Design and Characterization of Zeolite/Serpentine Nanocomposite Photocatalyst for Solar Hydrogen Generation
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. Method
2.2.1. Synthesis of the Hydrothermal Zeolite and Pure Serpentine Samples
2.2.2. Synthesis of Zeo/Serp (2:1) Nanocomposites
2.3. Characterization of the Hydrothermally Prepared Samples
3. Results and Discussion
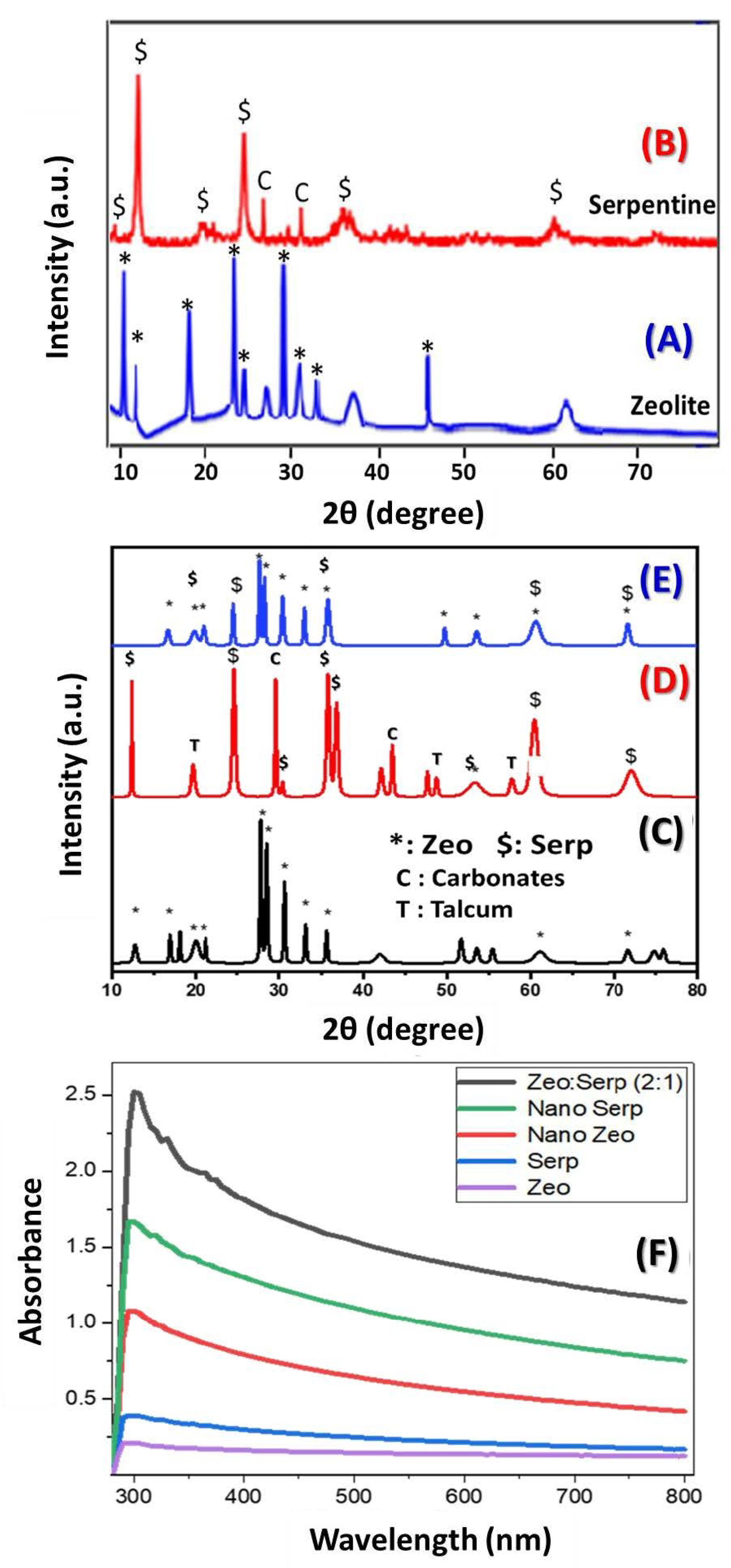
3.1. The XRD Study and Optical Absorption
3.2. FTIR Study
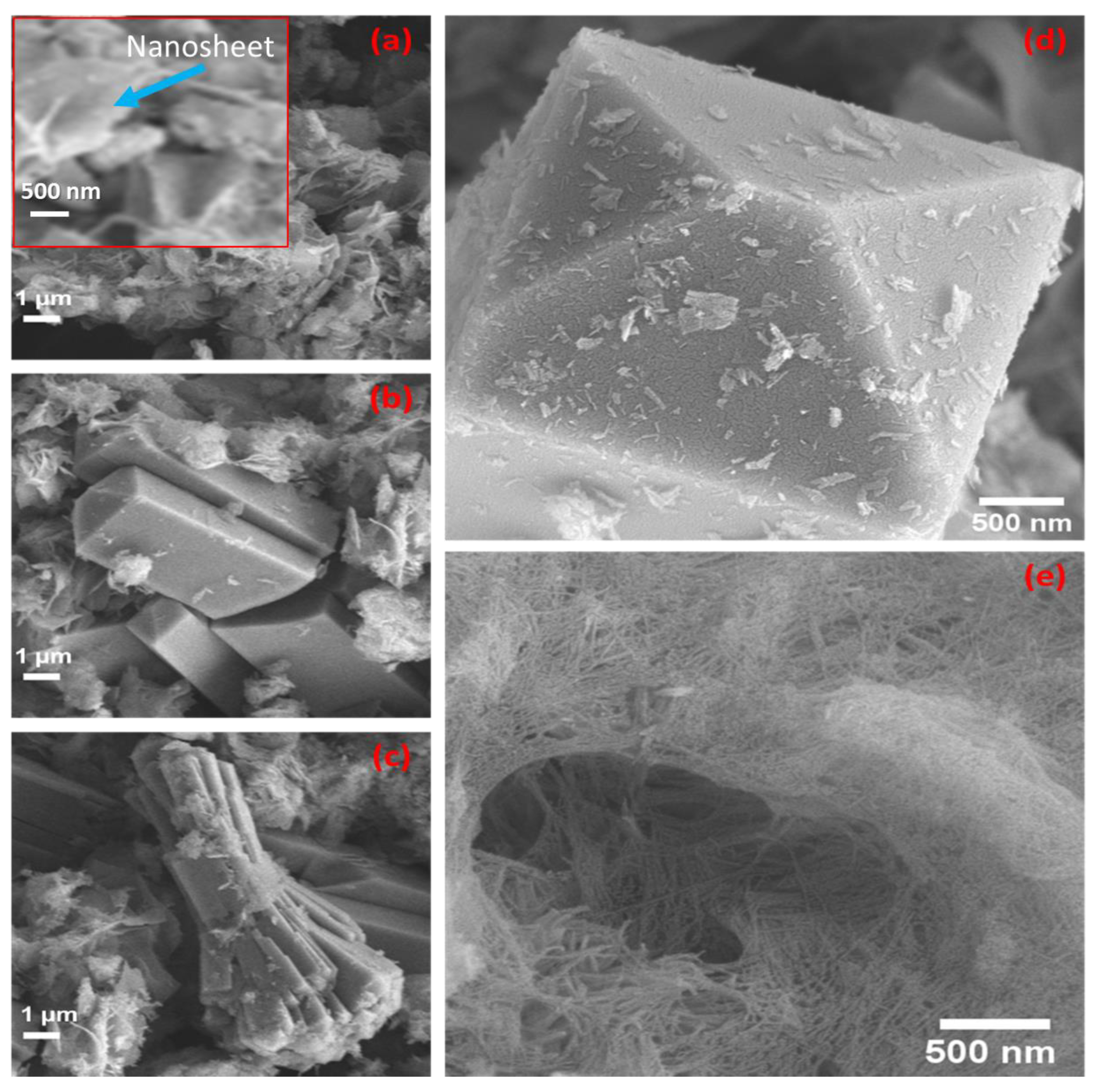
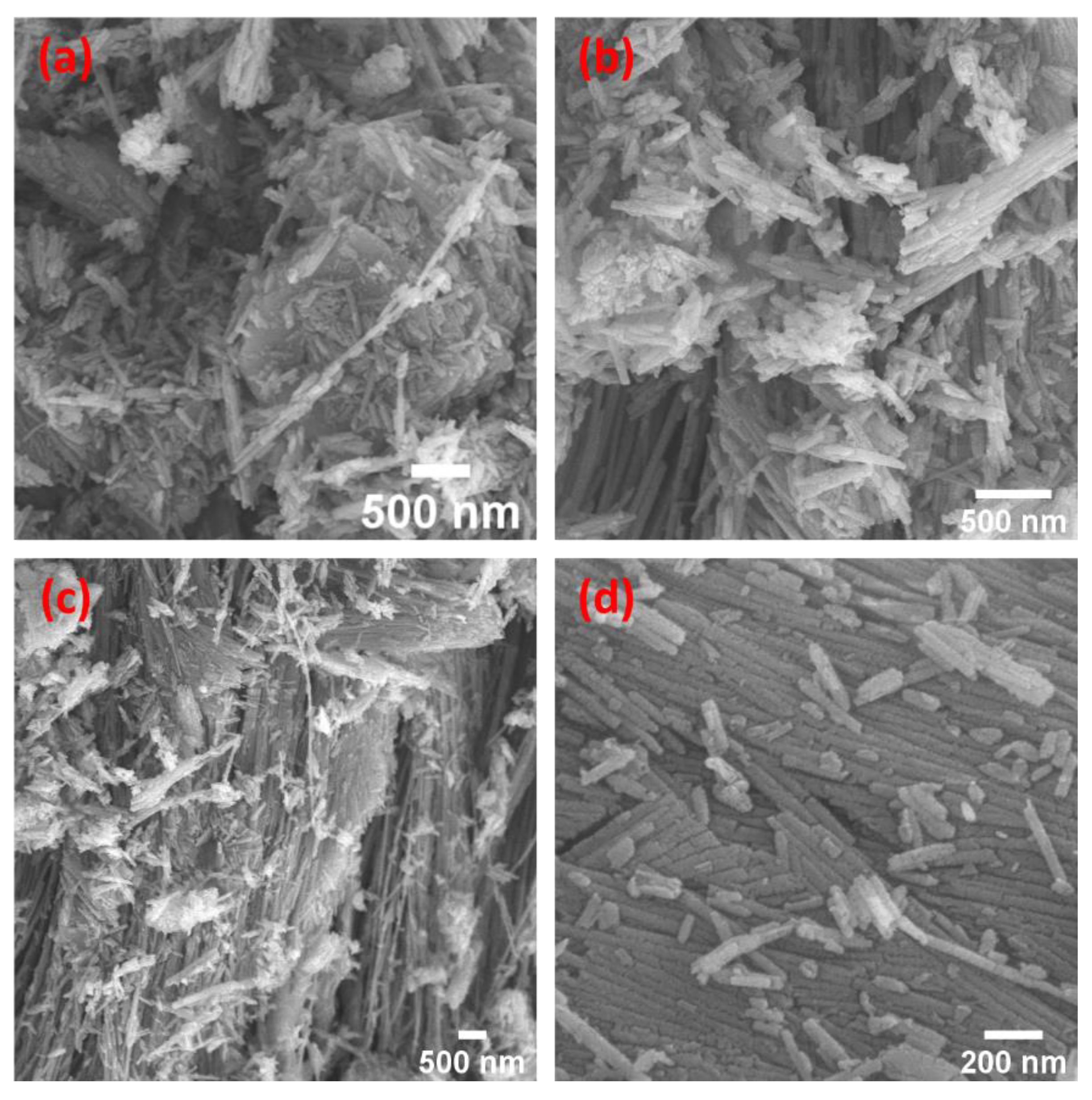
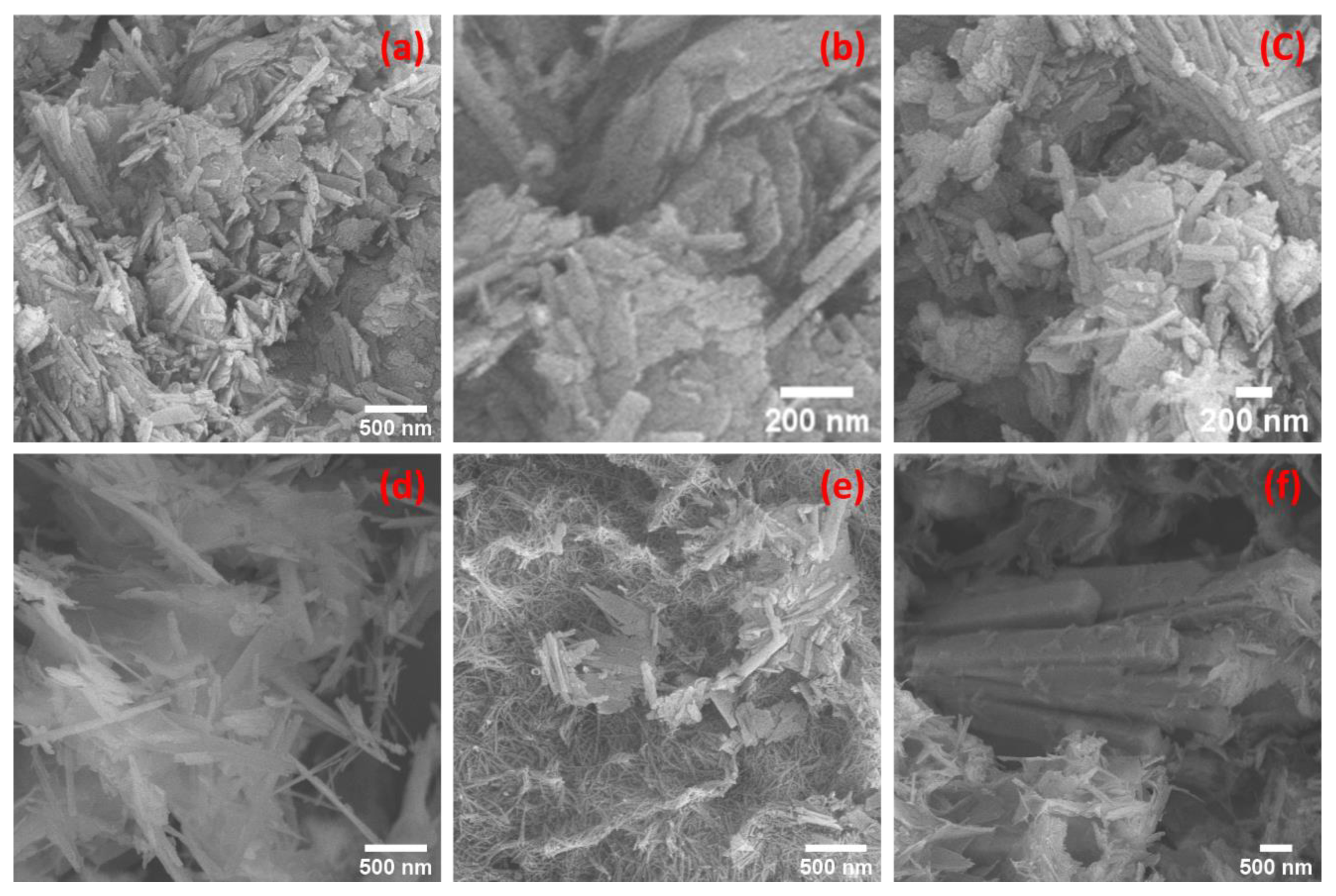
3.3. SEM Morphologies
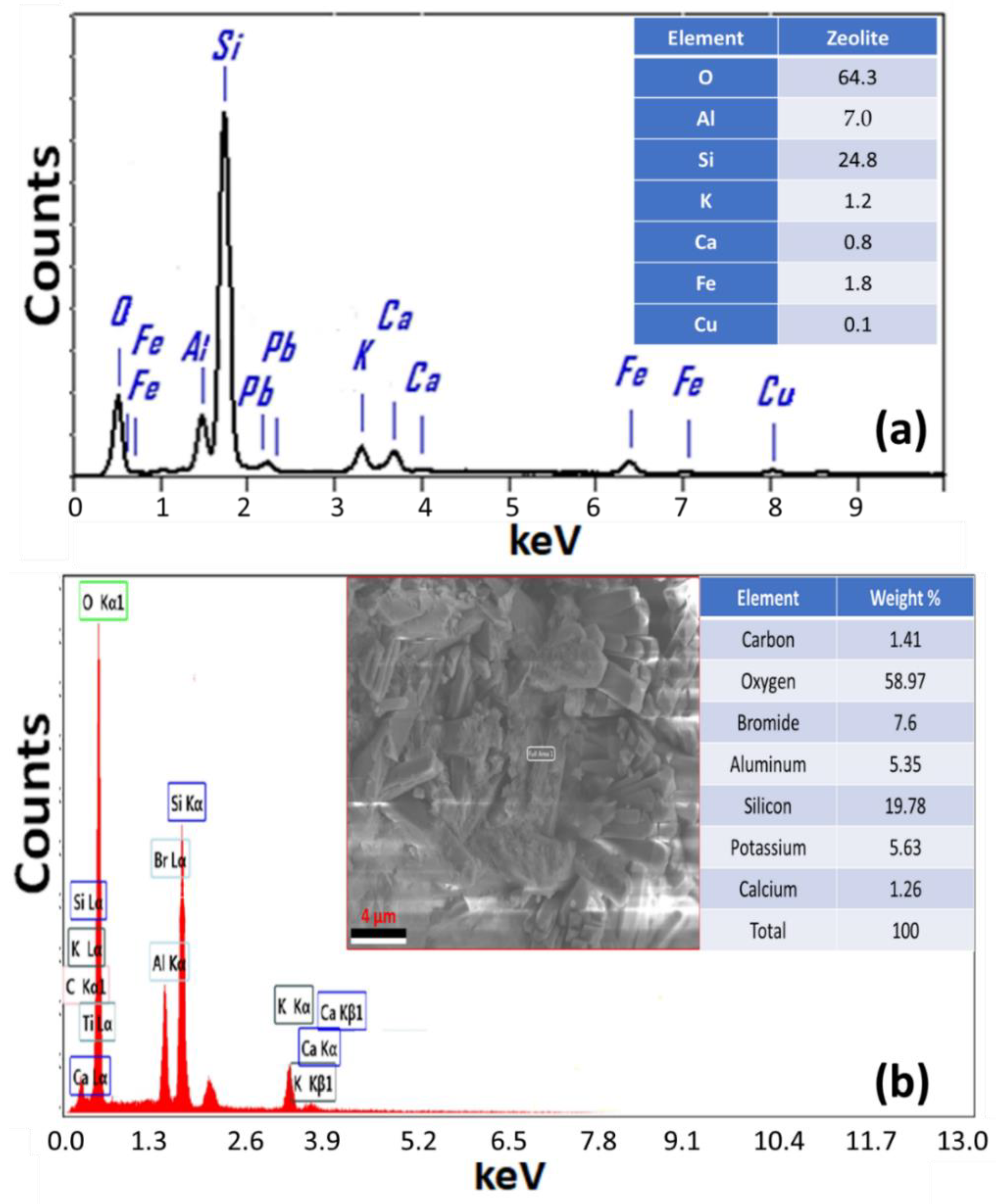
3.4. EDAX Study
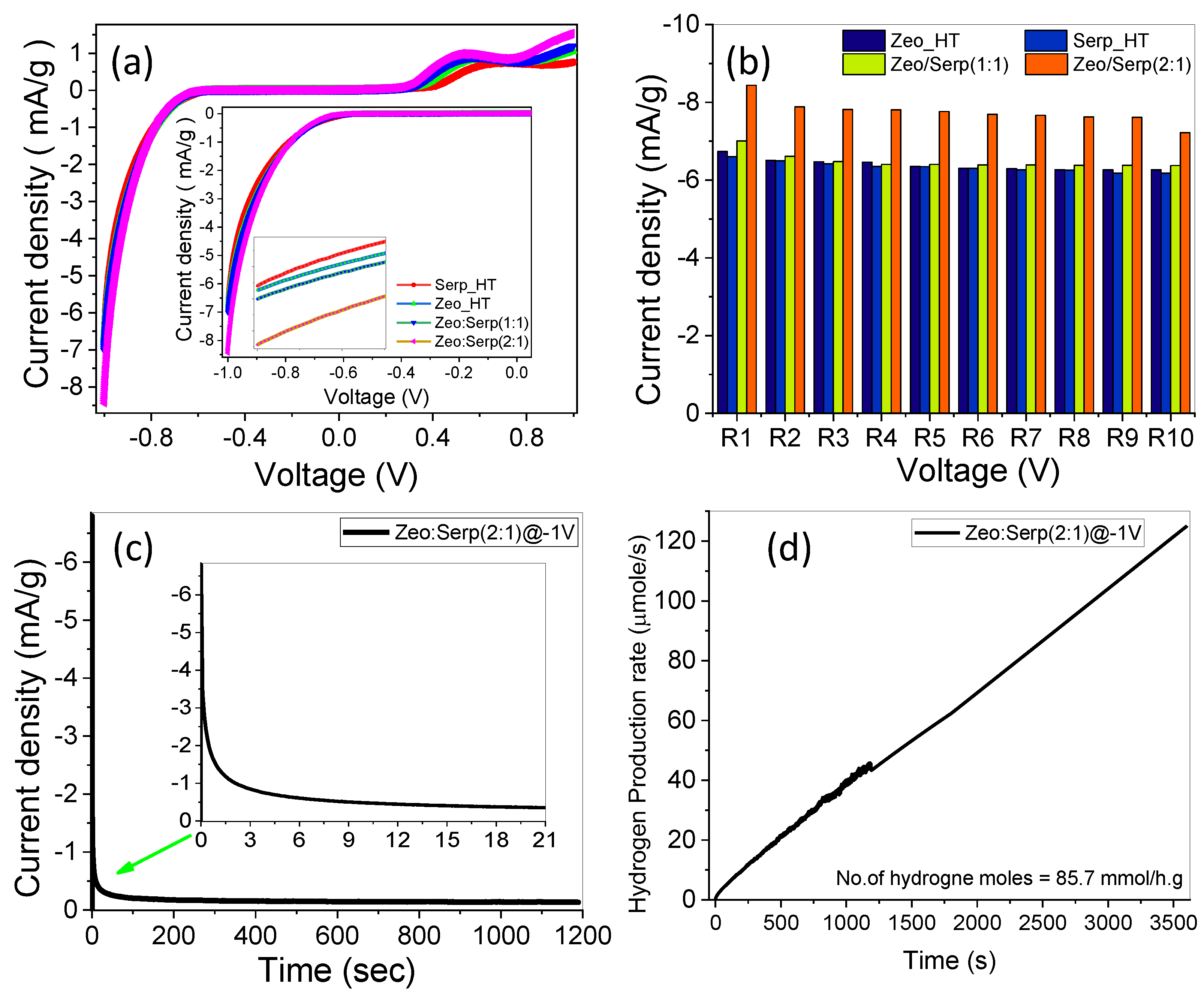
3.5. Photoelectrochemical (PEC) Water Splitting Measurements
3.5.1. Photoelectrochemical Stability and Behavior
3.5.2. The Effect of Monochromatic Light Illumination and Photoelectrochemical Efficiencies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdelmoneim, A.; Naji, A.; Wagenaars, E.; Shaban, M. Outstanding stability and photoelectrochemical catalytic performance of (Fe, Ni) co-doped Co3O4 photoelectrodes for solar hydrogen production. Int. J. Hydrogen Energy 2021, 46, 12915–12935. [Google Scholar] [CrossRef]
- Zayed, M.; Ahmed, A.M.; Shaban, M. Synthesis and characterization of nanoporous ZnO and Pt/ZnO thin films for dye degradation and water splitting applications. Int. J. Hydrogen Energy 2019, 44, 17630–17648. [Google Scholar] [CrossRef]
- Rabia, M.; Mohamed, S.H.; Zhao, H.; Shaban, M.; Lei, Y.; Ahmed, A.M. TiO2/TiOxNY hollow mushrooms-like nanocomposite photoanode for hydrogen electrogeneration. J. Porous Mater. 2020, 27, 133–139. [Google Scholar] [CrossRef]
- Król, M. Natural vs. Synthetic zeolites. Crystals 2020, 10, 622. [Google Scholar] [CrossRef]
- Nasief, F.; Shaban, M.; Alamry, K.A.; Abu Khadra, M.R.; Khan, A.A.P.; Asiri, A.M.; El-Salam, H.A. Hydrothermal synthesis and mechanically activated zeolite material for utilizing the removal of Ca/Mg from aqueous and raw groundwater. J. Environ. Chem. Eng. 2021, 9, 105834. [Google Scholar] [CrossRef]
- Hamd, A.; Dryaz, A.R.; Shaban, M.; AlMohamadi, H.; Abu Al-Ola, K.A.; Soliman, N.K.; Ahmed, S.A. Fabrication and application of zeolite/acanthophora spicifera nanoporous composite for adsorption of congo red dye from wastewater. Nanomaterials 2021, 11, 2441. [Google Scholar] [CrossRef]
- Reháková, M.; Čuvanová, S.; Dzivák, M.; Rimár, J.; Gaval’Ová, Z. Agricultural and agrochemical uses of natural zeolite of the clinoptilolite type. Curr. Opin. Solid State Mater. Sci. 2004, 8, 397–404. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Buszewski, B.; Terzyk, A.P.; Namieśnik, J. Study of the selection mechanism of heavy metal (Pb2+, Cu2+, Ni2+, and Cd2+) adsorption on clinoptilolite. J. Colloid Interface Sci. 2006, 304, 21–28. [Google Scholar] [CrossRef]
- Vassileva, P.; Voikova, D. Investigation on natural and pretreated Bulgarian clinoptilolite for ammonium ions removal from aqueous solutions. J. Hazard. Mater. 2009, 170, 948–953. [Google Scholar] [CrossRef]
- Radoor, S.; Karayil, J.; Parameswaranpillai, J.; Siengchin, S. Removal of anionic dye Congo red from aqueous environment using polyvinyl alcohol/sodium alginate/ZSM-5 zeolite membrane. Sci. Rep. 2020, 10, 15452. [Google Scholar] [CrossRef]
- Nassar, M.Y.; Abdelrahman, E.A. Hydrothermal tuning of the morphology and crystallite size of zeolite nanostructures for simultaneous adsorption and photocatalytic degradation of methylene blue dye. J. Mol. Liq. 2017, 242, 364–374. [Google Scholar] [CrossRef]
- You-Ji, L.; Wei, C. Photocatalytic degradation of Rhodamine B using nanocrystalline TiO2–zeolite surface composite catalysts: Effects of photocatalytic condition on degradation efficiency. Catal. Sci. Technol. 2011, 1, 802–809. [Google Scholar] [CrossRef]
- Zhao, L.; Cui, T.; Li, Y.; Wang, B.; Han, J.; Han, L.; Liu, Z. Efficient visible light photocatalytic activity of p–n junction CuO/TiO2 loaded on natural zeolite. Photodecolorization of methyl orange over α-Fe2O3-supported HY catalysts: The effects of catalyst preparation and dealumination. RSC Adv. 2015, 5, 64495–64502. [Google Scholar] [CrossRef]
- Jaafar, N.F.; Jalil, A.A.; Triwahyono, S.; Muhid, M.N.M.; Sapawe, N.; Satar, M.A.H.; Asaari, H. Photodecolorization of methyl orange over α-Fe2O3-supported HY catalysts: The effects of catalyst preparation and dealumination. Chem. Eng. J. 2012, 191, 112–122. [Google Scholar] [CrossRef]
- Shaban, M.; Abukhadra, M.R.; Shahien, M.G.; Ibrahim, S.S. Novel bentonite/zeolite-NaP composite efficiently removes methylene blue and Congo red dyes. Environ. Chem. Lett. 2018, 16, 275–280. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Shaban, M.; El Samad, M.A.A. Enhanced photocatalytic removal of Safranin-T dye under sunlight within minute time intervals using heulandite/polyaniline@ nickel oxide composite as a novel photocatalyst. Ecotoxicol. Environ. Saf. 2018, 162, 261–271. [Google Scholar] [CrossRef]
- Jothivenkatachalam, K.; Prabhu, S.; Nithya, A.; Jeganathan, K. Facile synthesis of WO3 with reduced particle size on zeolite and enhanced photocatalytic activity. RSC Adv. 2014, 4, 21221–21229. [Google Scholar] [CrossRef]
- Zhao, J.H.; Wang, Y.; Tang, X.; Li, Y.H.; Liu, F.T.; Zhang, Y.; Li, K. Enhanced photocatalytic hydrogen evolution over bimetallic zeolite imidazole framework-encapsulated CdS nanorods. Dalton Trans. 2019, 48, 3560–3565. [Google Scholar] [CrossRef]
- Yue, P.; Khan, F. Methods for increasing photo-assisted production of hydrogen over titanium exchanged zeolites. Int. J. Hydrogen Energy 1991, 16, 609–613. [Google Scholar] [CrossRef]
- Mendes, P.S.F.; Lapisardi, G.; Bouchy, C.; Rivallan, M.; Silva, J.M.; Ribeiro, M.F. Hydrogenating activity of Pt/zeolite catalysts focusing acid support and metal dispersion influence. Appl. Catal. A Gen. 2015, 504, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Zahmakıran, M.; Durap, F.; Özkar, S. Zeolite confined copper(0) nanoclusters as cost-effective and reusable catalyst in hydrogen generation from the hydrolysis of ammonia-borane. Int. J. Hydrogen Energy 2010, 35, 187–197. [Google Scholar] [CrossRef]
- Carmignano, O.R.R.D.; Vieira, S.S.; Brandão, P.R.G.; Bertoli, A.C.; Lago, R.M. Serpentinites: Mineral structure, properties and technological applications. J. Braz. Chem. Soc. 2020, 31, 2–14. [Google Scholar] [CrossRef]
- Shaban, M.; Abukhadra, M.R.; Khan, A.A.P.; Jibali, B.M. Removal of Congo red, methylene blue and Cr(VI) ions from water using natural serpentine. J. Taiwan Inst. Chem. Eng. 2018, 82, 102–116. [Google Scholar] [CrossRef]
- Farahat, M.M.; Sanad, M.M.S.; Abdel-Khalek, M.A. Decoration of serpentine with iron ore as an efficient low-cost magnetic adsorbent for Cr (VI) removal from tannery wastewater. Powder Technol. 2021, 388, 51–62. [Google Scholar] [CrossRef]
- Masoud, M.A.; Rashad, A.M.; Sakr, K.; Shahien, M.G.; Zayed, A.M. Possibility of using different types of Egyptian serpentine as fine and coarse aggregates for concrete production. Mater. Struct. 2020, 53, 87. [Google Scholar] [CrossRef]
- Treacy, M.M.J.; Higgins, J.B.; International Zeolite Association. Collection of Simulated XRD Powder Patterns for Zeolites; Structure Commission, Elsevier: Oxford, UK, 2007; p. 485. [Google Scholar]
- Mastinu, A.; Kumar, A.; Maccarinelli, G.; Bonini, S.A.; Premoli, M.; Aria, F.; Gianoncelli, A.; Memo, M. Zeolite Clinoptilolite: Therapeutic Virtues of an Ancient Mineral. Molecules 2019, 24, 1517. [Google Scholar] [CrossRef]
- León, E.R.; Rodríguez, E.L.; Beas, C.R.; Plascencia-Villa, G.; Palomares, R.A.I. Study of Methylene Blue Degradation by Gold Nanoparticles Synthesized within Natural Zeolites. J. Nanomater. 2016, 2016, 9541683. [Google Scholar] [CrossRef]
- Madejova, J.; Komadel, P. Baseline studies of the clay minerals society source clays: Infrared methods. Clays Clay Miner. 2001, 49, 410–432. [Google Scholar] [CrossRef]
- Masoudi, R.; Moghimi, H.; Azin, E.; Taheri, R.A. Adsorption of cadmium from aqueous solutions by novel Fe3O4- newly isolated Actinomucor sp. bio-nanoadsorbent: Functional group study. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S3), S1092–S1101. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, X.; Yi, H.; Yu, Q.; Zhang, Y.; Wei, J.; Yuan, Y. Synthesis, characterization and application of fe-zeolite: A review. Appl. Catal. A Gen. 2022, 630, 118467. [Google Scholar] [CrossRef]
- Mohamed, F.; Hassaballa, S.; Shaban, M.; Ahmed, A.M. Highly Efficient Photocatalyst Fabricated from the Chemical Recycling of Iron Waste and Natural Zeolite for Super Dye Degradation. Nanomaterials 2022, 12, 235. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Mohamed, F.; Ashraf, A.M.; Shaban, M.; Aslam, A.; Khan, P.; Asiri, A.M. Enhanced photoelectrochemical water splitting activity of carbon nanotubes@ TiO2 nanoribbons in different electrolytes. Chemosphere 2020, 238, 124554. [Google Scholar] [CrossRef]
- Choudhary, S.; Upadhyay, S.; Kumar, P.; Singh, N.; Satsangi, V.R.; Shrivastav, R.; Dass, S. Nanostructured bilayered thin films in photoelec-trochemical water splitting–A review. Int. J. Hydrogen Energy 2012, 37, 18713–18730. [Google Scholar] [CrossRef]
- Jiang, C.; Moniz, S.J.A.; Wang, A.; Zhang, T.A.; Tang, J. Photoelectrochemical devices for solar water splitting–materials and challenges. Chem. Soc. Rev. 2017, 46, 4645–4660. [Google Scholar] [CrossRef]
- Aboud, A.A.; Shaban, M.; Revaprasadu, N. Effect of Cu, Ni and Pb doping on the photo-electrochemical activity of ZnO thin films. RSC Adv. 2019, 9, 7729–7736. [Google Scholar] [CrossRef]
- Mohammadnezhad, G.; Momeni, M.M.; Nasiriani, F. Enhanced photoelectrochemical performance of tin oxide decorated tungsten oxide doped TiO2 nanotube by electrodeposition for water splitting. J. Electroanal. Chem. 2020, 876, 114505. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altowyan, A.S.; Shaban, M.; Faidey, Z.M.; Abdelkarem, K.; Al-Dossari, M.; Abd El-Gawaad, N.S.; Kordy, M.G.M. Design and Characterization of Zeolite/Serpentine Nanocomposite Photocatalyst for Solar Hydrogen Generation. Materials 2022, 15, 6325. https://doi.org/10.3390/ma15186325
Altowyan AS, Shaban M, Faidey ZM, Abdelkarem K, Al-Dossari M, Abd El-Gawaad NS, Kordy MGM. Design and Characterization of Zeolite/Serpentine Nanocomposite Photocatalyst for Solar Hydrogen Generation. Materials. 2022; 15(18):6325. https://doi.org/10.3390/ma15186325
Chicago/Turabian StyleAltowyan, Abeer S., Mohamed Shaban, Zeinab M. Faidey, Khaled Abdelkarem, Mawaheb Al-Dossari, N. S. Abd El-Gawaad, and Mohamed G. M. Kordy. 2022. "Design and Characterization of Zeolite/Serpentine Nanocomposite Photocatalyst for Solar Hydrogen Generation" Materials 15, no. 18: 6325. https://doi.org/10.3390/ma15186325
APA StyleAltowyan, A. S., Shaban, M., Faidey, Z. M., Abdelkarem, K., Al-Dossari, M., Abd El-Gawaad, N. S., & Kordy, M. G. M. (2022). Design and Characterization of Zeolite/Serpentine Nanocomposite Photocatalyst for Solar Hydrogen Generation. Materials, 15(18), 6325. https://doi.org/10.3390/ma15186325







