Evaluation of Photon Interaction Parameters of Some Antioxidants for Food Irradiation Applications
Abstract
:1. Introduction
2. Material and Method
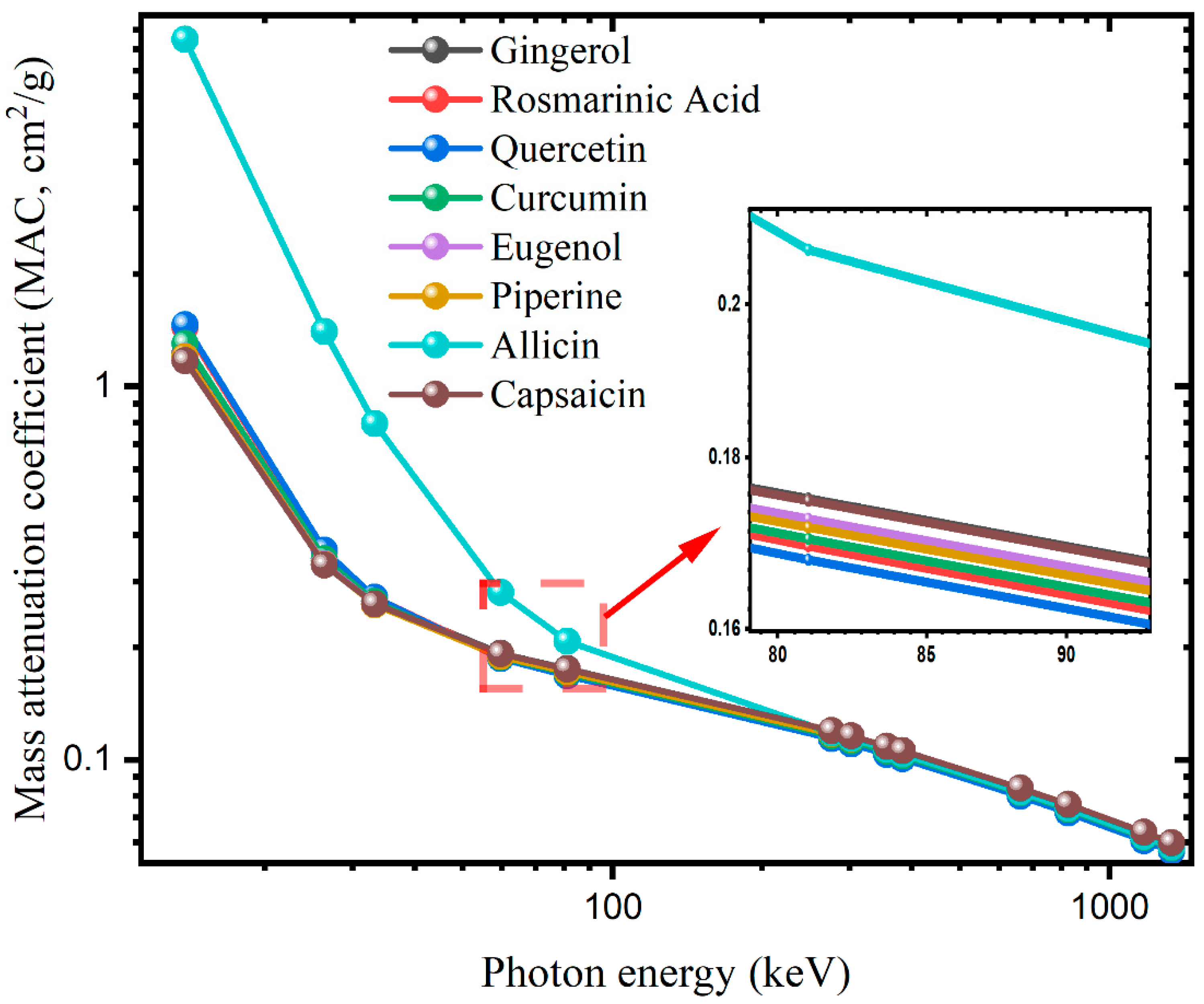
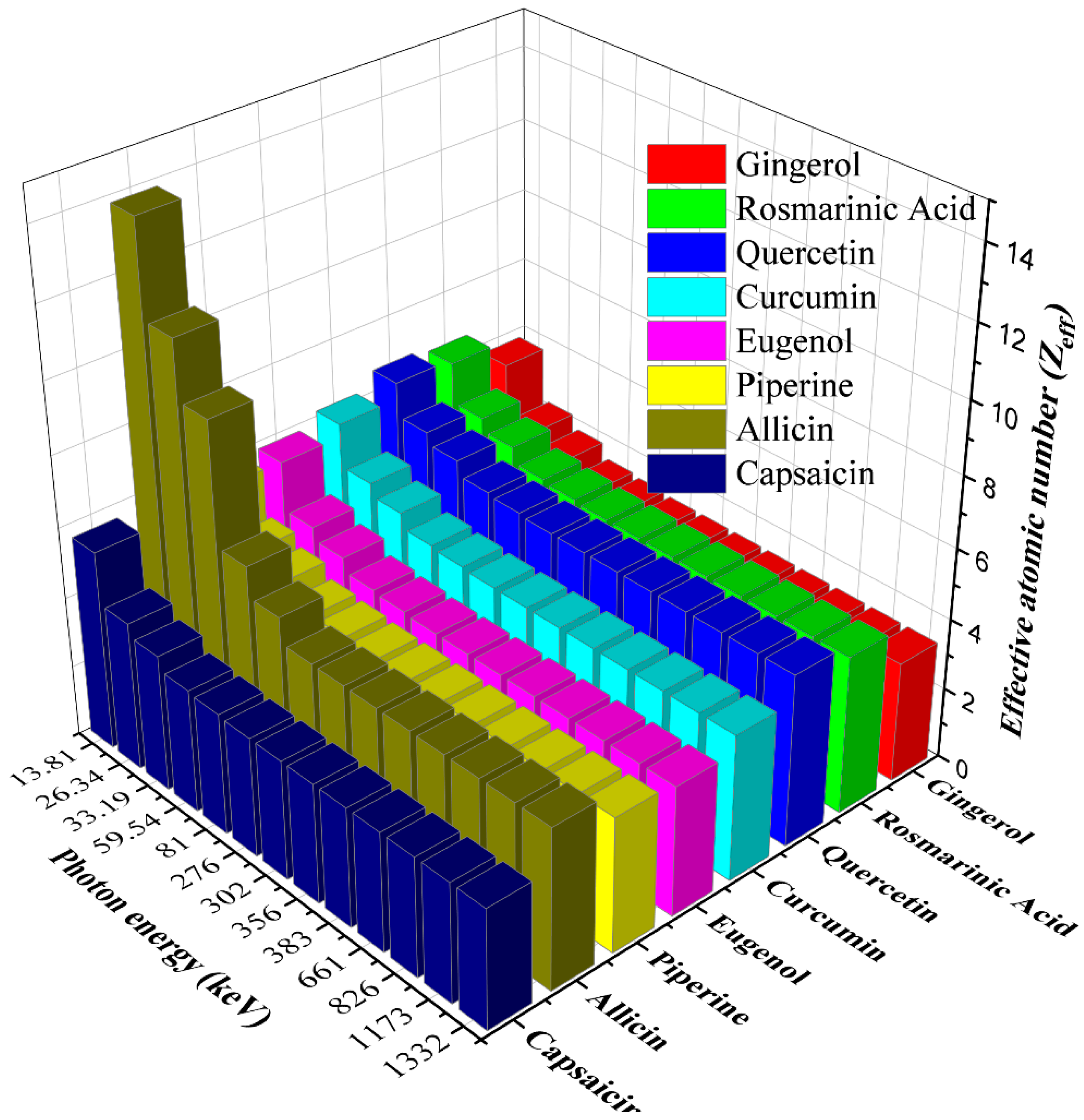
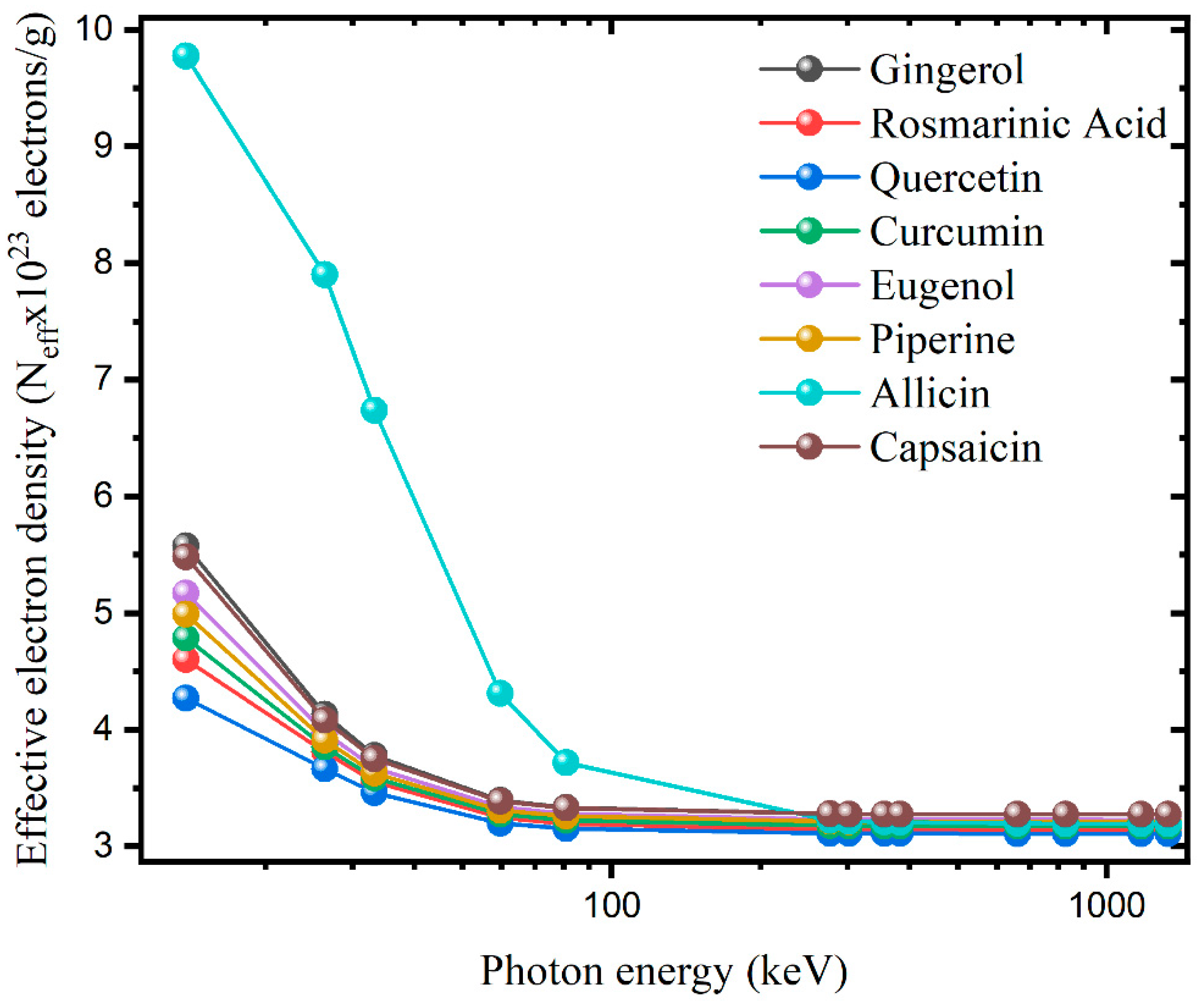
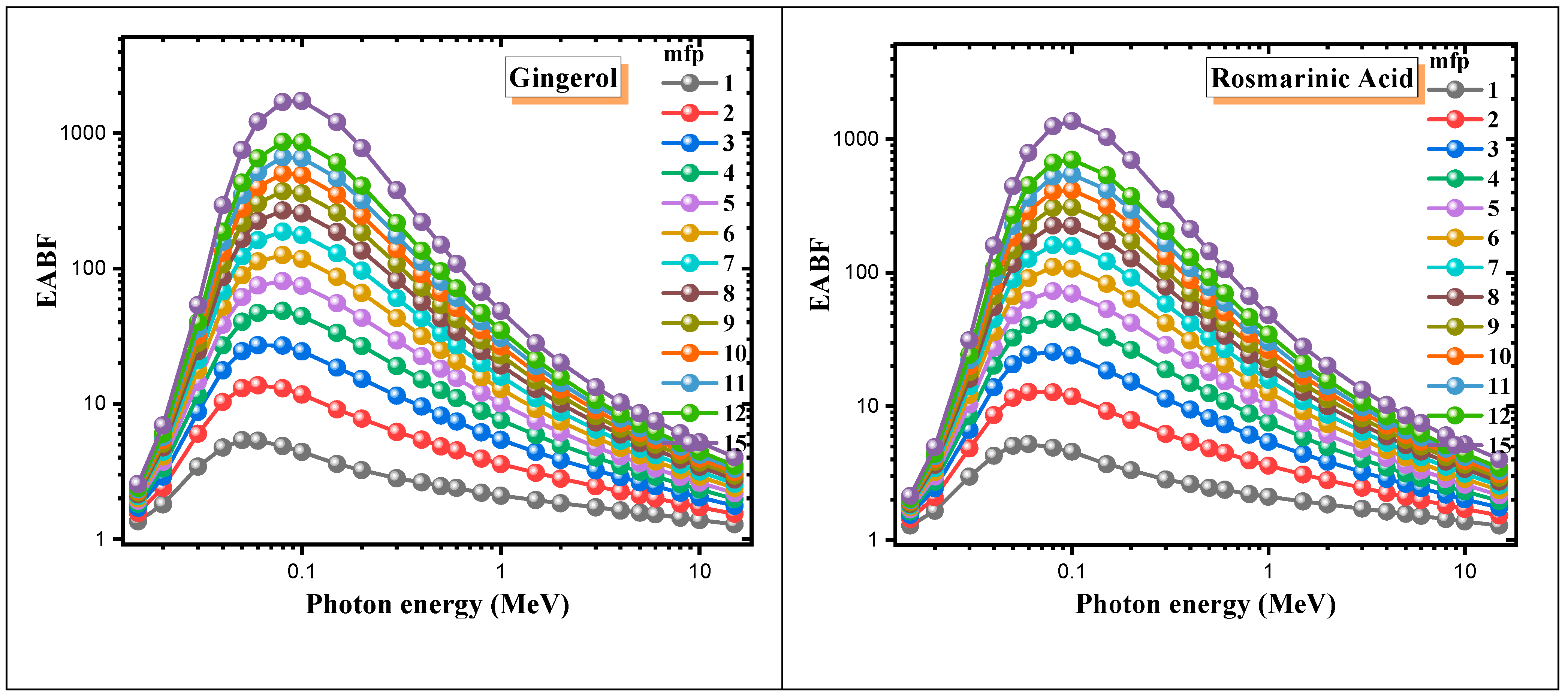
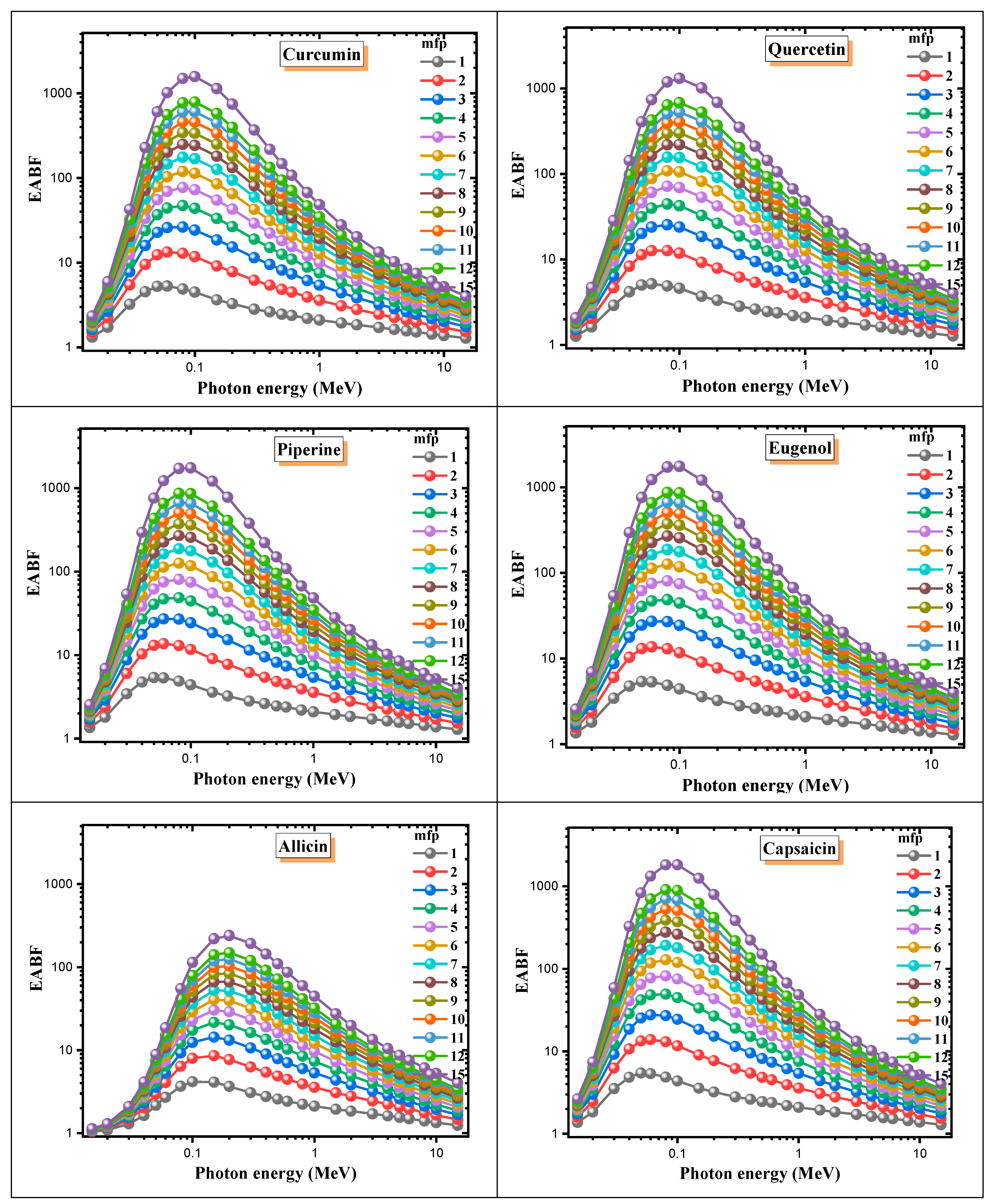
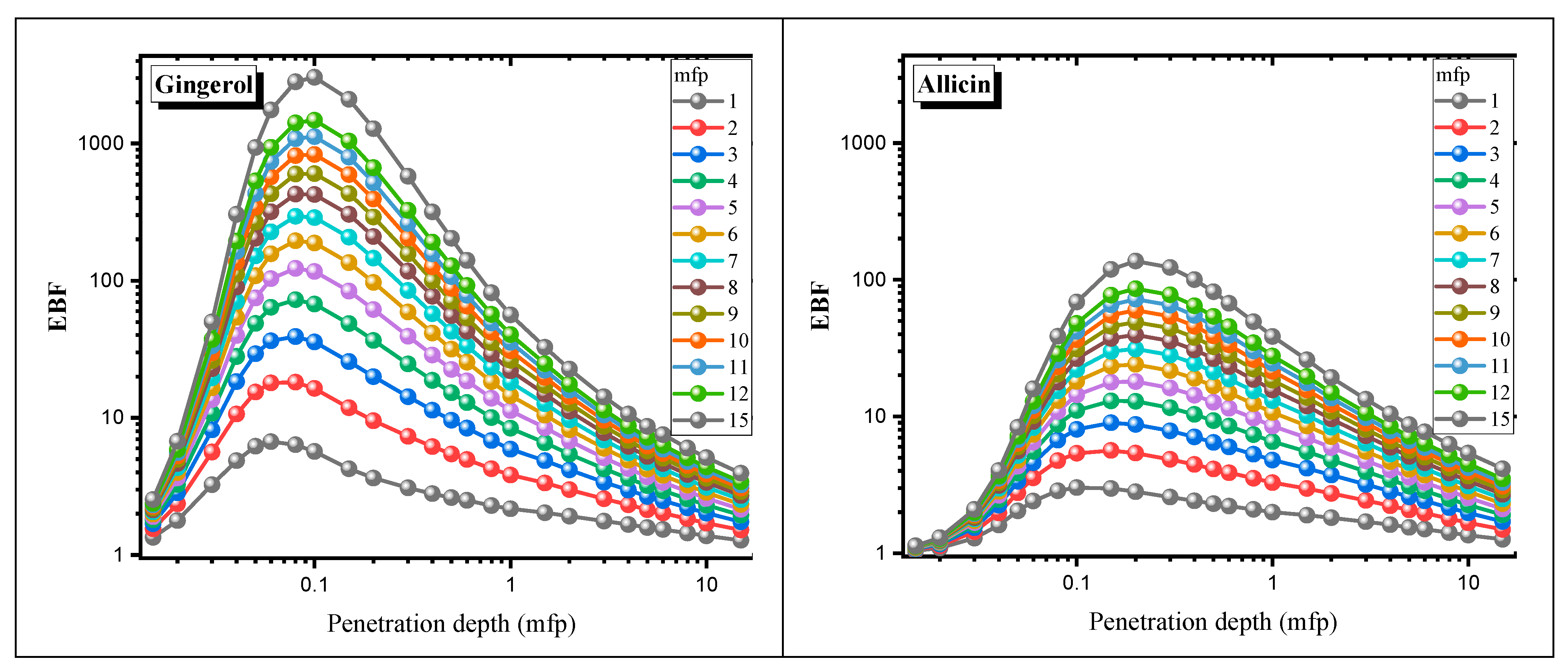
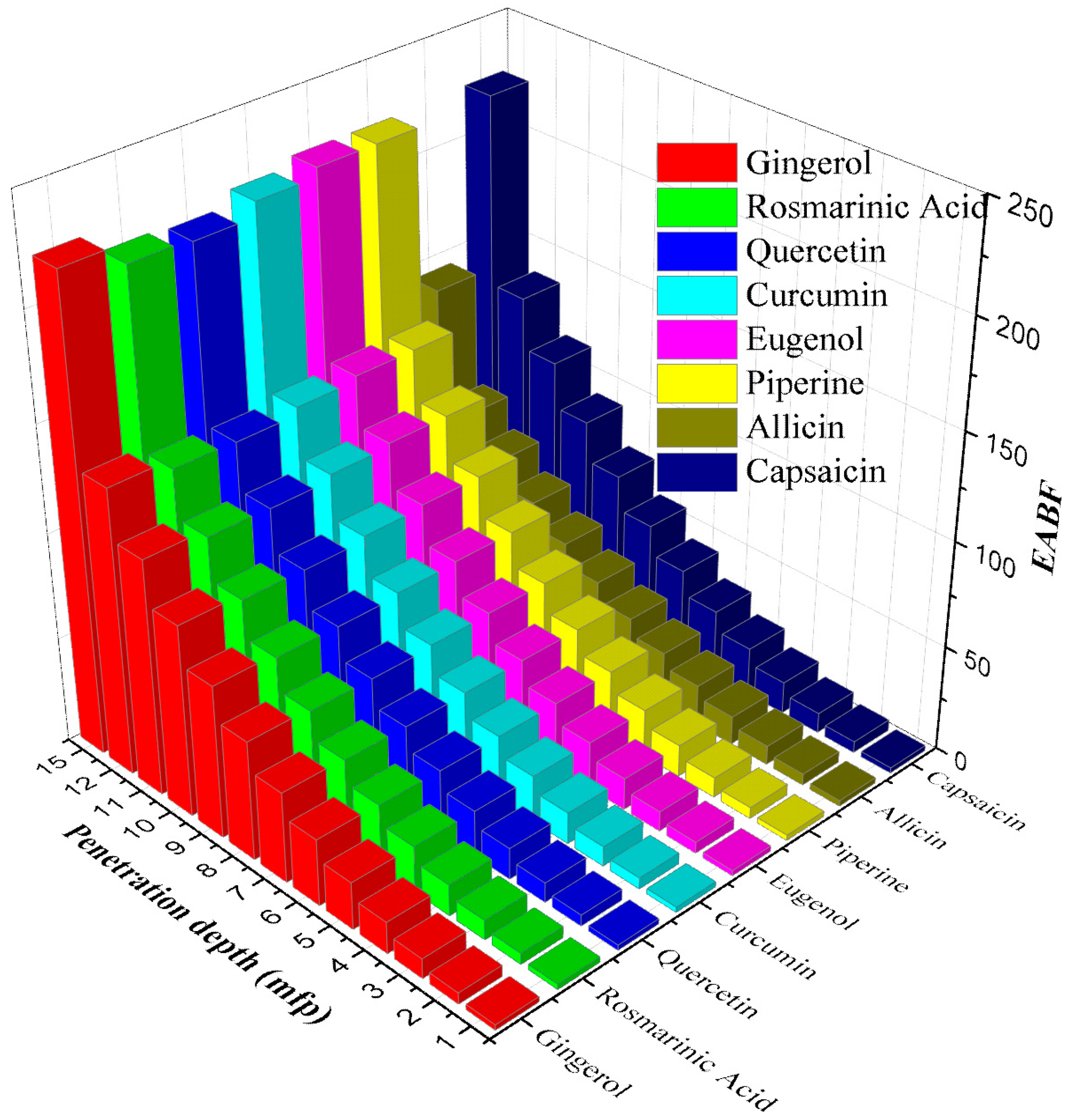
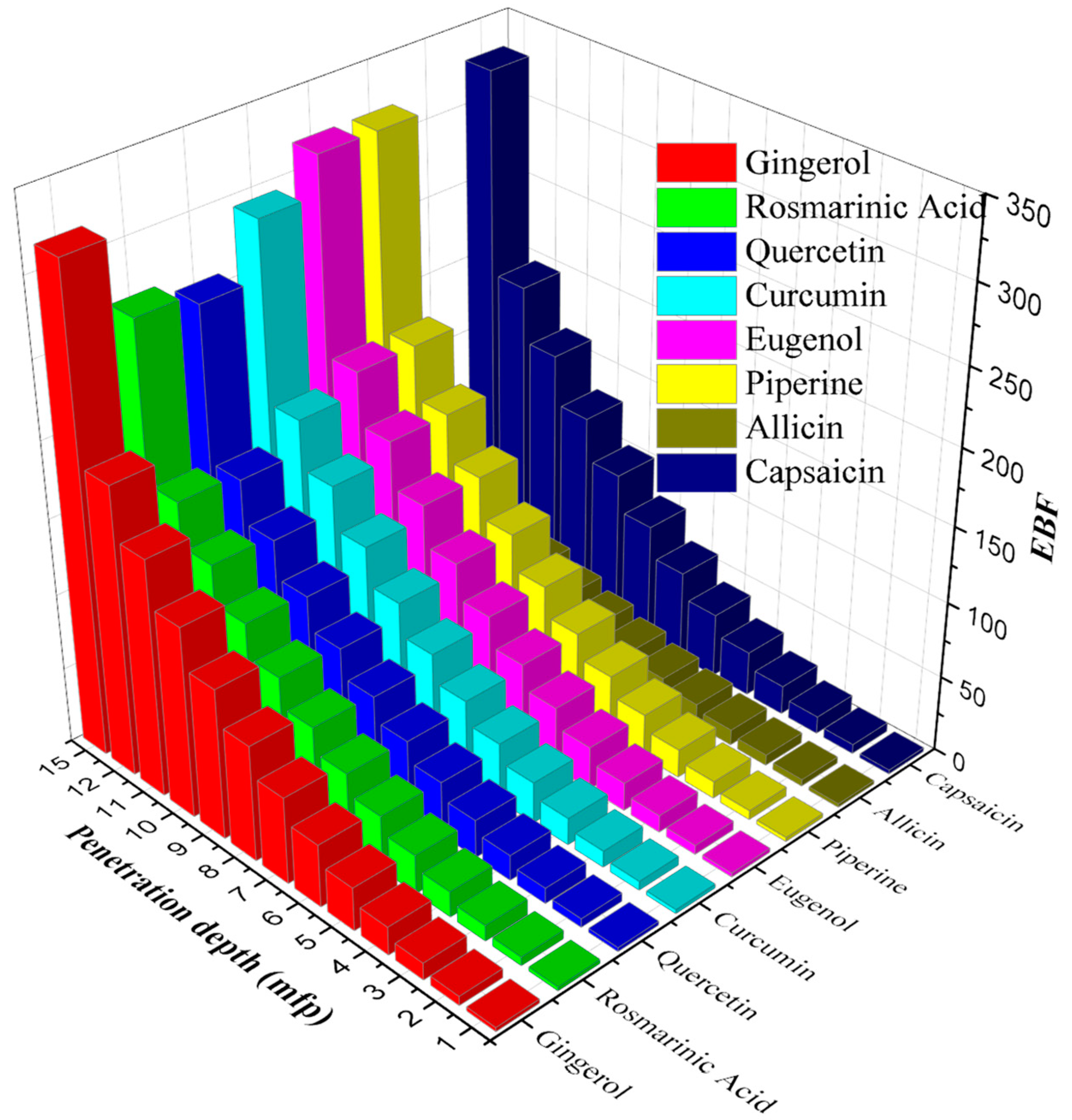
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akinloye, M.K.; Isola, G.A.; Olasunkanmi, S.K.; Okunade, D.A. Irradiation as a Food Preservation Method in Nigeria: Prospects and Problems. Int. J. Res. Appl. Sci. Eng. Technol. 2015, 3, 85–96. [Google Scholar]
- Kyriakos, A.G. Nonlinear Theory of Elementary Particles: II. Photon Theory. Prespacetime J. 2010, 1, 727–738. [Google Scholar]
- Pereira, E.; Barros, L.; Dueñas, M.; Antonio, A.L.; Santos-Buelga, C.; Ferreira, I.C.F.R. Gamma Irradiation Improves the Extractability of Phenolic Compounds in Ginkgo biloba L. Ind. Crops Prod. 2015, 74, 144–149. [Google Scholar] [CrossRef]
- ESA. ESA (European Spice Association) List of Culinary Herbs and Spices. 2020. Available online: https://www.Esa-Spices.Org/Index-Esa (accessed on 22 July 2022).
- Small, E. Culinary Herbs; NRC Research Press: Ottawa, ON, Canada, 2006. [Google Scholar]
- Peter, K.V. Handbook of Herbs and Spices; Woodhead Publishing: Sawston, UK, 2006. [Google Scholar]
- Wojtowicz, E.; Zawirska-Wojtasiak, R.; Przygoński, K. Influence of Steam Water Sterilization Process on the Content of Volatile Aroma Compounds in Marjoram (Origanum Majorana L.) Estimated with GC/MS and GC/O. Polish J. Food Nutr. Sci. 2007, 57, 151–155. [Google Scholar]
- Mathot, A.G.; Postollec, F.; Leguerinel, I. Bacterial Spores in Spices and Dried Herbs: The Risks for Processed Food. Compr. Rev. Food Sci. Food Saf. 2021, 20, 840–862. [Google Scholar] [CrossRef] [PubMed]
- Saudi, H.A.; Abedelkader, H.T.; Issa, S.A.M.; Diab, H.M.; Alharshan, G.A.; Uosif, M.A.M.; Bashter, I.I.; Ene, A.; Ghazaly, M.E.; Zakaly, H.M.H. An In-Depth Examination of the Natural Radiation and Radioactive Dangers Associated with Regularly Used Medicinal Herbs. Int. J. Environ. Res. Public Health 2022, 19, 8124. [Google Scholar] [CrossRef]
- Dogan, M.; Kayacier, A.; Ic, E. Rheological Characteristics of Some Food Hydrocolloids Processed with Gamma Irradiation. Food Hydrocoll. 2007, 21, 392–396. [Google Scholar] [CrossRef]
- Lacroix, M.; Ouattara, B. Combined Industrial Processes with Irradiation to Assure Innocuity and Preservation of Food Products —A Review. Food Res. Int. 2000, 33, 719–724. [Google Scholar] [CrossRef]
- Ali, A.M.; Issa, S.A.M.; Zakaly, H.M.H.; Pyshkina, M.; Somaily, H.H.; Algarni, H.; Rashad, M.; Saif, M.; Sidek, H.A.A.; Matori, K.A.; et al. Structural and Shielding Properties of NiO/XCo3O4 Nanocomposites Synthesized by Microwave Irradiation Method. Results Phys. 2020, 19, 103488. [Google Scholar] [CrossRef]
- Abd-Elnaiem, A.M.; Saudi, H.A.; Zakaly, H.M.H.; Issa, S.A.M.; Rashad, M. The Effect of Composition and γ-Irradiation on the Vickers Hardness, Structural and Optical Properties of xLiNbO3-25CaO-35PbO-(40-x) Waste Systems. Ceram. Int. 2021, 47, 18751–18760. [Google Scholar] [CrossRef]
- Piggott, J.R.; Othman, Z. Effect of Irradiation on Volatile Oils of Black Pepper. Food Chem. 1993, 46, 115–119. [Google Scholar] [CrossRef]
- Molins, R.A. Food Irradiation: Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2001; p. 469. [Google Scholar]
- WHO. High-Dose Irradiation: Wholesomeness of Food Irradiated with Doses above 10 KGy; WHO: Geneva, Switzerland, 1999.
- Karadaş, Ö.; Yılmaz, İ.; Geçgel, Ü. Determination of Physicochemical Properties of Irradiated Sumac (Rhus coriaria L.) Fruit Oils. Radiat. Phys. Chem. 2022, 198, 110210. [Google Scholar] [CrossRef]
- Swinehart, D.F. The Beer-Lambert Law. J. Chem. Educ. 1962, 39, 333. [Google Scholar] [CrossRef]
- Zakaly, H.M.H.; Saudi, H.A.; Issa, S.A.M.; Rashad, M.; Elazaka, A.I.; Tekin, H.O.; Saddeek, Y.B. Alteration of Optical, Structural, Mechanical Durability and Nuclear Radiation Attenuation Properties of Barium Borosilicate Glasses through BaO Reinforcement: Experimental and Numerical Analyses. Ceram. Int. 2021, 47, 5587–5596. [Google Scholar] [CrossRef]
- Kavaz, E. An Experimental Study on Gamma Ray Shielding Features of Lithium Borate Glasses Doped with Dolomite, Hematite and Goethite Minerals. Radiat. Phys. Chem. 2019, 160, 112–123. [Google Scholar] [CrossRef]
- Hila, F.C.; Asuncion-Astronomo, A.; Dingle, C.A.M.; Jecong, J.F.M.; Javier-Hila, A.M.V.; Gili, M.B.Z.; Balderas, C.V.; Lopez, G.E.P.; Guillermo, N.R.D.; Amorsolo, A.V. EpiXS: A Windows-Based Program for Photon Attenuation, Dosimetry and Shielding Based on EPICS2017 (ENDF/B-VIII) and EPDL97 (ENDF/B-VI.8). Radiat. Phys. Chem. 2021, 182, 109331. [Google Scholar] [CrossRef]
- Gerward, L.; Guilbert, N.; Jensen, K.B.; Levring, H. WinXCom—A Program for Calculating X-Ray Attenuation Coefficients. Radiat. Phys. Chem. 2004, 71, 653–654. [Google Scholar] [CrossRef]
- Cullen, D. EPDL97 The Evaluated Data Library,’97 Version. 1997. [Google Scholar]
- Tekin, H.O.; Erguzel, T.T.; Sayyed, M.I.; Singh, V.P.; Manici, T.; Altunsoy, E.E.; Agar, O. An Investigation on Shielding Properties of Different Granite Samples Using MCNPX Code. Dig. J. Nanomater. Biostruct. 2018, 13, 381–389. [Google Scholar]
- Obaid, S.S.; Sayyed, M.I.; Gaikwad, D.K.; Tekin, H.O.; Elmahroug, Y.; Pawar, P.P. Photon Attenuation Coefficients of Different Rock Samples Using MCNPX, Geant4 Simulation Codes and Experimental Results: A Comparison Study. Radiat. Eff. Defects Solids 2018, 173, 900–914. [Google Scholar] [CrossRef]
- Al-Buriahi, M.S.; Sriwunkum, C.; Arslan, H.; Tonguc, B.T.; Bourham, M.A. Investigation of Barium Borate Glasses for Radiation Shielding Applications. Appl. Phys. A Mater. Sci. Process. 2020, 126, 68. [Google Scholar] [CrossRef]
- Issa, S.A.M.; Tekin, H.O.; Elsaman, R.; Kilicoglu, O.; Saddeek, Y.B.; Sayyed, M.I. Radiation Shielding and Mechanical Properties of Al2O3-Na2O-B2O3-Bi2O3 Glasses Using MCNPX Monte Carlo Code. Mater. Chem. Phys. 2019, 223, 209–219. [Google Scholar] [CrossRef]
- Zakaly, H.M.H.; Saudi, H.A.; Tekin, H.O.; Rashad, M.; Issa, S.A.M.; Rammah, Y.S.; Elazaka, A.I.; Hessien, M.M.; Ene, A. Glass Fabrication Using Ceramic and Porcelain Recycled Waste and Lithium Niobate: Physical, Structural, Optical and Nuclear Radiation Attenuation Properties. J. Mater. Res. Technol. 2021, 15, 4074–4085. [Google Scholar] [CrossRef]
- Issa, S.A.M.; Rashad, M.; Zakaly, H.M.H.; Tekin, H.O.; Abouhaswa, A.S. Nb2O5-Li2O-Bi2O3-B2O3 Novel Glassy System: Evaluation of Optical, Mechanical, and Gamma Shielding Parameters. J. Mater. Sci. Mater. Electron. 2020, 31, 22039–22056. [Google Scholar] [CrossRef]
- Obaid, S.S.; Sayyed, M.I.; Gaikwad, D.K.; Pawar, P.P. Attenuation Coefficients and Exposure Buildup Factor of Some Rocks for Gamma Ray Shielding Applications. Radiat. Phys. Chem. 2018, 148, 86–94. [Google Scholar] [CrossRef]
- Zakaly, H.M.H.; Issa, S.A.M.; Tekin, H.O.; Badawi, A.; Saudi, H.A.; Henaish, A.M.A.; Rammah, Y.S. An Experimental Evaluation of CdO/PbO-B2O3 Glasses Containing Neodymium Oxide: Structure, Electrical Conductivity, and Gamma-Ray Resistance. Mater. Res. Bull. 2022, 151, 111828. [Google Scholar] [CrossRef]
- Saudi, H.A.; Zakaly, H.M.H.; Issa, S.A.M.; Tekin, H.O.; Hessien, M.M.; Rammah, Y.S.; Henaish, A.M.A. Fabrication, FTIR, Physical Characteristics and Photon Shielding Efficacy of CeO2 /Sand Reinforced Borate Glasses: Experimental and Simulation Studies. Radiat. Phys. Chem. 2022, 151, 109837. [Google Scholar] [CrossRef]
- Ekinci, N.; Kavaz, E.; Aygün, B.; Perişanoğlu, U. Gamma Ray Shielding Capabilities of Rhenium-Based Superalloys. Radiat. Eff. Defects Solids 2019, 174, 435–451. [Google Scholar] [CrossRef]
- Harima, Y. An Historical Review and Current Status of Buildup Factor Calculations and Applications. Radiat. Phys. Chem. 1993, 41, 631–672. [Google Scholar] [CrossRef]
- Kurtuluş, R.; Kavas, T.; Kavaz, E.; Tekin, H.O.; Kurucu, Y. Synthesis and Characterization of Waste CRT Glasses through Physical, Optical and Structural Properties: A Comprehensive Study on Recycling. Optik 2021, 248, 168167. [Google Scholar] [CrossRef]
- Abouhaswa, A.S.; Kalecik, S.; Kavaz, E. Newly Designed Borate Glass System for Optical and Radiation Shielding Applications: Multiple Effects of CdS on Structural, Magnetic, Optical, Mechanical and Photon Shielding Features. Ceram. Int. 2022, 48, 27120–27129. [Google Scholar] [CrossRef]
- Oto, B.; Gulebaglan, S.E.; Madak, Z.; Kavaz, E. Effective Atomic Numbers, Electron Densities and Gamma Rays Buildup Factors of Inorganic Metal Halide Cubic Perovskites CsBX3 (B = Sn, Ge; X = I, Br, Cl). Radiat. Phys. Chem. 2019, 159, 195–206. [Google Scholar] [CrossRef]
- Kurudirek, M.; Kurucu, Y. Estimation of Energy Absorption Buildup Factors of Some Human Tissues at Energies Relevant to Brachytherapy and External Beam Radiotherapy. Int. J. Radiat. Biol. 2019, 95, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Kurudirek, M.; Kurucu, Y. Investigation of Some Nuclear Engineering Materials in Terms of Gamma Ray Buildup Factors at Experimental Energies Used in Nuclear Physics Experiments. Radiat. Eff. Defects Solids 2020, 175, 640–656. [Google Scholar] [CrossRef]
- Perişanoğlu, U.; El-Agawany, F.I.; Kavaz, E.; Al-Buriahi, M.; Rammah, Y.S. Surveying of Na2O3–BaO–PbO–Nb2O5–SiO2–Al2O3 Glass-Ceramics System in Terms of Alpha, Proton, Neutron and Gamma Protection Features by Utilizing GEANT4 Simulation Codes. Ceram. Int. 2020, 46, 3190–3202. [Google Scholar] [CrossRef]
| Spice Name | Active Molecule Name | Chemical Formula | Molecular Structures |
|---|---|---|---|
| Ginger | Gingerol | C17H26O4 | 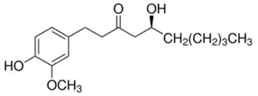 |
| Rosemary | Rosmarinic Acid | C18H16O8 | 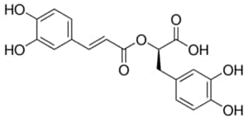 |
| Onion | Quercetin | C15H10O7 | 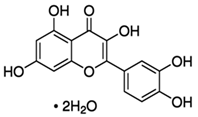 |
| Turmeric | Curcumin | C21H20O6 | 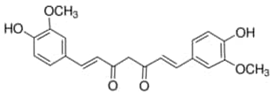 |
| Cloves | Eugenol | C10H12O2 | 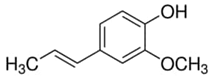 |
| Black Pepper | Piperine | C17H19 NO3 | 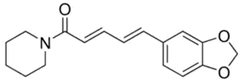 |
| Garlic | Allicin | C6H10OS2 |  |
| Red Pepper | Capsaicin | C18H27NO3 | 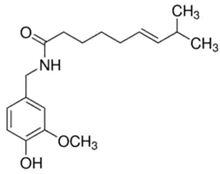 |
| Photon Energy (keV) | Gingerol | Rosmarinic Acid | Quercetin | Curcumin | ||||
| EpiXS | WinXCOM | EpiXS | WinXCOM | EpiXS | WinXCOM | EpiXS | WinXCOM | |
| 13.81 | 1.2225 | 1.2234 | 1.4324 | 1.4336 | 1.4597 | 1.4609 | 1.3008 | 1.3018 |
| 26.34 | 0.3394 | 0.3398 | 0.3614 | 0.3619 | 0.3634 | 0.3639 | 0.3449 | 0.3454 |
| 33.19 | 0.2649 | 0.2651 | 0.2720 | 0.2722 | 0.2720 | 0.2722 | 0.2647 | 0.2649 |
| 59.54 | 0.1926 | 0.1926 | 0.1881 | 0.1881 | 0.1866 | 0.1866 | 0.1881 | 0.1881 |
| 81 | 0.1750 | 0.1750 | 0.1694 | 0.1694 | 0.1678 | 0.1678 | 0.1703 | 0.1702 |
| 276 | 0.1196 | 0.1195 | 0.1149 | 0.1148 | 0.1137 | 0.1136 | 0.1160 | 0.1159 |
| 302 | 0.1157 | 0.1157 | 0.1112 | 0.1111 | 0.1100 | 0.1100 | 0.1122 | 0.1121 |
| 356 | 0.1087 | 0.1086 | 0.1044 | 0.1043 | 0.1033 | 0.1032 | 0.1054 | 0.1052 |
| 383 | 0.1057 | 0.1056 | 0.1015 | 0.1015 | 0.1004 | 0.1004 | 0.1024 | 0.1024 |
| 661 | 0.0840 | 0.0839 | 0.0806 | 0.0806 | 0.0798 | 0.0797 | 0.0814 | 0.0813 |
| 826 | 0.0758 | 0.0758 | 0.0728 | 0.0728 | 0.0720 | 0.0720 | 0.0735 | 0.0734 |
| 1173 | 0.0640 | 0.0638 | 0.0614 | 0.0613 | 0.0607 | 0.0606 | 0.0620 | 0.0619 |
| 1332 | 0.0599 | 0.0598 | 0.0575 | 0.0574 | 0.0569 | 0.0568 | 0.0581 | 0.0580 |
| Photon Energy (keV) | Eugenol | Piperine | Allicin | Capsaicin | ||||
| EpiXS | WinXCOM | EpiXS | WinXCOM | EpiXS | WinXCOM | EpiXS | WinXCOM | |
| 13.81 | 1.2020 | 1.2029 | 1.1978 | 1.1988 | 8.4826 | 8.4912 | 1.1679 | 1.1688 |
| 26.34 | 0.3343 | 0.3347 | 0.3327 | 0.3331 | 1.4016 | 1.4037 | 0.3319 | 0.3324 |
| 33.19 | 0.2611 | 0.2613 | 0.2597 | 0.2599 | 0.7921 | 0.7934 | 0.2612 | 0.2614 |
| 59.54 | 0.1899 | 0.1899 | 0.1888 | 0.1888 | 0.2797 | 0.2801 | 0.1920 | 0.1919 |
| 81 | 0.1726 | 0.1726 | 0.1716 | 0.1716 | 0.2074 | 0.2076 | 0.1748 | 0.1747 |
| 276 | 0.1180 | 0.1179 | 0.1173 | 0.1172 | 0.1179 | 0.1178 | 0.1196 | 0.1195 |
| 302 | 0.1141 | 0.1141 | 0.1134 | 0.1134 | 0.1139 | 0.1138 | 0.1157 | 0.1157 |
| 356 | 0.1072 | 0.1071 | 0.1066 | 0.1064 | 0.1067 | 0.1066 | 0.1087 | 0.1086 |
| 383 | 0.1042 | 0.1042 | 0.1036 | 0.1035 | 0.1036 | 0.1036 | 0.1057 | 0.1056 |
| 661 | 0.0828 | 0.0827 | 0.0823 | 0.0823 | 0.0821 | 0.0820 | 0.0840 | 0.0839 |
| 826 | 0.0748 | 0.0747 | 0.0743 | 0.0743 | 0.0741 | 0.0740 | 0.0758 | 0.0758 |
| 1173 | 0.0631 | 0.0630 | 0.0627 | 0.0626 | 0.0624 | 0.0623 | 0.0640 | 0.0638 |
| 1332 | 0.0591 | 0.0590 | 0.0587 | 0.0586 | 0.0585 | 0.0584 | 0.0599 | 0.0598 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kavaz Yüksel, A.; Zakaly, H.M.H.; Ene, A. Evaluation of Photon Interaction Parameters of Some Antioxidants for Food Irradiation Applications. Materials 2022, 15, 6376. https://doi.org/10.3390/ma15186376
Kavaz Yüksel A, Zakaly HMH, Ene A. Evaluation of Photon Interaction Parameters of Some Antioxidants for Food Irradiation Applications. Materials. 2022; 15(18):6376. https://doi.org/10.3390/ma15186376
Chicago/Turabian StyleKavaz Yüksel, Arzu, Hesham M. H. Zakaly, and Antoaneta Ene. 2022. "Evaluation of Photon Interaction Parameters of Some Antioxidants for Food Irradiation Applications" Materials 15, no. 18: 6376. https://doi.org/10.3390/ma15186376
APA StyleKavaz Yüksel, A., Zakaly, H. M. H., & Ene, A. (2022). Evaluation of Photon Interaction Parameters of Some Antioxidants for Food Irradiation Applications. Materials, 15(18), 6376. https://doi.org/10.3390/ma15186376








