Amino-Functionalized Titanate Nanotubes: pH and Kinetic Study of a Promising Adsorbent for Acid Dye in Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Sodium Titanate Nanotubes
2.3. Synthesis of Modified Nanotubes
2.4. Point of Zero Charge ()
2.5. Remazol Blue R Adsorption
2.6. Kinetic Models
2.7. Characterizations
3. Results and Discussion
3.1. Material Characterizations
3.2. Adsorption Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dos Santos Silva, L.; de Oliveira Carvalho, J.; de Sousa Bezerra, R.D.; da Silva, M.; Ferreira, F.; Osajima, J.; da Silva Filho, E. Potential of Cellulose Functionalized with Carboxylic Acid as Biosorbent for the Removal of Cationic Dyes in Aqueous Solution. Molecules 2018, 23, 743. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; He, Y.; Zhou, L.; Xu, T.; Hu, J.; Peng, C.; Liu, H. Fast adsorption of methylene blue, basic fuchsin, and malachite green by a novel sulfonic-grafted triptycene-based porous organic polymer. RSC Adv. 2018, 8, 41986–41993. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.S.; Silva, M.S.; Ferreira, F.J.L.; Lima, L.C.B.; Bezerra, R.D.S.; Citó, A.M.G.L.; Osajima, J.A.; Silva Filho, E.C. Effective Removal of the Remazol Yellow GR Dye Using Cellulose Functionalized by Basic Groups. Water Air Soil Pollut. 2018, 229, 218. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chatterjee, S.; Chatterjee, B.P.; Das, A.R.; Guha, A.K. Adsorption of a model anionic dye, eosin Y, from aqueous solution by chitosan hydrobeads. J. Colloid Interface Sci. 2005, 288, 30–35. [Google Scholar] [CrossRef]
- Subbaiah, M.V.; Kim, D.S. Adsorption of methyl orange from aqueous solution by aminated pumpkin seed powder: Kinetics, isotherms, and thermodynamic studies. Ecotoxicol. Environ. Saf. 2016, 128, 109–117. [Google Scholar] [CrossRef]
- Wang, L.; Liu, W.; Wang, T.; Ni, J. Highly efficient adsorption of Cr(VI) from aqueous solutions by amino-functionalized titanate nanotubes. Chem. Eng. J. 2013, 225, 153–163. [Google Scholar] [CrossRef]
- Niu, G.; Liu, W.; Wang, T.; Ni, J. Absorption of Cr(VI) onto amino-modified titanate nanotubes using 2-Bromoethylamine hydrobromide through SN2 reaction. J. Colloid Interface Sci. 2013, 401, 133–140. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, L.; Liu, G.; Zhou, Y. Enhanced adsorption of Ag+ on triethanolamine modified titanate nanotubes. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 537, 28–35. [Google Scholar] [CrossRef]
- Dobó, D.G.; Berkesi, D.; Kukovecz, Á. Morphology conserving aminopropyl functionalization of hollow silica nanospheres in toluene. J. Mol. Struct. 2017, 1140, 83–88. [Google Scholar] [CrossRef]
- Bergmann, C.P.; Machado, F.M. (Eds.) Carbon Nanomaterials as Adsorbents for Environmental and Biological Applications; Carbon Nanostructures; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Sandoval, A.; Hernández-Ventura, C.; Klimova, T.E. Titanate nanotubes for removal of methylene blue dye by combined adsorption and photocatalysis. Fuel 2017, 198, 22–30. [Google Scholar] [CrossRef]
- Subramaniam, M.N.; Goh, P.S.; Abdullah, N.; Lau, W.J.; Ng, B.C.; Ismail, A.F. Adsorption and photocatalytic degradation of methylene blue using high surface area titanate nanotubes (TNT) synthesized via hydrothermal method. J. Nanopart. Res. 2017, 19, 220. [Google Scholar] [CrossRef]
- Lee, C.K.; Liu, S.S.; Juang, L.C.; Wang, C.C.; Lyu, M.D.; Hung, S.H. Application of titanate nanotubes for dyes adsorptive removal from aqueous solution. J. Hazard. Mater. 2007, 148, 756–760. [Google Scholar] [CrossRef]
- Juang, L.C.; Lee, C.K.; Wang, C.C.; Hung, S.H.; Lyu, M.D. Adsorptive Removal of Acid Red 1 from Aqueous Solution with Surfactant Modified Titanate Nanotubes. Environ. Eng. Sci. 2008, 25, 519–528. [Google Scholar] [CrossRef]
- Marques, T.M.; Sales, D.A.; Silva, L.S.; Bezerra, R.D.; Silva, M.S.; Osajima, J.A.; Ferreira, O.P.; Ghosh, A.; Silva Filho, E.C.; Viana, B.C.; et al. Amino-functionalized titanate nanotubes for highly efficient removal of anionic dye from aqueous solution. Appl. Surf. Sci. 2020, 512, 145659. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; El-Bindary, A.A.; Ismail, A.; Morshidy, A.M. Sorptive removal of Remazol Brilliant Blue R from aqueous solution by diethylenetriamine functionalized magnetic macro-reticular hybrid material. RSC Adv. 2016, 6, 22395–22410. [Google Scholar] [CrossRef]
- Sheng, G.; Linghu, W.; Chen, Z.; Xu, D.; Alsaedi, A.; Shammakh, W.; Monaquel, S.; Sheng, J. Sequestration of selenate and selenite onto titanate nanotube: A combined classical batch and advanced EXAFS approach. Environ. Nanotechnol. Monit. Manag. 2016, 6, 152–158. [Google Scholar] [CrossRef]
- Silva, L.S.; Lima, L.C.; Silva, F.C.; Matos, J.M.E.; Santos, M.R.M.; Santos Júnior, L.S.; Sousa, K.S.; da Silva Filho, E.C. Dye anionic sorption in aqueous solution onto a cellulose surface chemically modified with aminoethanethiol. Chem. Eng. J. 2013, 218, 89–98. [Google Scholar] [CrossRef]
- Ma, J.; Li, F.; Qian, T.; Liu, H.; Liu, W.; Zhao, D. Natural organic matter resistant powder activated charcoal supported titanate nanotubes for adsorption of Pb(II). Chem. Eng. J. 2017, 315, 191–200. [Google Scholar] [CrossRef]
- Ferreira, F.J.; Silva, L.S.; da Silva, M.S.; Osajima, J.A.; Meneguin, A.B.; Santagneli, S.H.; Barud, H.S.; Bezerra, R.D.; Silva-Filho, E.C. Understanding kinetics and thermodynamics of the interactions between amitriptyline or eosin yellow and aminosilane-modified cellulose. Carbohydr. Polym. 2019, 225, 115246. [Google Scholar] [CrossRef]
- El-Hakam, S.; Ibrahim, A.A.; Elatwy, L.; El-Yazeed, W.A.; Salama, R.S.; El-Reash, Y.A.; Ahmed, A.I. Greener route for the removal of toxic heavy metals and synthesis of 14-aryl-14H dibenzo[a,j] xanthene using a novel and efficient Ag-Mg bimetallic MOF as a recyclable heterogeneous nanocatalyst. J. Taiwan Inst. Chem. Eng. 2021, 122, 176–189. [Google Scholar] [CrossRef]
- Bakry, A.M.; Alamier, W.M.; Salama, R.S.; El-Shall, M.S.; Awad, F.S. Remediation of water containing phosphate using ceria nanoparticles decorated partially reduced graphene oxide (CeO2-PRGO) composite. Surf. Interfaces 2022, 31, 102006. [Google Scholar] [CrossRef]
- Silva, M.S.; Silva, L.S.; Ferreira, F.J.; Bezerra, R.D.; Marques, T.M.; Meneguin, A.B.; Barud, H.S.; Osajima, J.A.; Silva Filho, E.C. Study of interactions between organic contaminants and a new phosphated biopolymer derived from cellulose. Int. J. Biol. Macromol. 2020, 146, 668–677. [Google Scholar] [CrossRef]
- Kukovecz, Á.; Kordás, K.; Kiss, J.; Kónya, Z. Atomic scale characterization and surface chemistry of metal modified titanate nanotubes and nanowires. Surf. Sci. Rep. 2016, 71, 473–546. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Y.; Jáuregui-Haza, U.; Guo, Z.; Campos, L.C. The impact of humic acid on metaldehyde adsorption onto powdered activated carbon in aqueous solution. RSC Adv. 2019, 9, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.P.; Santana, S.A.; Bezerra, C.W.; Silva, H.A.; de Melo, J.C.; da Silva Filho, E.C.; Airoldi, C. Copper sorption from aqueous solutions and sugar cane spirits by chemically modified babassu coconut (Orbignya speciosa) mesocarp. Chem. Eng. J. 2010, 161, 99–105. [Google Scholar] [CrossRef]
- Queiroga, L.N.; Pereira, M.B.; Silva, L.S.; Silva Filho, E.C.; Santos, I.M.; Fonseca, M.G.; Georgelin, T.; Jaber, M. Microwave bentonite silylation for dye removal: Influence of the solvent. Appl. Clay Sci. 2019, 168, 478–487. [Google Scholar] [CrossRef]
- De Castro Silva, F.; da Silva, M.M.F.; Lima, L.C.B.; Osajima, J.A.; da Silva Filho, E.C. Integrating chloroethyl phosphate with biopolymer cellulose and assessing their potential for absorbing brilliant green dye. J. Environ. Chem. Eng. 2016, 4, 3348–3356. [Google Scholar] [CrossRef]
- Lagergren, S.K. About the theory of so-called adsorption of soluble substances. Sven. Vetenskapsakad. Handingarl 1898, 24, 1–39. [Google Scholar]
- Ho, Y.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Elovich, S.Y.; Larinov, O.G. Theory of adsorption from solutions of non electrolytes on solid (I) equation adsorption from solutions and the analysis of its simplest form,(II) verification of the equation of adsorption isotherm from solutions. Izv. Akad. Nauk. SSSR Otd. Khim. Nauk 1962, 2, 209–216. [Google Scholar]
- Gusmão, S.B.; Ghosh, A.; Marques, T.M.; Gusmão, G.O.; Oliveira, T.G.; Cavalcante, L.C.D.; Vasconcelos, T.L.; Abreu, G.J.; Guerra, Y.; Peña-Garcia, R.; et al. Structural and magnetic properties of titanate nano-heterostructures decorated with iron based nanoparticles. J. Phys. Chem. Solids 2020, 145, 109561. [Google Scholar] [CrossRef]
- Sales, D.A.; Marques, T.M.; Ghosh, A.; Gusmão, S.B.; Vasconcelos, T.L.; Luz-Lima, C.; Ferreira, O.P.; Hollanda, L.M.; Lima, I.S.; Silva-Filho, E.C.; et al. Synthesis of silver-cerium titanate nanotubes and their surface properties and antibacterial applications. Mater. Sci. Eng. C 2020, 115, 111051. [Google Scholar] [CrossRef]
- Gusmão, S.B.; Ghosh, A.; Marques, T.M.; Vieira, L.H.S.; Ferreira, O.P.; Silva-Filho, E.C.; Lobo, A.O.; Osajima, J.A.; Viana, B.C. Titanate-based one-dimensional nano-heterostructure: Study of hydrothermal reaction parameters for improved photocatalytic application. Solid State Sci. 2019, 98, 106043. [Google Scholar] [CrossRef]
- Gusmão, S.B.S.; Ghosh, A.; Marques, T.M.F.; Ferreira, O.P.; Lobo, A.O.; Osajima, J.A.O.; Luz-Lima, C.; Sousa, R.R.M.; Matos, J.M.E.; Viana, B.C. One-Pot Synthesis of Titanate Nanotubes Decorated with Anatase Nanoparticles Using a Microwave-Assisted Hydrothermal Reaction. J. Nanomater. 2019, 2019, 4825432. [Google Scholar] [CrossRef]
- Chen, Q.; Du, G.; Zhang, S.; Peng, L.M. The structure of trititanate nanotubes. Acta Crystallogr. Sect. B Struct. Sci. 2002, 58, 587–593. [Google Scholar] [CrossRef]
- Viana, B.C.; Ferreira, O.P.; Souza Filho, A.G.; Hidalgo, A.A.; Mendes Filho, J.; Alves, O.L. Highlighting the mechanisms of the titanate nanotubes to titanate nanoribbons transformation. J. Nanopart. Res. 2011, 13, 3259–3265. [Google Scholar] [CrossRef]
- Pontón, P.I.; D’Almeida, J.R.; Marinkovic, B.A.; Savić, S.M.; Mancic, L.; Rey, N.A.; Morgado, E.; Rizzo, F.C. The effects of the chemical composition of titanate nanotubes and solvent type on 3-aminopropyltriethoxysilane grafting efficiency. Appl. Surf. Sci. 2014, 301, 315–322. [Google Scholar] [CrossRef]
- Plodinec, M.; Gajović, A.; Iveković, D.; Tomašić, N.; Zimmermann, B.; Macan, J.; Haramina, T.; Su, D.S.; Willinger, M. Study of thermal stability of (3-aminopropyl)trimethoxy silane-grafted titanate nanotubes for application as nanofillers in polymers. Nanotechnology 2014, 25, 435601. [Google Scholar] [CrossRef]
- Brnardić, I.; Huskić, M.; Umek, P.; Fina, A.; Grgurić, T.H. Synthesis of silane functionalized sodium titanate nanotubes and their influence on thermal and mechanical properties of epoxy nanocomposite. Phys. Status Solidi 2013, 210, 2284–2291. [Google Scholar] [CrossRef]
- Maleki, A.; Hamesadeghi, U.; Daraei, H.; Hayati, B.; Najafi, F.; McKay, G.; Rezaee, R. Amine functionalized multi-walled carbon nanotubes: Single and binary systems for high capacity dye removal. Chem. Eng. J. 2017, 313, 826–835. [Google Scholar] [CrossRef]
- Silva, L.S.; Lima, L.C.B.; Ferreira, F.J.L.; Silva, M.S.; Osajima, J.A.; Bezerra, R.D.S.; Filho, E.C.S. Sorption of the anionic reactive red RB dye in cellulose: Assessment of kinetic, thermodynamic, and equilibrium data. Open Chem. 2015, 13. [Google Scholar] [CrossRef]
- Silva, L.S.; Ferreira, F.J.; Silva, M.S.; Citó, A.M.; Meneguin, A.B.; Sábio, R.M.; Barud, H.S.; Bezerra, R.D.; Osajima, J.A.; Silva Filho, E.C. Potential of amino-functionalized cellulose as an alternative sorbent intended to remove anionic dyes from aqueous solutions. Int. J. Biol. Macromol. 2018, 116, 1282–1295. [Google Scholar] [CrossRef]
- Monsef Khoshhesab, Z.; Modaresnia, N. Adsorption of Acid Black 210 and Remazol Brilliant Blue R onto magnetite nanoparticles. Inorg. Nano-Met. Chem. 2019, 49, 231–239. [Google Scholar] [CrossRef]
- Monsef Khoshhesab, Z.; Souhani, S. Adsorptive removal of reactive dyes from aqueous solutions using zinc oxide nanoparticles. J. Chin. Chem. Soc. 2018, 65, 1482–1490. [Google Scholar] [CrossRef]
- Silva, T.L.; Ronix, A.; Pezoti, O.; Souza, L.S.; Leandro, P.K.; Bedin, K.C.; Beltrame, K.K.; Cazetta, A.L.; Almeida, V.C. Mesoporous activated carbon from industrial laundry sewage sludge: Adsorption studies of reactive dye Remazol Brilliant Blue R. Chem. Eng. J. 2016, 303, 467–476. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S.; Kashi, E.; Yaseen, Z.M.; ALOthman, Z.A.; Khan, M.R. Cross-Linked Chitosan-Glyoxal/Kaolin Clay Composite: Parametric Optimization for Color Removal and COD Reduction of Remazol Brilliant Blue R Dye. J. Polym. Environ. 2022, 30, 164–178. [Google Scholar] [CrossRef]
- Reghioua, A.; Barkat, D.; Jawad, A.H.; Abdulhameed, A.S.; Rangabhashiyam, S.; Khan, M.R.; ALOthman, Z.A. Magnetic Chitosan-Glutaraldehyde/Zinc Oxide/Fe3O4 Nanocomposite: Optimization and Adsorptive Mechanism of Remazol Brilliant Blue R Dye Removal. J. Polym. Environ. 2021, 29, 3932–3947. [Google Scholar] [CrossRef]
- Bouzikri, S.; Ouasfi, N.; Benzidia, N.; Salhi, A.; Bakkas, S.; Khamliche, L. Marine alga “Bifurcaria bifurcata”: Biosorption of Reactive Blue 19 and methylene blue from aqueous solutions. Environ. Sci. Pollut. Res. 2020, 27, 33636–33648. [Google Scholar] [CrossRef]
- Guerritore, M.; Castaldo, R.; Silvestri, B.; Avolio, R.; Cocca, M.; Errico, M.E.; Avella, M.; Gentile, G.; Ambrogi, V. Hyper-Crosslinked Polymer Nanocomposites Containing Mesoporous Silica Nanoparticles with Enhanced Adsorption Towards Polar Dyes. Polymers 2020, 12, 1388. [Google Scholar] [CrossRef]
- Mate, C.J.; Mishra, S. Synthesis of borax cross-linked Jhingan gum hydrogel for remediation of Remazol Brilliant Blue R (RBBR) dye from water: Adsorption isotherm, kinetic, thermodynamic and biodegradation studies. Int. J. Biol. Macromol. 2020, 151, 677–690. [Google Scholar] [CrossRef]
- Chinoune, K.; Bentaleb, K.; Bouberka, Z.; Nadim, A.; Maschke, U. Adsorption of reactive dyes from aqueous solution by dirty bentonite. Appl. Clay Sci. 2016, 123, 64–75. [Google Scholar] [CrossRef]
- Shanehsaz, M.; Seidi, S.; Ghorbani, Y.; Shoja, S.M.R.; Rouhani, S. Polypyrrole-coated magnetic nanoparticles as an efficient adsorbent for RB19 synthetic textile dye: Removal and kinetic study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 481–486. [Google Scholar] [CrossRef]
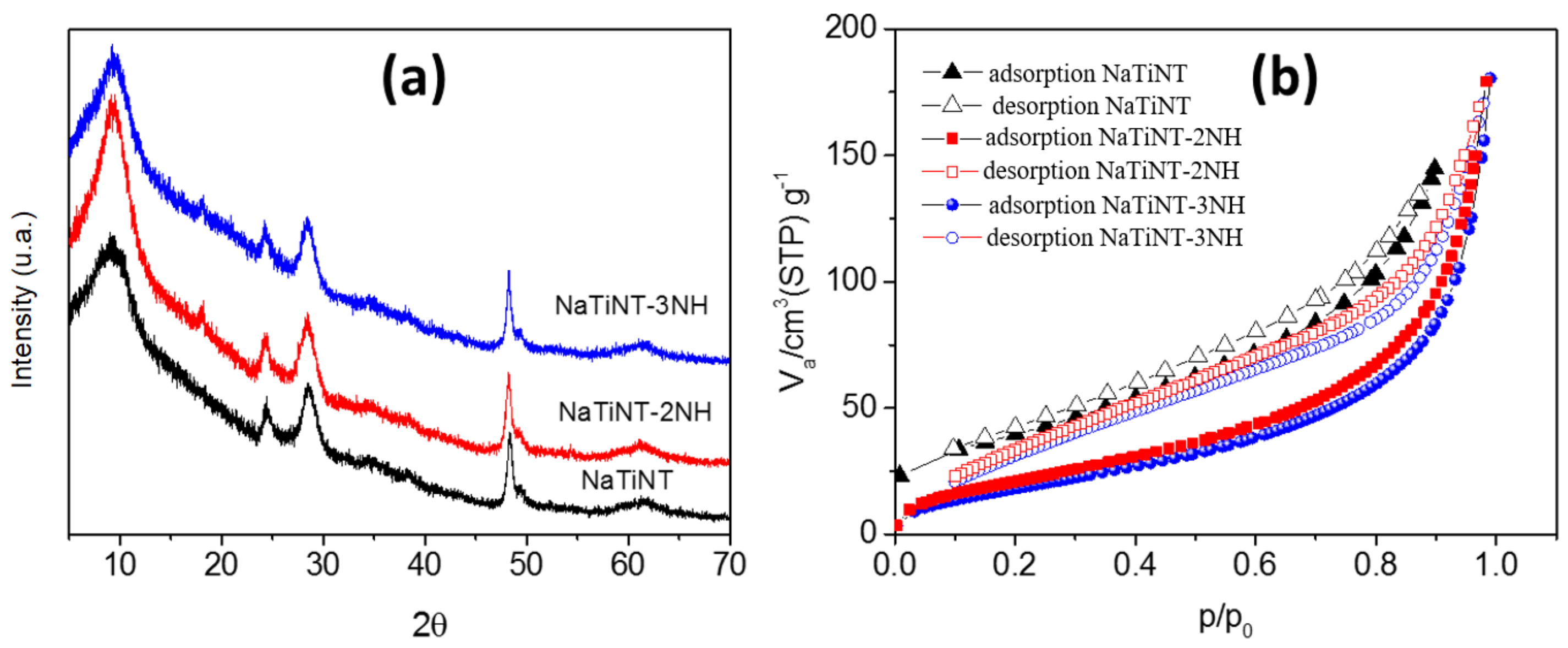
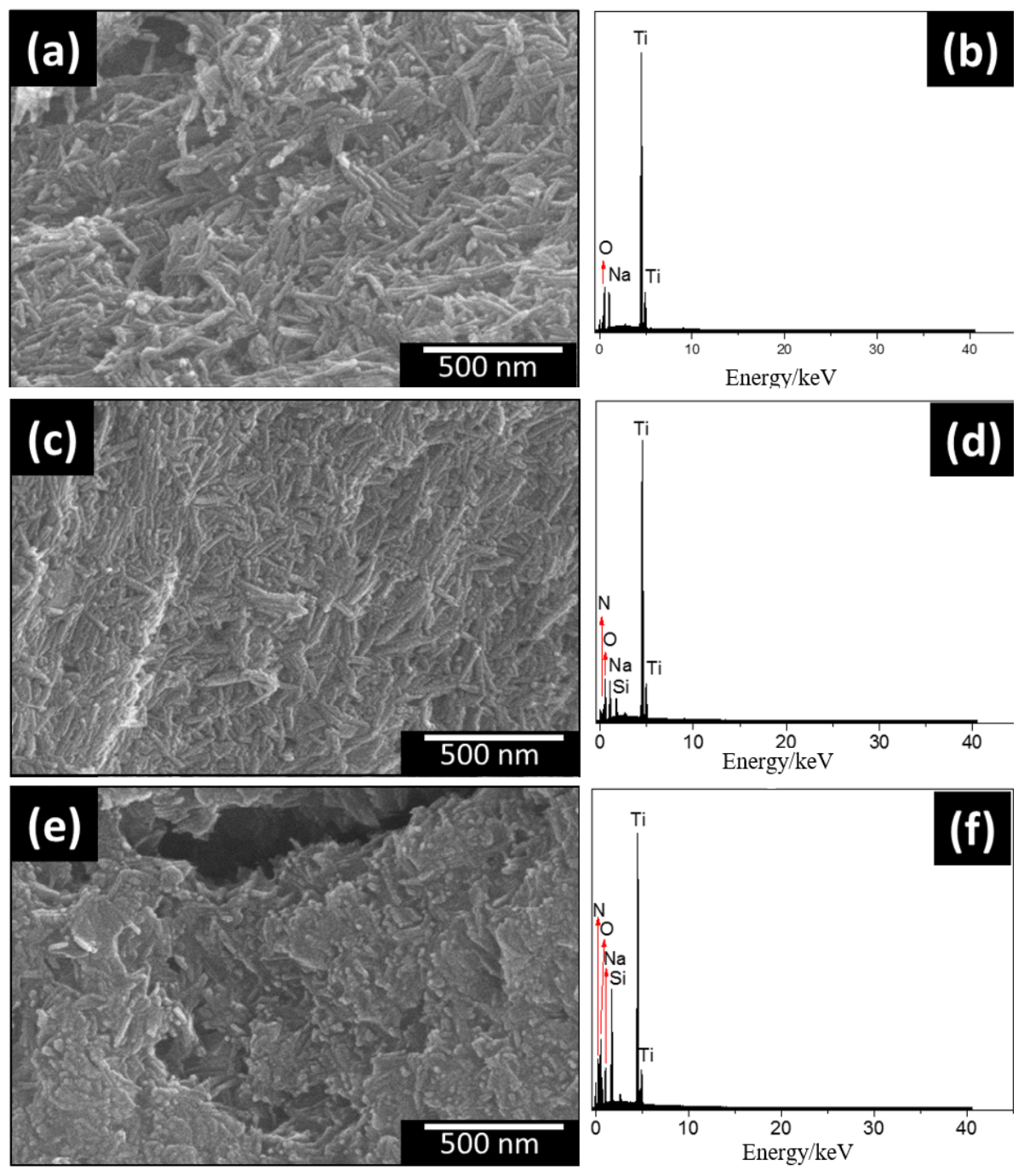

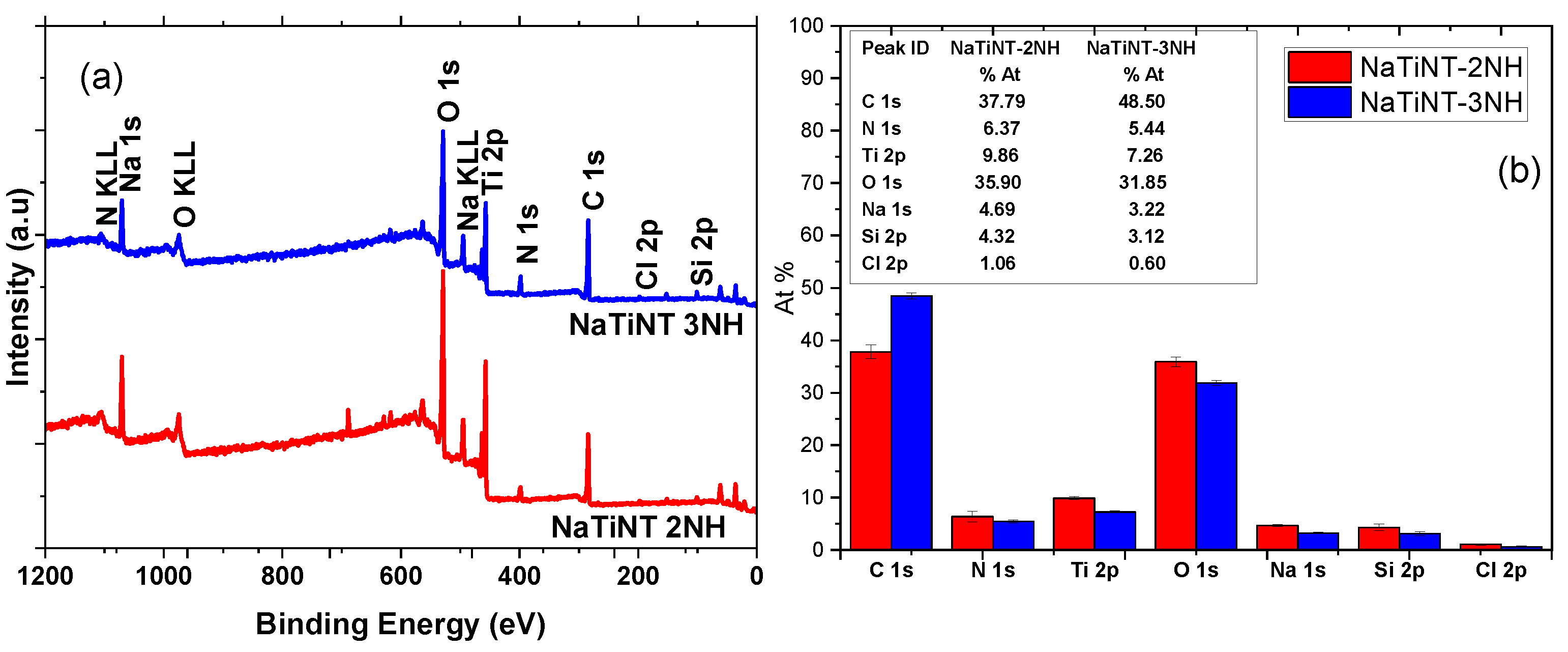
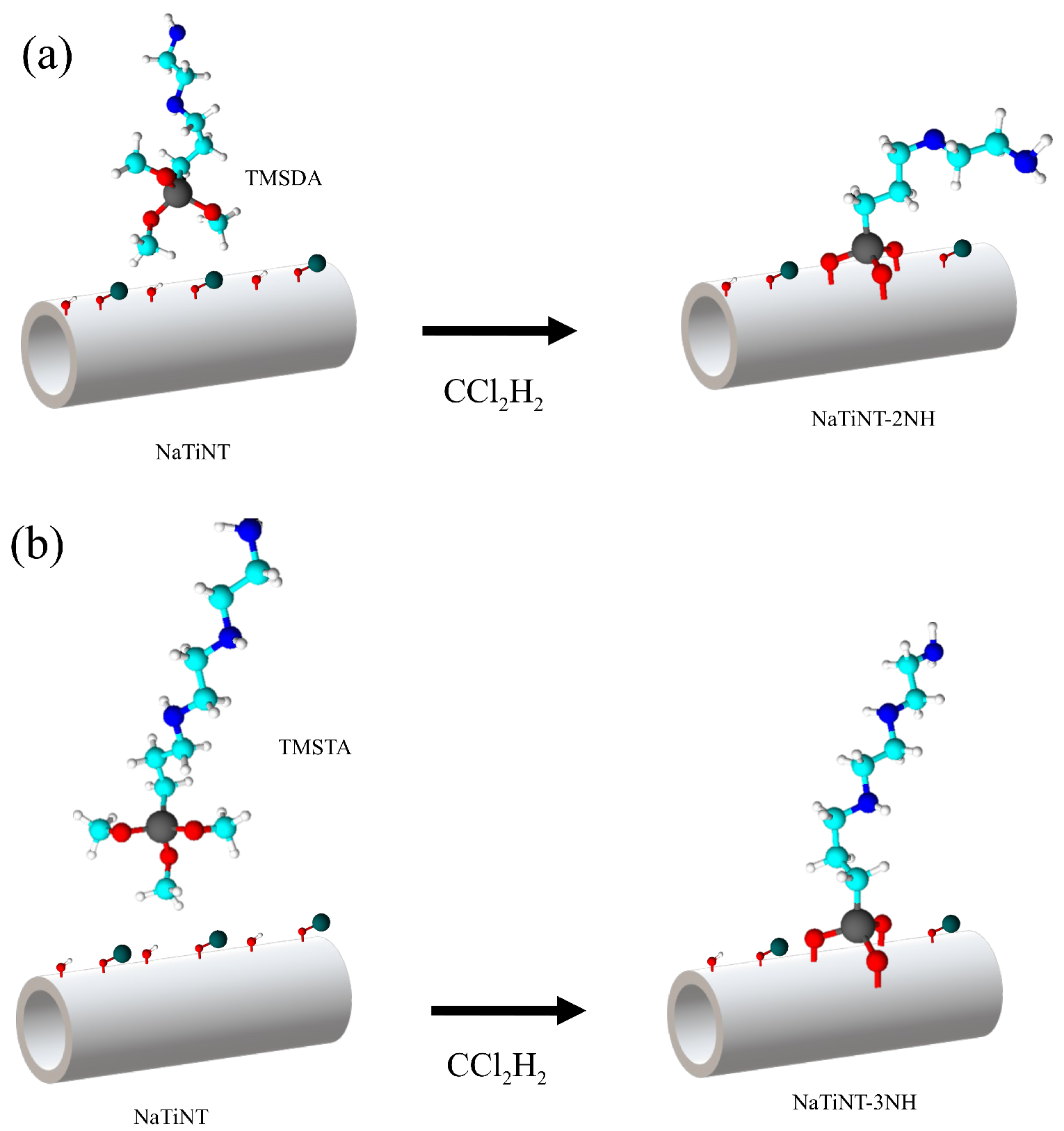
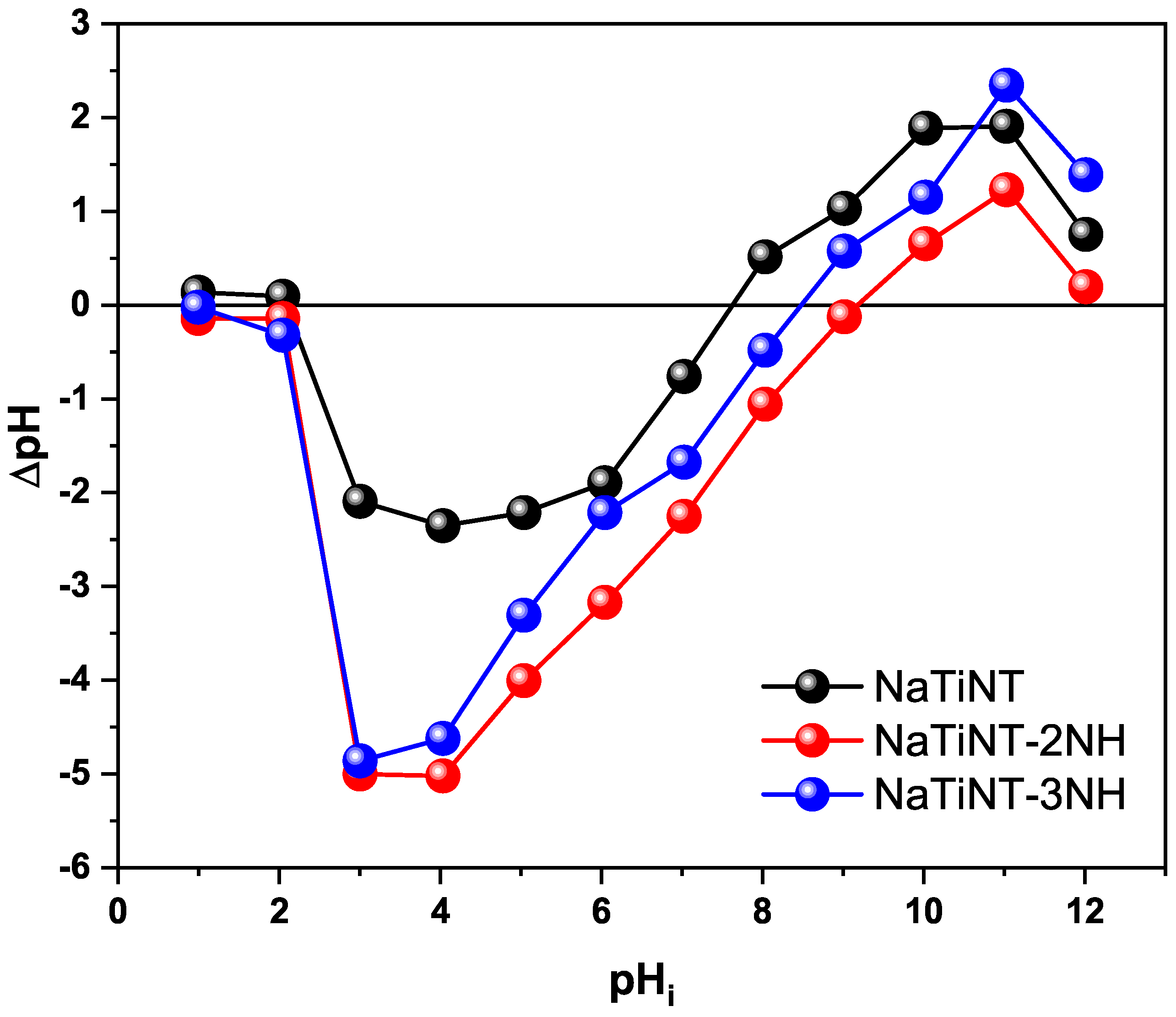

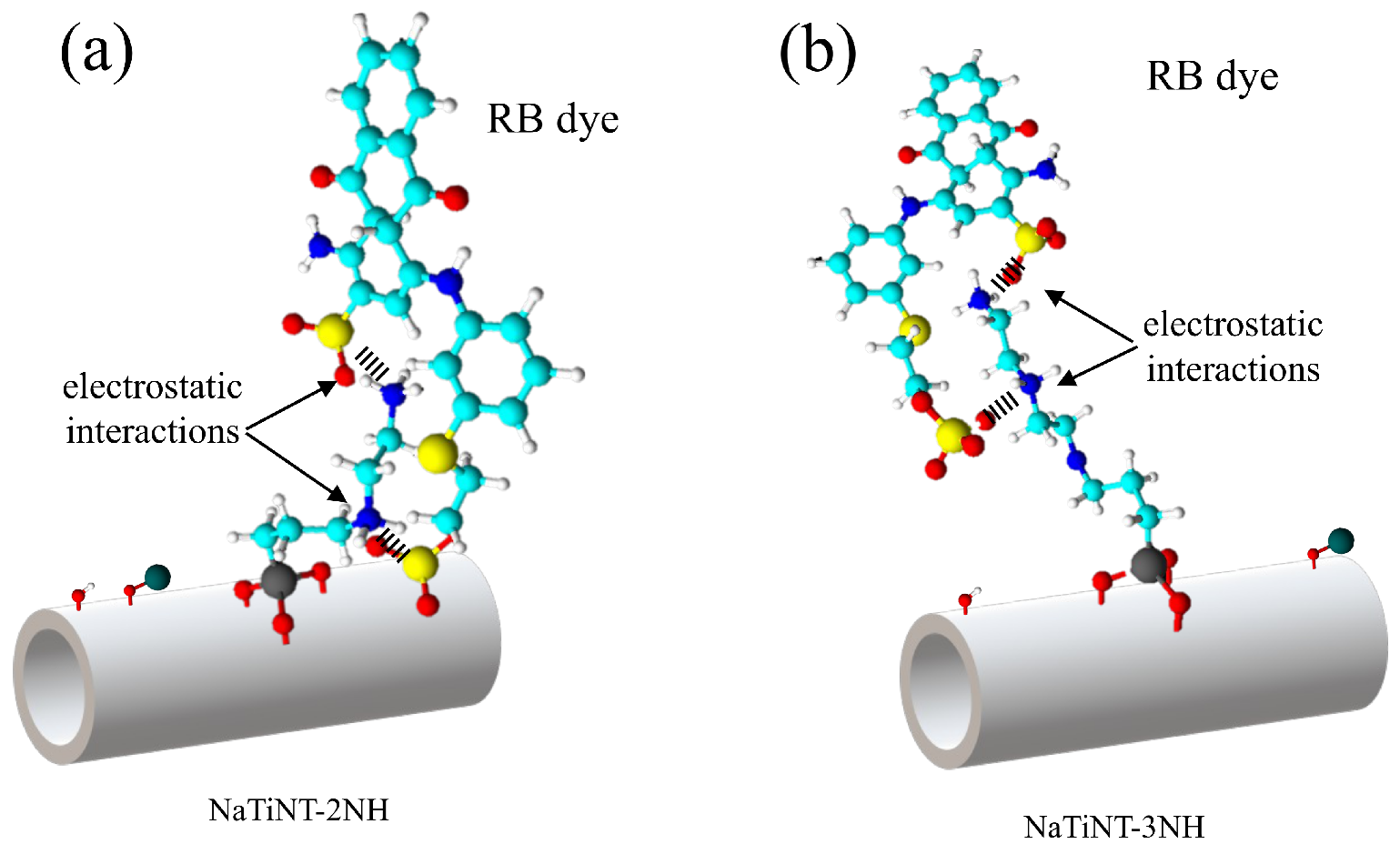
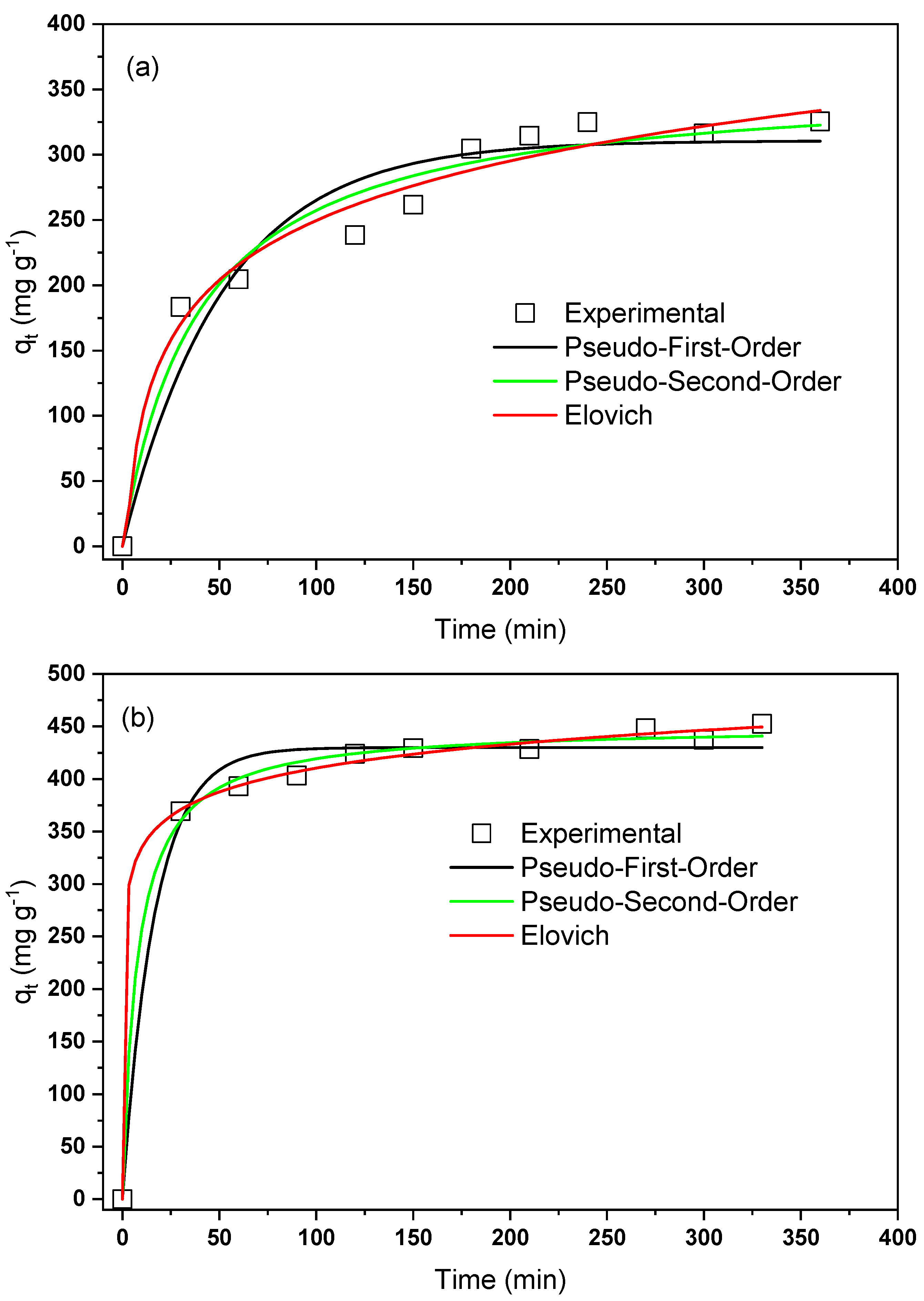
| Samples | (m g) | (nm) | (cm g) |
|---|---|---|---|
| NaTiNT | 147.5 | 10.9 | 0.401 |
| NaTiNT−2NH | 84.2 | 13.2 | 0.277 |
| NaTiNT−3NH | 74.6 | 14.9 | 0.278 |
| Pseudo-First-Order | |||
|---|---|---|---|
| Adsorbent | (min) | (mg g) | |
| NaTiNT−2NH | 0.019 | 310.623 | 0.9309 |
| NaTiNT−3NH | 0.059 | 429.958 | 0.9845 |
| Pseudo-Second-Order | |||
| Adsorbent | (g mg min) | (mg g) | |
| NaTiNT−2NH | 357.477 | 0.9635 | |
| NaTiNT−3NH | 450.960 | 0.9961 | |
| Elovich | |||
| Adsorbent | A (g mg min) | (mg g) | |
| NaTiNT−2NH | 29.20 | 0.015 | 0.9757 |
| NaTiNT−3NH | 88149.07 | 0.030 | 0.9982 |
| Adsorbent | Maximum Amount Adsorbed (mg g) | Reference |
|---|---|---|
| Magnetite nanoparticles | 74.40 | [44] |
| ZnO nanoparticles | 38.02 | [45] |
| Mesoporous activated carbon | 33.50 | [46] |
| Chitosan-Glyoxal/Kaolin Clay Composite | 284.90 | [47] |
| Magnetic chitosan-Glutaraldehyde/Zinc Oxide/Fe3O4 Nanocomposite | 179.70 | [48] |
| Marine alga “Bifurcaria bifurcata” | 88.70 | [49] |
| Mesoporous Silica Nanoparticles | 225.0 | [50] |
| Borax cross-linked Jhingan gum hydrogel | 9.88 | [51] |
| Magnesium hydroxide coated bentonite | 47.21 | [52] |
| Polypyrrole-coated magnetic nanoparticles | 112.36 | [53] |
| NaTiNT−2NH | 365.84 | This work |
| NaTiNT−3NH | 440.70 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sales, D.A.; Lima, P.N.S.; Silva, L.S.; Marques, T.M.F.; Gusmão, S.B.S.; Ferreira, O.P.; Ghosh, A.; Guerra, Y.; Morais, A.Í.S.; Bezerra, R.D.S.; et al. Amino-Functionalized Titanate Nanotubes: pH and Kinetic Study of a Promising Adsorbent for Acid Dye in Aqueous Solution. Materials 2022, 15, 6393. https://doi.org/10.3390/ma15186393
Sales DA, Lima PNS, Silva LS, Marques TMF, Gusmão SBS, Ferreira OP, Ghosh A, Guerra Y, Morais AÍS, Bezerra RDS, et al. Amino-Functionalized Titanate Nanotubes: pH and Kinetic Study of a Promising Adsorbent for Acid Dye in Aqueous Solution. Materials. 2022; 15(18):6393. https://doi.org/10.3390/ma15186393
Chicago/Turabian StyleSales, Débora A., Paloma N. S. Lima, Lucinaldo S. Silva, Thalles M. F. Marques, Suziete B. S. Gusmão, Odair P. Ferreira, Anupama Ghosh, Yuset Guerra, Alan Í. S. Morais, Roosevelt D. S. Bezerra, and et al. 2022. "Amino-Functionalized Titanate Nanotubes: pH and Kinetic Study of a Promising Adsorbent for Acid Dye in Aqueous Solution" Materials 15, no. 18: 6393. https://doi.org/10.3390/ma15186393
APA StyleSales, D. A., Lima, P. N. S., Silva, L. S., Marques, T. M. F., Gusmão, S. B. S., Ferreira, O. P., Ghosh, A., Guerra, Y., Morais, A. Í. S., Bezerra, R. D. S., Silva-Filho, E. C., & Viana, B. C. (2022). Amino-Functionalized Titanate Nanotubes: pH and Kinetic Study of a Promising Adsorbent for Acid Dye in Aqueous Solution. Materials, 15(18), 6393. https://doi.org/10.3390/ma15186393










