Rare-Earth-Doped Barium Molybdate Up-Conversion Phosphor with Potential Application in Optical Temperature Sensing
Abstract
:1. Introduction
2. Materials and Methods
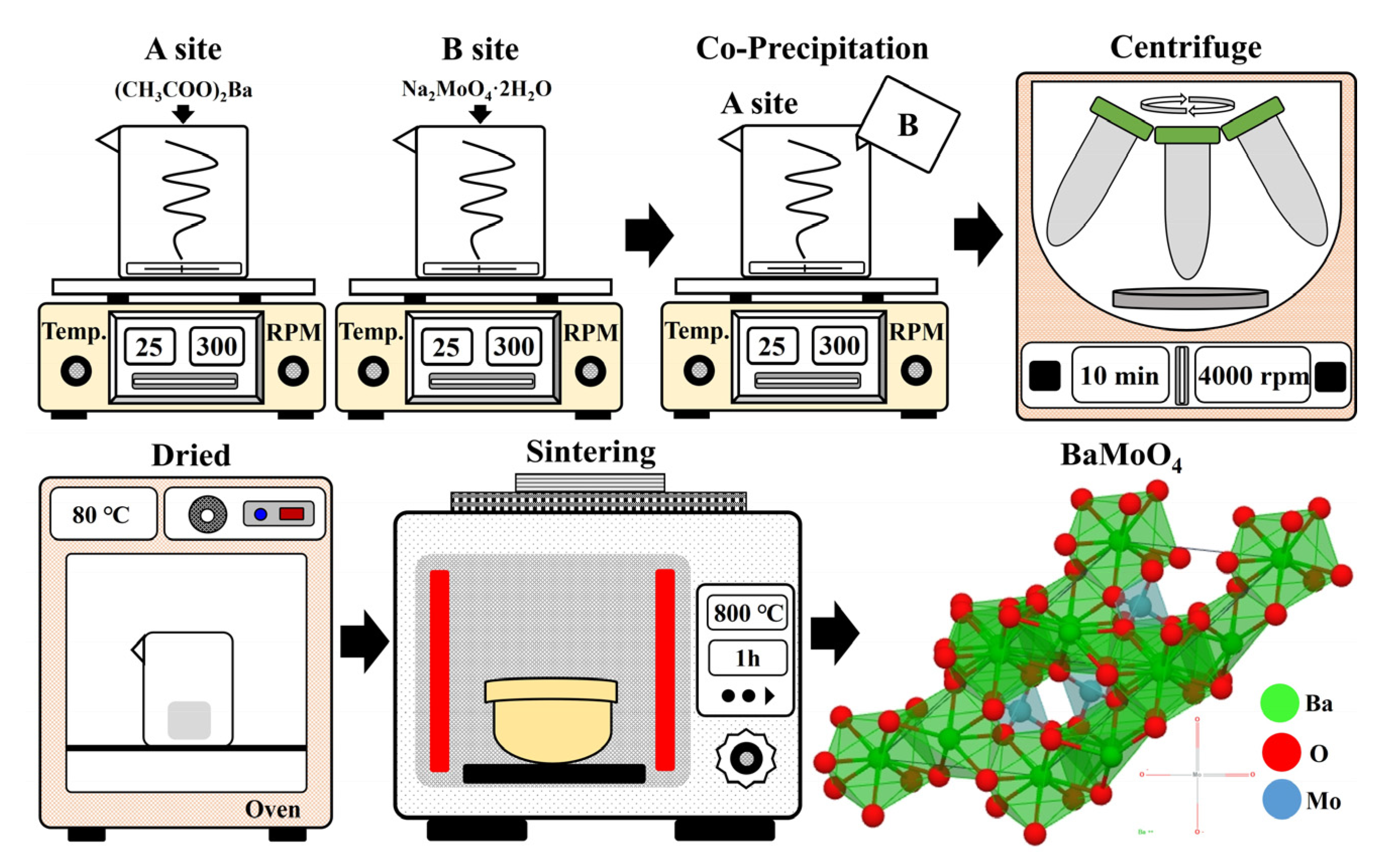
2.1. Synthesis of BaMoO4:[Er3+]/[Yb3+] UC Phosphor
2.2. Characterization
2.3. Fabricated Pellet and Flexible Composite
3. Results and Discussion
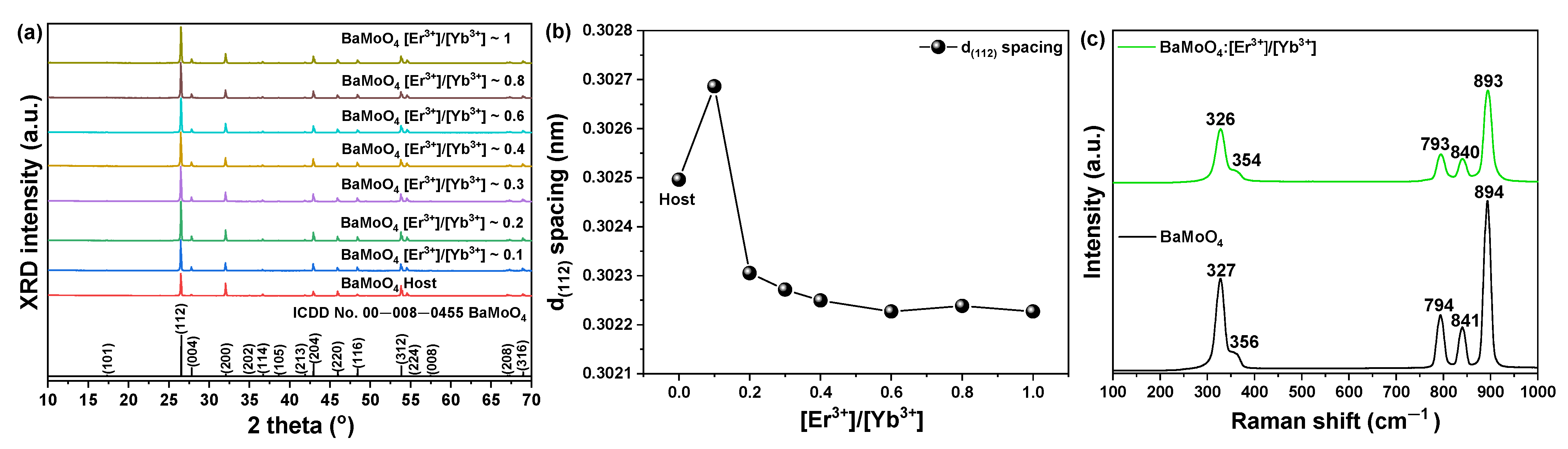
3.1. Structure and Surface Morphology of BaMoO4 UC Phosphor
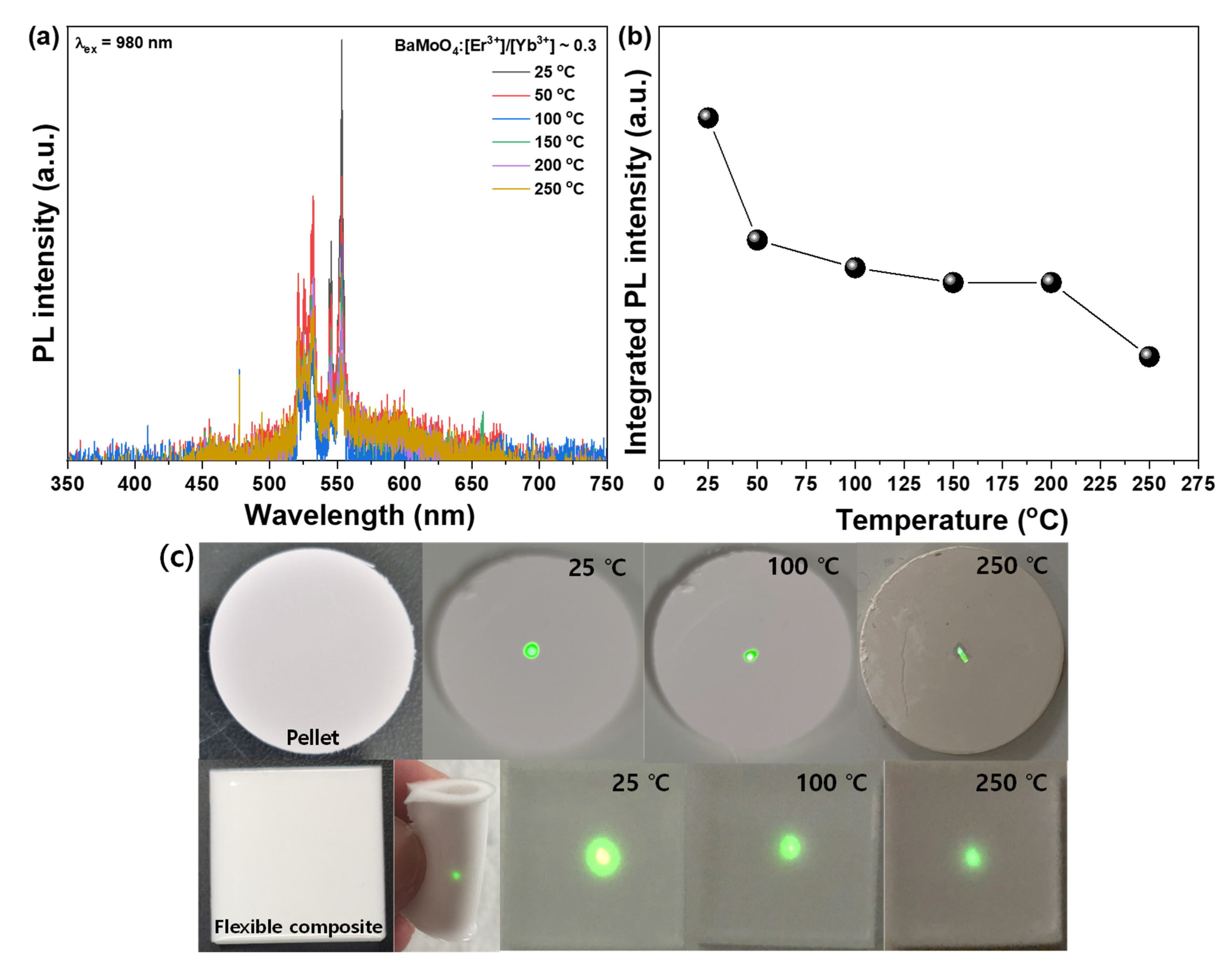
3.2. Luminescence Properties of BaMoO4 UC Phosphor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hirotaka, T.; Yuta, M. Investigation on Luminescent Properties of Rare Earth Doped Mullite Phosphors and the Occupation Site of the Doped Rare Earths. J. Electrochem. Soc. 2019, 166, B3209. [Google Scholar] [CrossRef]
- Jung, J. White luminescent calcium molybdate phosphor synthesized at room temperature via the Co-precipitation method used in a LED flexible composite. Opt. Mater. 2022, 132, 112830. [Google Scholar] [CrossRef]
- Secu, M.; Secu, C.; Bartha, C. Optical Properties of Transparent Rare-Earth Doped Sol-Gel Derived Nano-Glass Ceramics. Materials 2021, 14, 6871. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Shim, Y.; Son, C.S.; Kim, Y.; Hwang, D. Boron Nitride Nanoparticle Phosphors for Use in Transparent Films for Deep-UV Detection and White Light-Emitting Diodes. ACS Appl. Nano Mater. 2021, 4, 3529–3536. [Google Scholar] [CrossRef]
- Al-Waisawy, S.; George, A.F.; Jadwisienczak, W.M.; Rahman, F. Preparation of balanced trichromatic white phosphors for solid-state white lighting. Luminescence 2017, 32, 791–799. [Google Scholar] [CrossRef]
- Smet, P.F.; Parmentier, A.B.; Poelman, D.; Liu, R. Selecting Conversion Phosphors for White Light-Emitting Diodes. J. Electrochem. Soc. 2011, 158, R37. [Google Scholar] [CrossRef] [Green Version]
- Marques, A.P.A.; Motta, F.V.; Cruz, M.A.; Varela, J.A.; Longo, E.; Rosa, I.L.V. BaMoO4:Tb3+ Phosphor Properties: Synthesis, Characterization and Photophysical Studies. Solid State Ion. 2011, 202, 54–59. [Google Scholar] [CrossRef]
- Yuhang, X.; Xiangyu, Z.; Hongbo, Z.; Mengjie, Z.; Shuo, M.; Chunhui, S.; Shao, J. Luminescence properties of Eu3+ doped BaMoO4 transparent glass ceramics. J. Non-Cryst. Solids 2018, 500, 243–248. [Google Scholar] [CrossRef]
- Jena, P.; Gupta, S.K.; Natarajan, V.; Sahu, M.; Satyanarayana, N.; Venkateswarlu, M. Structural characterization and photoluminescence properties of sol–gel derived nanocrystalline BaMoO4:Dy3+. J. Lumin. 2015, 158, 203–210. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Cao, J.; Jiang, Y.; Zhao, X.; Meng, Z. Hydrothermal synthesis and luminescent properties of BaMoO4:Sm3+ red phosphor. J. Rare Earths 2016, 34, 143. [Google Scholar] [CrossRef]
- Jung, J. Changes in structure and luminescence properties according to the Tb3+ doping concentration of crystalline BaMoO4 synthesized at room temperature and application in LED color filters. MRS Commun. 2022, 12, 762–767. [Google Scholar] [CrossRef]
- Yi, S.; Jung, J. Barium molybdate white emitting phosphor synthesized at room temperature by co-precipitation. RSC Adv. 2022, 12, 21827–21835. [Google Scholar] [CrossRef] [PubMed]
- Zijlmans, H.J.M.A.A.; Bonnet, J.; Burton, J.; Kardos, K.; Vail, T.; Niedbala, R.S.; Tanke, H.J. Detection of Cell and Tissue Surface Antigens Using Up-Converting Phosphors: A New Reporter Technology. Anal. Biochem. 1999, 267, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Rapaport, A.; Milliez, J.; Bass, M.; Cassanho, A.; Jenssen, H. Review of the properties of up-conversion phosphors for new emissive displays. JDT 2006, 2, 68–78. [Google Scholar] [CrossRef]
- Kundu, J.; Ghosh, Y.; Dennis, A.M.; Htoon, H.; Hollingsworth, J.A. Giant Nanocrystal Quantum Dots: Stable Down-Conversion Phosphors that Exploit a Large Stokes Shift and Efficient Shell-to-Core Energy Relaxation. Nano Lett. 2012, 12, 3031–3037. [Google Scholar] [CrossRef]
- Richards, B.S. Luminescent layers for enhanced silicon solar cell performance: Down-conversion. Sol. Energy Mater. Sol. Cells 2006, 90, 1189–1207. [Google Scholar] [CrossRef]
- Soni, A.; Rai, V. Upconversion luminescence in BaMoO4:Pr3+ phosphor for display devices. Adv. Mater. Radiat. Phys. 2015, 1675, 030073. [Google Scholar] [CrossRef]
- Chung, J.H.; Lee, S.Y.; Kang, S.H.; Kim, S.A.; Shim, K.B.; Cho, H.; Lee, J.; Ryu, J.H. Green Lighting Upconversion Luminescence of Yb3+, Er3+ Co-Doped BaMoO4. J. Nanosci. Nanotechnol. 2013, 13, 6089–6091. [Google Scholar] [CrossRef]
- Jung, J.; Kim, J.; Shim, Y.; Hwang, D.; Son, C.S. Structure and Photoluminescence Properties of Rare-Earth (Dy3+, Tb3+, Sm3+)-Doped BaWO4 Phosphors Synthesized via Co-Precipitation for Anti-Counterfeiting. Materials 2020, 13, 4165. [Google Scholar] [CrossRef]
- Jung, J. Fabicated Flexible Composite for a UV-LED Colir Filter and Anti-Counterfieting Application of Calcium Molybdate Phosphor Synthesized at Room Temperature. Materials 2022, 15, 2078. [Google Scholar] [CrossRef]
- Yi, S.; Jung, J. Up-conversion luminescence properties with temperature change of strontium tungstate phosphors. RSC Adv. 2022, 12, 24752–24759. [Google Scholar] [CrossRef] [PubMed]
- Jung, J. Synthesis of Barium Tungstate Up-Conversion Phosphor Applied in Temperature Sensing and Anti-Counterfeiting. ECS J. Solid State Sci. Technol. 2022, 11, 076005. [Google Scholar] [CrossRef]
- Pope, C.G. X-Ray Diffraction and the Bragg Equation. J. Chem. Educ. 1997, 74, 129. [Google Scholar] [CrossRef]
- Lovisa, L.X.; Fernandes, Y.L.R.L.; Garcia, L.M.P.; Barros, B.S.; Longo, E.; Paskocimas, C.A.; Bomio, M.R.D.; Motta, F.V. Tb3+/Pr3+ co-doped ZnMoO4 phosphor with tunable photoluminescence and energy transfer processes. Opt. Mater. 2019, 96, 109332. [Google Scholar] [CrossRef]
- Lim, C.S. Synthesis of BaMoO4:Er3+/Yb3+ particles by an MAM method and their upconversion photoluminescence properties. Mater. Chem. Phys. 2013, 140, 154–158. [Google Scholar] [CrossRef]
- Kumar, A.; Tiwari, S.P.; Swart, H.C.; Esteves da Silva, J.C.G. Infrared interceded YF3: Er3+/Yb3+ upconversion phosphor for crime scene and anti-counterfeiting applications. Opt. Mater. 2019, 92, 347–351. [Google Scholar] [CrossRef]
- Lu, W.; Cheng, L.; Zhong, H.; Sun, J.; Wan, J.; Tian, Y.; Chen, B. Dependence of upconversion emission intensity on Yb3+ concentration in Er3+/Yb3+ co-doped flake shaped Y2(MoO4)3 phosphors. J. Phys. D Appl. Phys. 2010, 43, 085404. [Google Scholar] [CrossRef]
- Solis, D.; De La Rosa, E.; Meza, O.; Diaz-Torres, L.A.; Salas, P.; Solis, D.; De La Rosa, E.; Meza, O.; Diaz-Torres, L.A.; Salas, P.; et al. Ultrabroadband terahertz conductivity of Si nanocrystal films. J. Appl. Phys 2010, 108, 211107. [Google Scholar] [CrossRef]
- Jung, J. Applied anti-counterfeiting with strontium molybdate up-conversion phosphor synthesized via co-precipitation at low sintering temperature. Bull. Mater. Sci. 2022, 45, 152. [Google Scholar] [CrossRef]
- Choudhary, A.K.; Dwivedi, A.; Bahadur, A.; Rai, S.B. Effect of the concentration of the dopants (Er3+, Yb3+ and Zn2+) and temperature on the upconversion emission behavior of Er3+/Yb3+ co-doped SrAl2O4 phosphor. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 185, 155–162. [Google Scholar] [CrossRef]
- Boyer, J.; Vetrone, F.; Cuccia, L.A.; Capobianco, J.A. Synthesis of Colloidal Upconverting NaYF4 Nanocrystals Doped with Er3+, Yb3+ and Tm3+, Yb3+ via Thermal Decomposition of Lanthanide Trifluoroacetate Precursors. J. Am. Chem. Soc. 2006, 128, 7444–7445. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.W.; Kwak, M.; Yang, H.K. Improvement of luminescence properties of NaYF4:Yb3+/Er3+ upconversion materials by a cross-relaxation mechanism based on co-doped Ho3+ ion concentrations. Luminescence 2021, 36, 812–818. [Google Scholar] [CrossRef]
- Vetrone, F.; Naccache, R.; Mahalingam, V.; Morgan, C.G.; Capobianco, J.A. The Active-Core/Active-Shell Approach: A Strategy to Enhance the Upconversion Luminescence in Lanthanide-Doped Nanoparticles. Adv. Funct. Mater. 2009, 19, 2924–2929. [Google Scholar] [CrossRef]
- Liao, J.; Wang, M.; Lin, F.; Han, Z.; Fu, B.; Tu, D.; Chen, X.; Qiu, B.; Wen, H. Thermally boosted upconversion and downshifting luminescence in Sc2(MoO4)3:Yb/Er with two-dimensional negative thermal expansion. Nat. Commun. 2022, 13, 2090. [Google Scholar] [CrossRef] [PubMed]
- Ryszczyńska, S.; Trejgis, K.; Marciniak, Ł.; Grzyb, T. Upconverting SrF2:Er3+ Nanoparticles for Optical Temperature Sensors. ACS Appl. Nano Mater. 2021, 4, 10438–10448. [Google Scholar] [CrossRef]



| BaMoO4 Up-Conversion Phosphor Synthesis | ||||
|---|---|---|---|---|
| Reagents | (CH3COO)2Ba | Na2MoO4·2H2O | Yb(NO3)3·5H2O | Er(NO3)3·5H2O |
| Molecular Weight (g/mol) | 255.42 | 241.95 | 449.13 | 443.35 |
| Used mole (mmol) | 10 | 10 | 0.5 | 0.05~0.5 |
| [Er3+]/[Yb3+] Ratio | ||||
| Reagents | (CH3COO)2Ba | Na2MoO4·2H2O | Yb(NO3)3·5H2O | Er(NO3)3·5H2O |
| Used mole (mmol) | 10 | 10 | 0.5 | 0.05 |
| 10 | 10 | 0.5 | 0.1 | |
| 10 | 10 | 0.5 | 0.15 | |
| 10 | 10 | 0.5 | 0.2 | |
| 10 | 10 | 0.5 | 0.3 | |
| 10 | 10 | 0.5 | 0.4 | |
| 10 | 10 | 0.5 | 0.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wi, J.-H.; Park, S.-G.; Shim, Y.-S.; Lee, K.; Jung, J.-Y. Rare-Earth-Doped Barium Molybdate Up-Conversion Phosphor with Potential Application in Optical Temperature Sensing. Materials 2022, 15, 7917. https://doi.org/10.3390/ma15227917
Wi J-H, Park S-G, Shim Y-S, Lee K, Jung J-Y. Rare-Earth-Doped Barium Molybdate Up-Conversion Phosphor with Potential Application in Optical Temperature Sensing. Materials. 2022; 15(22):7917. https://doi.org/10.3390/ma15227917
Chicago/Turabian StyleWi, Jung-Hyun, Sang-Geon Park, Young-Seok Shim, Kwangjae Lee, and Jae-Yong Jung. 2022. "Rare-Earth-Doped Barium Molybdate Up-Conversion Phosphor with Potential Application in Optical Temperature Sensing" Materials 15, no. 22: 7917. https://doi.org/10.3390/ma15227917





