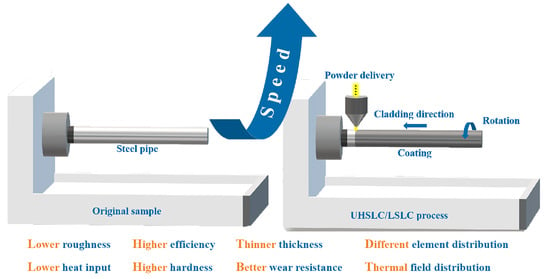
A Comparative Study on Microstructure and Properties of Ultra-High-Speed Laser Cladding and Traditional Laser Cladding of Inconel625 Coatings
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials Preparation
2.2. Laser Cladding
2.3. Characterization
3. Results and Discussion
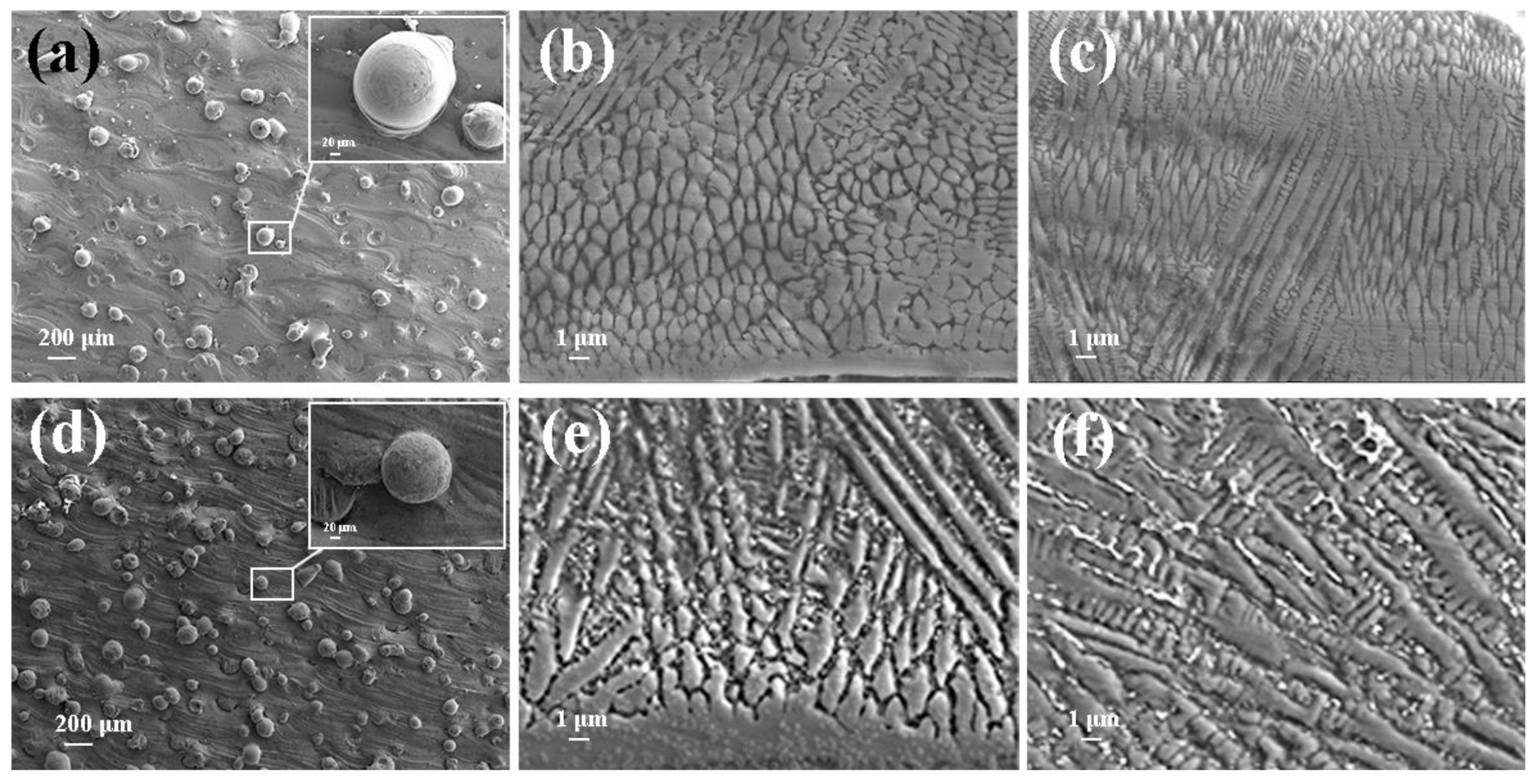
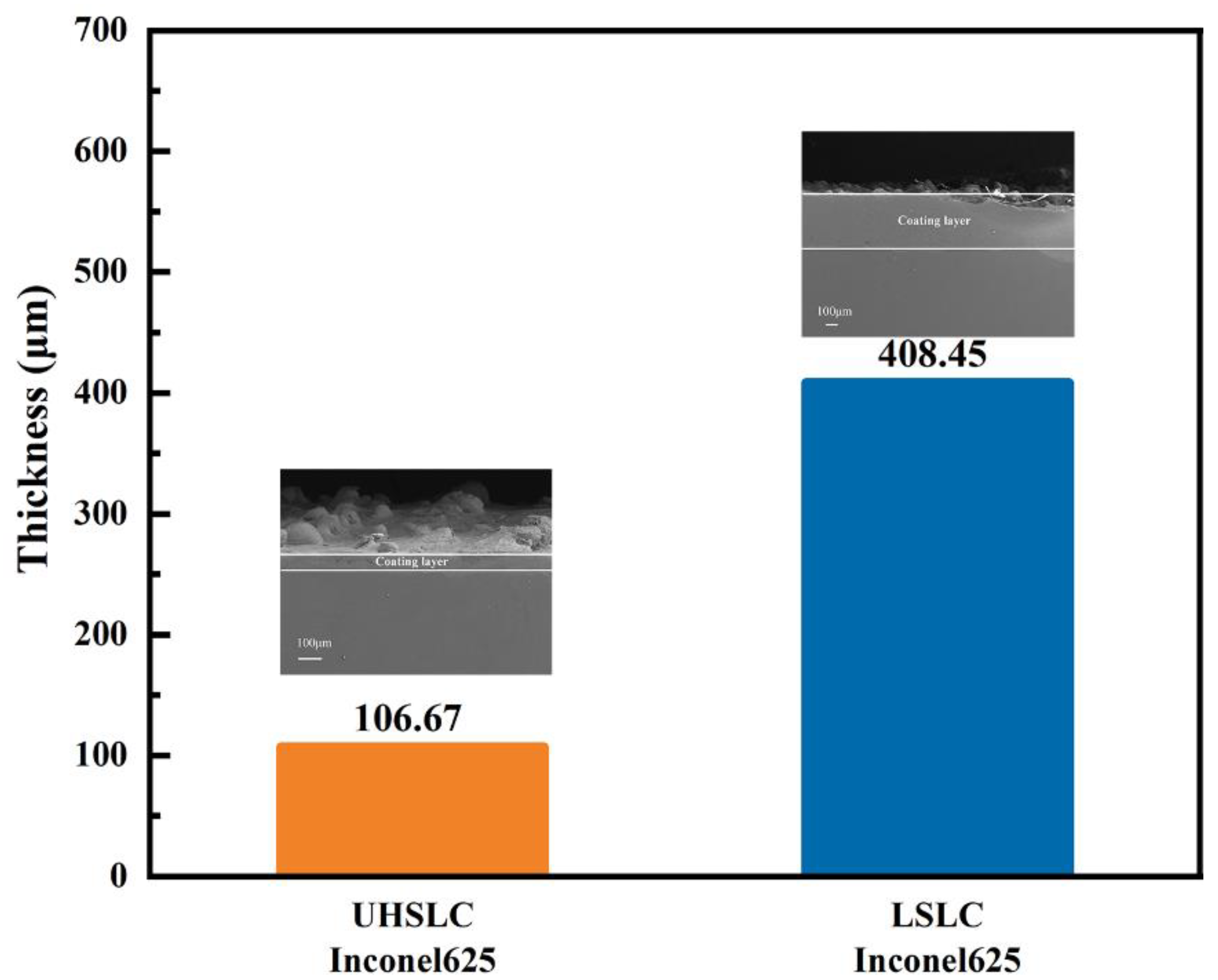
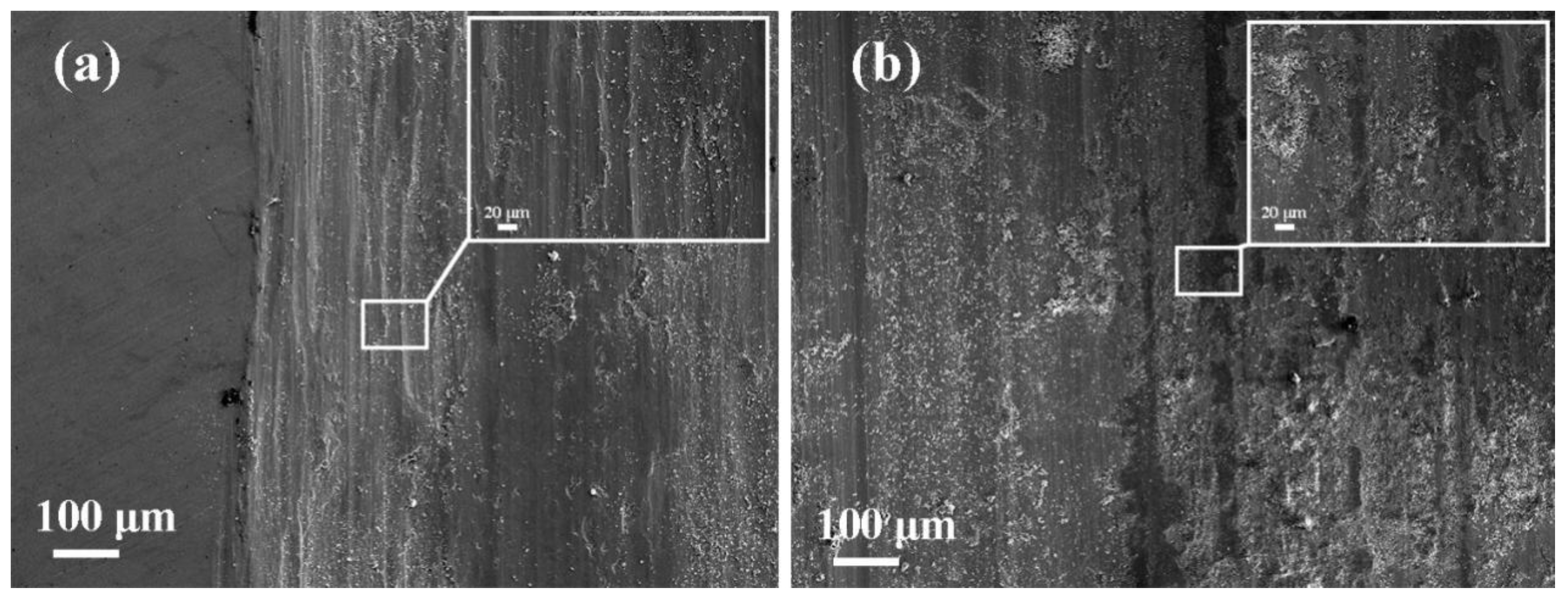
3.1. Surface and Cross-Sectional Microstructure Feature
3.2. Phase Composition Characterization
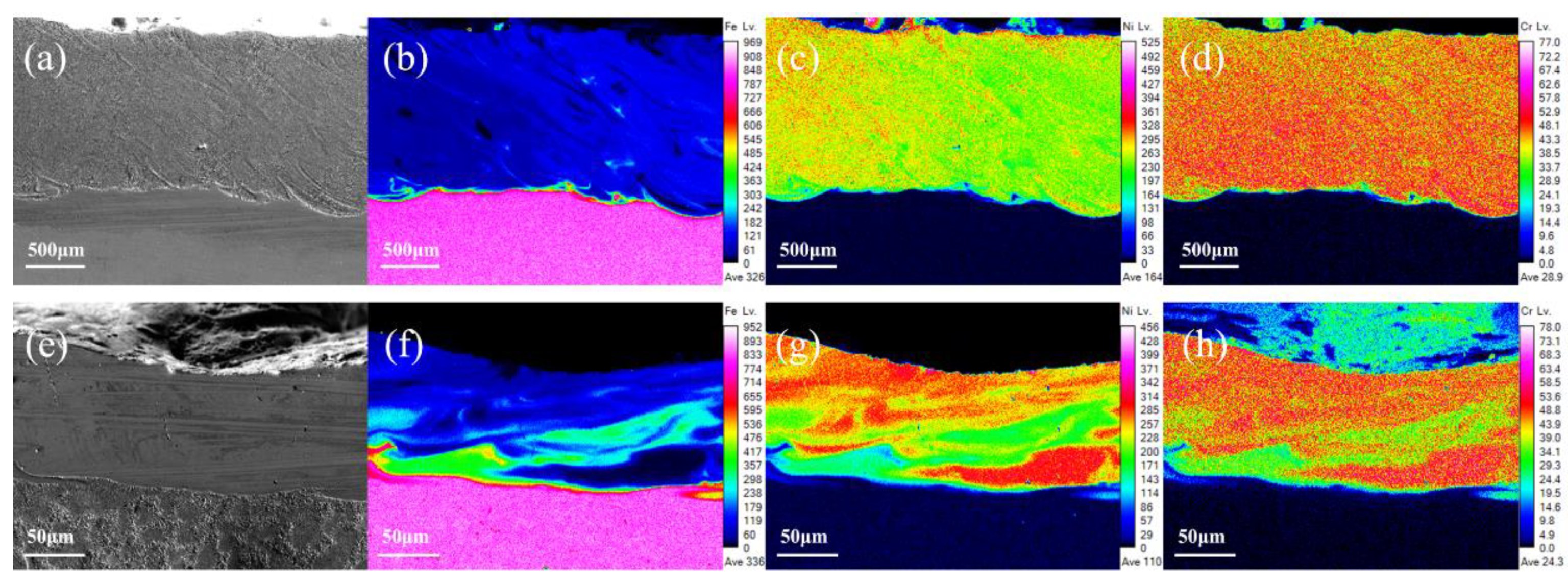
3.3. Elements Distribution of Coatings Characterization
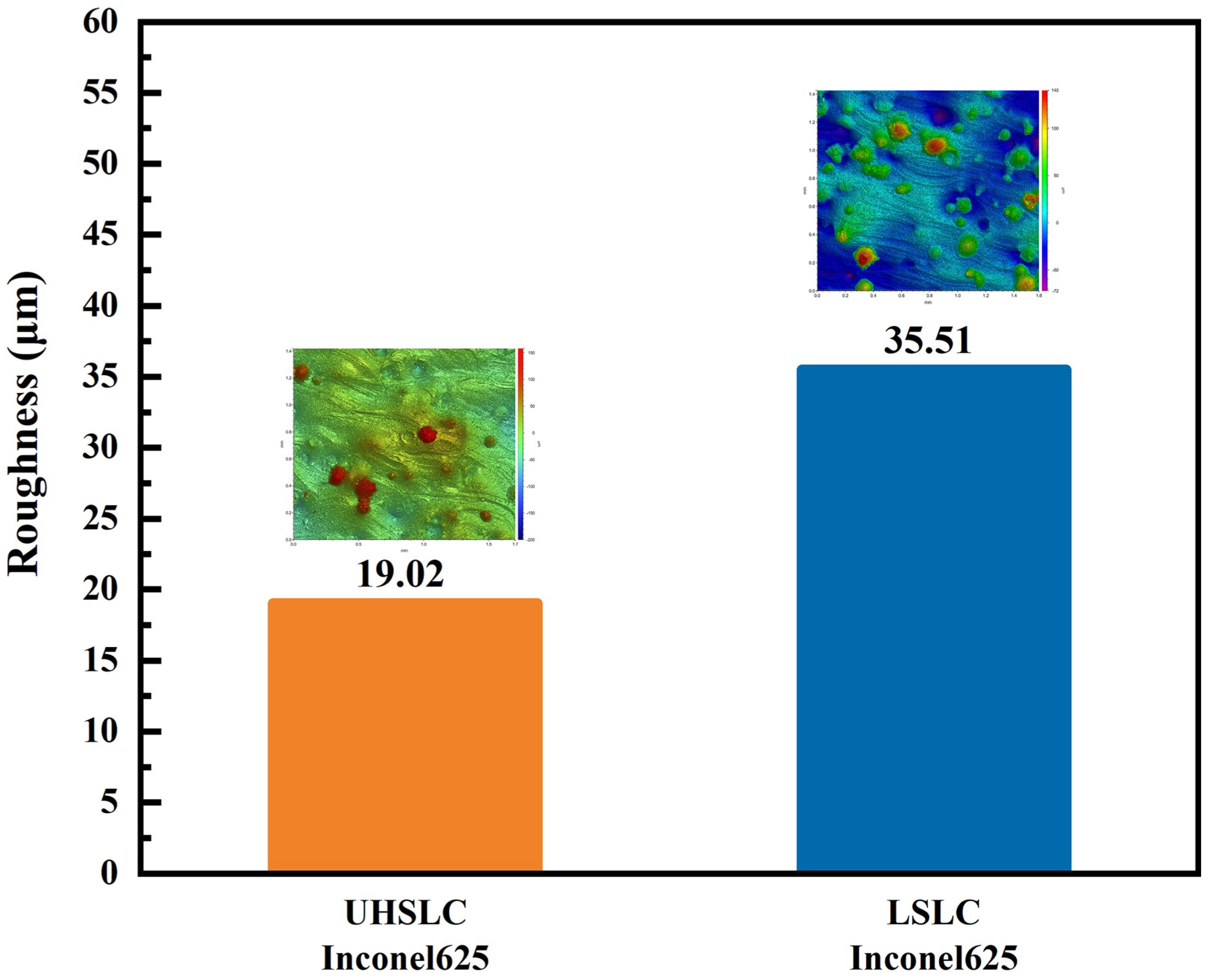
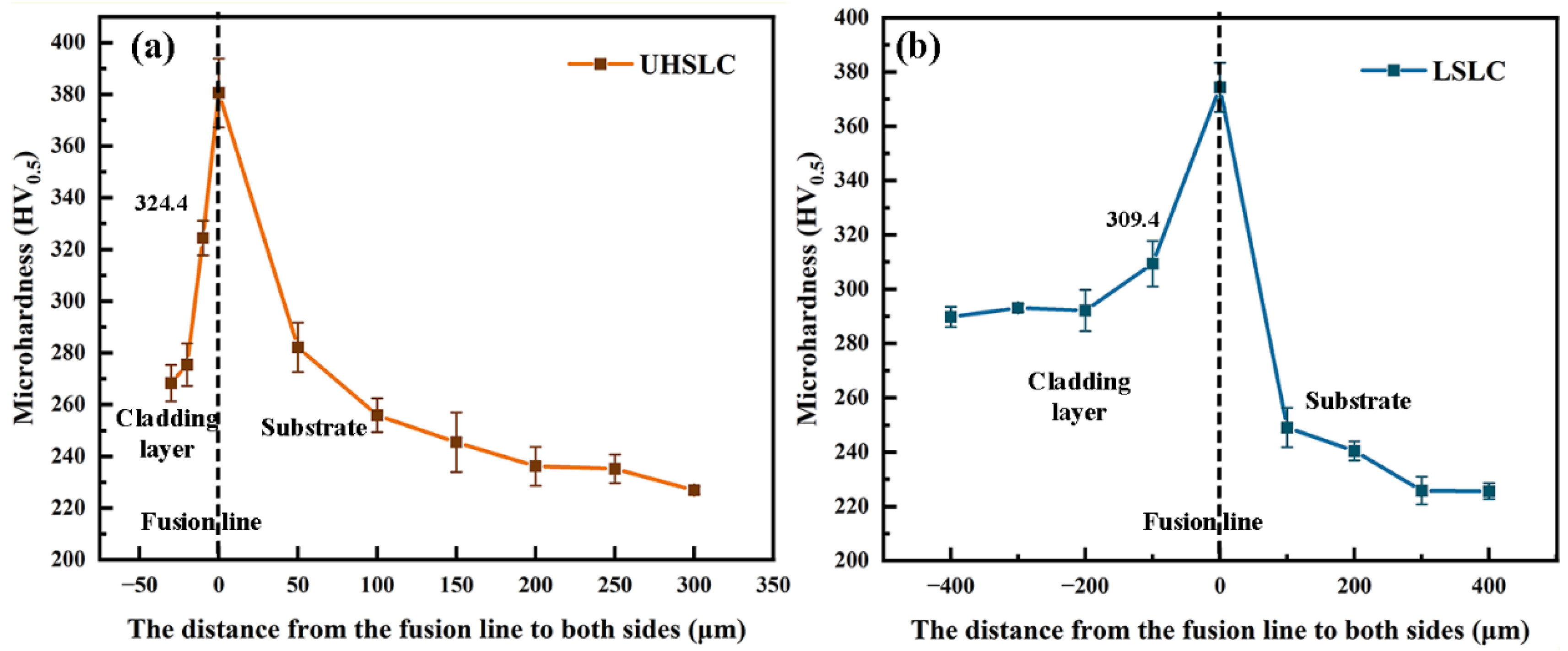
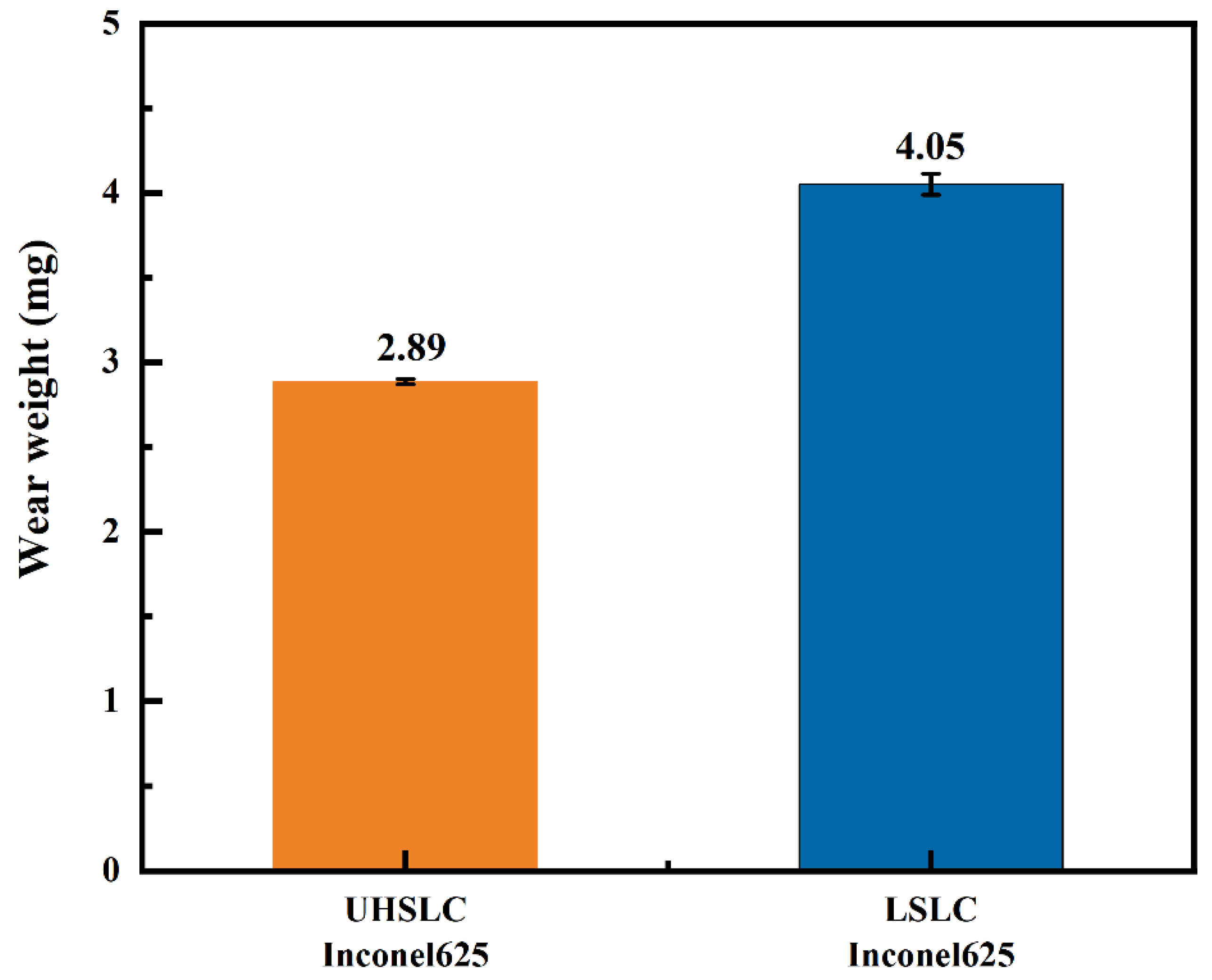
3.4. Surface Mechanical Properties
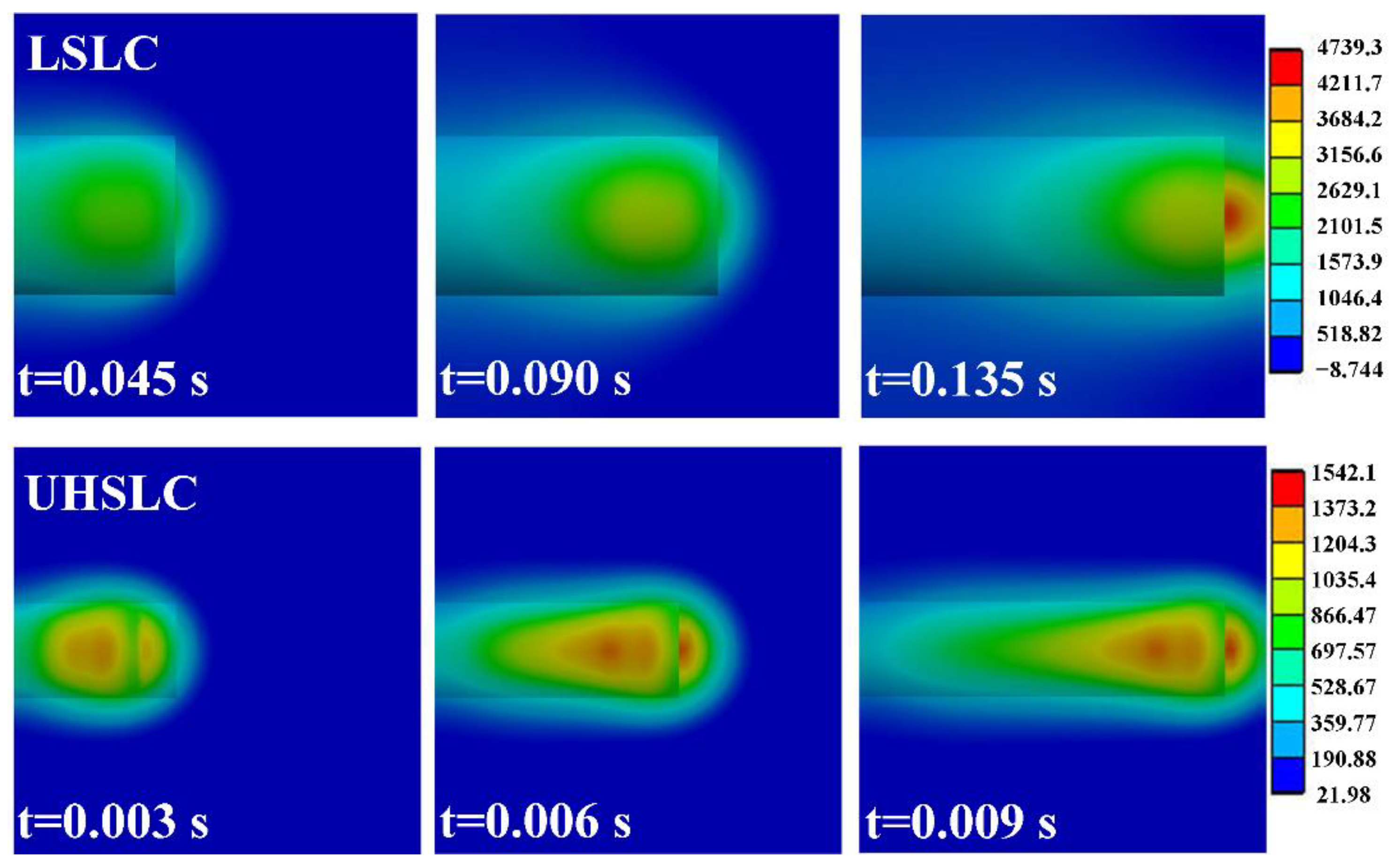
3.5. Numerical Simulation of Temperature Field in Laser Cladding Process
4. Conclusions
- (1)
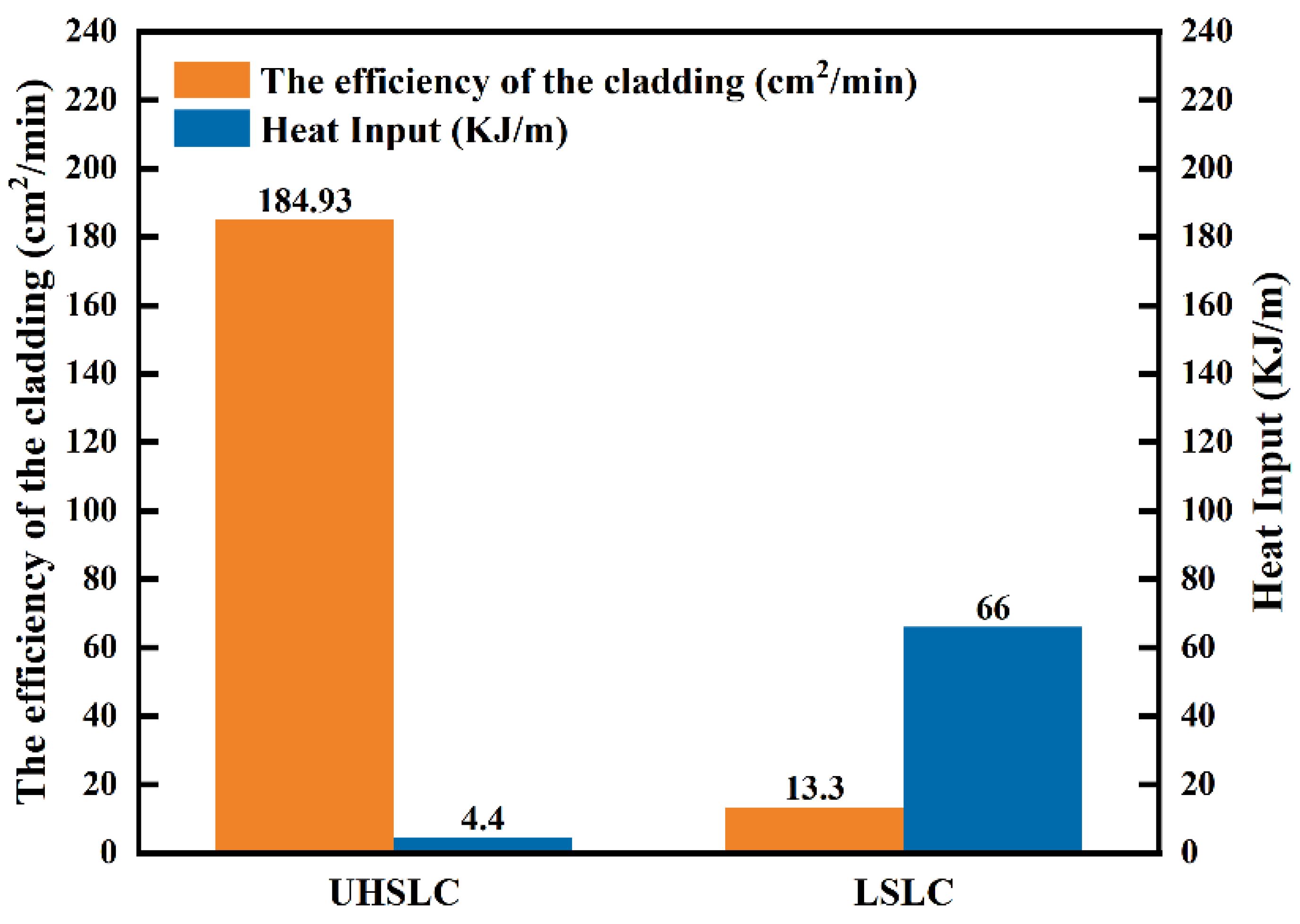
- As compared to LSLC, UHSLC Inconel625 alloy coating exhibits reduced powder sticking, roughness and coating thickness. The smoother surface and more efficient production process make UHSLC technology more suitable for practical applications;
- (2)
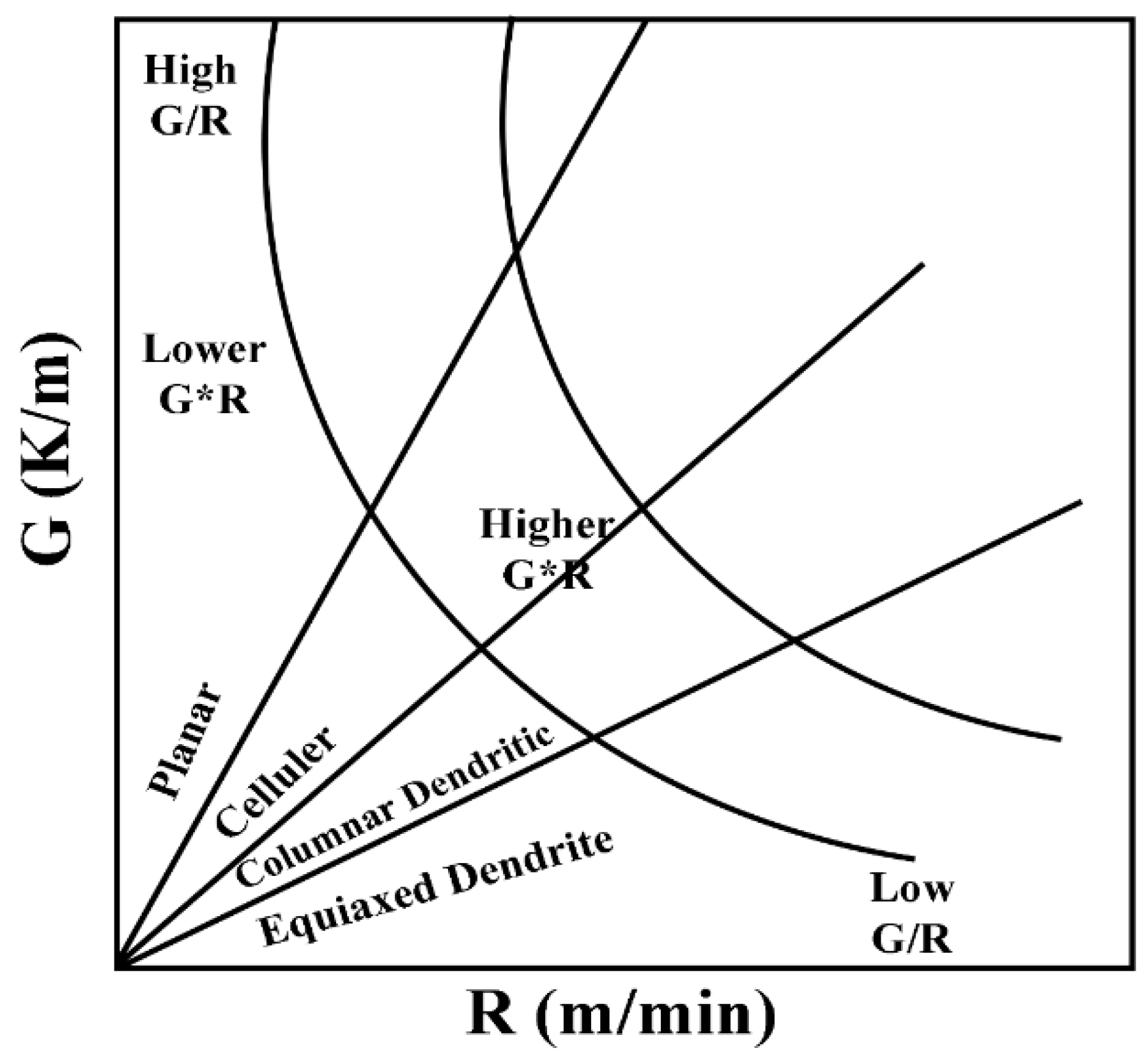
- A higher speed of cladding leads to a faster cooling rate of the coating. A high laser cladding rate increased the value of R (growth rate of dendritic crystal tips), and a high laser cladding rate increased the value of R (growth rate of dendrite tips), which resulted in a decrease in G/R (microstructure supercooling) and an equiaxed dendrite structure in the microstructure;
- (3)
- By increasing the laser cladding speed, the cladding structure is continuously refined, and coarse columnar crystals are transformed into fine dendrites. Hardness of the coating has been greatly improved, as well as wear resistance and corrosion resistance.
- (4)
- Despite the improvement in UHSLC coating performance, the melted coating elements are unevenly distributed and solidified due to the strong centrifugal effect and very high cooling rate, which should be addressed in the future;
- (5)
- A comparison of the heat input and cladding efficiency of UHSLC and LSLC was conducted. Simulations based on finite elements were conducted for the two, allowing the distributions of their temperature fields to be determined.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yanfei, C.; Chen, Y.; Fu, P.; Liu, H.; Li, D. The Experimental Characterization and Numerical Simulation of A-Segregates in 27SiMn Steel. Metall. Mater. Trans. A 2017, 48, 2260–2273. [Google Scholar] [CrossRef]
- Merlo, A.; Léonard, G. Magnetron Sputtering vs. Electrodeposition for Hard Chrome Coatings: A Comparison of Environmental and Economic Performances. Materials 2021, 14, 3823. [Google Scholar] [CrossRef] [PubMed]
- Lampa, C.; Smirnov, I. High Speed Laser Cladding of an Iron Based Alloy Developed for Hard Chrome Replacement. J. Laser Appl. 2019, 31, 022511. [Google Scholar] [CrossRef]
- Shen, F.; Tao, W.; Li, L.; Zhou, Y.; Wang, W.; Wang, S. Effect of Microstructure on the Corrosion Resistance of Coatings by Extreme High Speed Laser Cladding. Appl. Surf. Sci. 2020, 517, 146085. [Google Scholar] [CrossRef]
- Li, L.; Shen, F.; Zhou, Y.; Tao, W. Comparative Study of Stainless Steel AISI 431 Coatings Prepared by Extreme-High-Speed and Conventional Laser Cladding. J. Laser Appl. 2019, 31, 042009. [Google Scholar] [CrossRef]
- Zhu, L.; Xue, P.; Lan, Q.; Meng, G.; Ren, Y.; Yang, Z.; Xu, P.; Liu, Z. Recent Research and Development Status of Laser Cladding: A Review. Opt. Laser Technol. 2021, 138, 106915. [Google Scholar] [CrossRef]
- Chen, L.; Bai, S.-L. The Anti-Corrosion Behavior under Multi-Factor Impingement of Hastelloy C22 Coating Prepared by Multilayer Laser Cladding. Appl. Surf. Sci. 2018, 437, 1–12. [Google Scholar] [CrossRef]
- Song, B.; Voisey, K.T.; Hussain, T. High Temperature Chlorine-Induced Corrosion of Ni50Cr Coating: HVOLF, HVOGF, Cold Spray and Laser Cladding. Surf. Coat. Technol. 2018, 337, 357–369. [Google Scholar] [CrossRef]
- Feng, K.; Chen, Y.; Deng, P.; Li, Y.; Zhao, H.; Lu, F.; Li, R.; Huang, J.; Li, Z. Improved High-Temperature Hardness and Wear Resistance of Inconel 625 Coatings Fabricated by Laser Cladding. J. Mater. Process. Technol. 2017, 243, 82–91. [Google Scholar] [CrossRef]
- Ren, Y.; Li, L.; Zhou, Y.; Wang, S. In Situ Synthesized VC Reinforced Fe-Based Coating by Using Extreme High-Speed Laser Cladding. Mater. Lett. 2022, 315, 131962. [Google Scholar] [CrossRef]
- Schopphoven, T.; Gasser, A.; Wissenbach, K.; Poprawe, R. Investigations on Ultra-High-Speed Laser Material Deposition as Alternative for Hard Chrome Plating and Thermal Spraying. J. Laser Appl. 2016, 28, 022501. [Google Scholar] [CrossRef]
- Schopphoven, T.; Gasser, A.; Backes, G. EHLA: Extreme High-Speed Laser Material Deposition. Laser Tech. J. 2017, 14, 45. [Google Scholar] [CrossRef]
- Liu, M.; Jiang, H.; Chang, G.; Xu, Y.; Ma, F.; Xu, K. Effect of Laser Remelting on Corrosion and Wear Resistance of Fe82Cr16SiB Alloy Coatings Fabricated by Extreme High-Speed Laser Cladding. Mater. Lett. 2022, 325, 132823. [Google Scholar] [CrossRef]
- Ding, Y.; Du, C.; Wang, X.; Zhang, B. Microstructure and Interfacial Metallurgical Bonding of 1Cr17Ni2/Carbon Steel Extreme High-Speed Laser Cladding Coating. Adv. Compos. Hybrid Mater. 2021, 4, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Li, R.; Chen, Z.; Gu, J.; Tian, Y. A Comparative Study on Microstructure and Properties of Traditional Laser Cladding and High-Speed Laser Cladding of Ni45 Alloy Coatings. Surf. Coat. Technol. 2021, 405, 126582. [Google Scholar] [CrossRef]
- Ge, T.; Chen, L.; Gu, P.; Ren, X.; Chen, X. Microstructure and Corrosion Resistance of TiC/Inconel 625 Composite Coatings by Extreme High Speed Laser Cladding. Opt. Laser Technol. 2022, 150, 107919. [Google Scholar] [CrossRef]
- Du, C.; Hu, L.; Ren, X.; Li, Y.; Zhang, F.; Liu, P.; Li, Y. Cracking Mechanism of Brittle FeCoNiCrAl HEA Coating Using Extreme High-Speed Laser Cladding. Surf. Coat. Technol. 2021, 424, 127617. [Google Scholar] [CrossRef]
- Vilar, R. Laser Cladding. J. Laser Appl. 1999, 11, 64–79. [Google Scholar] [CrossRef]
- Lu, J.Z.; Cao, J.; Lu, H.F.; Zhang, L.Y.; Luo, K.Y. Wear Properties and Microstructural Analyses of Fe-Based Coatings with Various WC Contents on H13 Die Steel by Laser Cladding. Surf. Coat. Technol. 2019, 369, 228–237. [Google Scholar] [CrossRef]
- Xiao, Q.; Sun, W.L.; Yang, K.X.; Xing, X.F.; Chen, Z.H.; Zhou, H.N.; Lu, J. Wear Mechanisms and Micro-Evaluation on WC Particles Investigation of WC-Fe Composite Coatings Fabricated by Laser Cladding. Surf. Coat. Technol. 2021, 420, 127341. [Google Scholar] [CrossRef]
- Bartkowski, D.; Bartkowska, A.; Jurči, P. Laser Cladding Process of Fe/WC Metal Matrix Composite Coatings on Low Carbon Steel Using Yb: YAG Disk Laser. Opt. Laser Technol. 2021, 136, 106784. [Google Scholar] [CrossRef]
- Ouyang, C.; Bai, Q.; Yan, X.; Chen, Z.; Han, B.; Liu, Y. Microstructure and Corrosion Properties of Laser Cladding Fe-Based Alloy Coating on 27SiMn Steel Surface. Coatings 2021, 11, 552. [Google Scholar] [CrossRef]
- Wang, Q.; Qian, R.; Yang, J.; Niu, W.; Zhou, L.; Pan, X.; Su, C. Effect of High-Speed Powder Feeding on Microstructure and Tribological Properties of Fe-Based Coatings by Laser Cladding. Coatings 2021, 11, 1456. [Google Scholar] [CrossRef]
- Bai, Q.; Ouyang, C.; Zhao, C.; Han, B.; Liu, Y. Microstructure and Wear Resistance of Laser Cladding of Fe-Based Alloy Coatings in Different Areas of Cladding Layer. Materials 2021, 14, 2839. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Zhuang, S.-G.; Liu, X.-B.; OuYang, C.-S.; Zhu, Y.; Meng, Y. Microstructure Evolution and High-Temperature Tribological Behavior of Ti3SiC2 Reinforced Ni60 Composite Coatings on 304 Stainless Steel by Laser Cladding. Surf. Coat. Technol. 2021, 420, 127335. [Google Scholar] [CrossRef]
- Gui, W.; Zhong, C.; Gu, J.; Ding, Y.; Wang, X.; Wu, T.; Liang, Y.; Qin, J.; Qu, Y.; Lin, J. Laser-Clad Inconel 625 Coatings on Q245R Structure Steel: Microstructure, Wear and Corrosion Resistance. NPJ Mater. Degrad. 2022, 6, 37. [Google Scholar] [CrossRef]
- Scendo, M.; Staszewska-Samson, K.; Danielewski, H. Corrosion Behavior of Inconel 625 Coating Produced by Laser Cladding. Coatings 2021, 11, 759. [Google Scholar] [CrossRef]
- Yan, Q.; Yang, K.; Wang, Z.; Chen, M.; Sun, G.; Ni, Z. Surface Roughness Optimization and High-Temperature Wear Performance of H13 Coating Fabricated by Extreme High-Speed Laser Cladding. Opt. Laser Technol. 2022, 149, 107823. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, X.; Wu, Y.; Chen, C.; Li, Y.; Zhou, W.; Ren, X. Effect of Surface Morphology and Microstructure on the Hot Corrosion Behavior of TiC/IN625 Coatings Prepared by Extreme High-Speed Laser Cladding. Corros. Sci. 2022, 201, 110271. [Google Scholar] [CrossRef]
- Kou, S. Welding Metallurgy. MRS Bull. 2003, 28, 674–675. [Google Scholar] [CrossRef] [Green Version]
- Hao, J.; Hu, F.; Le, X.; Liu, H.; Yang, H.; Han, J. Microstructure and High-Temperature Wear Behaviour of Inconel 625 Multi-Layer Cladding Prepared on H13 Mould Steel by a Hybrid Additive Manufacturing Method. J. Mater. Process. Technol. 2021, 291, 117036. [Google Scholar] [CrossRef]
- Meng, L.; Sheng, P.; Zeng, X. Comparative Studies on the Ni60 Coatings Deposited by Conventional and Induction Heating Assisted Extreme-High-Speed Laser Cladding Technology: Formability, Microstructure and Hardness. J. Mater. Res. Technol. 2022, 16, 1732–1746. [Google Scholar] [CrossRef]
- Wu, Z.; Qian, M.; Brandt, M.; Matthews, N. Ultra-High-Speed Laser Cladding of Stellite® 6 Alloy on Mild Steel. JOM 2020, 72, 4632–4638. [Google Scholar] [CrossRef]
- Abioye, T.E.; McCartney, D.G.; Clare, A.T. Laser Cladding of Inconel 625 Wire for Corrosion Protection. J. Mater. Process. Technol. 2015, 217, 232–240. [Google Scholar] [CrossRef]
- Xu, X.; Lu, H.; Su, Y.; Peng, M.; Xing, F.; Luo, K.; Lu, J. Comparing Corrosion Behavior of Additively Manufactured Cr-Rich Stainless Steel Coating between Conventional and Extreme High-Speed Laser Metal Deposition. Corros. Sci. 2022, 195, 109976. [Google Scholar] [CrossRef]
- Salaheldeen, M.; Garcia-Gomez, A.; Ipatov, M.; Corte-Leon, P.; Zhukova, V.; Blanco, J.M.; Zhukov, A. Fabrication and Magneto-Structural Properties of Co2-Based Heusler Alloy Glass-Coated Microwires with High Curie Temperature. Chemosensors 2022, 10, 225. [Google Scholar] [CrossRef]
- Saleem, M.; Fang, L.; Ruan, H.B.; Wu, F.; Huang, Q.L.; Xu, C.L.; Kong, C.Y. Effect of Zinc Acetate Concentration on the Structural and Optical Properties of ZnO Thin Films Deposited by Sol-Gel Method. IJPS 2012, 7, 2971–2979. [Google Scholar] [CrossRef]
- Bindu, P.; Thomas, S. Estimation of Lattice Strain in ZnO Nanoparticles: X-Ray Peak Profile Analysis. J. Theor. Appl. Phys. 2014, 8, 123–134. [Google Scholar] [CrossRef]
- Iida, T.; Guthrie, R. The Physical Properties of Liquid Metals; Clarendon Press: Oxford, UK, 1988. [Google Scholar]
- Huang, R.; Zhang, Q.; Zhang, X.; Li, J.; Cao, T.; Yao, J.; Xue, Y.; Gao, H.; Li, X. Dynamic Recrystallization-Induced Temperature Insensitivity of Yield Stress in Single-Crystal Al1.2CrFeCoNi Micropillars. Sci. China Technol. Sci. 2021, 64, 11–22. [Google Scholar] [CrossRef]
- Chen, J.; Dong, J.; Zhang, M.; Yao, Z. Deformation Mechanisms in a Fine-Grained Udimet 720LI Nickel-Base Superalloy with High Volume Fractions of Γ′ Phases. Mater. Sci. Eng. A 2016, 673, 122–134. [Google Scholar] [CrossRef]
- Li, R.; Yuan, W.; Yue, H.; Zhu, Y. Study on Microstructure and Properties of Fe-Based Amorphous Composite Coating by High-Speed Laser Cladding. Opt. Laser Technol. 2022, 146, 107574. [Google Scholar] [CrossRef]
- Qian, S.; Dai, Y.; Guo, Y.; Zhang, Y. Microstructure and Wear Resistance of Multi-Layer Ni-Based Alloy Cladding Coating on 316L SS under Different Laser Power. Materials 2021, 14, 781. [Google Scholar] [CrossRef]
- Singh, R. 2-Physics of Welding. In Applied Welding Engineering, 3rd ed.; Singh, R., Ed.; Butterworth-Heinemann: Oxford, UK, 2020; pp. 125–155. ISBN 978-0-12-821348-3. [Google Scholar]
- Choi, J.; Han, L.; Hua, Y. Modeling and Experiments of Laser Cladding With Droplet Injection. J. Heat Transf. 2005, 127, 978–986. [Google Scholar] [CrossRef]
- Qiao, X.; Xia, T.; Chen, P. Numerical Research on Effect of Overlap Ratio on Thermal-Stress Behaviors of the High-Speed Laser Cladding Coating. Chin. Phys. B 2021, 30, 018104. [Google Scholar] [CrossRef]
- Ya, W.; Pathiraj, B.; Liu, S. 2D Modelling of Clad Geometry and Resulting Thermal Cycles during Laser Cladding. J. Mater. Process. Technol. 2016, 230, 217–232. [Google Scholar] [CrossRef]
- Huang, Y.; Khamesee, M.B.; Toyserkani, E. A Comprehensive Analytical Model for Laser Powder-Fed Additive Manufacturing. Addit. Manuf. 2016, 12, 90–99. [Google Scholar] [CrossRef]
- Khamidullin, B.A.; Tsivilskiy, I.V.; Gorunov, A.I.; Gilmutdinov, A.K. Modeling of the Effect of Powder Parameters on Laser Cladding Using Coaxial Nozzle. Surf. Coat. Technol. 2019, 364, 430–443. [Google Scholar] [CrossRef]
- Zhong, C.; Kittel, J.; Gasser, A.; Schleifenbaum, J.H. Study of Nickel-Based Super-Alloys Inconel 718 and Inconel 625 in High-Deposition-Rate Laser Metal Deposition. Opt. Laser Technol. 2019, 109, 352–360. [Google Scholar] [CrossRef]
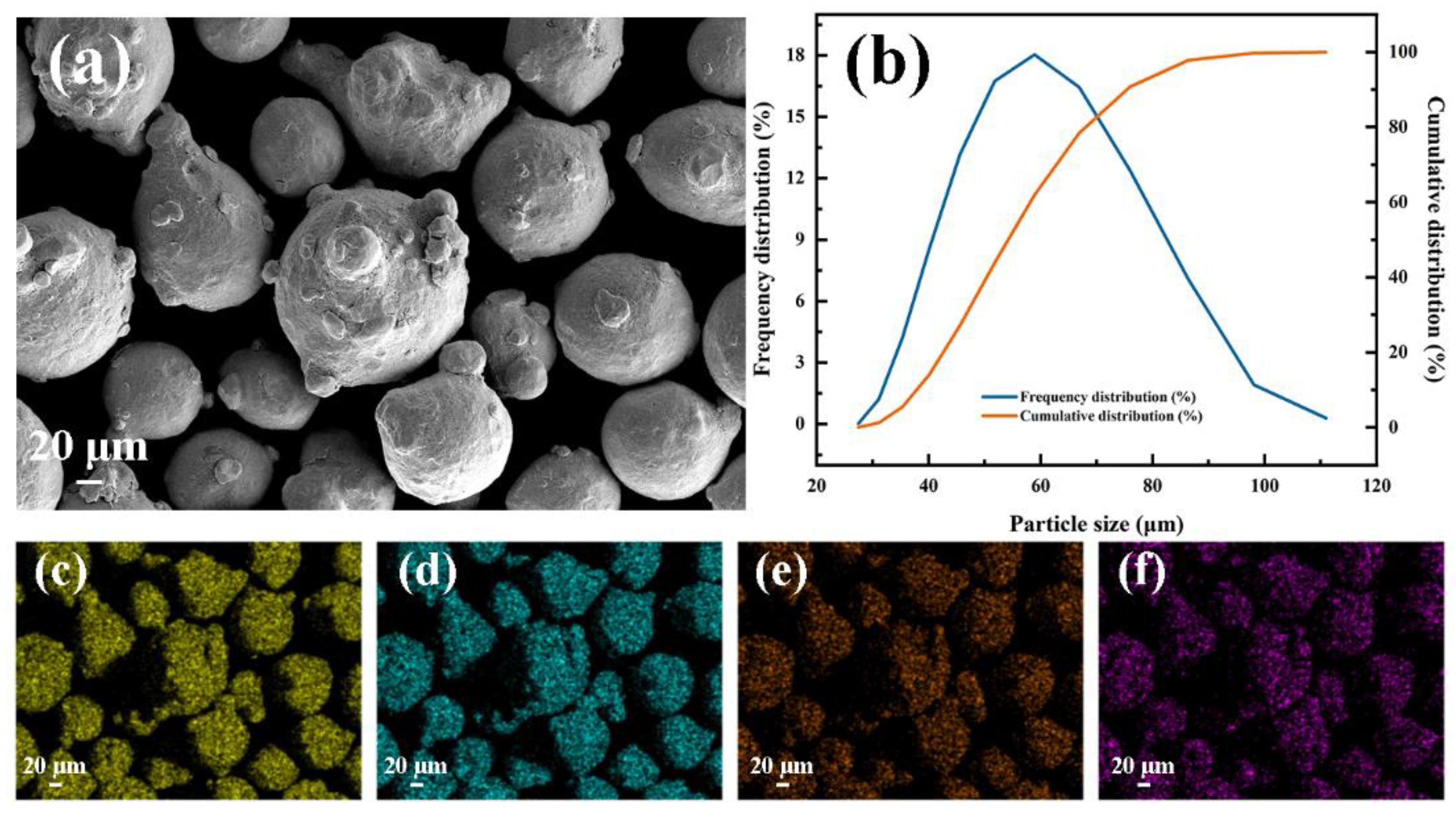
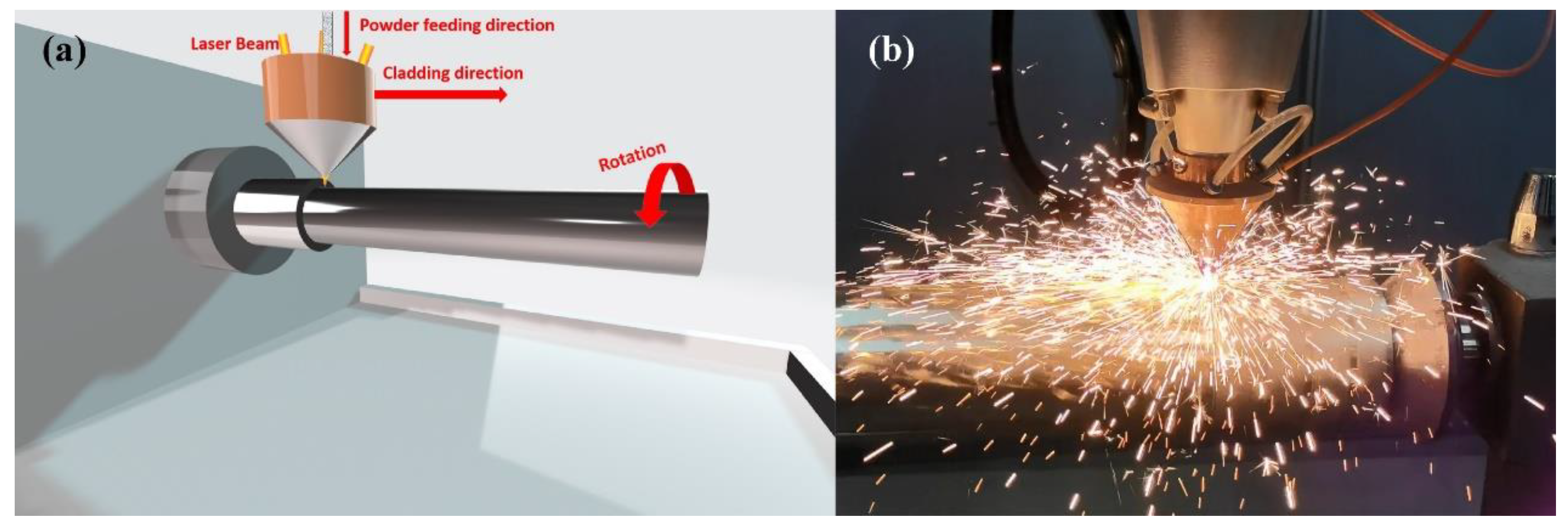



| Material | Cr | C | Mn | Ni | Si | Mo | Fe | V | Co | Nb | Cu | Sd | Sn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 27SiMn | <0.30 | 0.24–0.32 | 1.01 | <0.30 | 0.96 | Bal | |||||||
| Inconel625 | 21.87 | 0.39 | 63.35 | 9.5 | 0.75 | 0.06 | 3.64 | 0.32 | 0.023 | 0.05 |
| Form | Laser Power (W) | Cladding Rate (m/min) | Powder Feeding Speed (g/min) | Overlap Rate |
|---|---|---|---|---|
| UHSLC LSLC | 2200 | 30 | 32.3 | 85% |
| 2200 | 2 | 32.3 | 85% |
| Form | Peak Position 2θ (°) | FWHM (°) | Crystallite Size (nm) | Dislocation Density δ × 10−3 (nm−2) | a (Å) |
|---|---|---|---|---|---|
| UHSLC LSLC | 50.84 | 0.41 | 21.58 | 2.15 | 3.59 |
| 43.62 | 0.34 | 25.33 | 1.56 | 3.11 |
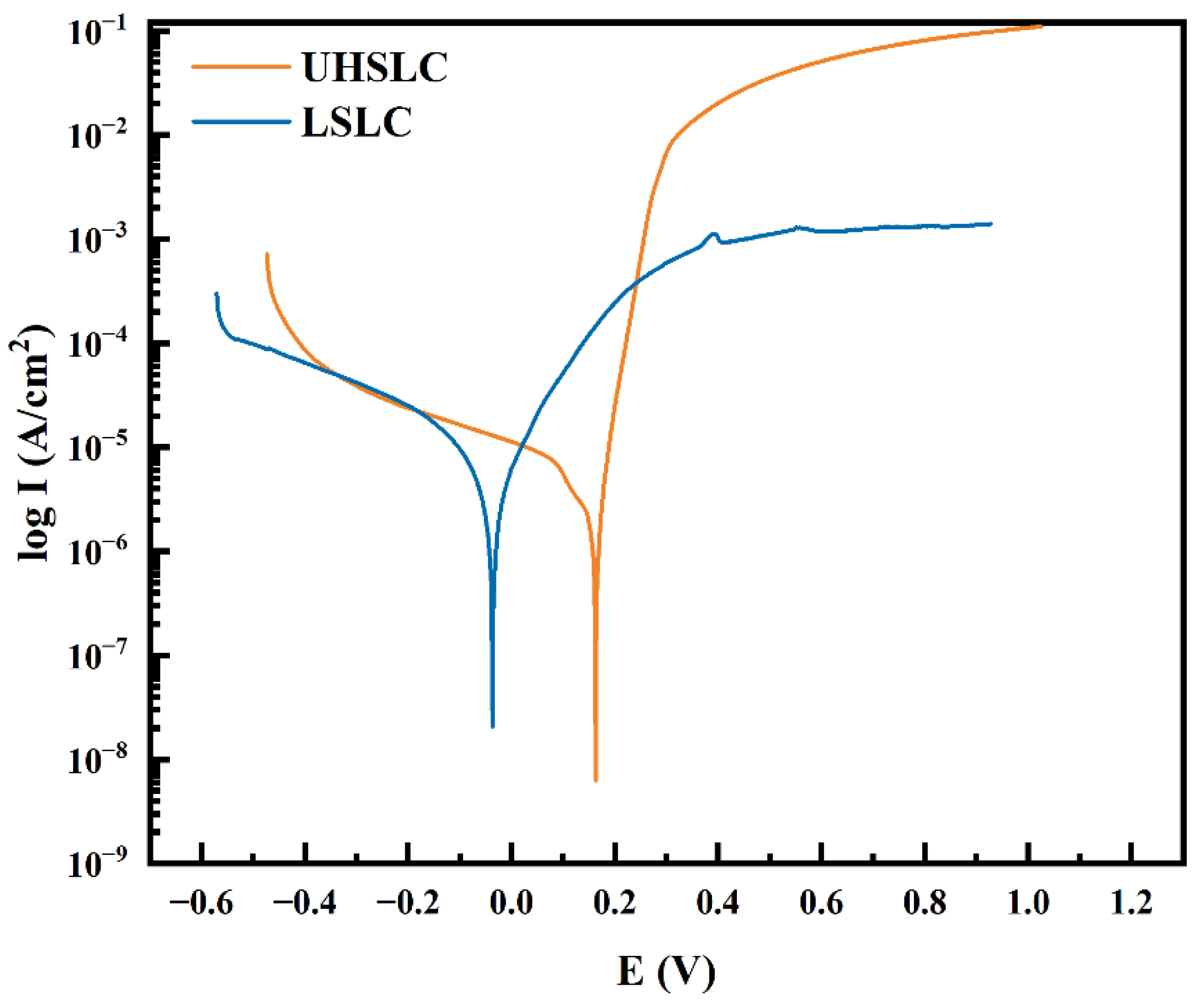
| Form | Corrosion Current Density (A/cm2) | Self-Corrosion Potential (V) | CR (mm/year) |
|---|---|---|---|
| UHSLC | 1.99 × 10−6 | 0.19 | 2.09 × 10−2 |
| LSLC | 2.64 × 10−6 | −0.04 | 2.78 × 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Bi, W.; Zhong, C.; Wu, T.; Gui, W. A Comparative Study on Microstructure and Properties of Ultra-High-Speed Laser Cladding and Traditional Laser Cladding of Inconel625 Coatings. Materials 2022, 15, 6400. https://doi.org/10.3390/ma15186400
Ding Y, Bi W, Zhong C, Wu T, Gui W. A Comparative Study on Microstructure and Properties of Ultra-High-Speed Laser Cladding and Traditional Laser Cladding of Inconel625 Coatings. Materials. 2022; 15(18):6400. https://doi.org/10.3390/ma15186400
Chicago/Turabian StyleDing, Yuhang, Wenya Bi, Cheng Zhong, Tao Wu, and Wanyuan Gui. 2022. "A Comparative Study on Microstructure and Properties of Ultra-High-Speed Laser Cladding and Traditional Laser Cladding of Inconel625 Coatings" Materials 15, no. 18: 6400. https://doi.org/10.3390/ma15186400
APA StyleDing, Y., Bi, W., Zhong, C., Wu, T., & Gui, W. (2022). A Comparative Study on Microstructure and Properties of Ultra-High-Speed Laser Cladding and Traditional Laser Cladding of Inconel625 Coatings. Materials, 15(18), 6400. https://doi.org/10.3390/ma15186400