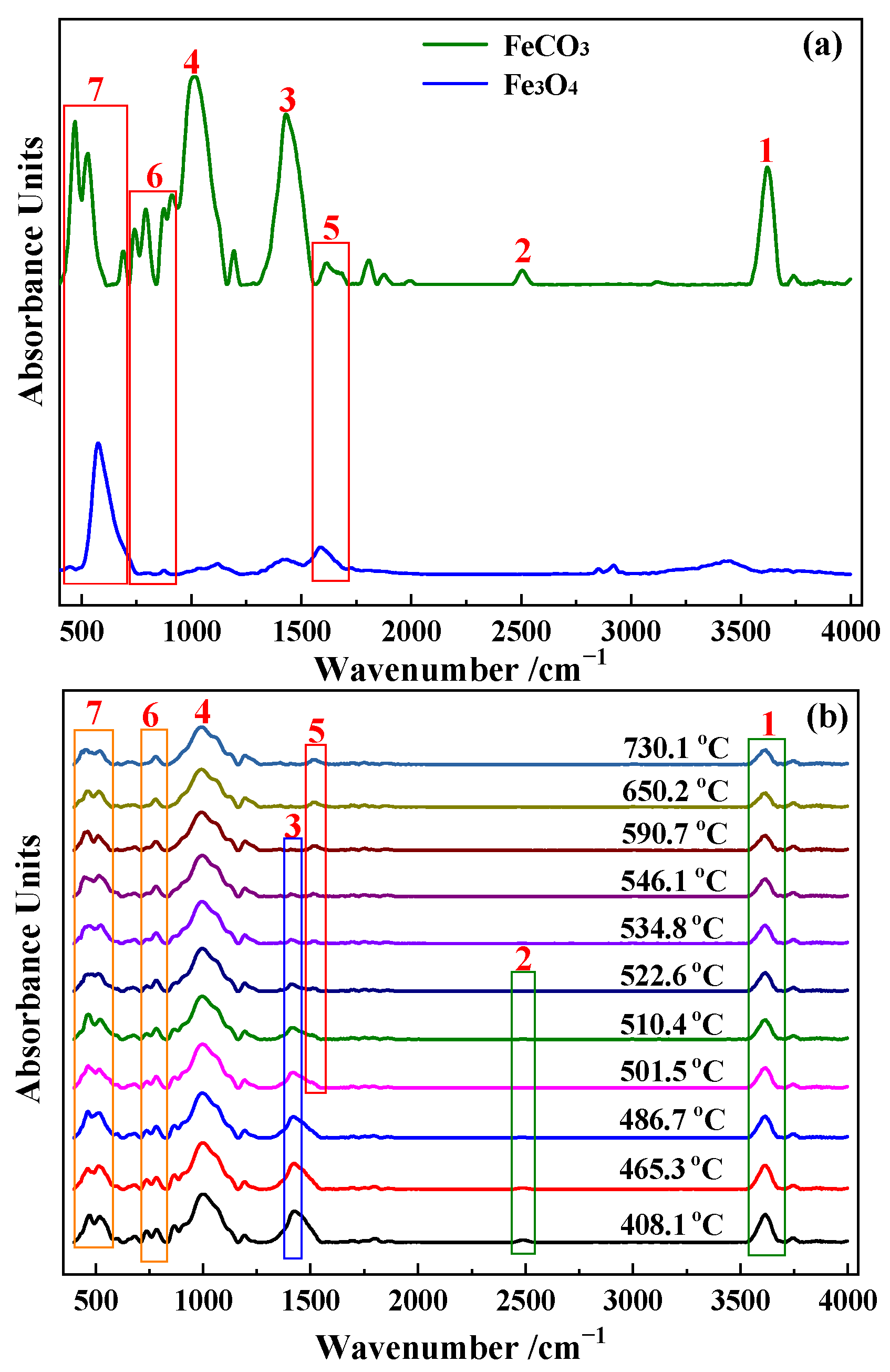
2.2. Reaction Mechanism Analysis
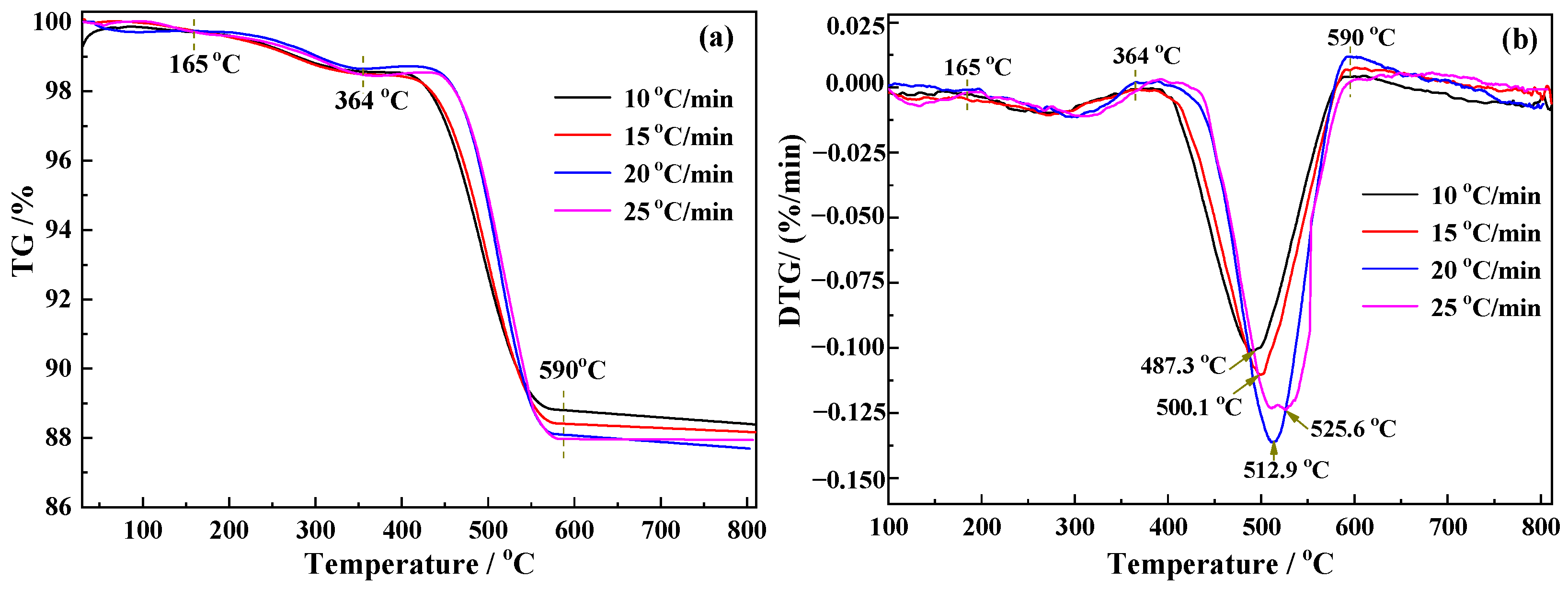
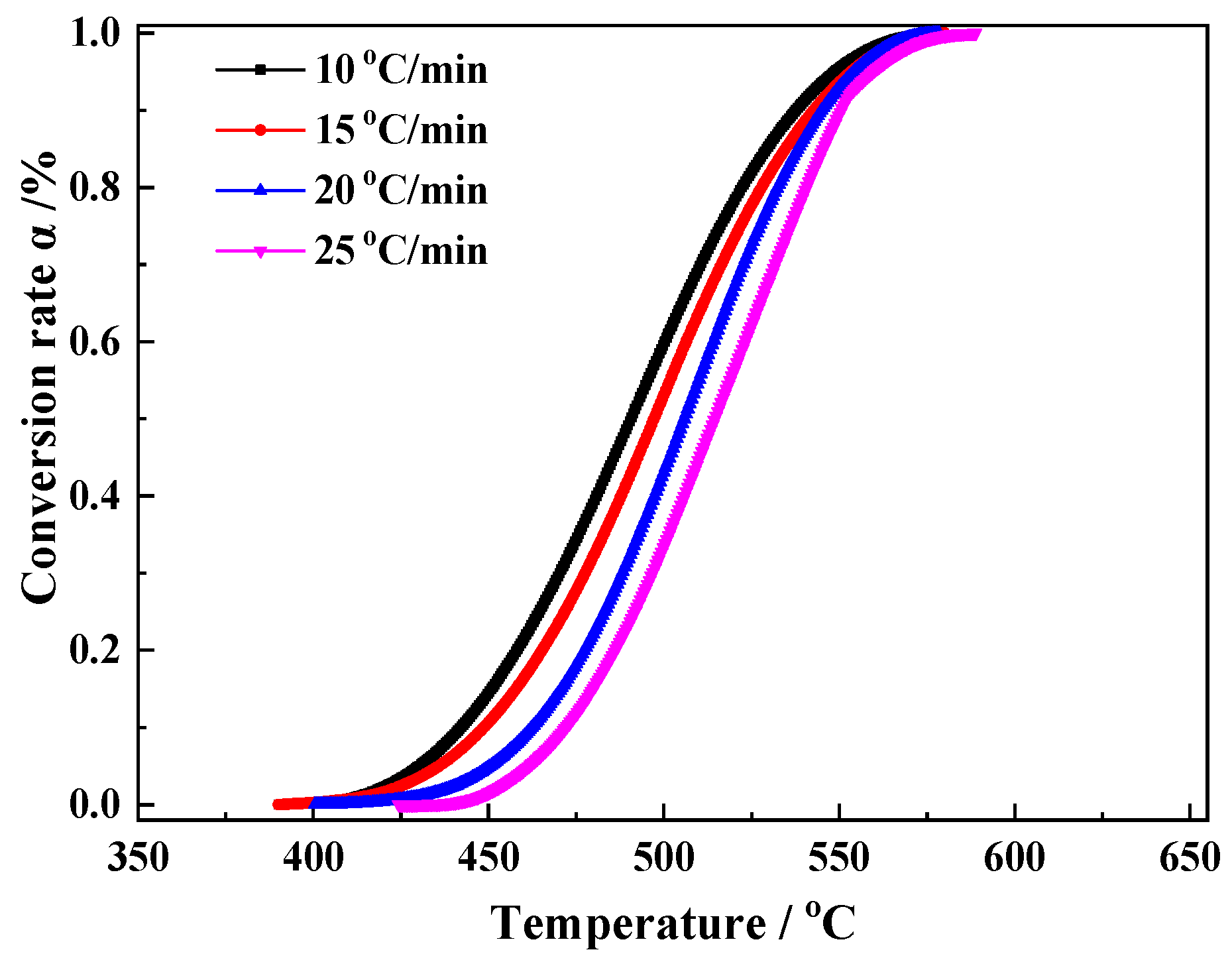
2.3. Kinetics Analysis Method
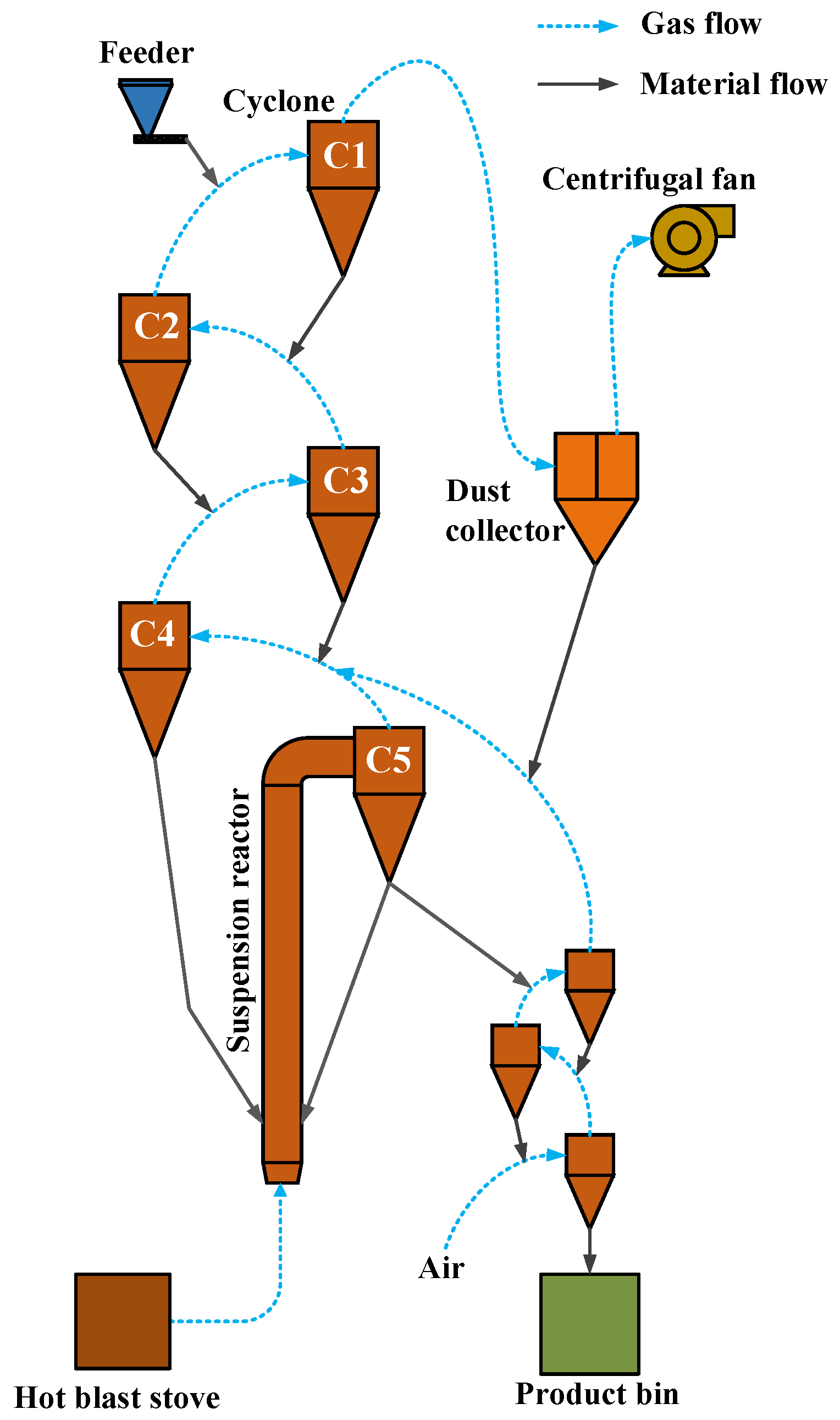
2.4. Conveyor Bed Magnetization Roasting Pilot Test System
2.5. Characterization of the Roasted Ores
2.6. Magnetic Separation Analysis
3. Results and Discussion
3.1. Reaction Mechanism Analysis
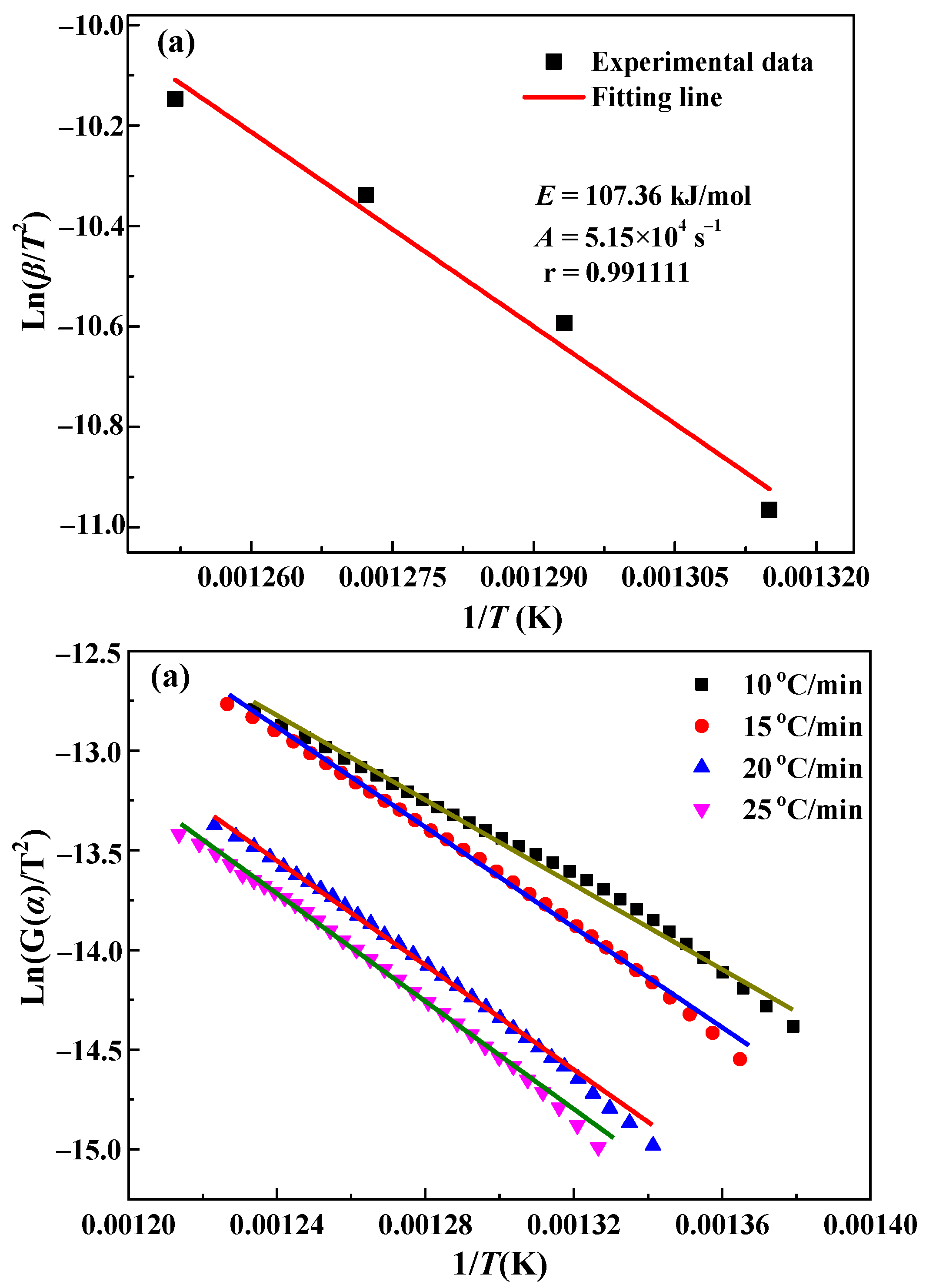
3.2. Kinetics Analysis
3.3. Characterization of Roasted Ore
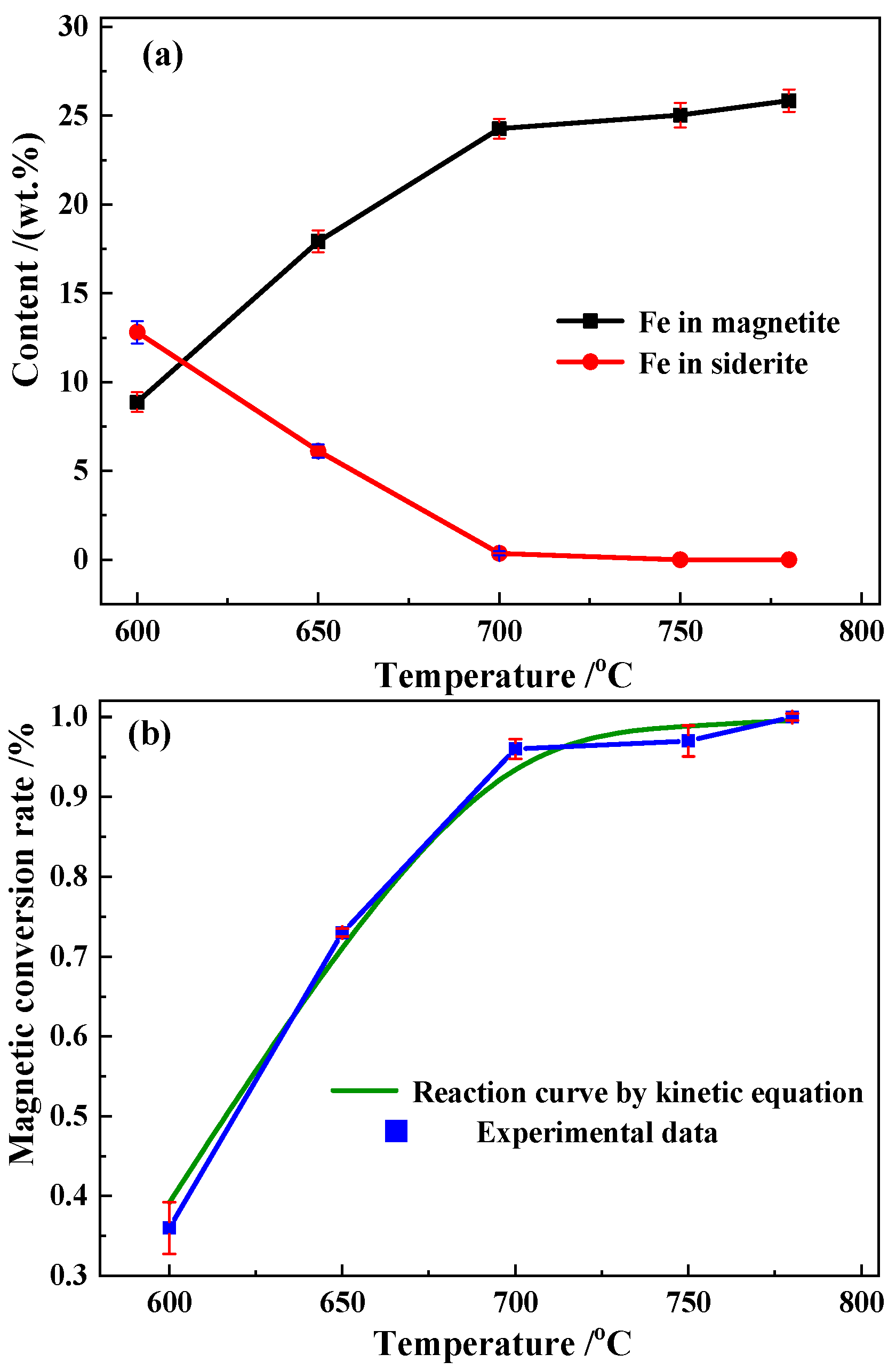
3.3.1. Magnetic Conversion Analysis
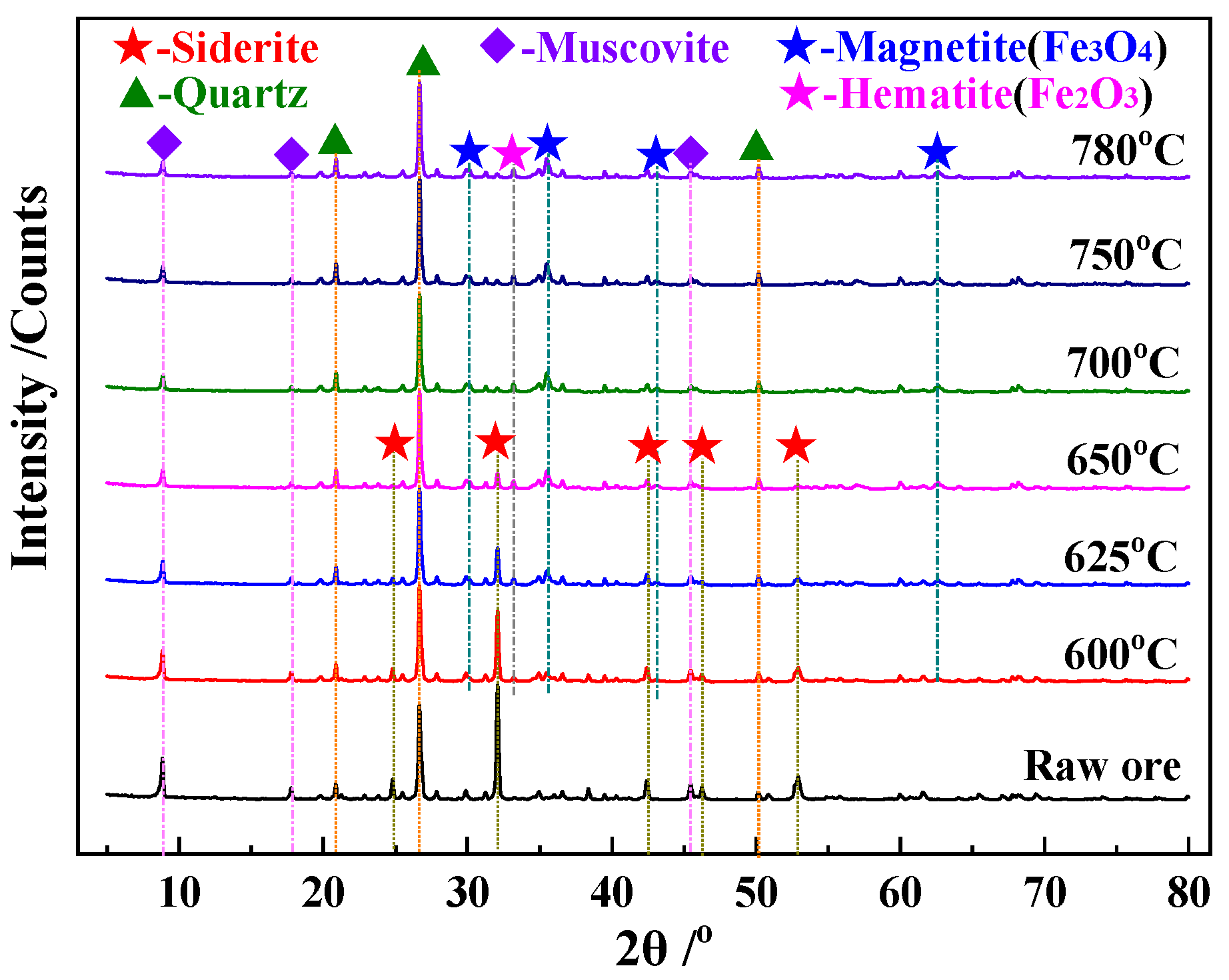
3.3.2. XRD Analysis
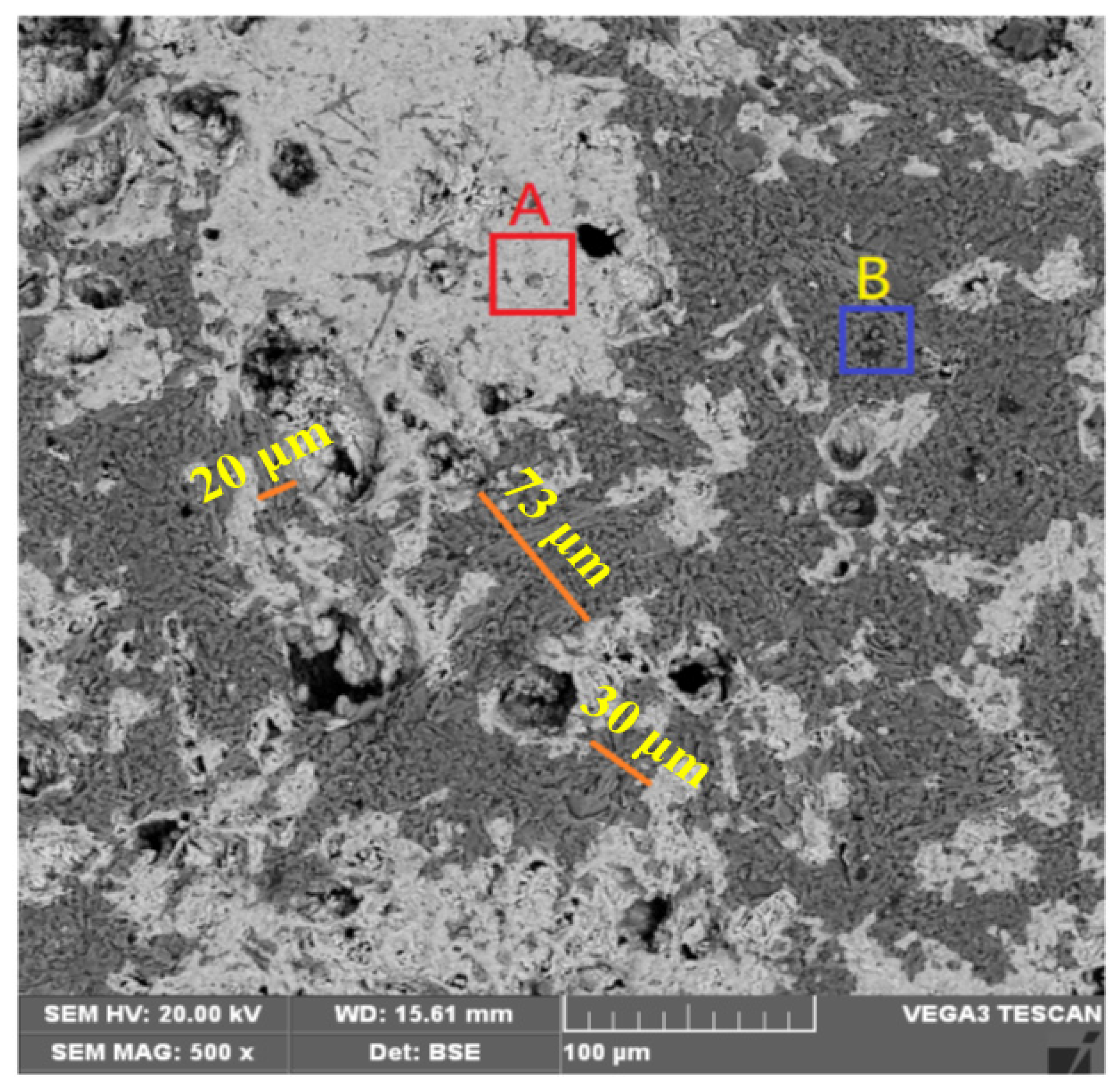
3.3.3. BSE-EDS Analysis
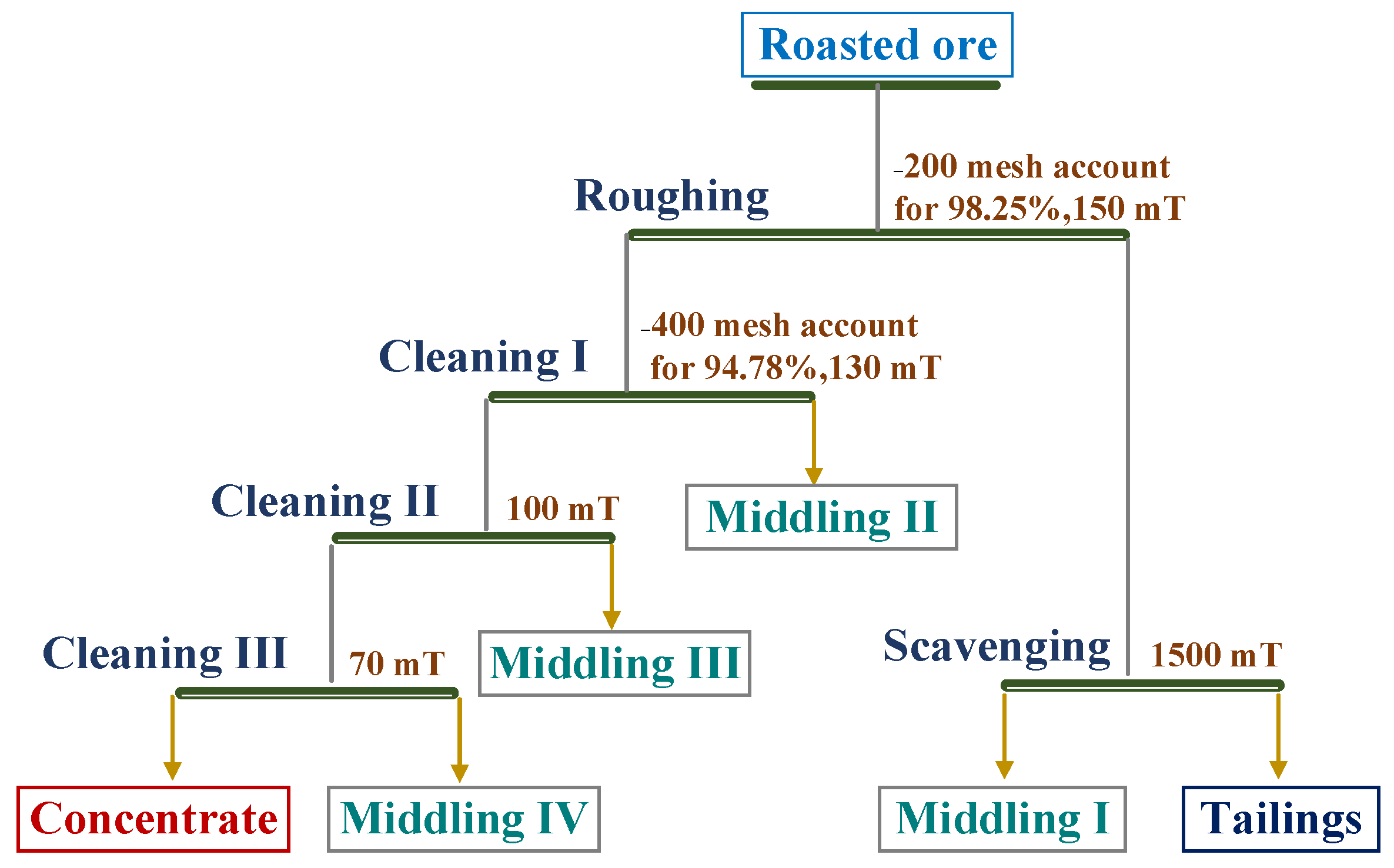
3.4. Magnetic Separation Analysis
4. Conclusions
- (1)
- Magnetite was prepared by roasting siderite under an inert and reducing atmosphere, which was almost synchronous with the decomposition of siderite. The reaction mechanism of the magnetization roasting of magnetite was determined to be random nucleation and subsequent growth, with a reaction activation energy of 106.1 kJ/mol.
- (2)
- Roasted ore with a magnetic conversion of ≥0.99 was obtained using a conveyor bed system at 750 °C and 780 °C for around 3.5 s. Magnetite oxidation was prevented via the adoption of a three-stage suspended state air cooling process. The kinetic model predictions were consistent with the experimental results.
- (3)
- The conveyor bed magnetization roasting and magnetic separation process were suitable for the upgrading and utilization of low-grade iron ore. For the iron grade of Daxigou siderite with 21.42 wt.% original ore, a concentrate with an iron grade of 62.80 wt.% and a recovery rate of 68.83% was obtained after conducting the conveyor bed magnetization roasting and magnetic separation process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ponomar, V.; Dudchenko, N.; Brik, A. Phase transformations of siderite ore by the thermomagnetic analysis data. J. Magn. Magn. Mater. 2017, 423, 373–378. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, X.; Han, Y.; Li, Y. Green magnetization roasting technology for refractory iron ore using siderite as a reductant. J. Clean. Prod. 2019, 206, 40–50. [Google Scholar] [CrossRef]
- Wang, D.; Pan, J.; Zhu, D.; Guo, Z.; Yang, C.; Yuan, Z. An efficient process to upgrade siderite ore by preoxidation-magnetization roasting-magnetic separation-acid leaching. J. Mater. Res. Technol. 2022, 19, 4296–4307. [Google Scholar] [CrossRef]
- Zhu, X.; Han, Y.; Sun, Y.; Li, Y.; Wang, H. Siderite as a novel reductant for clean utilization of refractory iron ore. J. Clean. Prod. 2020, 245, 118704. [Google Scholar] [CrossRef]
- Ponomar, V.; Dudchenko, N.; Brik, A. Reduction roasting of hematite to magnetite using carbohydrates. Int. J. Miner. Process. 2017, 164, 21–25. [Google Scholar] [CrossRef]
- Subhnit, K.; Deepak, N.; Swagat, S. A review on the enrichment of iron values of low-grade Iron ore resources using reduction roasting-magnetic separation. Powder Technol. 2020, 367, 796–808. [Google Scholar]
- Rath, S.; Dhawan, N.; Rao, D.; Das, B.; Mishra, B. Beneficiation studies of a difficult to treat iron ore using conventional and microwave roasting. Powder Technol. 2016, 301, 1016–1024. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Han, Y.; Li, Y. Pyrolysis behavior of a green and clean reductant for suspension magnetization roasting. J. Clean. Prod. 2020, 268, 122173. [Google Scholar] [CrossRef]
- Ponomar, V.; Dudchenko, N.; Brik, A. Synthesis of magnetite powder from the mixture consisting of siderite and hematite iron ores. Miner. Eng. 2018, 122, 277–284. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Han, Y.; Li, Y.; Gao, P. Producing magnetite concentrate via self-magnetization roasting in N2 atmosphere: Phase and structure transformation, and extraction kinetics. J. Ind. Eng. Chem. 2021, 104, 571–581. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Han, Y.; Gao, P.; Li, Y. Thermal decomposition kinetics of siderite ore during magnetization roasting. Min. Metall. Explor. 2021, 38, 1497–1508. [Google Scholar] [CrossRef]
- Kamariah, N.; Kalebic, D.; Xanthopoulos, P.; Blannin, R.; Araujo, F.; Koelewijn, S.; Dehaen, W.; Binnemans, K.; Spooren, J. Conventional versus microwave-assisted roasting of sulfidic tailings: Mineralogical transformation and metal leaching behavior. Miner. Eng. 2022, 183, 107587. [Google Scholar] [CrossRef]
- Zhou, W.; Sun, Y.; Han, Y.; Gao, P.; Li, Y. Recycling iron from oolitic hematite via microwave fluidization roasting and magnetic separation. Miner. Eng. 2021, 164, 106851. [Google Scholar] [CrossRef]
- Yuan, S.; Xiao, H.; Wang, R.; Li, Y.; Gao, P. Improved iron recovery from low-grade iron ore by efficient suspension magnetization roasting and magnetic separation. Miner. Eng. 2022, 186, 107761. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, Q.; Sun, Y.; Gao, P.; Han, Y. Pilot-scale extraction of iron from flotation tailings via suspension magnetization roasting in a mixture of CO and H2 followed by magnetic separation. Resour. Conserv. Recycl. 2021, 172, 105680. [Google Scholar] [CrossRef]
- Zhang, X.; Han, Y.; Sun, Y.; Li, Y. Innovative utilization of refractory iron ore via suspension magnetization roasting: A pilot-scale study. Powder Technol. 2019, 352, 16–24. [Google Scholar] [CrossRef]
- Yuan, S.; Ding, H.; Wang, R.; Zhang, Q.; Li, Y.; Gao, P. The mechanism of suspension reduction on Fe enrichment with low-grade carbonate-containing iron ore. Adv. Powder Technol. 2022, 33, 103643. [Google Scholar] [CrossRef]
- Coenen, K.; Gallucci, F.; Mezari, B.; Hensen, E.; Van Sint Annaland, M. An in-situ IR study on the adsorption of CO2 and H2O on hydrotalcites. J. CO2 Util. 2018, 24, 228–239. [Google Scholar] [CrossRef]
- Song, Y.; Lei, S.; Li, J.; Yin, N.; Zhou, J.; Lan, X. In situ FT-IR analysis of coke formation mechanism during Copyrolysis of low-rank coal and direct coal liquefaction residue. Renew. Energy 2021, 179, 2048–2062. [Google Scholar] [CrossRef]
- Ma, W.; Dong, X.; Wang, Y.; He, W.; Zhang, W.; Liang, Y.; Wang, Y.; Fu, W.; Liao, J.; Dong, F. Highly enhanced photocatalytic toluene degradation and in situ FT-IR investigation on designed Sn-doped BiOCl nanosheets. Appl. Surf. Sci. 2022, 578, 152002. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Wight, C. Isothermal and nonisothermal kinetics of thermally stimulated reactions of solids. Int. Rev. Phys. Chem. 1998, 3, 407–433. [Google Scholar] [CrossRef]
- Budrugeac, P. On the use of the model-free way method for kinetic analysis of thermoanalytical data–advantages and limitations. Thermochim. Acta 2021, 706, 179063. [Google Scholar] [CrossRef]
- Hu, R.Z.; Shi, Q.Z. Thermal Analysis Kinetics, 1st ed.; Science Press: Beijing, China, 2001; pp. 127–154. [Google Scholar]
- Blaine, R.; Kissinger, H. Homer Kissinger and the Kissinger equation. Thermochim. Acta 2012, 540, 1–6. [Google Scholar] [CrossRef]
- Juan, J.; Francisco, P.; Guillermo, R. On the integration of kinetic models using a high-order Taylor series method. J. Chemom. 1992, 6, 231–246. [Google Scholar]
- Tokiwai, K.; Nakashima, S. Dehydration kinetics of muscovite by in situ infrared microspectroscopy. Phys. Chem. Miner. 2010, 37, 91–101. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds Part A: Theory and Applications in Inorganic Chemistry, 6th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 149–299. [Google Scholar]
- Zhu, X.; Han, Y.; Sun, Y.; Gao, P.; Li, Y. Magnetite oxidation mechanism of the air-cooling stage for limonite ore magnetization roasting. Miner. Eng. 2022, 186, 107720. [Google Scholar] [CrossRef]
- Nunna, V.; Hapugoda, S.; Pownceby, M.; Sparrow, G. Beneficiation of low-grade, goethite-rich iron ore using microwave-assisted magnetizing roasting. Miner. Eng. 2021, 166, 106826. [Google Scholar] [CrossRef]
- Ravisankar, V.; Venugopal, R.; Bhat, H. Investigation on beneficiation of goethite-rich iron ores using reduction roasting fol lowed by magnetic separation. Miner. Process. Extr. Metall. 2019, 128, 175–182. [Google Scholar]
| Al2O3 | SiO2 | Fe2O3 | CaO | MgO | MnO | K2O | SO3 | Mass loss | TFe |
| 11.18 | 36.82 | 40.12 | 0.81 | 1.95 | 0.69 | 2.60 | 0.38 | 11.63 | 21.42 |
| Method | β/(°C/min) | E/(kJ/mol) | A | r |
|---|---|---|---|---|
| General integral method | 10 | 98.1 | 7.58 × 104 | 0.995680 |
| 15 | 103.4 | 2.35 × 105 | 0.995052 | |
| 20 | 108.8 | 3.58 × 105 | 0.995351 | |
| 30 | 114.0 | 7.37 × 105 | 0.997411 | |
| Average | 106.1 | 3.51 × 105 | 0.995874 | |
| Kissinger method | - | 107.4 | 5.15 × 105 | 0.991111 |
| Temperature /(°C) | α = 0.80 | α = 0.90 | α = 0.95 | α = 0.98 | α = 0.999 |
|---|---|---|---|---|---|
| 600 | 9.06 | 11.85 | 14.44 | 17.64 | 27.02 |
| 650 | 4.11 | 5.37 | 6.54 | 7.99 | 12.24 |
| 700 | 2.02 | 2.64 | 3.22 | 3.93 | 6.02 |
| 750 | 1.06 | 1.39 | 1.69 | 2.07 | 3.17 |
| 780 | 0.75 | 0.97 | 1.19 | 1.45 | 2.22 |
| Temperature (°C) | Fe in Magnetite (wt.%) | Fe in Siderite (wt.%) | Fe in Hematite (wt.%) | Fe in Silicate (wt.%) | Fe in Sulfide (wt.%) | Total (wt.%) | Standard Deviation | Magnetic Conversion Rate (%) |
|---|---|---|---|---|---|---|---|---|
| Raw ore | 0.21 | 19.58 | 0.84 | 0.56 | 0.23 | 21.42 | 0.0299 | 0.0 |
| 600 | 8.87 | 12.81 | 0.76 | 0.59 | 0.18 | 23.21 | 0.0294 | 0.39 |
| 650 | 17.92 | 6.12 | 0.56 | 0.63 | 0.12 | 25.35 | 0.0258 | 0.72 |
| 700 | 24.27 | 0.35 | 0.32 | 0.64 | 0.02 | 25.60 | 0.0275 | 0.97 |
| 750 | 25.83 | 0 | 0.27 | 0.64 | 0 | 26.74 | 0.0321 | 0.99 |
| 780 | 25.75 | 0 | 0.11 | 0.62 | 0 | 26.48 | 0.0327 | 1.00 |
| Region A | Region B | ||||
|---|---|---|---|---|---|
| Element | Mass Percentage (%) | Atomic Percentage (%) | Element | Mass Percentage (%) | Atomic Percentage (%) |
| O | 26.81 | 49.52 | O | 43.06 | 57.93 |
| Fe | 62.6 | 32.84 | Si | 48.25 | 36.97 |
| Si | 5.15 | 5.42 | Fe | 3.23 | 1.24 |
| Mg | 0.87 | 1.05 | K | 2.03 | 1.12 |
| Ca | 0.30 | 0.22 | Al | 3.43 | 2.74 |
| Product Type | Yield (%) | Iron Grade (wt.%) | Iron Recovery (%) |
|---|---|---|---|
| Concentrate | 28.79 | 62.80 | 68.83 |
| Middling IV | 2.76 | 58.04 | 6.10 |
| Middling III | 2.22 | 44.70 | 3.78 |
| Middling II | 13.90 | 12.82 | 6.78 |
| Middling I | 22.92 | 12.97 | 11.32 |
| Tailings | 29.41 | 2.85 | 3.19 |
| Roasted ore | 100.00 | 26.32 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiu, S.; Zhao, B.; Yang, C.; Chen, Y.; Cheng, F. High-Efficiency Iron Extraction from Low-Grade Siderite via a Conveyor Bed Magnetization Roasting–Magnetic Separation Process: Kinetics Research and Applications. Materials 2022, 15, 6260. https://doi.org/10.3390/ma15186260
Jiu S, Zhao B, Yang C, Chen Y, Cheng F. High-Efficiency Iron Extraction from Low-Grade Siderite via a Conveyor Bed Magnetization Roasting–Magnetic Separation Process: Kinetics Research and Applications. Materials. 2022; 15(18):6260. https://doi.org/10.3390/ma15186260
Chicago/Turabian StyleJiu, Shaowu, Bo Zhao, Chao Yang, Yanxin Chen, and Fuan Cheng. 2022. "High-Efficiency Iron Extraction from Low-Grade Siderite via a Conveyor Bed Magnetization Roasting–Magnetic Separation Process: Kinetics Research and Applications" Materials 15, no. 18: 6260. https://doi.org/10.3390/ma15186260






