Frost Resistance and Mechanism of Circulating Fluidized Bed Fly Ash-Blast Furnace Slag-Red Mud-Clinker Based Cementitious Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Physicochemical Performances of Raw Materials
2.1.1. Raw Materials
2.1.2. Chemical Compositions
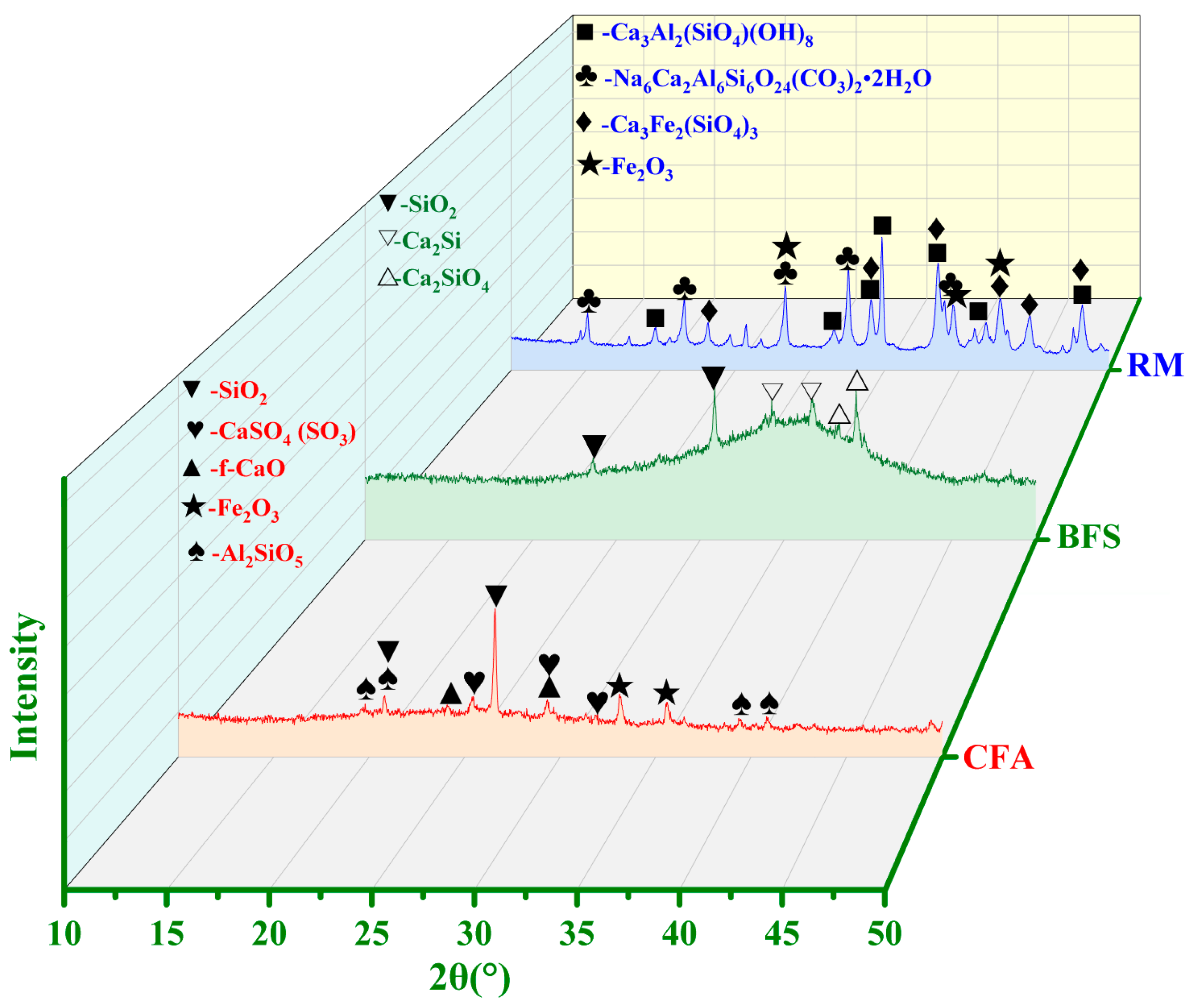
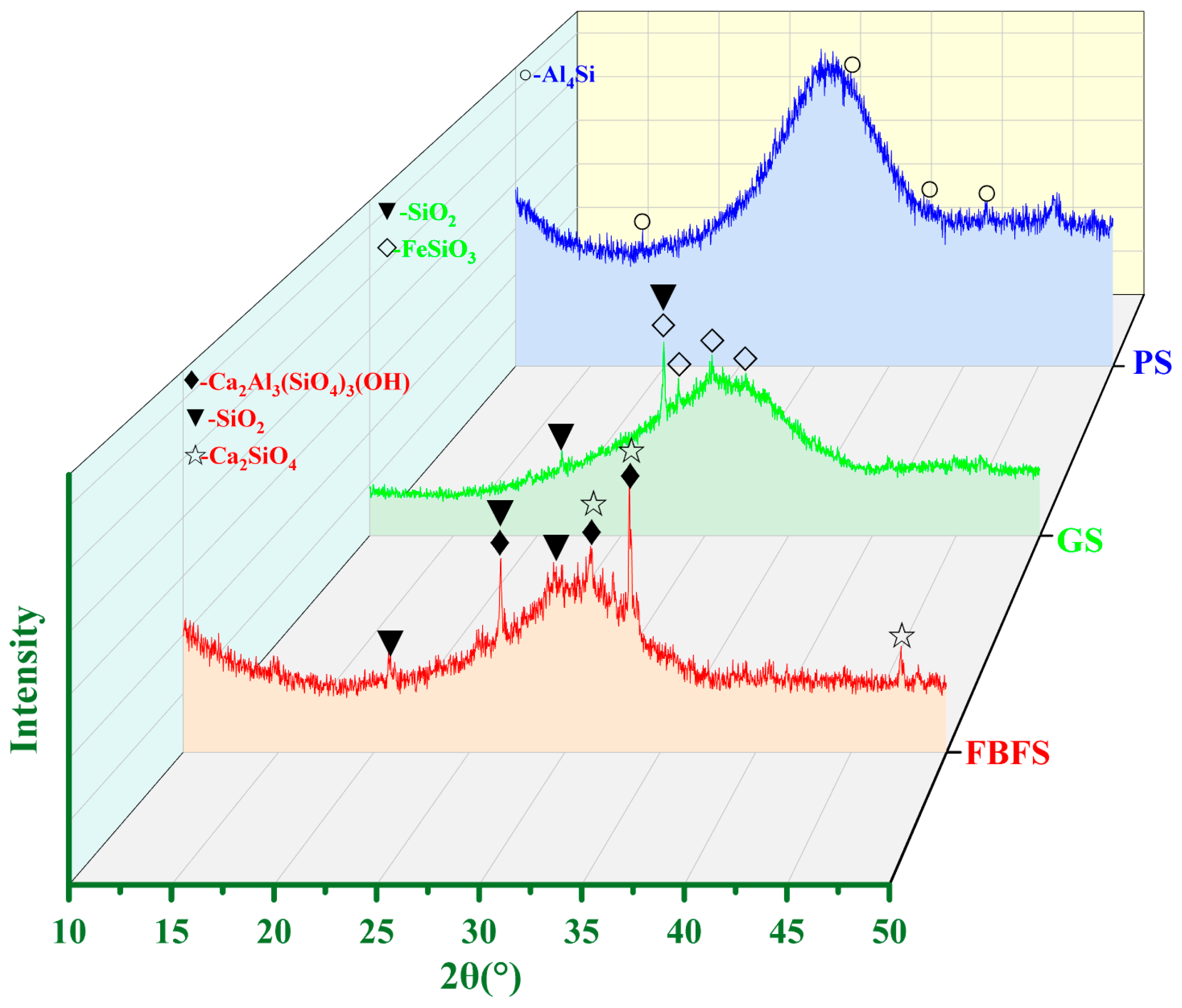
2.1.3. Phase Composition
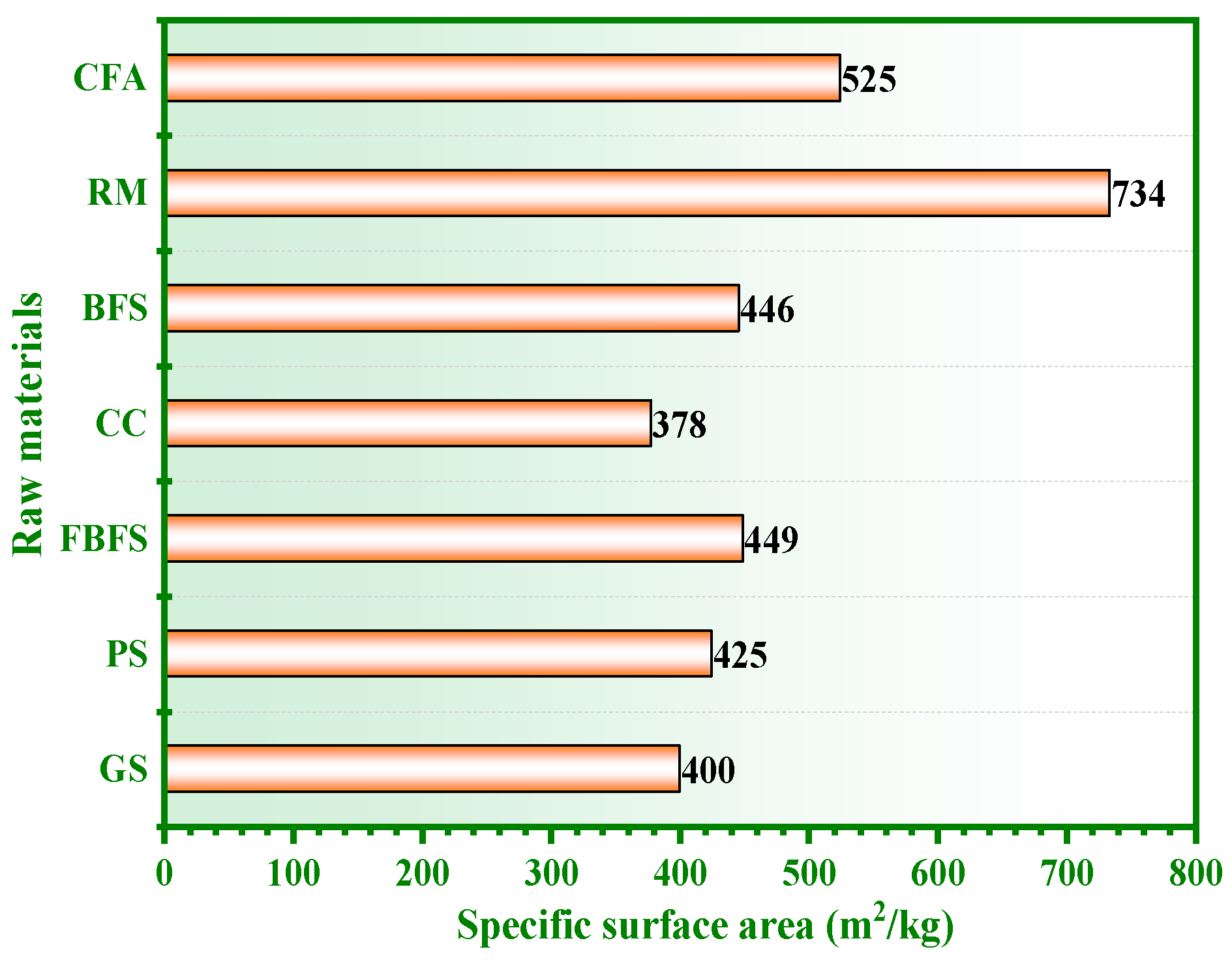
2.1.4. Specific Surface Area
2.2. Experimental Design of CSM
2.3. Technical Framework of CSM
- S—Strength loss rate of CSM after i freeze–thaw cycles (%);
- e0—Compressive strength value of CSM before freeze–thaw cycling (MPa);
- ei—Compressive strength value of CSM after i freeze–thaw cycles (MPa).
- ω—Mass loss rate of CSM after i freeze–thaw cycles (%);
- m0—Mass value of CSM before freeze–thaw cycle (MPa);
- mi—Mass value of CSM after i freeze–thaw cycles (MPa).
2.4. Test Methods
2.4.1. Performance Test
2.4.2. Microstructure Analysis
3. Results and Discussion
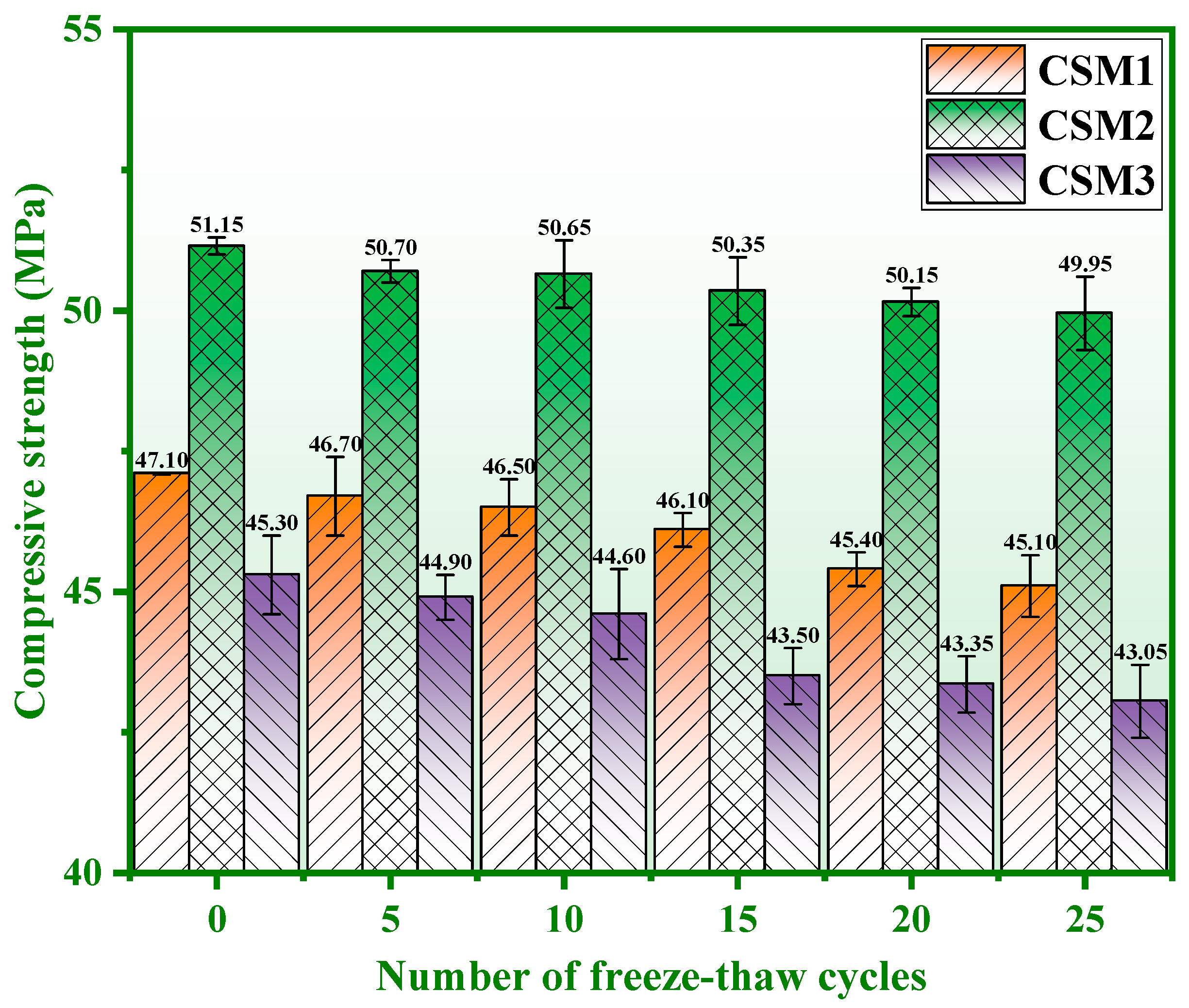
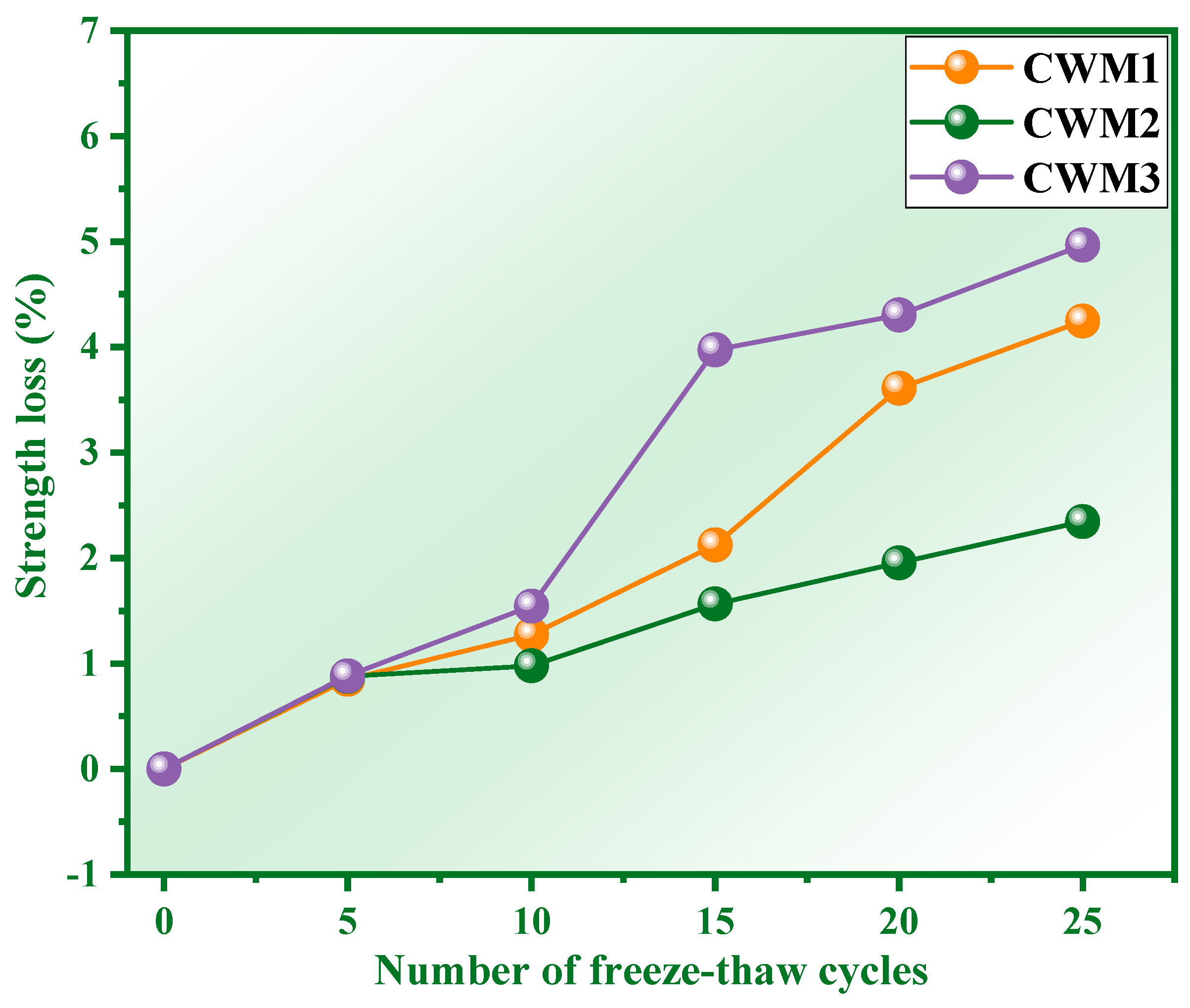
3.1. Strength Loss and Mass Loss
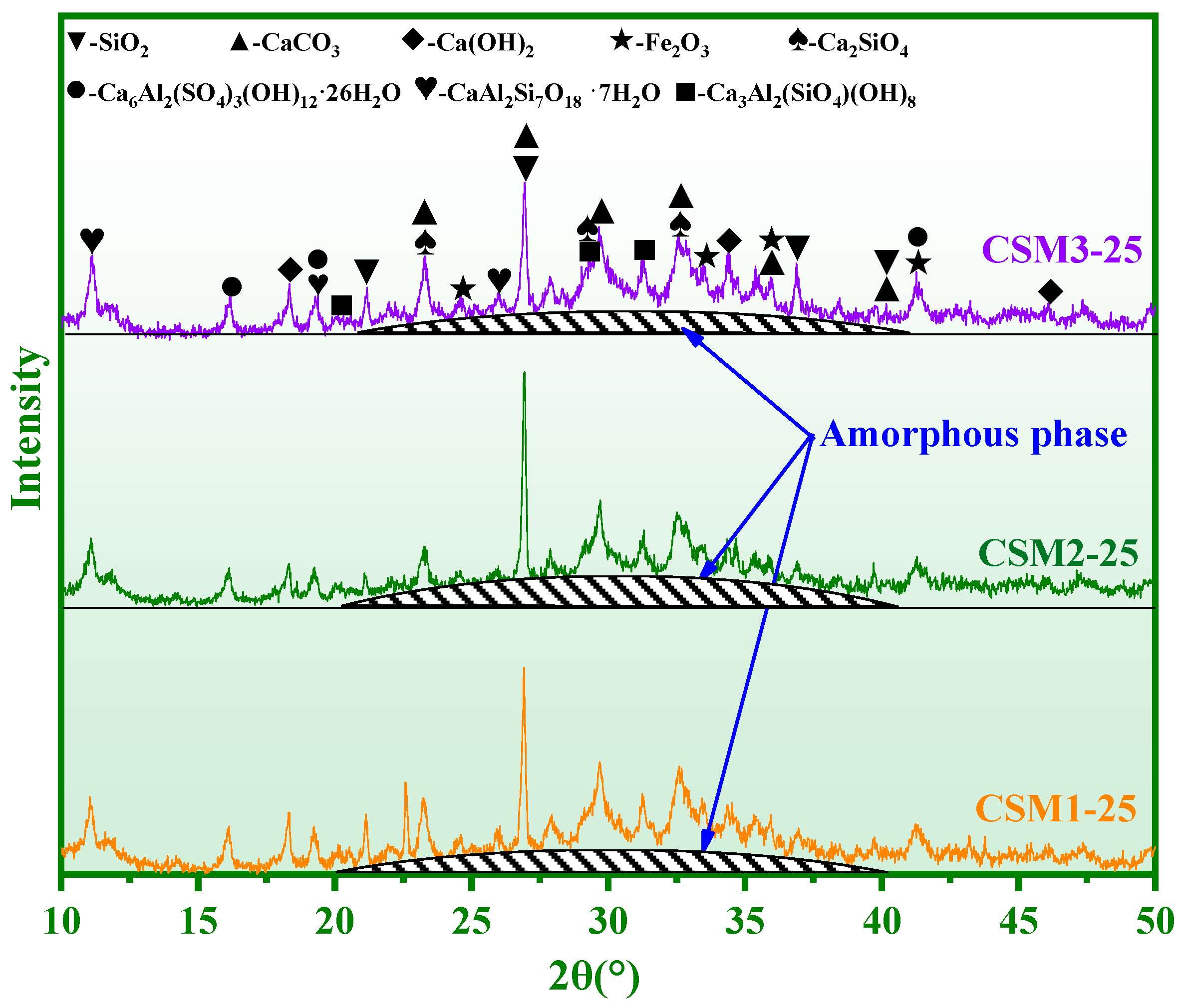
3.2. Phase Composition Analysis
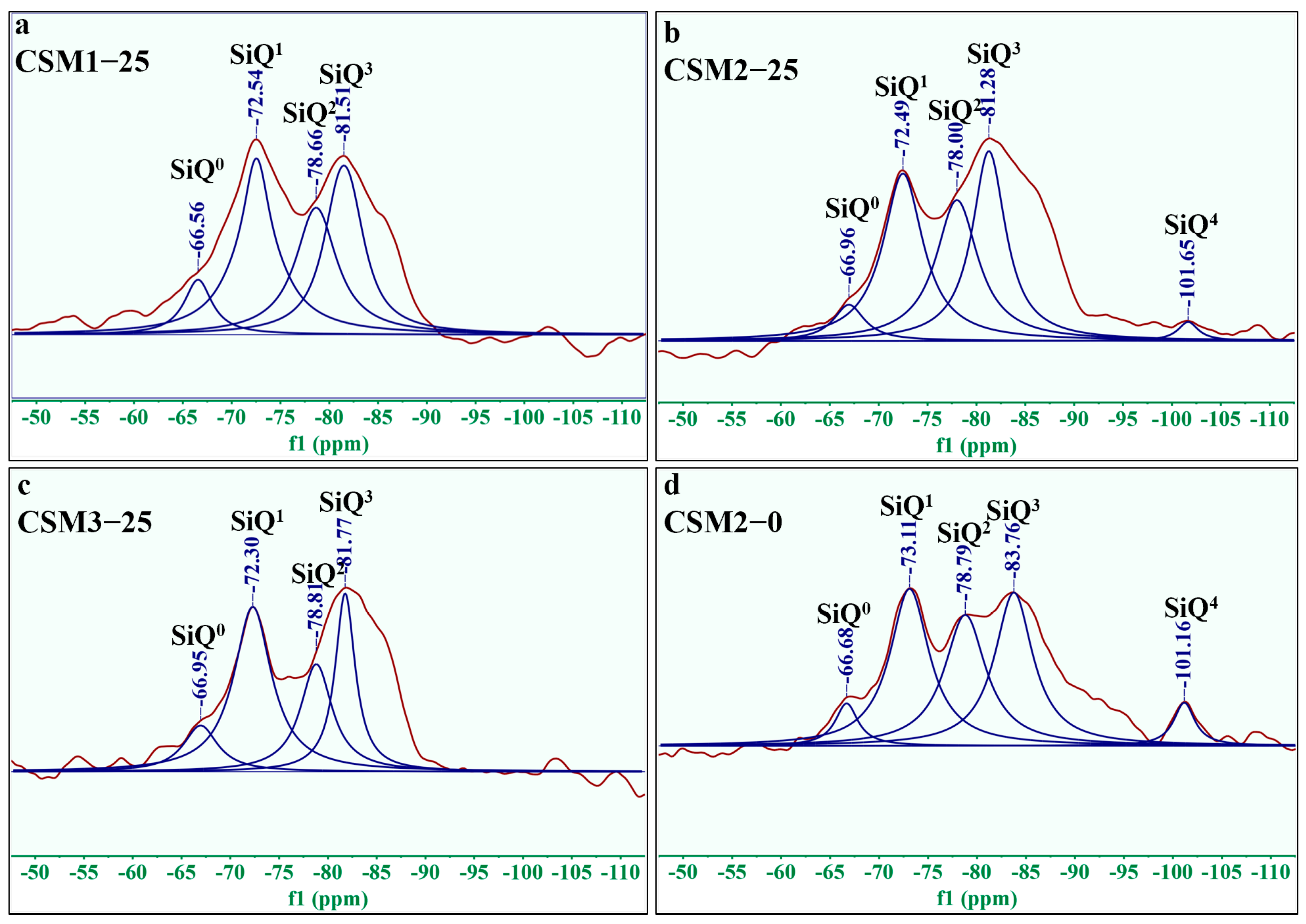
3.3. Si-Al Structure Analysis
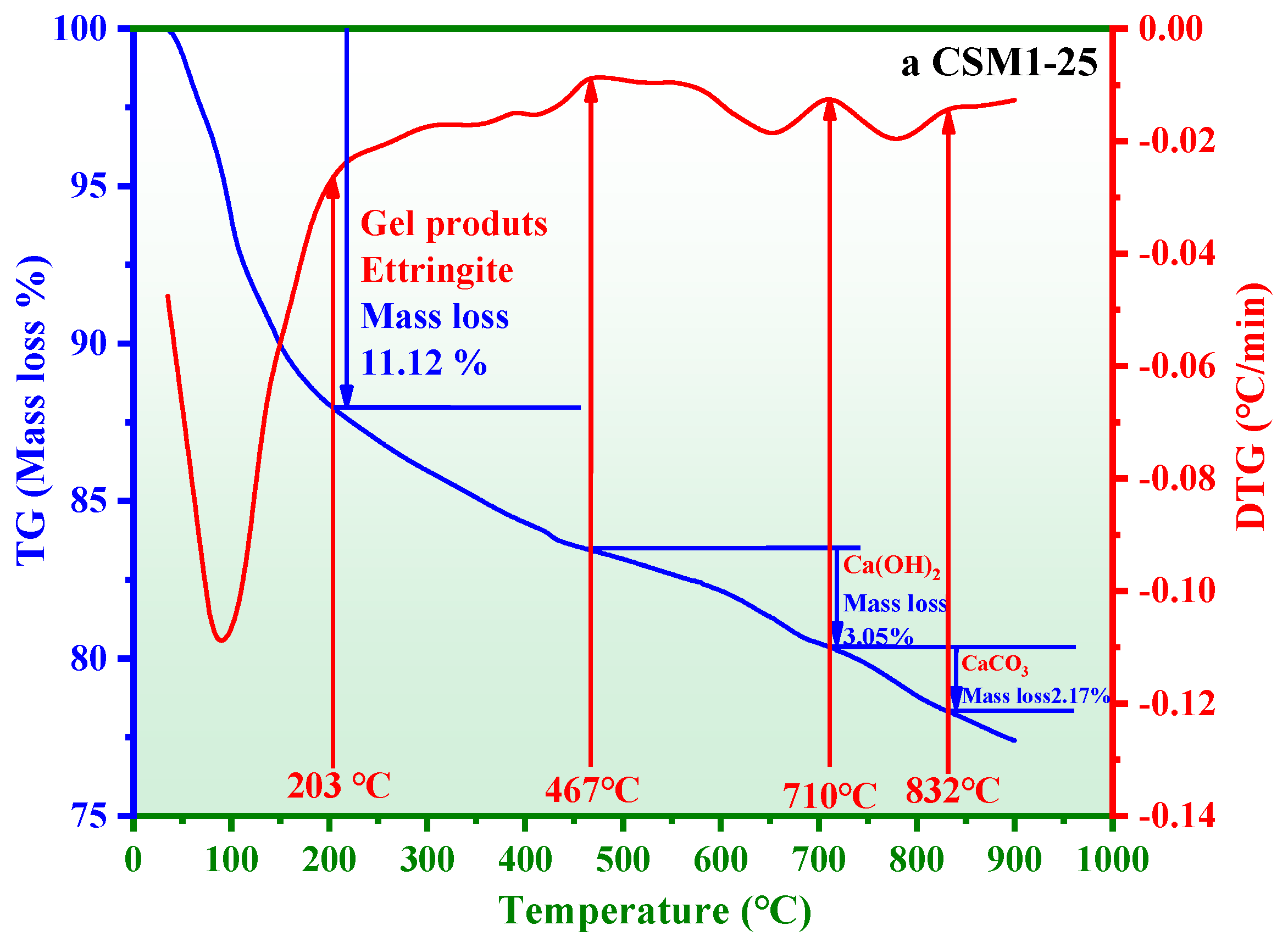
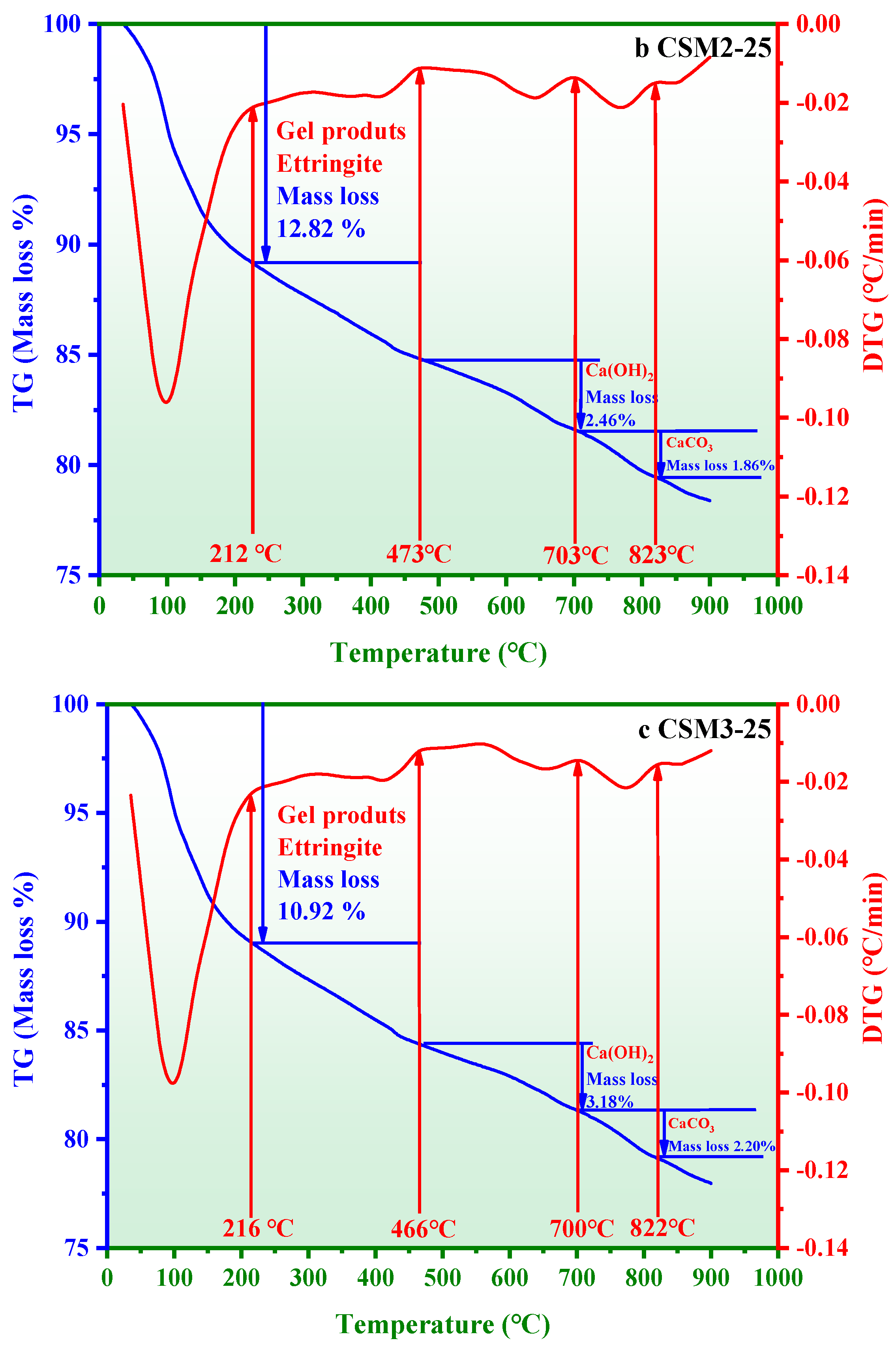
3.4. Thermogravimetric Loss Analysis
3.5. Microstructure Analysis
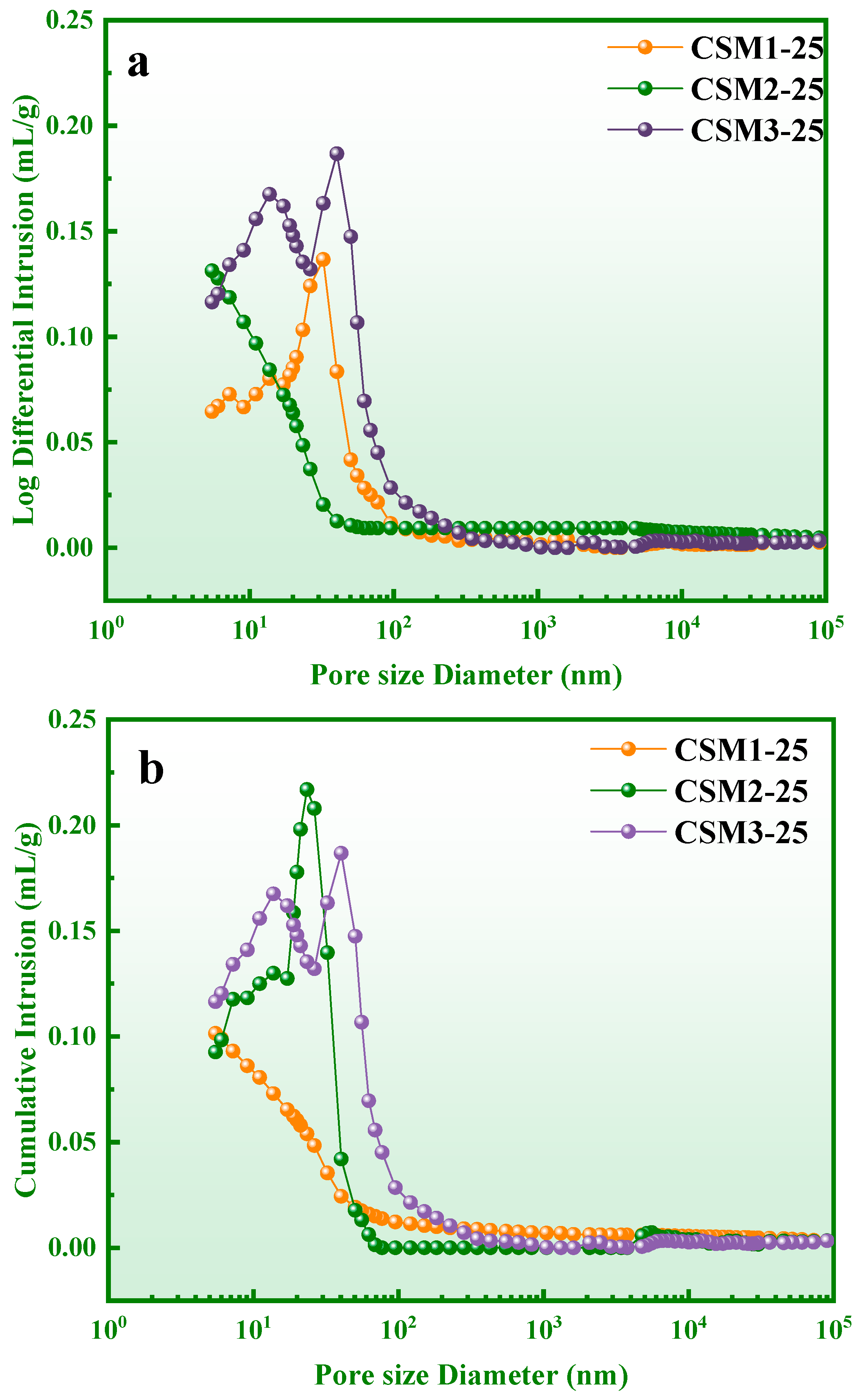
3.6. Pore Structure Analysis
3.7. Leaching of Harmful Elements
4. Conclusions
- (1)
- The strength loss, mass loss, and strength of CSM2 after 25 freeze–thaw cycles (CSM2-25) are 2.35%, 0.36%, and 49.95 MPa, respectively, which meets the performance requirements of fly ash Portland cement (42.5#). The frost resistance of CSM2 is excellent based on synergy of CFA, BFS, RM, and PS.
- (2)
- The thermogravimetric loss and polymerization degree of hydration products in CSM2-25 are 12.82 wt.% and 50.65%, respectively, which are higher than those of other CSMs. The reason is that the amount of hydration products (C-S-H gel, C/N-A-S-H gel, and ettringite) in CSM2 is the maximum during the freeze–thaw cycle.
- (3)
- The interface between paste and aggregate, micromorphology, and pore structure of CSM2 are the densest based on the degree of high polymerization. These characteristics are beneficial to the stable development of long-term frost resistance in CSM2.
- (4)
- The leaching concentrations of Na, As, Cd, and Hg in CSM2-25 are 82.6927 mg/L, 0.0025 mg/L, 0.0005 mg/L, and <0.0001 mg/L, respectively, during the action of 25 freeze–thaw cycles, which still meet the WHO standard of drinking water. Therefore, CSM2 is a green cementitious material with long-term frost resistance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, H.; Shi, C.; Guan, X.; Zhu, J.; Ding, Y.; Ling, T.; Zhang, H.; Wang, Y. Durability of recycled aggregate concrete—A review. Cem. Concr. Compos. 2018, 89, 251–259. [Google Scholar] [CrossRef]
- Dvorkin, L. Design estimation of concrete frost resistance. Constr. Build. Mater. 2019, 211, 779–784. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, X.; Chen, S.; Lin, H.; Li, Z.; Lin, X. Hydrophobic or superhydrophobic modification of cement-based materials: A systematic review. Compos. Part B Eng. 2022, 243, 110104. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, G.; Wu, C.; Li, J.; Wu, S.; Jiang, W.; Wang, X.; Wang, W.; Feng, M. Investigation of hierarchical porous cold bonded lightweight aggregates produced from red mud and solid-waste-based cementitious material. Constr. Build. Mater. 2021, 308, 124990. [Google Scholar] [CrossRef]
- Tarangini, D.; Sravana, P.; Rao, P.S. Effect of nano silica on frost resistance of pervious concrete. Mater. Today Proc. 2022, 51, 2185–2189. [Google Scholar] [CrossRef]
- Liu, L.; Guan, Q.; Zhang, L.; Liu, C.; Chen, X.; Cai, X. Evaluation of the compressive-strength reducing behavior of concrete containing rubber aggregate. Clean. Mater. 2022, 4, 100057. [Google Scholar] [CrossRef]
- Şahin, Y.; Akkaya, Y.; Taşdemir, M.A. Effects of freezing conditions on the frost resistance and microstructure of concrete. Constr. Build. Mater. 2021, 270, 121458. [Google Scholar] [CrossRef]
- Han, F.; Zhang, H.; Liu, J.; Song, S. Influence of iron tailing powder on properties of concrete with fly ash. Powder Technol. 2022, 398, 117132. [Google Scholar] [CrossRef]
- Cantero, B.; del Bosque, I.F.S.; de Rojas, M.I.S.; Matías, A.; Medina, C. Durability of concretes bearing construction and demolition waste as cement and coarse aggregate substitutes. Cem. Concr. Compos. 2022, 134, 104722. [Google Scholar] [CrossRef]
- Zhang, N.; Wu, L.; Liu, X.; Zhang, Y. Structural characteristics and cementitious behavior of basic oxygen furnace slag mud and electric arc furnace slag. Constr. Build. Mater. 2019, 219, 11–18. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, A.; Orlando, A.; Montegrossi, G.; Huet, B.; Virgili, G.; Vaselli, O. Experimental analysis on the carbonation rate of Portland cement at room temperature and CO2 partial pressure from 1 to 51 bar. Cem. Concr. Compos. 2021, 124, 104271. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, C.; Wang, W.; Wu, S.; Li, J.; Wang, X.; Wu, C. Preparation and physico-chemical performance optimization of sintering-free lightweight aggregates with high proportions of red mud. Materials 2021, 14, 218. [Google Scholar] [CrossRef] [PubMed]
- Siddique, R. Utilization of municipal solid waste (MSW) ash in cement and mortar. Resour. Conserv. Recycl. 2010, 54, 1037–1047. [Google Scholar] [CrossRef]
- Kaliyavaradhan, S.K.; Ling, T.; Mo, K.H. Valorization of waste powders from cement-concrete life cycle: A pathway to circular future. J. Clean. Prod. 2020, 268, 122358. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, X.; Wang, Y.; Li, Z.; Li, Y.; Ren, Y. Binary reaction behaviors of red mud based cementitious material: Hydration characteristics and Na+ utilization. J. Hazard. Mater. 2021, 410, 124592. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Wang, W.; Liu, M.; Yao, Y.; Wu, S. Synergistic use of industrial solid waste mixtures to prepare ready-to-use lightweight porous concrete. J. Clean. Prod. 2019, 211, 1034–1043. [Google Scholar] [CrossRef]
- Huang, L.; Han, L.; Liu, Z.; Wang, J.; Zhang, Y.; Wang, D. In-situ crystallization and characteristics of alkali-activated materials-supported analcime-C from a by-product of the lithium carbonate Industry. Materials 2022, 15, 1261. [Google Scholar] [CrossRef]
- Zhang, N.; Tang, B.; Liu, X. Cementitious activity of iron ore tailing and its utilization in cementitious materials, bricks and concrete. Constr. Build. Mater. 2021, 288, 123022. [Google Scholar] [CrossRef]
- Zhang, W.; Gu, J.; Zhou, X.; Li, Y.; Wang, Y.; Xue, Y.; Liu, X. Circulating fluidized bed fly ash based multi-solid wastes road base materials: Hydration characteristics and utilization of SO3 and f -CaO. J. Clean. Prod. 2021, 316, 128355. [Google Scholar] [CrossRef]
- Wang, M.; Liu, X. Applications of red mud as an environmental remediation material: A review. J. Hazard. Mater. 2021, 408, 124420. [Google Scholar] [CrossRef]
- Fan, D.; Ni, W.; Yan, A.; Wang, J.; Cui, W. Orthogonal experiments on direct reduction of carbon-bearing pellets of bayer red mud. J. Iron Steel Res. Int. 2015, 22, 686–693. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, C.; Jiang, W.; Li, J.; Wang, X.; Wu, S.; Yang, S.; Wang, W. Preparation and properties of alkali and sulfate coactivated ternary cementitious matrix and its application in high-strength cold bonded lightweight aggregates. Constr. Build. Mater. 2022, 346, 128430. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, X.; Zhang, Z.; Li, Y.; Gu, J. Synergic effects of circulating fluidized bed fly ash-red mud-blast furnace slag in green cementitious materials: Hydration products and environmental performance. J. Build. Eng. 2022, 58, 105007. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, X.; Zhang, Z. Mechanical, expansion and rheological properties of circulating fluidized bed fly ash based ecological cement: A critical review. Int. J. Miner. Metall. Mater. 2022, 29, 1670–1682. [Google Scholar] [CrossRef]
- Ting, L.; Qiang, W.; Shiyu, Z. Effects of ultra-fine ground granulated blast-furnace slag on initial setting time, fluidity and rheological properties of cement pastes. Powder Technol. 2019, 345, 54–63. [Google Scholar] [CrossRef]
- Jia, R.; Wang, Q.; Luo, T. Understanding the workability of alkali-activated phosphorus slag pastes: Effects of alkali dose and silicate modulus on early-age hydration reactions. Cem. Concr. Compos. 2022, 133, 104649. [Google Scholar] [CrossRef]
- Xie, F.; Liu, Z.; Zhang, D.; Wang, J.; Wang, D.; Ni, J. The effect of NaOH content on rheological properties, microstructures and interfacial characteristic of alkali activated phosphorus slag fresh pastes. Constr. Build. Mater. 2020, 252, 119132. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, P.; Mi, G. Effect of blended steel slag–GBFS mineral admixture on hydration and strength of cement. Constr. Build. Mater. 2012, 35, 8–14. [Google Scholar] [CrossRef]
- GB 175-2007, Common Portland Cement. 2007. Available online: https://www.antpedia.com/standard/5156435.html (accessed on 11 November 2007).
- GB/T 41060-2021, Test Method for Determining Frost Resistance of Cement Mortar. 2021. Available online: https://www.yjssishisi.com/jcbz/4312.html (accessed on 31 December 2021).
- EN 451-1-2017, Method of Testing Fly Ash—Part 1: Determination of Free Calcium Oxide Content. 2017. Available online: https://www.doc88.com/p-9189147009468.html (accessed on 3 May 2017).
- Zhang, W.; Liu, X.; Zhang, Z.; Wang, Y.; Xue, Y.; Hao, X.; Lu, Y. Circulating fluidized bed fly ash mixed functional cementitious materials: Shrinkage compensation of f-CaO, autoclaved hydration characteristics and environmental performance. Materials 2021, 14, 6004. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, H.; Sun, Y.; Zhang, N. Correlation between ~(29)Si polymerization and cementitious activity of coal gangue. J. Zhejiang Univ. 2009, 10, 1334–12240. [Google Scholar] [CrossRef]
- Walkley, B.; San Nicolas, R.; Sani, M.A.; Rees, G.J.; Hanna, J.V.; van Deventer, J.S.; Provis, J.L. Phase evolution of C-(N)-A-S-H/N-A-S-H gel blends investigated via alkali-activation of synthetic calcium aluminosilicate precursors. Cem. Concr. Res. 2016, 89, 120–135. [Google Scholar] [CrossRef]
- Bian, Y.; Li, Z.; Zhao, J.; Wang, Y. Synergistic enhancement effect of recycled fine powder (RFP) cement paste and carbonation on recycled aggregates performances and its mechanism. J. Clean. Prod. 2022, 344, 130848. [Google Scholar] [CrossRef]
- Kapeluszna, E.; Kotwica, Ł.; Różycka, A.; Gołek, Ł. Incorporation of Al in C-A-S-H gels with various Ca/Si and Al/Si ratio: Microstructural and structural characteristics with DTA/TG, XRD, FTIR and TEM analysis. Constr. Build. Mater. 2017, 155, 643–653. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Y.; Zhang, Z.; Huang, J.; Zhang, P.; Ma, Y.; Wang, H. Study of acidic degradation of alkali-activated materials using synthetic C-(N)-A-S-H and N-A-S-H gels. Compos. Part B Eng. 2022, 230, 109510. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, J.; Wang, D.; Xu, C.; Zhai, M.; Ma, X. Comparative study on the properties of three hydraulic lime mortar systems: Natural hydraulic lime mortar, cement-aerial lime-based mortar and slag-aerial lime-based mortar. Constr. Build. Mater. 2018, 186, 42–52. [Google Scholar] [CrossRef]
- Hao, X.; Liu, X.; Zhang, Z.; Zhang, W.; Lu, Y.; Wang, Y.; Yang, T. In-depth insight into the cementitious synergistic effect of steel slag and red mud on the properties of composite cementitious materials. J. Build. Eng. 2022, 52, 104449. [Google Scholar] [CrossRef]
- Ma, Y.; Li, W.; Jin, M.; Liu, J.; Zhang, J.; Huang, J.; Lu, C.; Zeng, H.; Wang, J.; Zhao, H.; et al. Influences of leaching on the composition, structure and morphology of calcium silicate hydrate (C–S–H) with different Ca/Si ratios. J. Build. Eng. 2022, 58, 105017. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, J.; Wu, A.; Wang, H. Coupled effects of superplasticizers and glazed hollow beads on the fluidy performance of cemented paste backfill containing alkali-activated slag and MSWI fly ash. Powder Technol. 2022, 399, 116726. [Google Scholar] [CrossRef]
- Tang, X.; Tao, S.; Li, P.; Rutqvist, J.; Hu, M.; Sun, L. The propagation and interaction of cracks under freeze-thaw cycling in rock-like material. Int. J. Rock Mech. Min. Sci. 2022, 154, 105112. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, Y.; Zhang, L.; Yang, K.; Guan, X.; Zhao, R. Insights on substitution preference of Pb Ions in sulfoaluminate cement clinker phases. Materials 2021, 14, 44. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, Z.; Wang, M.; Lu, S.; Chi, L. Functionalized PP fiber to improve compressive strength and solidification/stabilization performance of cement with heavy metals. Constr. Build. Mater. 2021, 278, 122412. [Google Scholar] [CrossRef]
- GB 5086.1-1997, Test Method Standard for Leaching Toxicity of Solid Wastes--Roll Over Leaching Procedure. 1997. Available online: https://www.antpedia.com/standard/351944.html (accessed on 22 December 1997).
- World Health Organization Drinking Water Quality Indicators. 2017. Available online: https://max.book118.com/html/2017/0122/86028975.shtm (accessed on 22 January 2017).





| Oxide | T-CaO | f-CaO | SiO2 | Al2O3 | SO3 | Fe2O3 | MgO | TiO2 | P2O5 | F | K2O | Na2O | LOI | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CFA | 12.73 | 4.10 | 34.15 | 24.04 | 6.67 | 5.31 | 1.25 | 0.76 | 0.23 | - | 0.82 | 0.20 | 10.38 | 96.54 |
| RM | 21.09 | - | 19.02 | 22.46 | 0.29 | 15.15 | 0.46 | 4.33 | 0.67 | - | 0.57 | 6.01 | 8.84 | 98.89 |
| BFS | 34.14 | - | 34.64 | 18.64 | 1.66 | 0.86 | 6.96 | 0.77 | 0.04 | - | 0.63 | 0.62 | 0.50 | 99.46 |
| CC | 63.87 | - | 22.75 | 5.76 | 0.38 | 3.17 | 2.06 | 0.24 | 0.19 | - | 0.82 | 0.33 | 0.03 | 99.60 |
| FBFS | 39.74 | - | 29.19 | 15.02 | 2.73 | 0.82 | 9.38 | 0.81 | 0.02 | - | 0.50 | 0.47 | 0.60 | 99.28 |
| PS | 46.67 | - | 36.79 | 2.93 | 1.30 | 0.13 | 1.32 | 0.20 | 3.34 | 3.04 | 0.69 | 0.98 | 1.85 | 99.24 |
| GS | 26.41 | - | 34.79 | 15.96 | 0.49 | 9.53 | 0.98 | 0.89 | 0.07 | - | 0.70 | 4.89 | 3.94 | 98.65 |
| Sample | CFA | RM | BFS | CC | Silicon-Aluminum-Based Solid Wastes | Ca/(Si + Al) Mass Ratio |
|---|---|---|---|---|---|---|
| CSM1 | 30 | 10 | 20 | 30 | 10 (FBFS) | 0.79 |
| CSM2 | 30 | 10 | 20 | 30 | 10 (PS) | 0.81 |
| CSM3 | 30 | 10 | 20 | 30 | 10 (GS) | 0.75 |
| Sample | Peak Position (PPM) | Assign | Relative | Polymerization Degree of RBO |
|---|---|---|---|---|
| CSM1-25 | −66.56 | SiQ0 | 22.22 | 48.95% |
| −72.54 | SiQ1 | 100.00 | ||
| −78.66 | SiQ2 (1Al) | 77.78 | ||
| −81.51 | SiQ3 (2Al) | 88.89 | ||
| CSM2-25 | −66.96 | SiQ0 | 16.25 | 50.65% |
| −72.49 | SiQ1 | 100.00 | ||
| −78.00 | SiQ2 (1Al) | 88.75 | ||
| −81.28 | SiQ3 (2Al) | 95.00 | ||
| −101.65 | SiQ4 | 6.25 | ||
| CSM3-25 | −65.95 | SiQ0 | 25.49 | 44.91% |
| −72.30 | SiQ1 | 100.00 | ||
| −78.81 | SiQ2 (1Al) | 54.90 | ||
| −81.77 | SiQ3 (2Al) | 56.86 | ||
| CSM2-0 | −66.68 | SiQ0 | 12.96 | 52.16% |
| −73.11 | SiQ1 | 100.00 | ||
| −78.79 | SiQ2 (1Al) | 88.89 | ||
| −83.76 | SiQ3 (2Al) | 96.30 | ||
| −101.16 | SiQ4 | 14.81 |
| Samples | Total Pore Volume (mL/g) | Average Pore Diameter (nm) | Porosity (%) | Bulk Density (g/mL) | Apparent Density (g/mL) |
|---|---|---|---|---|---|
| CSM1-25 | 0.1014 | 18.0600 | 17.59 | 1.7339 | 2.1040 |
| CSM2-25 | 0.1312 | 15.1200 | 23.14 | 1.7636 | 2.2947 |
| CSM3-25 | 0.1814 | 17.3200 | 27.1100 | 1.4941 | 2.0497 |
| Samples | Total Pore Volume (mL/g) | Average Pore Diameter (nm) | Porosity (%) | Bulk Density (g/mL) | Apparent Density (g/mL) |
|---|---|---|---|---|---|
| CSM2-5 | 0.1524 | 14.9700 | 23.84 | 1.5637 | 2.0531 |
| CSM2-15 | 0.1374 | 15.3900 | 23.19 | 1.6881 | 2.1976 |
| CSM2-25 | 0.1312 | 15.1200 | 23.14 | 1.7636 | 2.2947 |
| Sample (mg/L) | Na | As | Cd | Hg |
|---|---|---|---|---|
| CFA | 5.2753 | 0.0441 | 0.0015 | <0.0001 |
| RM | 685.6372 | 0.0491 | 0.0016 | 0.0022 |
| PS | 12.5648 | 0.0007 | <0.0001 | <0.0001 |
| GS | 37.3006 | 0.0046 | 0.0005 | <0.0001 |
| CSM1-25 | 96.3796 | 0.0025 | 0.0003 | <0.0001 |
| CSM2-25 | 82.6927 | 0.0025 | 0.0005 | <0.0001 |
| CSM3-25 | 105.5107 | 0.0012 | 0.0007 | <0.0001 |
| WHO drinking water standard [46] | ≤200.0000 | ≤0.0100 | ≤0.0030 | ≤0.0010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Wei, C.; Liu, X.; Zhang, Z. Frost Resistance and Mechanism of Circulating Fluidized Bed Fly Ash-Blast Furnace Slag-Red Mud-Clinker Based Cementitious Materials. Materials 2022, 15, 6311. https://doi.org/10.3390/ma15186311
Zhang W, Wei C, Liu X, Zhang Z. Frost Resistance and Mechanism of Circulating Fluidized Bed Fly Ash-Blast Furnace Slag-Red Mud-Clinker Based Cementitious Materials. Materials. 2022; 15(18):6311. https://doi.org/10.3390/ma15186311
Chicago/Turabian StyleZhang, Wei, Chao Wei, Xiaoming Liu, and Zengqi Zhang. 2022. "Frost Resistance and Mechanism of Circulating Fluidized Bed Fly Ash-Blast Furnace Slag-Red Mud-Clinker Based Cementitious Materials" Materials 15, no. 18: 6311. https://doi.org/10.3390/ma15186311
APA StyleZhang, W., Wei, C., Liu, X., & Zhang, Z. (2022). Frost Resistance and Mechanism of Circulating Fluidized Bed Fly Ash-Blast Furnace Slag-Red Mud-Clinker Based Cementitious Materials. Materials, 15(18), 6311. https://doi.org/10.3390/ma15186311