A Study of Early-Stage Corrosion Behavior of AZ91 Alloy and MAO-Coated Alloy in 3.5% NaCl Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Experimental Techniques
3. Results
3.1. Microstructure
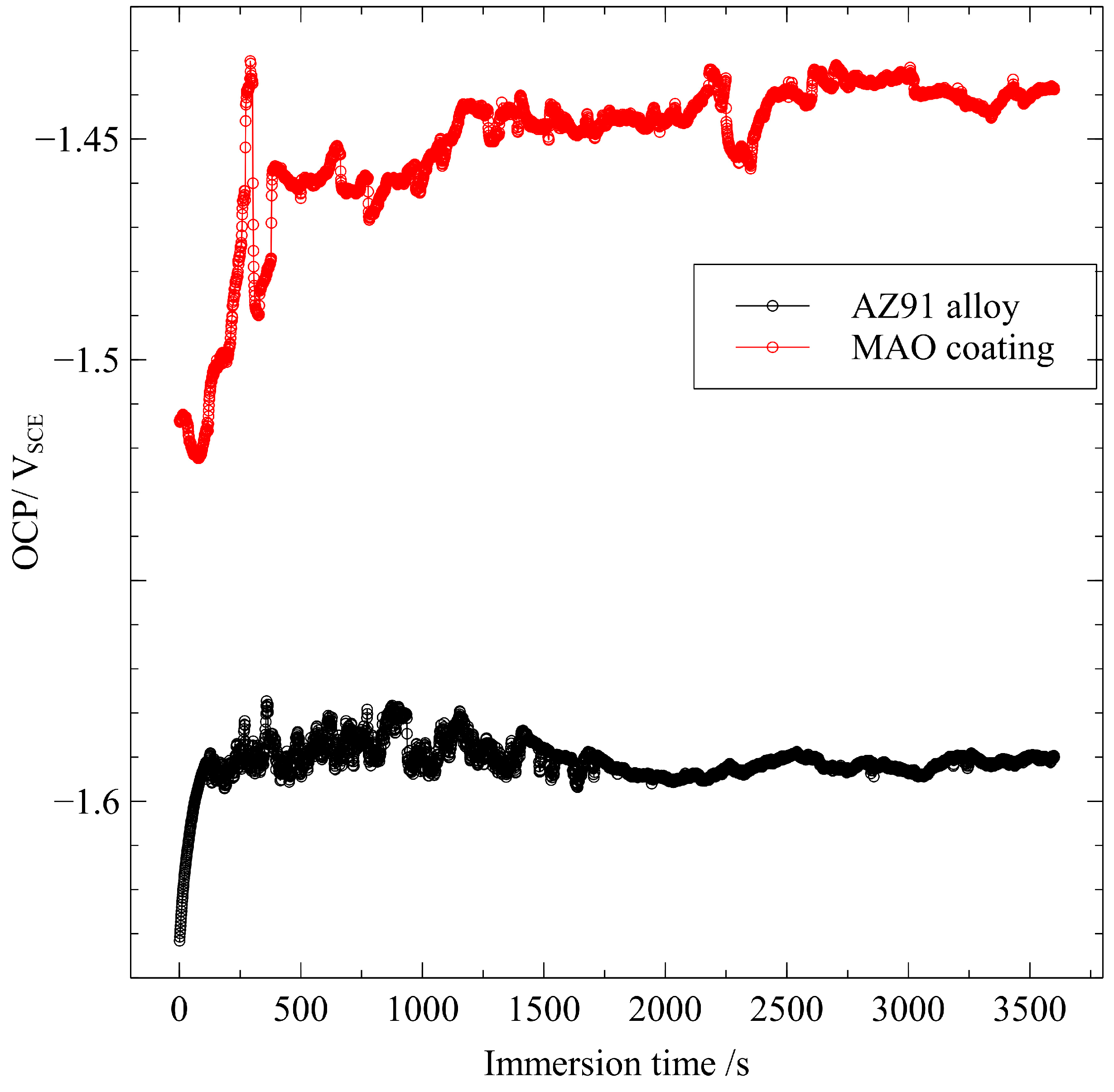
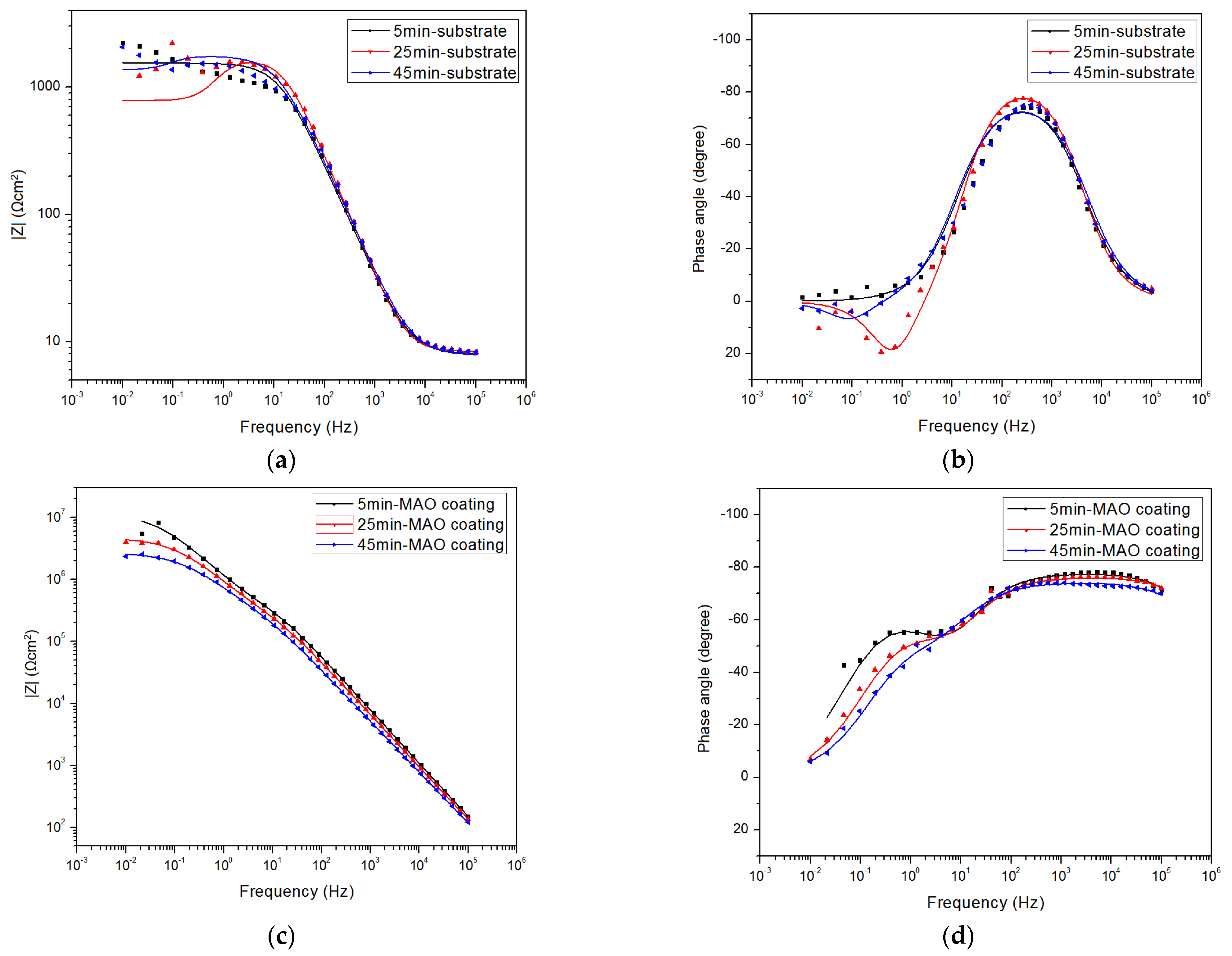
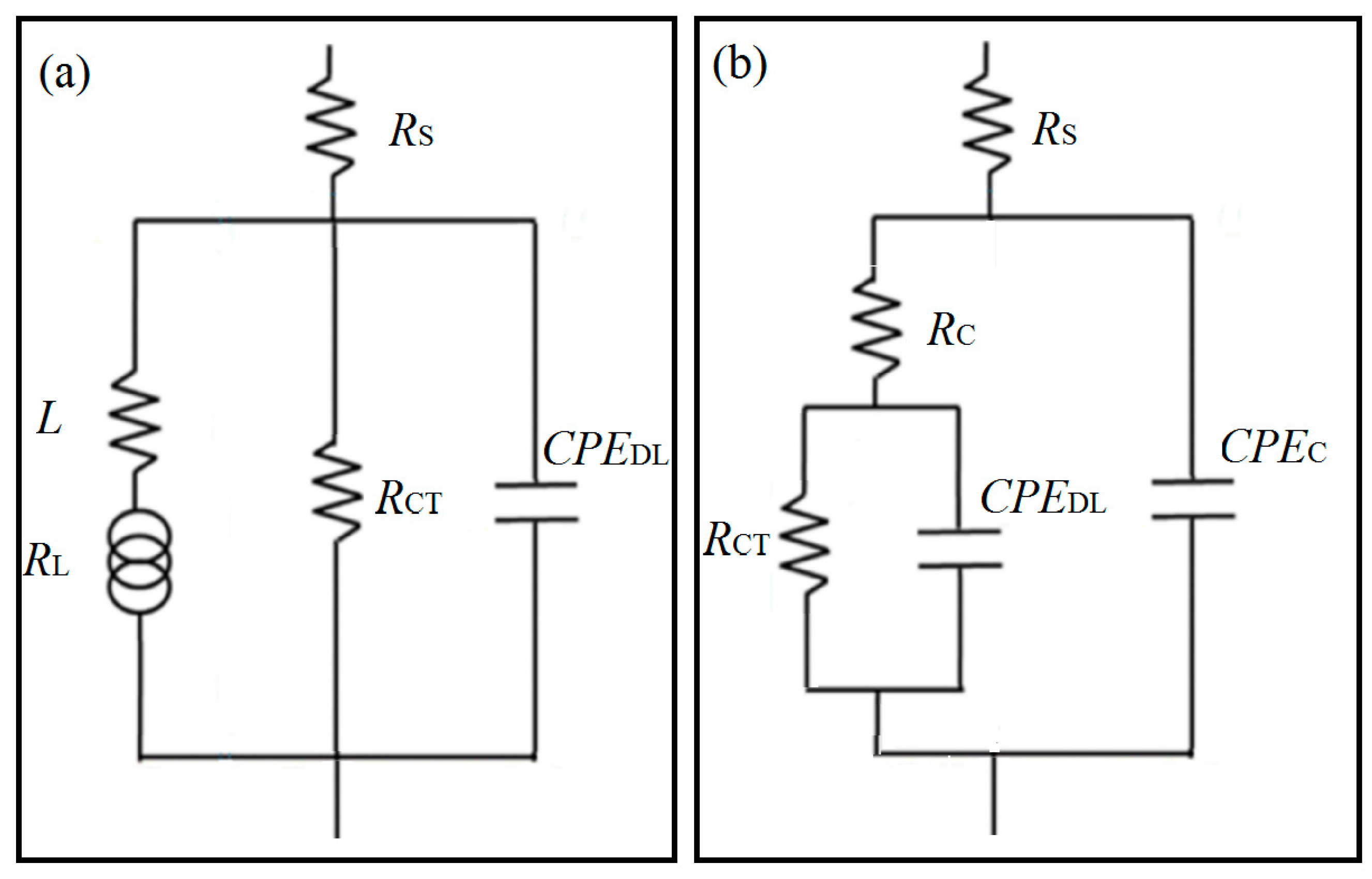
3.2. Electrochemical Measurements
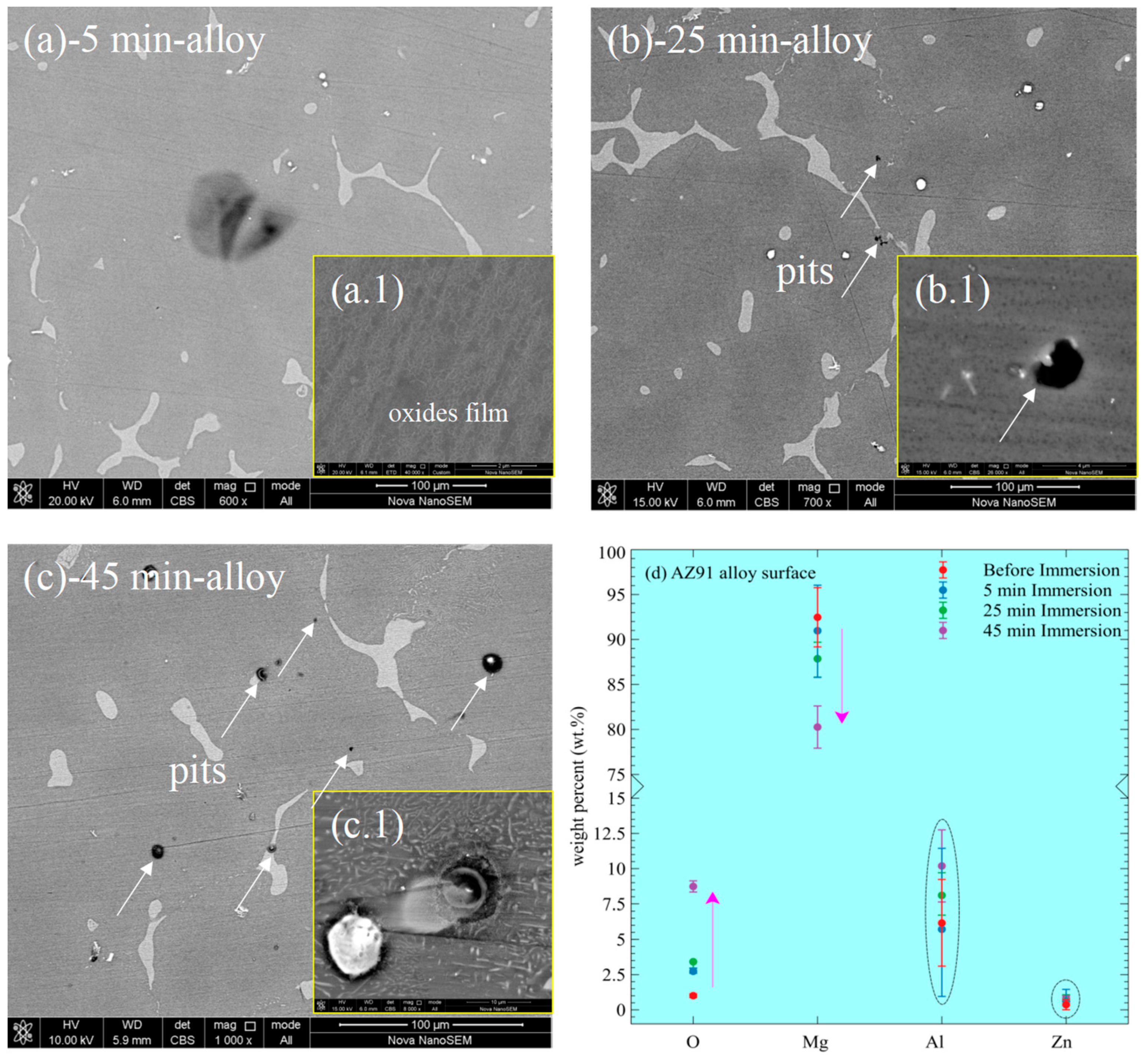
3.3. Evolution of Corrosion Morphology and Corrosion Products
4. Discussion
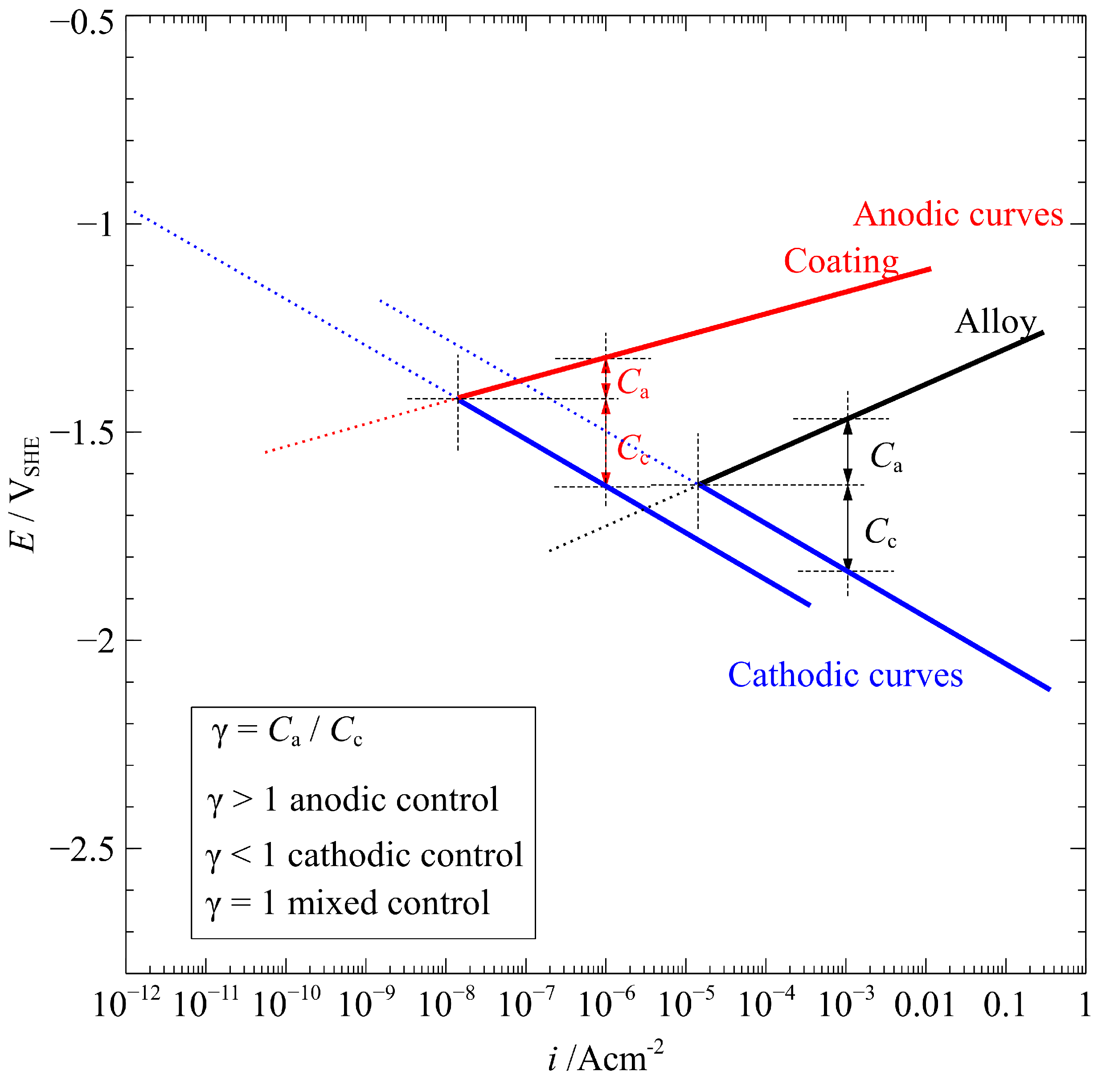
4.1. Corrosion Rate
4.2. Corrosion Behavior
4.2.1. Mg-Based Alloys
4.2.2. MAO-Coated Alloy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- AhadiParsa, M.; Mohammadloo, H.E.; Mirabedini, S.M.; Roshan, S. Bio-corrosion assessment and surface study of hydroxyapatite-coated AZ31 Mg alloy pre-treated with vinyl tri-ethoxy silane. Mater. Chem. Phys. 2022, 287, 126147. [Google Scholar] [CrossRef]
- Ocal, E.B.; Esen, Z.; Aydinol, K.; Dericioğlu, A.F. Comparison of the short and long-term degradation behaviors of as-cast pure Mg, AZ91 and WE43 alloys. Mater. Chem. Phys. 2021, 241, 112350. [Google Scholar]
- Song, G.-L. Corrosion and Protection of Magnesium Alloys: An Overview of Research Undertaken by CAST. Mater. Sci. Forum 2005, 488–489, 649–652. [Google Scholar] [CrossRef]
- Jin, Z.Z.; Zha, M.; Wang, S.Q.; Wang, S.C.; Wang, C.; Jia, H.L.; Wang, H.Y. Alloying design and microstructural control strategies towards developing Mg alloys with enhanced ductility. J. Magensium Alloy. 2022, 10, 1191–1206. [Google Scholar] [CrossRef]
- McCafferty, E. Introduction to Corrosion Science; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Miyake, M.; Fujii, H.; Hirato, T. Electroplating of Al on Mg alloy in a dimethyl sulfone–aluminum chloride bath. Surf. Coat. Technol. 2015, 277, 160–164. [Google Scholar] [CrossRef]
- Liu, Y.X.; Liu, Z.; Xu, A.Y.; Liu, X. Understanding pitting corrosion behavior of AZ91 alloy and its MAO coating in 3.5% NaCl solution by cyclic potentiodynamic polarization. J. Magensium Alloy. 2022, 10, 1368–1380. [Google Scholar] [CrossRef]
- Qin, B.D.; Zhou, S.G.; Chen, H.; Wang, M. Superior corrosion and wear resistance of AZ91D Mg alloy via electrodeposited SiO2-Ni-based composite coating. Mater. Chem. Phys. 2022, 283, 126001. [Google Scholar] [CrossRef]
- Ly, X.; Yang, S.; Nguyen, T. Effect of equal channel angular pressing as the pretreatment on microstructure and corrosion behavior of micro-arc oxidation (MAO) composite coating on biodegradable Mg-Zn-Ca alloy. Surf. Coat. Technol. 2020, 395, 125923. [Google Scholar] [CrossRef]
- Dou, J.; Wang, C.; Gu, G.; Chen, C. Formation of silicon-calcium-phosphate-containing coating on Mg-Zn-Ca alloy by a two-step micro-arc oxidation technique. Mater. Lett. 2018, 212, 37–40. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Yarmand, B.; Sharifi-Asl, S.; Mozafari, M. Improved corrosion performance of biodegradable magnesium in simulated inflammatory condition via drug-loaded plasma electrolytic oxidation coatings. Mater. Chem. Phys. 2020, 239, 122003. [Google Scholar] [CrossRef]
- Yao, W.H.; Wu, L.; Wang, J.F.; Jiang, B.; Zhang, D.; Serdechnova, M.; Shulha, T.; Blawert, C.; Zheludkevich, M.L.; Pan, F. Micro-arc oxidation of magnesium alloys: A review. J. Mater. Sci. Technol. 2022, 118, 158–180. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, T.; Yu, X.; Sun, X.; Yang, H. Functionalization treatment of micro-arc oxidation coatings on magnesium alloys: A review. J. Alloys Compd. 2021, 879, 160453. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, N.; Ling, N.; Zhang, J.; Wang, L. Enhanced long-term corrosion resistance of Mg alloys by superhydrophobic and self-healing composite coating. Chem. Eng. J. 2018, 449, 137778. [Google Scholar] [CrossRef]
- Liu, S.; Li, G.; Qi, Y.; Peng, Z.; Ye, Y.; Liang, J. Corrosion and tribocorrosion resistance of MAO-based composite coating on AZ31 magnesium alloy. J. Magnes. Alloys, 2021, in press. [CrossRef]
- Jiang, D.; Zhou, H.; Wan, S.; Cai, G.-Y.; Dong, Z.-H. Fabrication of superhydrophobic coating on magnesium alloy with improved corrosion resistance by combining micro-arc oxidation and cyclic assembly. Surf. Coat. Technol. 2018, 339, 155–166. [Google Scholar] [CrossRef]
- Luo, D.; Liu, Y.; Yin, X.; Wang, H.; Han, Z.; Ren, L. Corrosion inhibition of hydrophobic coatings fabricated by micro-arc oxidation on an extruded Mg–5Sn–1Zn alloy substrate. J. Alloys Compd. 2018, 731, 731–738. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A.; John, D.S.; Wu, X.; Nairn, J. The anodic dissolution of magnesium in chloride and sulphate solutions. Corros. Sci. 1997, 39, 1981–2004. [Google Scholar] [CrossRef]
- Curioni, M.; Salamone, L.; Scenini, F.; Santamaria, M.; Di Natale, M. A mathematical description accounting for the superfluous hydrogen evolution and the inductive behaviour observed during electrochemical measurements on magnesium. Electrochim. Acta 2018, 274, 343–352. [Google Scholar] [CrossRef]
- Birbilis, N.; King, A.D.; Thomas, S.; Frankel, G.S.; Scully, J.R. Evidence for enhanced catalytic activity of magnesium arising from anodic dissolution. Electrochim. Acta 2014, 132, 277–283. [Google Scholar] [CrossRef]
- Curioni, M. The behaviour of magnesium during free corrosion and potentiodynamic polarization investigated by real-time hydrogen measurement and optical imaging. Electrochim. Acta 2014, 120, 284–292. [Google Scholar] [CrossRef]
- Taheri, M.; Kish, J.R.; Birbilis, N.; Danaie, M.; McNally, E.A.; McDermid, J.R. Towards a Physical Description for the Origin of Enhanced Catalytic Activity of Corroding Magnesium Surfaces. Electrochim. Acta 2014, 116, 396–403. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Hussein, R.O.; Zhang, P.; Nie, X.; Xia, Y.; Northwood, D.O. The effect of current mode and discharge type on the corrosion resistance of plasma electrolytic oxidation (PEO) coated magnesium alloy AJ62. Surf. Coat. Technol. 2011, 206, 1990–1997. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, M.; Atrens, A. Measurement of the corrosion rate of magnesium alloys using Tafel extrapolation. Corros. Sci. 2010, 52, 579–588. [Google Scholar] [CrossRef]
- Huang, J.; Song, G.-L.; Atrens, A.; Dargusch, M. What activates the Mg surface—A comparison of Mg dissolution mechanisms. J. Mater. Sci. Technol. 2020, 57, 204–220. [Google Scholar] [CrossRef]
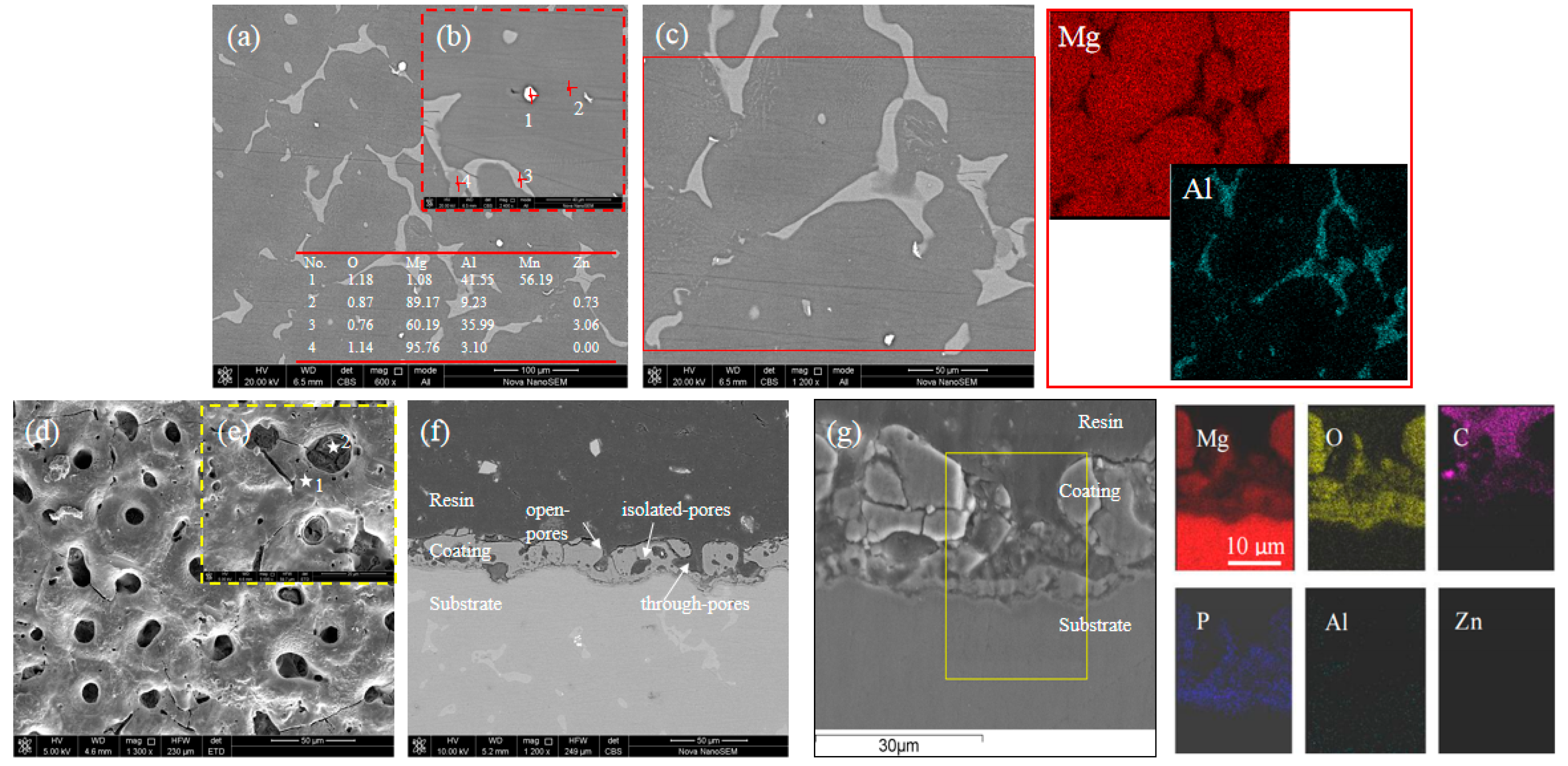
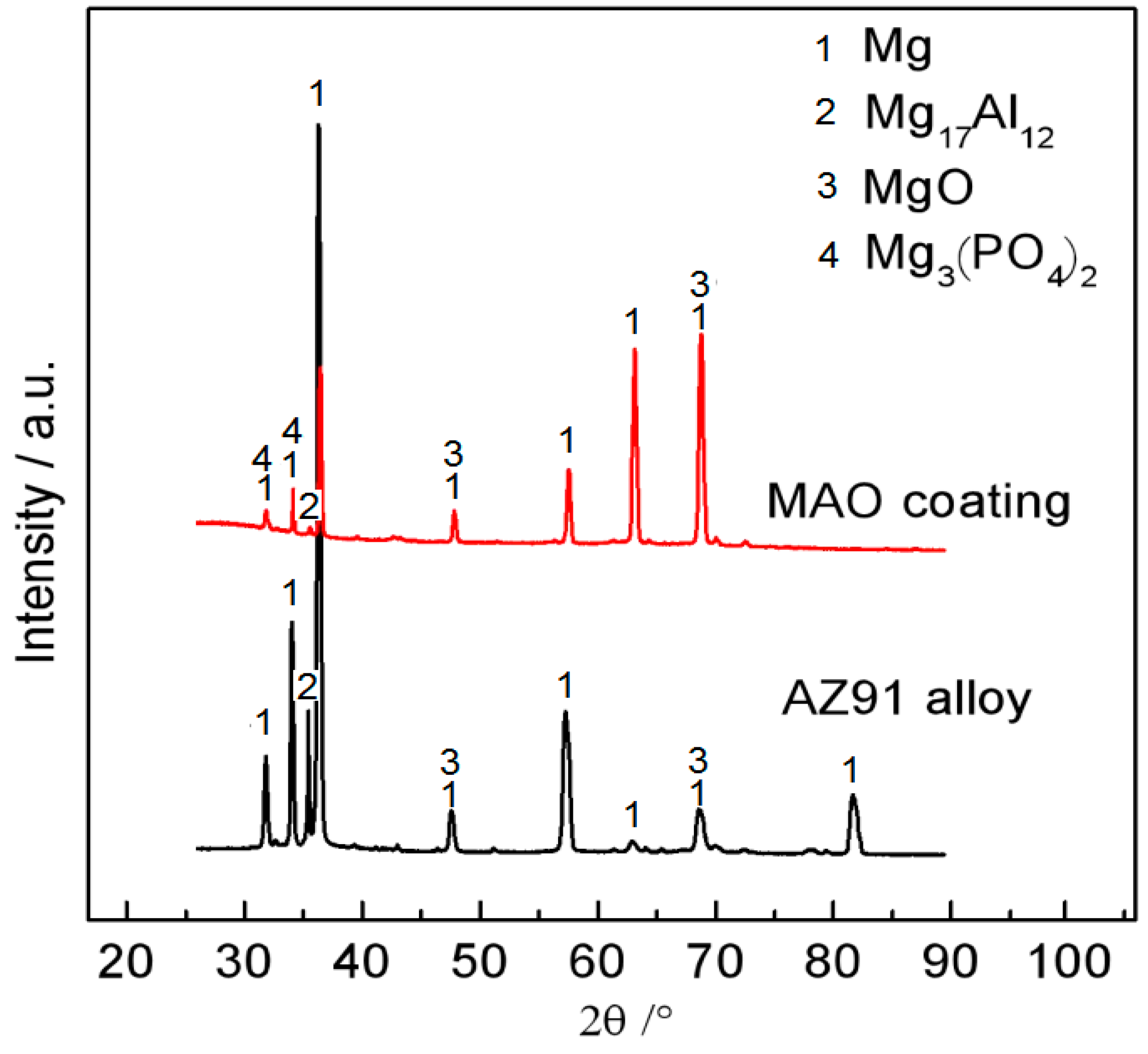



| Al | Zn | Mn | Si | Cu | Ni | Fe | Mg |
|---|---|---|---|---|---|---|---|
| 8.5–9.3 | 0.4–1.0 | 0.15 | 0.1 | 0.03 | 0.002 | 0.005 | Bal. |
| t/min | RCT (Ω cm2) | RL (Ω cm2) | RC (Ω cm2) | RZF (Ω cm2) | ||
|---|---|---|---|---|---|---|
| Alloy | Coating | Alloy | Coating | Alloy | Coating | |
| 5 | 1650 (10.6) | 1.100 × 107 (27.9) | 2.706 × 105 (33.9) | 9.500 × 105 (39.1) | 1640 (13.6) | 1.195 × 107 (49.3) |
| 25 | 1710 (14.1) | 1.193 × 106 (38.9) | 1501 (12.6) | 6.280 × 105 (43.8) | 799.3 (6.62) | 4.821 × 106 (28.6) |
| 45 | 1830 (22.3) | 2.174 × 106 (21.4) | 6542 (36.2) | 5.820 × 105 (42.4) | 1430 (27.4) | 2.756 × 106 (39.2) |
| t/min | 5 | 15 | 25 | 35 | 45 | 60 |
|---|---|---|---|---|---|---|
| [Mg2+] | 6.502 × 10−6 | 6.8313 × 10−6 | 7.2428 × 10−6 | 7.8601 × 10−6 | 8.0247 × 10−6 | 8.7243 × 10−6 |
| pH | 7.04 | 7.11 | 7.12 | 7.24 | 7.38 | 7.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, X. A Study of Early-Stage Corrosion Behavior of AZ91 Alloy and MAO-Coated Alloy in 3.5% NaCl Solutions. Materials 2022, 15, 7909. https://doi.org/10.3390/ma15227909
Liu Y, Liu X. A Study of Early-Stage Corrosion Behavior of AZ91 Alloy and MAO-Coated Alloy in 3.5% NaCl Solutions. Materials. 2022; 15(22):7909. https://doi.org/10.3390/ma15227909
Chicago/Turabian StyleLiu, Yuxiang, and Xiaoting Liu. 2022. "A Study of Early-Stage Corrosion Behavior of AZ91 Alloy and MAO-Coated Alloy in 3.5% NaCl Solutions" Materials 15, no. 22: 7909. https://doi.org/10.3390/ma15227909
APA StyleLiu, Y., & Liu, X. (2022). A Study of Early-Stage Corrosion Behavior of AZ91 Alloy and MAO-Coated Alloy in 3.5% NaCl Solutions. Materials, 15(22), 7909. https://doi.org/10.3390/ma15227909






