Investigation of the Performance of Ti6Al4V Lattice Structures Designed for Biomedical Implants Using the Finite Element Method
Abstract
:1. Introduction
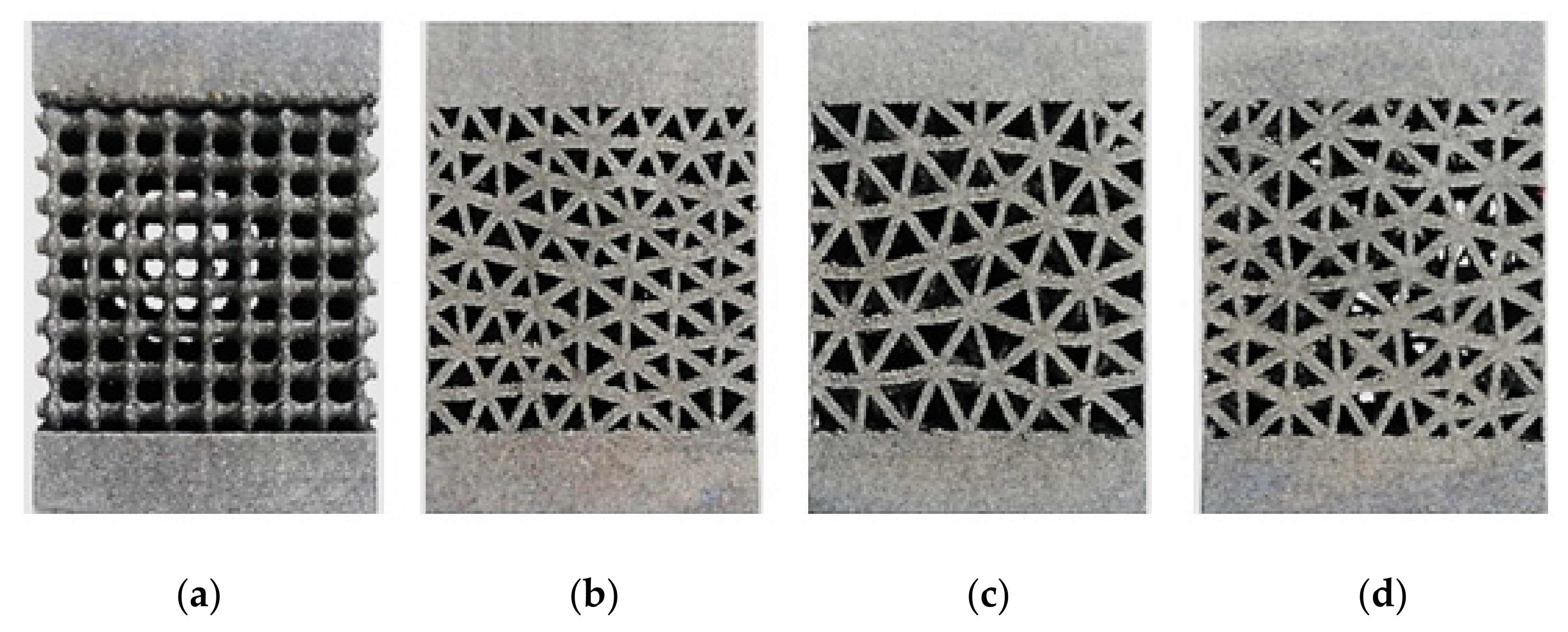
- Low-strut-diameter lattice structures were successfully manufactured using SLM.
- On the parallel building platform, the surfaces were smoother and better finished compared to the side surfaces.
- The octahedral lattice structure had the best dimensional accuracy among all types.
- The dimensions of the 3D-printed structures were smaller than the nominal CAD values, proving the shrinkage of the material upon solidification.
2. Materials and Methods
2.1. Raw Material
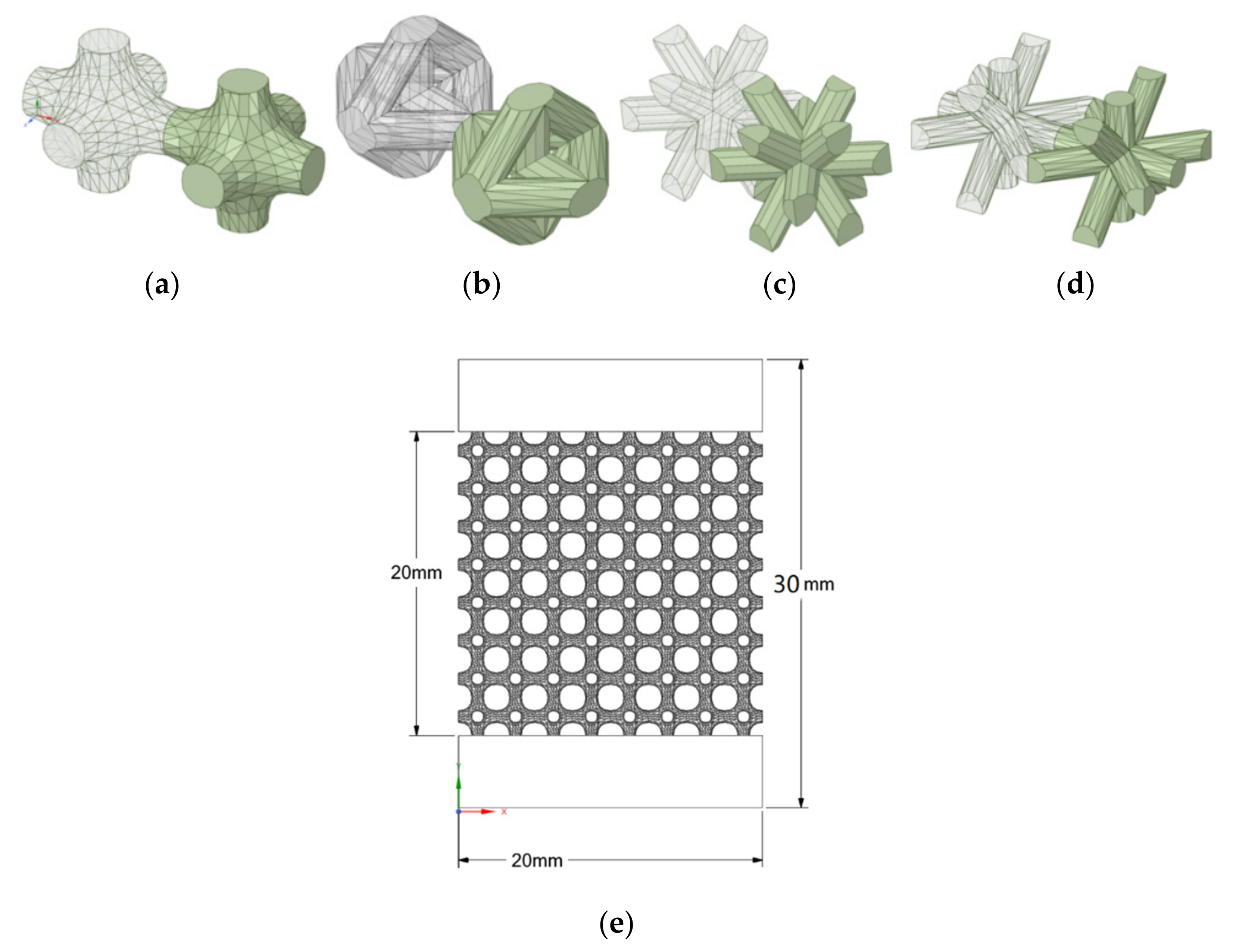
2.2. Lattice Structure Design
2.3. Manufacturing of Specimens
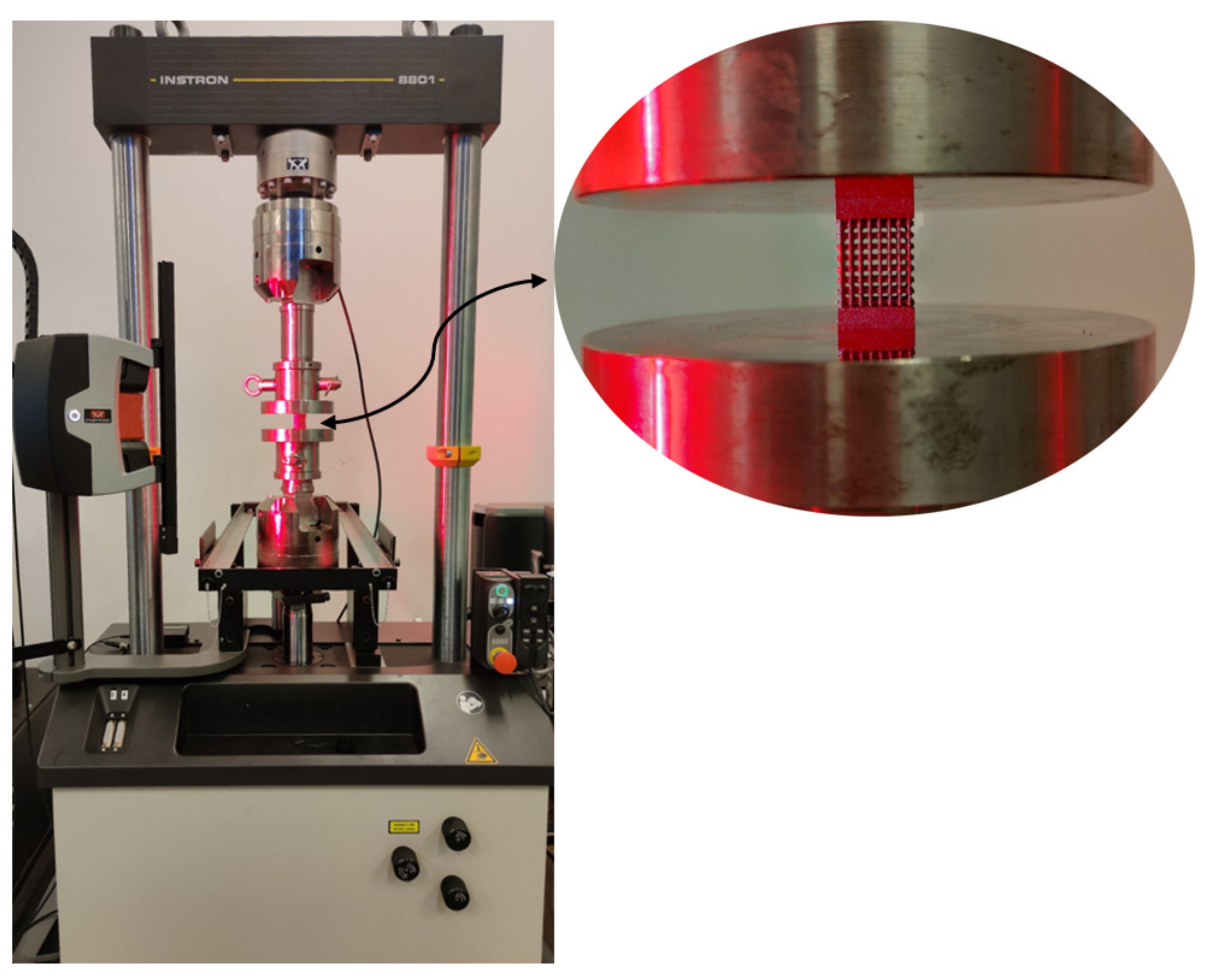
2.4. Compression Test
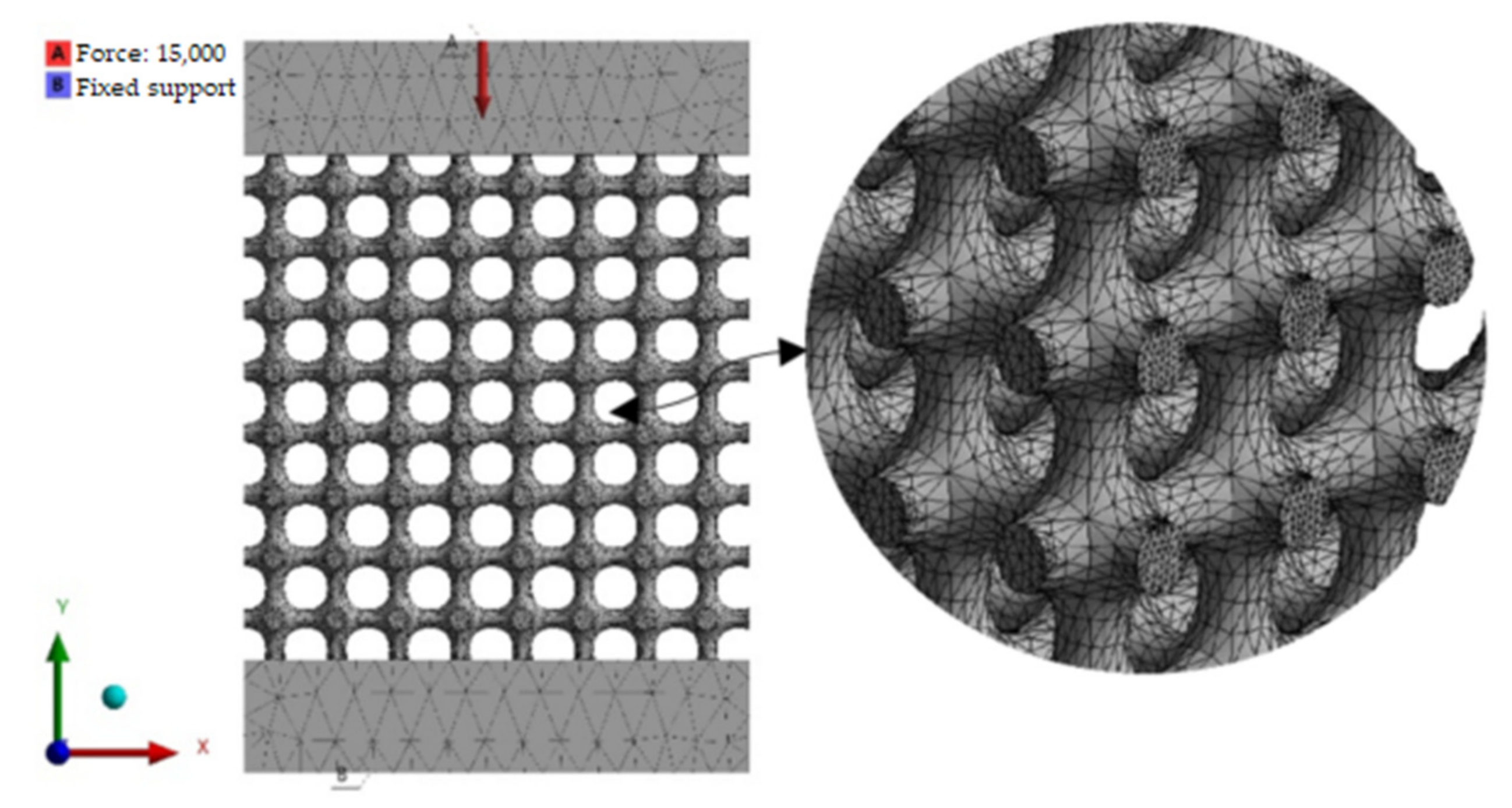
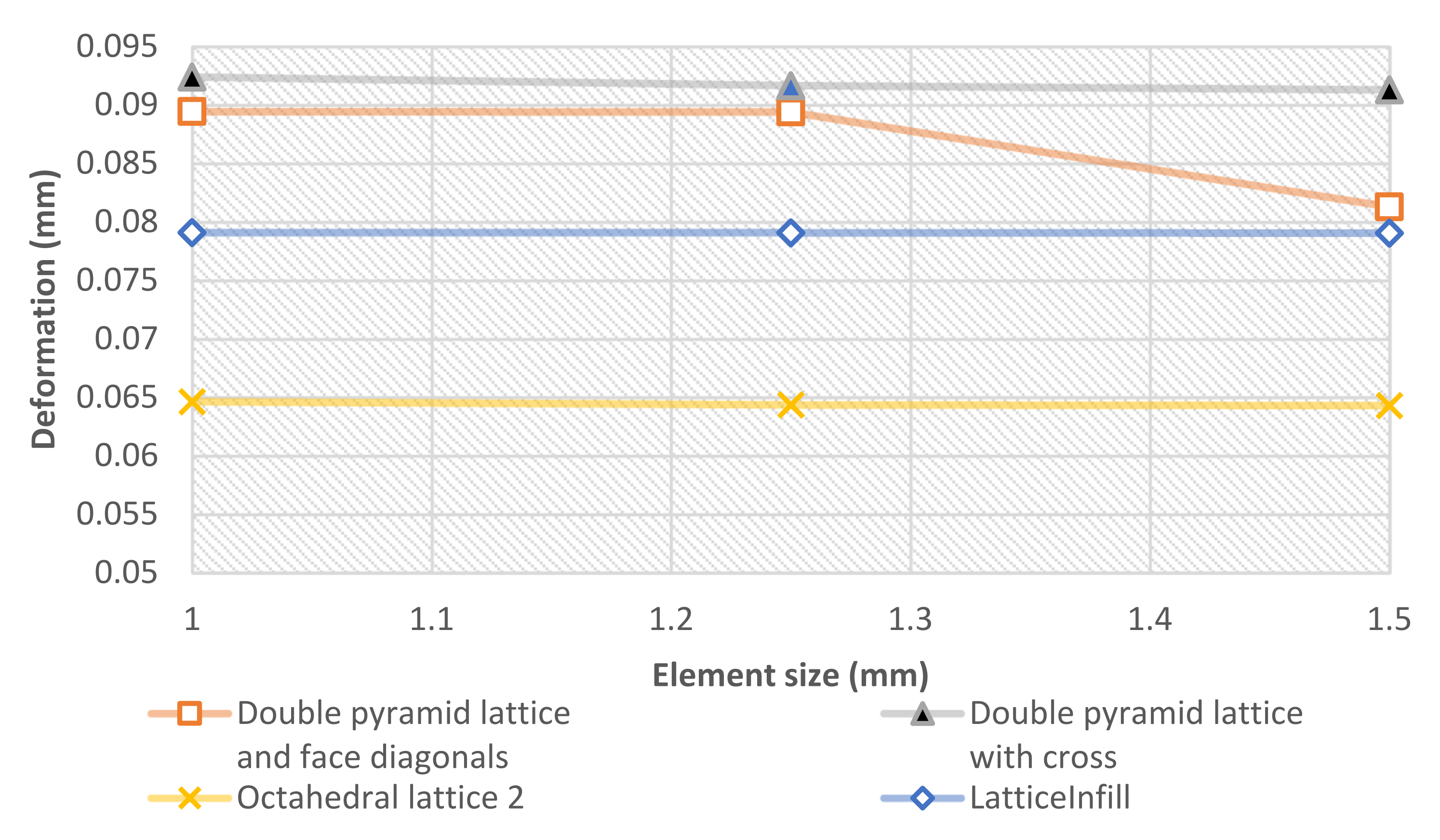
2.5. FEM Simulation
3. Results
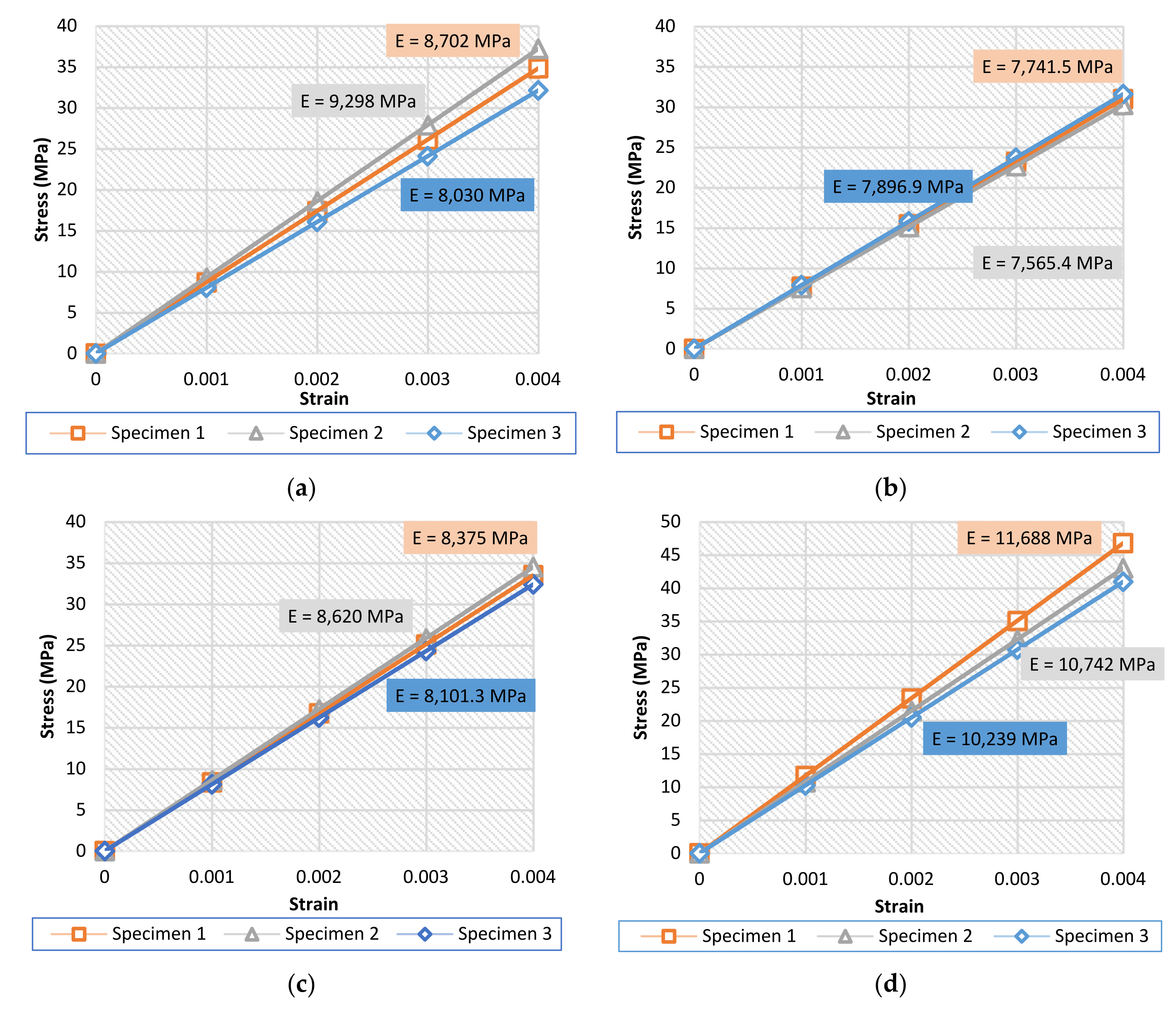
3.1. Compression Tests
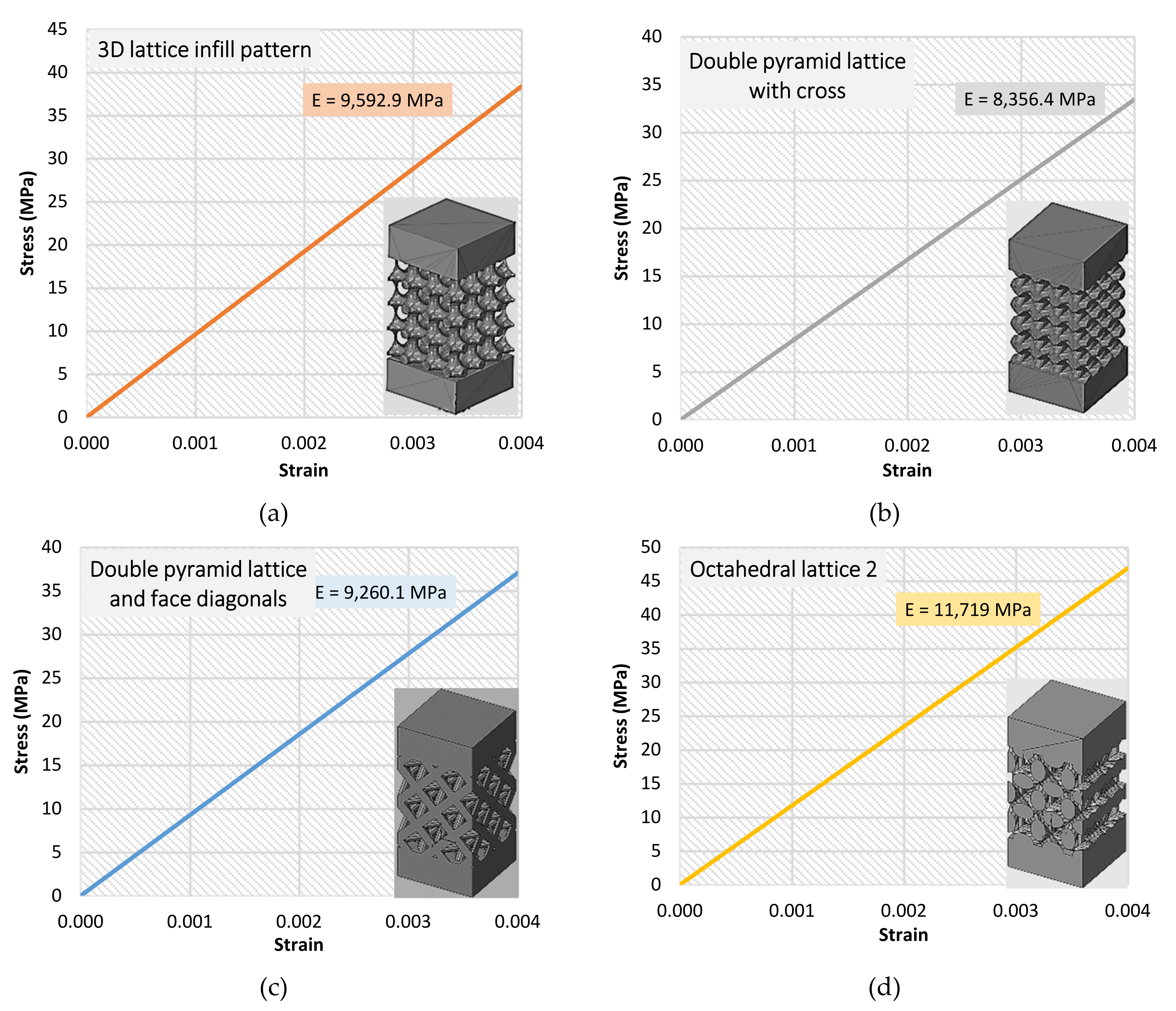
3.2. FEM Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herzog, D.; Seyda, V.; Wycisk, E.; Emmelmann, C. Additive manufacturing of metals. Acta Mater. 2016, 117, 371–392. [Google Scholar] [CrossRef]
- Tan, J.H.; Wong, W.L.E.; Dalgarno, K.W. An overview of powder granulometry on feedstock and part performance in the selective laser melting process. Addit. Manuf. 2017, 18, 228–255. [Google Scholar] [CrossRef]
- Liu, S.; Shin, Y.C. Additive manufacturing of Ti6Al4V alloy: A review. Mater. Des. 2019, 164, 107552. [Google Scholar] [CrossRef]
- Vrána, R.; Koutecký, T.; Červinek, O.; Zikmund, T.; Pantělejev, L.; Kaiser, J.; Koutný, D. Deviations of the SLM Produced Lattice Structures and Their Influence on Mechanical Properties. Materials 2022, 15, 3144. [Google Scholar] [CrossRef]
- Khaing, M.W.; Fuh, J.Y.H.; Lu, L. Direct metal laser sintering for rapid tooling: Processing and characterisation of EOS parts. J. Mater. Process. Technol. 2001, 113, 269–272. [Google Scholar] [CrossRef]
- Spierings, A.B.; Schneider, M.; Eggenberger, R. Comparison of density measurement techniques for additive manufactured metallic parts. Rapid Prototyp. J. 2011, 17, 380–386. [Google Scholar] [CrossRef]
- Murr, L.E.; Quinones, S.A.; Gaytan, S.M.; Lopez, M.I.; Rodela, A.; Martinez, E.Y.; Hernandez, D.H.; Martinez, E.; Medina, F.; Wicker, R.B. Microstructure and mechanical behavior of Ti-6Al-4V produced by rapid-layer manufacturing, for biomedical applications. J. Mech. Behav. Biomed. Mater. 2009, 2, 20–32. [Google Scholar] [CrossRef]
- Olli, N.; Andre, D.; Michae, F. Direct metal laser sintering. DMLS of Titanium. 2010. Available online: www.titanium.org (accessed on 25 June 2022).
- Terrier, A. Adaptation of Bone to Mechanical Stress: Theoretical Model, Experimental Identification and Orthopedic Applications. Ph.D. Thesis, École Polytechnique Fédérale de Lausanne, Écublens, Switzerland, 1999. [Google Scholar]
- Reimeringer, M.; Nuño, N. The influence of contact ratio and its location on the primary stability of cementless total hip arthroplasty: A finite element analysis. J. Biomech. 2016, 49, 1064–1070. [Google Scholar] [CrossRef]
- Kuiper, J.H. Numerical Optimization of Artificial Hip Joint Designs. Ph.D. Thesis, University Nijmegen, Nijmegen, The Netherlands, 1993. [Google Scholar]
- Cilla, M.; Checa, S.; Duda, G.N. Strain shielding inspired re-design of proximal femoral stems for total hip arthroplasty. J. Orthop. Res. 2017, 35, 2534–2544. [Google Scholar] [CrossRef]
- Arabnejad, S.; Johnston, B.; Tanzer, M.; Pasini, D. Fully porous 3D printed titanium femoral stem to reduce stress-shielding following total hip arthroplasty. J. Orthop. Res. 2017, 35, 1774–1783. [Google Scholar] [CrossRef] [Green Version]
- Abdulhadi, H.S.; Mian, A. Effect of strut length and orientation on elastic mechanical response of modified body-centered cubic lattice structures. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2019, 233, 2219–2233. [Google Scholar] [CrossRef]
- Arabnejad, S.; Burnett Johnston, R.; Pura, J.A.; Singh, B.; Tanzer, M.; Pasini, D. High-strength porous biomaterials for bone replacement: A strategy to assess the interplay between cell morphology, mechanical properties, bone ingrowth and manufacturing constraints. Acta Biomater. 2016, 30, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Pobloth, A.-M.; Checa, S.; Razi, H.; Petersen, A.; Weaver, J.C.; Schmidt-Bleek, K.; Windolf, M.; Tatai, A.Á.; Roth, C.P.; Schaser, K.-D. Mechanobiologically optimized 3D titanium-mesh scaffolds enhance bone regeneration in critical segmental defects in sheep. Sci. Transl. Med. 2018, 10, eaam8828. [Google Scholar] [CrossRef]
- Lancea, C.; Campbell, I.; Chicos, L.-A.; Zaharia, S.-M. Compressive Behaviour of Lattice Structures Manufactured by Polyjet Technologies. Polymers 2020, 12, 2767. [Google Scholar] [CrossRef]
- Riva, L.; Ginestra, P.S.; Ceretti, E. Mechanical characterization and properties of laser-based powder bed–fused lattice structures: A review. Int. J. Adv. Manuf. Technol. 2021, 113, 649–671. [Google Scholar] [CrossRef]
- Jin, Y.; Kong, H.; Zhou, X.; Li, G.; Du, J. Design and Characterization of Sheet-Based Gyroid Porous Structures with Bioinspired Functional Gradients. Materials 2020, 13, 3844. [Google Scholar] [CrossRef] [PubMed]
- Gürkan, D. Additively Manufactured Ti6Al4V Lattice Structures for Biomedical Applications. Int. J. 3D Print. Technol. Digit. Ind. 2021, 5, 155–163. [Google Scholar] [CrossRef]
- Bartolomeu, F.; Gasik, M.; Silva, F.S.; Miranda, G. Mechanical Properties of Ti6Al4V Fabricated by Laser Powder Bed Fusion: A Review Focused on the Processing and Microstructural Parameters Influence on the Final Properties. Metals 2022, 12, 986. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; van der Heide, E. In Silico Contact Pressure of Metal-on-Metal Total Hip Implant with Different Materials Subjected to Gait Loading. Metals 2022, 12, 1241. [Google Scholar] [CrossRef]
- He, Y.; Burkhalter, D.; Durocher, D.; Gilbert, J.M. Solid-Lattice Hip Prosthesis Design: Applying Topology and Lattice Optimization to Reduce Stress Shielding from Hip Implants; American Society of Mechanical Engineers: New York, NY, USA, 2018. [Google Scholar] [CrossRef] [Green Version]
- Corona-Castuera, J.; Rodriguez-Delgado, D.; Henao, J.; Castro-Sandoval, J.C.; Poblano-Salas, C.A. Design and Fabrication of a Customized Partial Hip Prosthesis Employing CT-Scan Data and Lattice Porous Structures. ACS Omega 2021, 6, 6902–6913. [Google Scholar] [CrossRef]
- Teng, F.; Sun, Y.; Guo, S.; Gao, B.; Yu, G. Topological and Mechanical Properties of Different Lattice Structures Based on Additive Manufacturing. Micromachines 2022, 13, 1017. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.; van Arkel, R.J. Topology Optimisation for Compliant Hip Implant Design and Reduced Strain Shielding. Materials 2021, 14, 7184. [Google Scholar] [CrossRef] [PubMed]
- Mahshid, R.; Hansen, H.N.; Højbjerre, K.L. Strength analysis and modeling of cellular lattice structures manufactured using selective laser melting for tooling applications. Mater. Des. 2016, 104, 276–283. [Google Scholar] [CrossRef]
- Cheng, X.Y.; Li, S.J.; Murr, L.E.; Zhang, Z.B.; Hao, Y.L.; Yang, R.; Medina, F.; Wicker, R.B. Compression deformation behavior of Ti–6Al–4V alloy with cellular structures fabricated by electron beam melting. J. Mech. Behav. Biomed. Mater. 2012, 16, 153–162. [Google Scholar] [CrossRef]
- Jamshidinia, M.; Kong, F.; Kovacevic, R. The Numerical Modeling of Fatigue Properties of a Bio-Compatible Dental Implant Produced by Electron Beam Melting® (EBM); University of Texas at Austin: Austin, TX, USA, 2013. [Google Scholar]
- Zhao, M.; Liu, F.; Fu, G.; Zhang, D.Z.; Zhang, T.; Zhou, H. Improved Mechanical Properties and Energy Absorption of BCC Lattice Structures with Triply Periodic Minimal Surfaces Fabricated by SLM. Materials 2018, 11, 2411. [Google Scholar] [CrossRef]
- Helou, M.; Vongbunyong, S.; Kara, S. Finite Element Analysis and Validation of Cellular Structures. Procedia CIRP 2016, 50, 94–99. [Google Scholar] [CrossRef]
- Juarez, C.Z.J.; Koay, S.C.; Chan, M.Y.; Choo, H.L.; Pang, M.M.; Ong, T.K. Finite Element Analysis Study on Lattice Structure Fabricated Recycled Polystyrene from Post-used Styrofoam Waste. MATEC Web Conf. 2021, 335, 03007. [Google Scholar] [CrossRef]
- Kladovasilakis, N.; Tsongas, K.; Tzetzis, D. Finite Element Analysis of Orthopedic Hip Implant with Functionally Graded Bioinspired Lattice Structures. Biomimetics 2020, 5, 44. [Google Scholar] [CrossRef]
- Ghani, S.A.C.; Harun, W.S.W.; Mat Taib, Z.A.; Ab Rashid, F.F.; Hazlen, R.M.; Omar, M.A. Finite Element Analysis of Porous Medical Grade Cobalt Chromium Alloy Structures Produced by Selective Laser Melting. Adv. Mater. Res. 2016, 1133, 113–118. [Google Scholar] [CrossRef]
- Gok, M.G. Creation and finite-element analysis of multi-lattice structure design in hip stem implant to reduce the stress-shielding effect. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2021, 236, 429–439. [Google Scholar] [CrossRef]
- Park, K.-M.; Min, K.-S.; Roh, Y.-S. Design Optimization of Lattice Structures under Compression: Study of Unit Cell Types and Cell Arrangements. Materials 2022, 15, 97. [Google Scholar] [CrossRef] [PubMed]
- EOS GmbH. EOS Titanium Ti64-Material Data Sheet. 2017. Available online: https://www.eos.info/material-m (accessed on 25 June 2022).
- EOS.info. Technical Data EOS M 290. 2022. Available online: https://www.eos.info/en/additive-manufacturing/3d-printing-metal/eos-metal-systems/eos-m-290 (accessed on 25 June 2022).
- Carretta, R.; Luisier, B.; Bernoulli, D.; Stüssi, E.; Müller, R.; Lorenzetti, S. Novel method to analyze post-yield mechanical properties at trabecular bone tissue level. J. Mech. Behav. Biomed. Mater. 2013, 20, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Jirousek, O.; Nemecek, J.; Kytyr, D.; Kunecky, J.; Zlamal, P.; Doktor, T. Nanoindentation of Trabecular Bone-Comparison with Uniaxial Testing of Single Trabecula. Chem. Listy 2011, 105, S668–S671. [Google Scholar]
- Hong, J.; Cha, H.; Park, Y.; Lee, S.; Khang, G.; Kim, Y. Elastic Moduli and Poisson’s Ratios of Microscopic Human Femoral Trabeculae BT. In Proceedings of the 11th Mediterranean Conference on Medical and Biomedical Engineering and Computing 2007, Ljubljana, Slovenia, 26–30 June 2007; pp. 274–277. [Google Scholar]

| Element | Chemical Composition Percentage % |
|---|---|
| Al | 5.50–6.50 |
| V | 3.50–4.50 |
| O | 0.13 |
| N | 0.05 |
| C | 0.08 |
| H | 0.012 |
| Fe | 0.25 |
| Y | 0.005 |
| Other elements each | 0.1 |
| Other elements total | 0.4 |
| Property | Value | Unit |
|---|---|---|
| Elastic modulus | 106,247 | MPa |
| Mass density | 4.4 | g/cm3 |
| Poisson’s ration | 0.34 |
| Cell Type | Porosity Percentage (%) | Actual Volume of Latticed Body (mm3) | Bulk Volume (mm3) | Beam (Strut) Thickness (mm) |
|---|---|---|---|---|
| 3D lattice infill pattern | 74 | 2079.17 | 8000 | 0.7 |
| Double-pyramid lattice with cross | 74 | 2067.58 | 8000 | 0.85 |
| Double-pyramid lattice and face diagonals | 71 | 2313.96 | 8000 | 0.6 |
| Octahedral lattice 2 | 70 | 2431.76 | 8000 | 0.8 |
| Laser Type | Scanning Speed | Focus Diameter | Power Supply | Building Volume |
|---|---|---|---|---|
| Yb-fiber laser; 400 W | Up to 7.0 m/s (23 ft./s) | 100 μm | 32 A/400 V | 250 × 250 × 325 mm |
| Unit Cell | Average Length (mm) | Average Width (mm) | Average Height (mm) | Weight (g) |
|---|---|---|---|---|
| 3D lattice infill pattern | 19.99 | 19.97 | 30.29 | 26.61 |
| Double-pyramid lattice with cross | 20.00 | 20.00 | 30.24 | 26.98 |
| Double-pyramid lattice and face diagonals | 19.96 | 20.03 | 30.24 | 28.34 |
| Octahedral lattice 2 | 20.04 | 20.03 | 30.26 | 28.44 |
| Cell Type | Effective Young’s Modulus (MPa) (Measurement) | Effective Young’s Modulus (MPa) (Numerical Analysis) | Deviation % |
|---|---|---|---|
| 3D lattice infill pattern | 8676.67 | 9592.9 | 9.6 |
| Double-pyramid lattice with cross | 7734.60 | 8356.4 | 7.4 |
| Double-pyramid lattice and face diagonals | 8332.00 | 9260.1 | 10.0 |
| Octahedral lattice 2 | 10,889.67 | 11,719 | 7.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkentar, R.; Máté, F.; Mankovits, T. Investigation of the Performance of Ti6Al4V Lattice Structures Designed for Biomedical Implants Using the Finite Element Method. Materials 2022, 15, 6335. https://doi.org/10.3390/ma15186335
Alkentar R, Máté F, Mankovits T. Investigation of the Performance of Ti6Al4V Lattice Structures Designed for Biomedical Implants Using the Finite Element Method. Materials. 2022; 15(18):6335. https://doi.org/10.3390/ma15186335
Chicago/Turabian StyleAlkentar, Rashwan, File Máté, and Tamás Mankovits. 2022. "Investigation of the Performance of Ti6Al4V Lattice Structures Designed for Biomedical Implants Using the Finite Element Method" Materials 15, no. 18: 6335. https://doi.org/10.3390/ma15186335





