Material and Process Tests of Heterogeneous Membranes Containing ZIF-8, SiO2 and POSS-Ph
Abstract
:1. Introduction
2. Materials and Methods
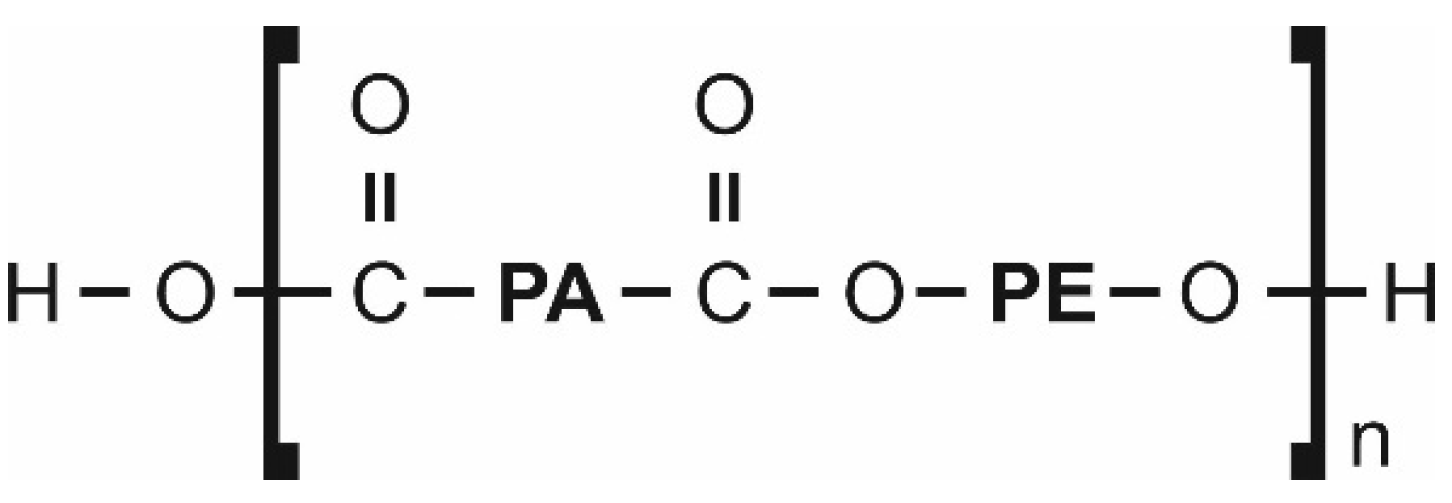
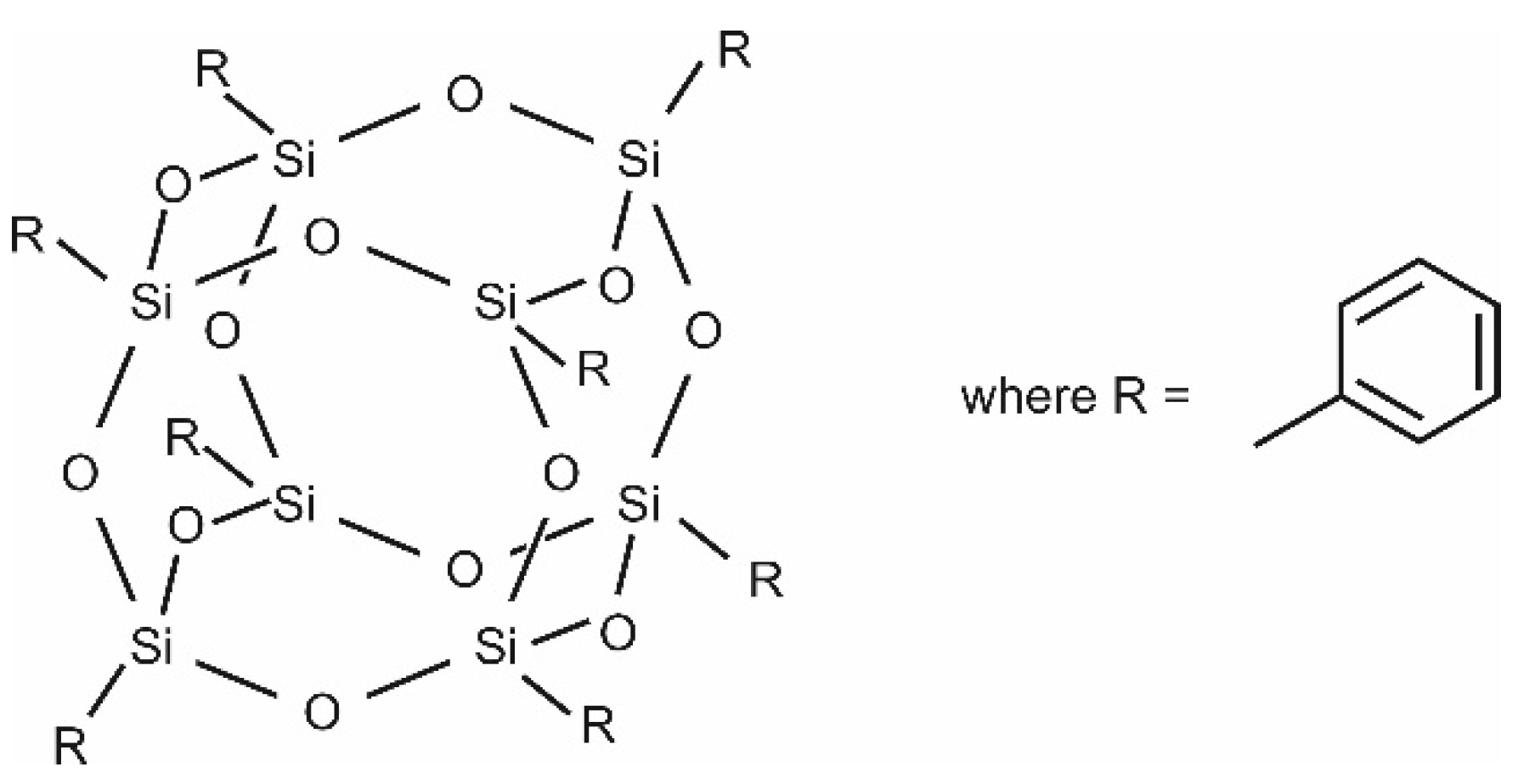
2.1. Polymer and Fillers
2.2. Membrane Preparation
2.3. Analytical Techniques
3. Results and Discussions
3.1. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Spectroscopy (EDX)
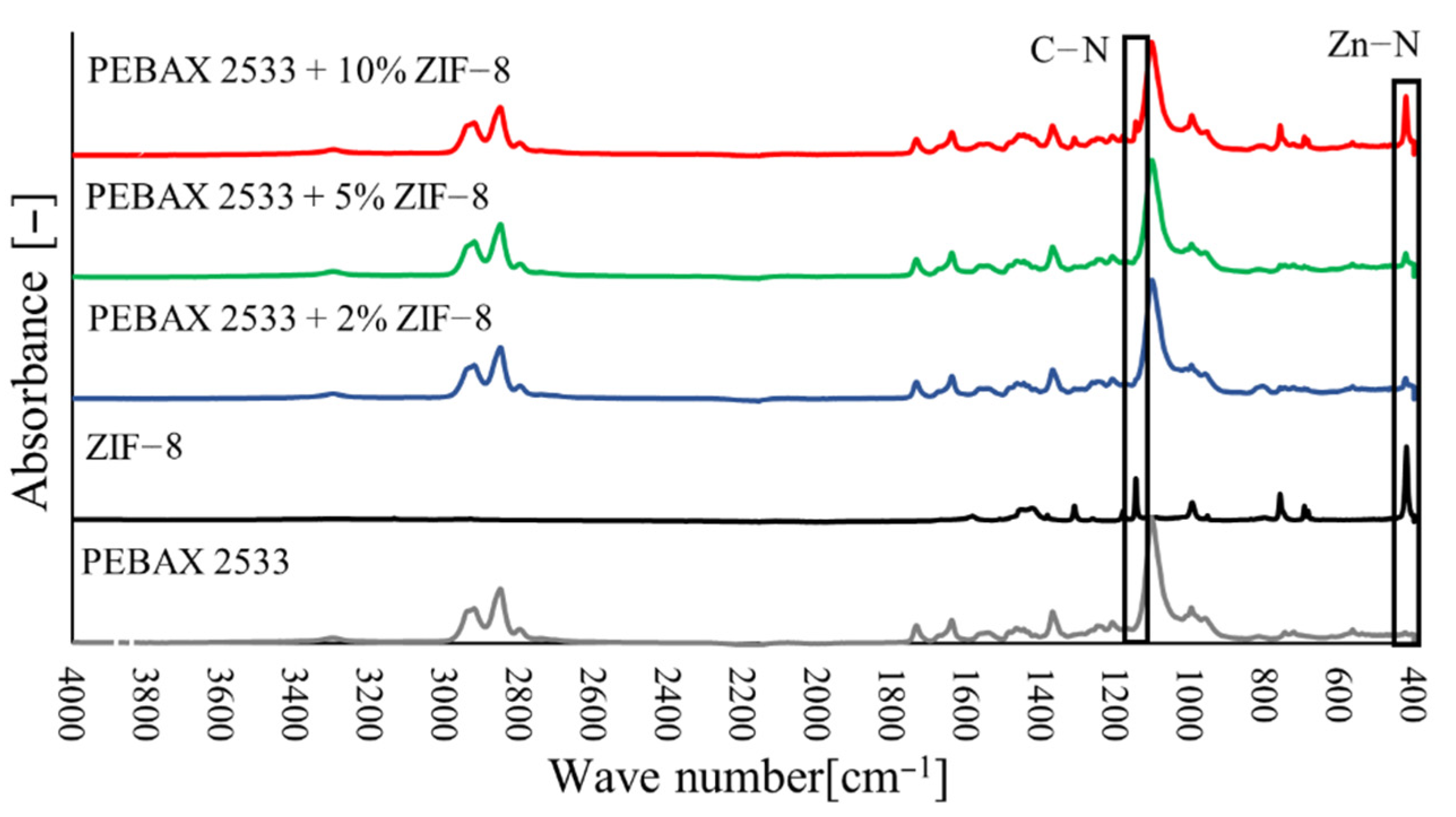
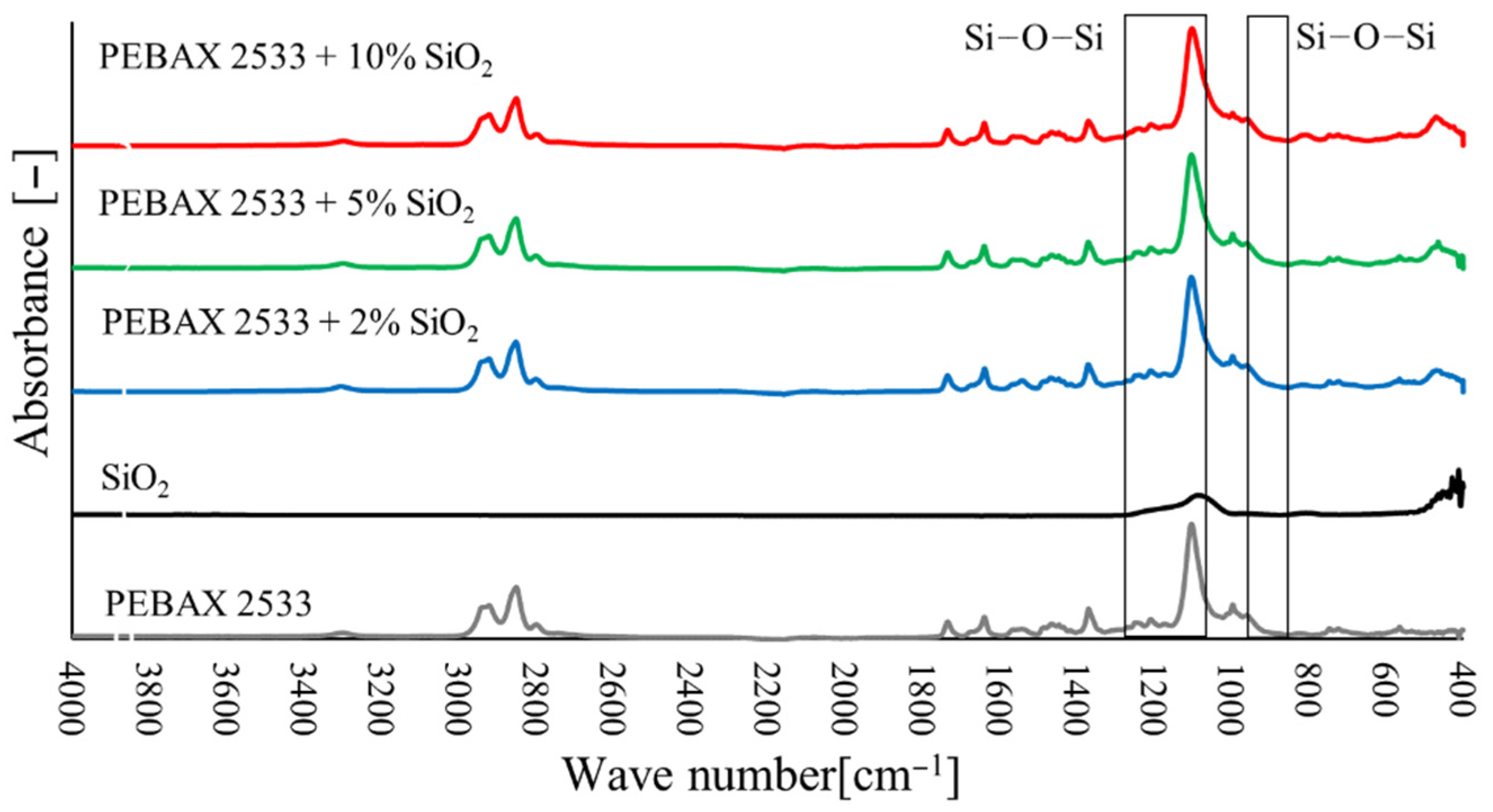
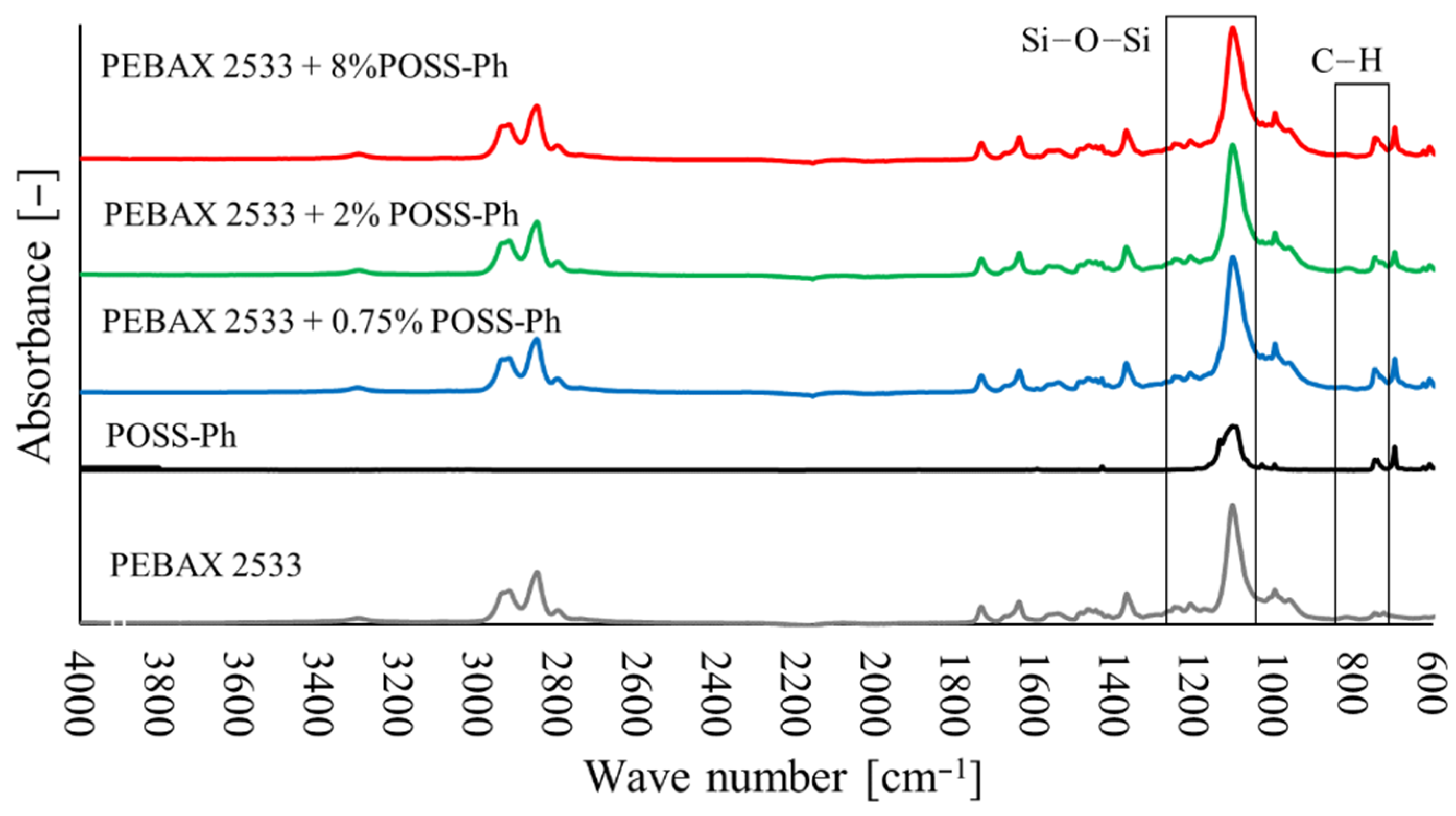
3.2. Fourier Transform Infrared Spectroscopy (FTIR)
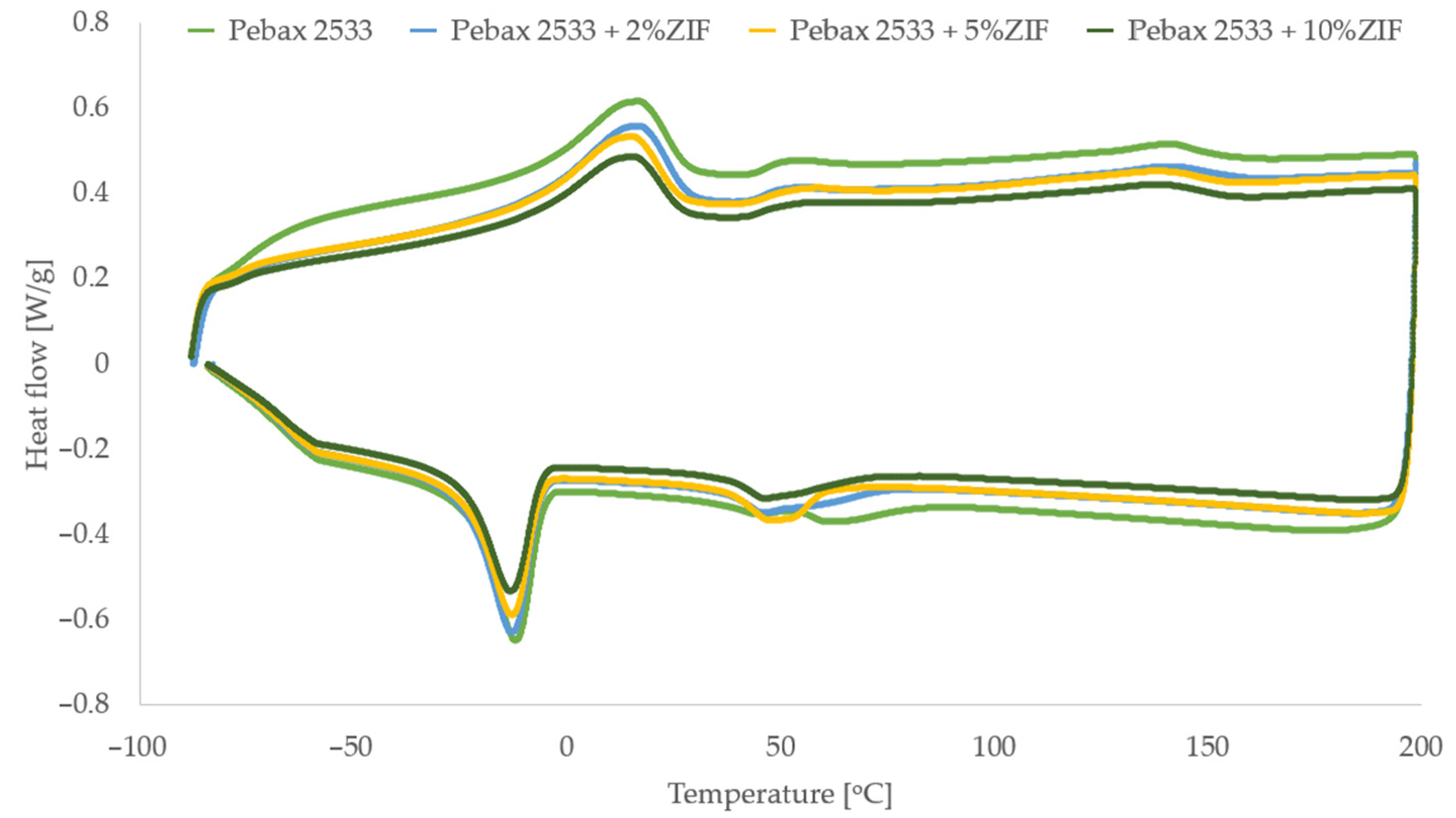
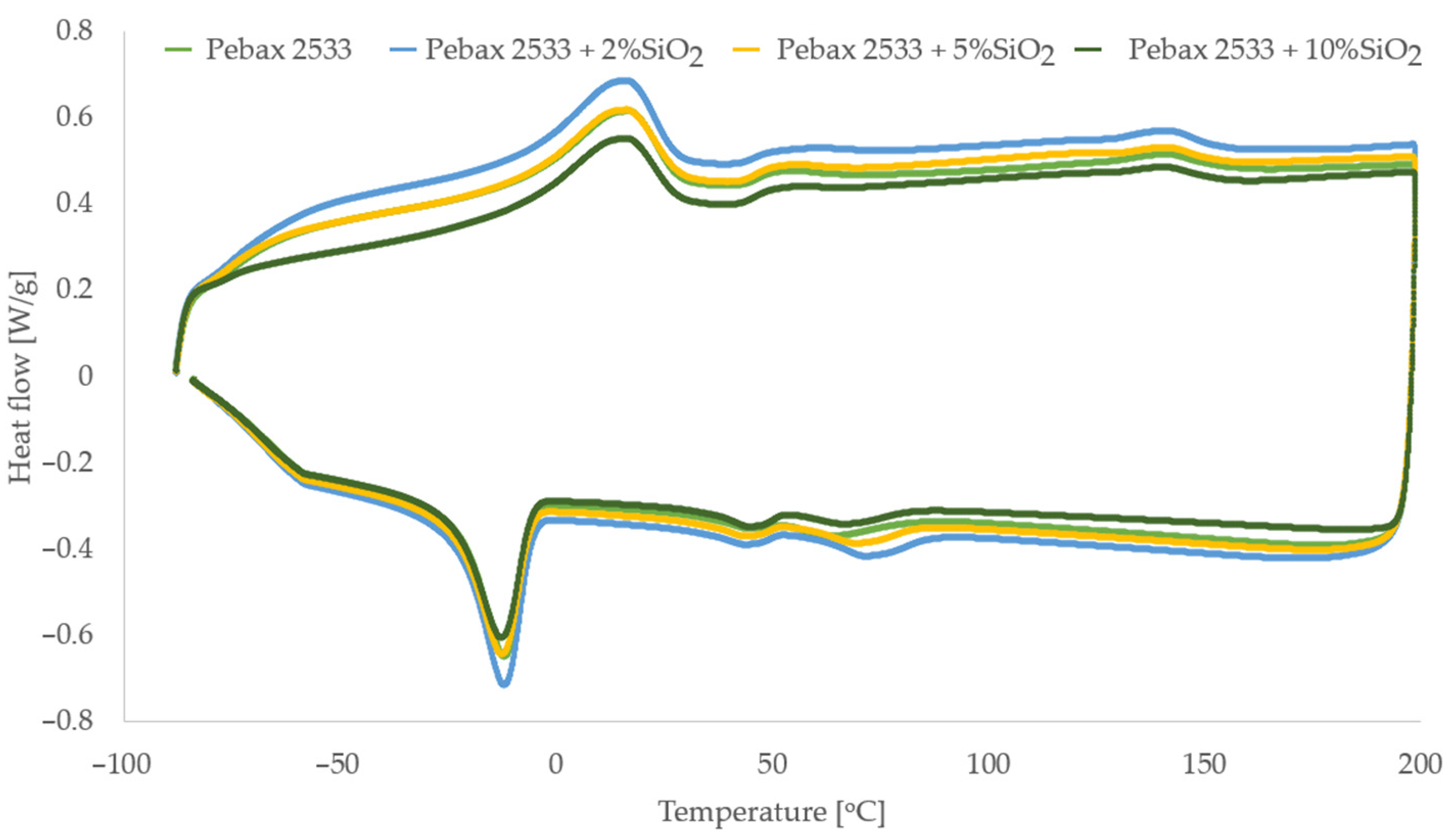
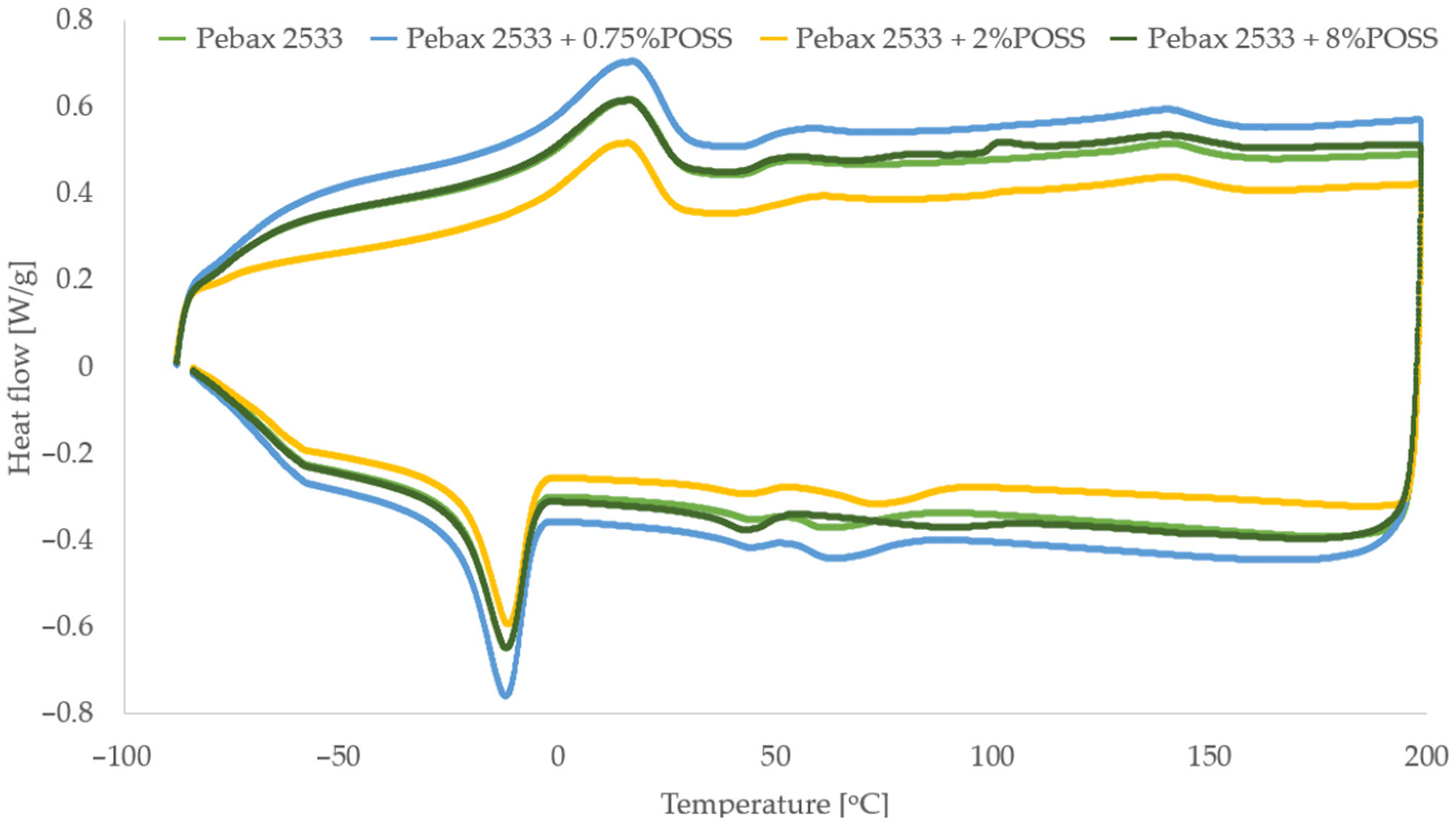
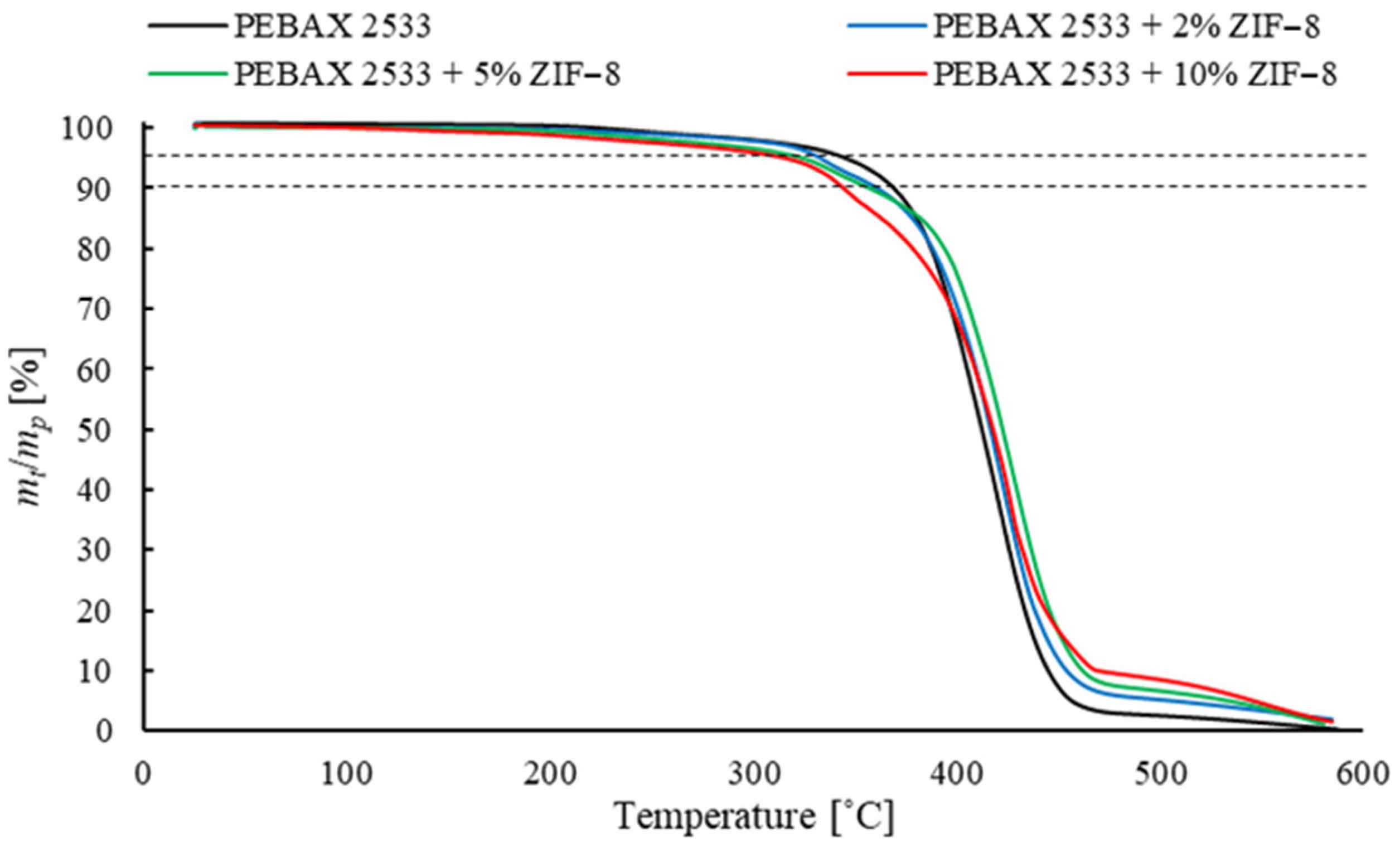
3.3. Differential Scanning Calorimetry (DSC), Thermogravimetric Analysis (TGA) and Process Parameters (P, D, S, α)
3.4. Membranes with ZIF-8
3.5. Membranes with SiO2
3.6. Membranes with POSS-Ph
4. Summary
- There is a boundary concentration of a filler, above which the process properties of the membrane decrease. A further increase in the content of the dispersed phase also causes deterioration of the membrane’s mechanical properties;
- the presence of the test fillers improves the solubility of the test gases. This is connected with expanding the membrane surface, i.e., increasing the gas–membrane contact surface. This increase is even 2.13 times for membranes containing 8 wt% POSS-Ph in relation to pure polymer membranes. In addition, interactions occur between CO2 molecules and chemical groups connected with filler particles, which improve the sorption of this gas on the membrane surface. This effect improves the selectivity of the membrane. There was an increase in CO2/N2 selectivity of 121% for the membrane containing 2 wt% POSS-Ph;
- the presence of the dispersed phase reduces the selectivity of the membrane, taking into account only the ratios of diffusion coefficients, which is associated with the formation of defects in the material structure. The higher reduction (2.06 times) was noted for the membrane containing 10 wt% ZIF-8. For sufficiently high filler concentrations, these effects occur intensively enough to deteriorate the membrane selectivity. For initial concentrations of the additives, the adverse phenomena that emerge in the membrane structure—which may cause deterioration of the membrane selectivity—are reduced by a greater improvement in the solubility of CO2 than other gases;
- the tests confirmed the validity of selecting inorganic fillers, as they improve the process properties of the membranes;
- for the developed heterogeneous membranes, improvement of the process parameters (P, α) can be noticed. Considering the gas permeability through the membrane and the selectivity of the membrane, among the tested membranes, both for the separation of the CO2/CH4 mixture and the CO2/N2 mixture, the membrane made of Pebax®2533 and containing 5 wt% SiO2 seems to be the best.
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Td5% [°C] | Td10% [°C] | |
|---|---|---|
| PEBAX 2533 | 345.1 | 369.5 |
| PEBAX 2533 + 2 wt% ZIF-8 | 332.7 | 360.9 |
| PEBAX 2533 + 5 wt% ZIF-8 | 324.1 | 357.0 |
| PEBAX 2533 + 10 wt% ZIF-8 | 315.5 | 344.1 |
| Td5% [°C] | Td10% [°C] | |
|---|---|---|
| PEBAX 2533 | 345.1 | 369.5 |
| PEBAX 2533 + 2 wt% SiO2 | 365.6 | 378.9 |
| PEBAX 2533 + 5 wt% SiO2 | 364.3 | 379.4 |
| PEBAX 2533 + 10 wt% SiO2 | 367.9 | 380.3 |
| Td5% [°C] | Td10% [°C] | |
|---|---|---|
| PEBAX 2533 | 345.1 | 369.5 |
| PEBAX 2533 + 0.75 wt% POSS-Ph | 372.4 | 382.6 |
| PEBAX 2533 + 2 wt% POSS-Ph | 375.4 | 385.7 |
| PEBAX 2533 + 8 wt% POSS-Ph | 368.0 | 382.6 |
| CmZIF-8 [wt%] | 0 | 2 | 5 | 10 |
|---|---|---|---|---|
| SCO2 * | 0.375 ± 0.023 | 0.428 ± 0.025 | 0.465 ± 0.028 | 0.520 ± 0.031 |
| SCH4 * | 0.224 ± 0.008 | 0.216 ± 0.009 | 0.227 ± 0.010 | 0.235 ± 0.010 |
| SN2 * | 0.145 ± 0.009 | 0.145 ± 0.009 | 0.139 ± 0.009 | 0.158 ± 0.010 |
| CmZIF-8 [wt%] | 0 | 2 | 5 | 10 |
|---|---|---|---|---|
| DCO2 * | 1.88 ± 0.11 | 2.14 ± 0.12 | 2.22 ± 0.12 | 2.82 ± 0.16 |
| DCH4 * | 0.56 ± 0.01 | 0.76 ± 0.01 | 0.82 ± 0.01 | 1.10 ± 0.02 |
| DN2 * | 0.27 ± 0.02 | 0.34 ± 0.02 | 0.37 ± 0.03 | 0.77 ± 0.05 |
| CmZIF-8 [wt%] | 0 | 2 | 5 | 10 |
|---|---|---|---|---|
| αSCO2/CH4 [-] | 1.68 ± 0.08 | 1.98 ± 0.09 | 2.05 ± 0.09 | 2.21 ± 0.10 |
| αSCO2/N2 [-] | 2.58 ± 0.23 | 2.93 ± 0.26 | 3.33 ± 0.29 | 3.30 ± 0.40 |
| αDCO2/CH4 [-] | 3.38 ± 0.22 | 2.82 ±0.23 | 2.71 ± 0.21 | 2.56 ± 0.18 |
| αDCO2/N2 [-] | 6.96 ± 0.55 | 6.29 ± 0.50 | 6.00 ± 0.42 | 3.37 ± 0.27 |
| CmSiO2 [wt%] | 0 | 2 | 5 | 10 |
|---|---|---|---|---|
| SCO2 * | 0.375 ± 0.023 | 0.521 ± 0.0312 | 0.536 ± 0.0322 | 0.598 ± 0.0200 |
| SCH4 * | 0.224 ± 0.008 | 0.310 ± 0.0096 | 0.325 ± 0.0101 | 0.319 ± 0.0064 |
| SN2 * | 0.145 ± 0.009 | 0.186 ± 0.0117 | 0.184 ± 0.0116 | 0.184 ± 0.0046 |
| CmSiO2 [wt%] | 0 | 2 | 5 | 10 |
|---|---|---|---|---|
| DCO2 * | 1.88 ± 0.11 | 1.53 ± 0.09 | 1.62 ± 0.09 | 1.40 ± 0.08 |
| DCH4 * | 0.56 ± 0.01 | 0.41 ± 0.01 | 0.41 ± 0.01 | 0.43 ± 0.01 |
| DN2 * | 0.27 ± 0.02 | 0.22 ± 0.02 | 0.22 ± 0.02 | 0.23 ± 0.02 |
| CmSiO2 [wt%] | 0 | 2 | 5 | 10 |
|---|---|---|---|---|
| αSCO2/CH4 [-] | 1.68 ± 0.08 | 1.68 ± 0.10 | 1.65 ± 0.10 | 1.88 ± 0.11 |
| αSCO2/N2 [-] | 2.58 ± 0.23 | 2.80 ± 0.25 | 2.91 ± 0.26 | 3.25 ± 0.29 |
| αDCO2/CH4 [-] | 3.38 ± 0.22 | 3.72 ± 0.22 | 3.94 ± 0.23 | 3.27 ± 0.19 |
| αDCO2/N2 [-] | 6.96 ± 0.55 | 6.95 ± 0.55 | 7.19 ± 0.57 | 5.81 ± 0.46 |
| CmPOSS-Ph [wt%] | 0 | 0.75 | 2 | 8 |
|---|---|---|---|---|
| SCO2 * | 0.375 ± 0.023 | 0.482 ± 0.027 | 0.564 ± 0.034 | 0.799 ± 0.048 |
| SCH4 * | 0.224 ± 0.008 | 0.264 ± 0.007 | 0.270 ± 0.007 | 0.299 ± 0.009 |
| SN2 * | 0.145 ± 0.009 | 0.144 ± 0.008 | 0.148 ± 0.009 | 0.179 ± 0.011 |
| CmPOSS-Ph [wt%] | 0 | 0.75 | 2 | 8 |
|---|---|---|---|---|
| DCO2 * | 1.88 ± 0.11 | 1.70 ± 0.10 | 1.45 ± 0.08 | 0.73 ± 0.04 |
| DCH4 * | 0.56 ± 0.01 | 0.51 ± 0.02 | 0.49 ± 0.02 | 0.40 ± 0.01 |
| DN2 * | 0.27 ± 0.02 | 0.27 ± 0.02 | 0.26 ± 0.02 | 0.20 ± 0.01 |
| CmPOSS-Ph [wt%] | 0 | 0.75 | 2 | 8 |
|---|---|---|---|---|
| αSCO2/CH4 [-] | 1.68 ± 0.08 | 1.83 ± 0.14 | 2.09 ± 0.16 | 2.68 ± 0.17 |
| αSCO2/N2 [-] | 2.58 ± 0.23 | 3.35 ± 0.31 | 3.82 ± 0.37 | 4.46 ± 0.40 |
| αDCO2/CH4 [-] | 3.38 ± 0.22 | 3.33 ± 0.18 | 2.96 ± 0.14 | 1.79 ± 0.11 |
| αDCO2/N2 [-] | 6.96 ± 0.55 | 6.30 ± 0.45 | 5.80 ± 0.42 | 3.64 ± 0.29 |
References
- Scholes, C.A.; Stevens, G.W.; Kentish, S.E. Membrane gas separation applications in natural gas processing. Fuel 2012, 96, 15–28. [Google Scholar] [CrossRef]
- Sridhar, S.; Smitha, B.; Aminabhavi, T.M. Separation of carbon dioxide from natural gas mixtures through polymeric membranes—A review. Sep. Purif. Rev. 2007, 36, 113–174. [Google Scholar] [CrossRef]
- Ackley, M.W. Medical oxygen concentrators: A review of progress in air separation technology. Adsorption 2019, 25, 1437–1474. [Google Scholar] [CrossRef]
- Han, J.; Bai, L.; Yang, B.; Bai, Y.; Luo, S.; Zeng, S.; Gao, H.; Nie, Y.; Ji, X.; Zhang, S.; et al. Highly selective oxygen/nitrogen separation membrane engineered using a porphyrin-based oxygen carrier. Membranes 2019, 9, 115. [Google Scholar] [CrossRef]
- Lu, J.; Wang, L.; Sun, X.; Li, J.; Liu, X. Absorption of CO2 into aqueous solutions of methyldiethanolamine and activated methyldiethanolamine from a gas mixture in a hollow fiber contactor. Ind. Eng. Chem. Res. 2005, 44, 9230–9238. [Google Scholar] [CrossRef]
- Taheri, M.; Mohebbi, A.; Hashemipour, H.; Rashidi, A.M. Simultaneous absorption of carbon dioxide (CO2) and hydrogen sulfide (H2S) from CO2–H2S–CH4 gas mixture using amine-based nanofluids in a wetted wall column. J. Nat. Gas Sci. Eng. 2016, 28, 410–417. [Google Scholar] [CrossRef]
- Goetz, V.; Pupier, O.; Guillot, A. Carbon dioxide-methane mixture adsorption on activated carbon. Adsorption 2006, 12, 55–63. [Google Scholar] [CrossRef]
- Sircar, S.; Golden, T.C. Purification of hydrogen by pressure swing adsorption. Sep. Sci. Technol. 2000, 35, 667–687. [Google Scholar] [CrossRef]
- Hart, A.; Gnanendran, N. Cryogenic CO2 capture in natural gas. Energy Procedia 2009, 1, 697–706. [Google Scholar] [CrossRef]
- Tengku Hassan, T.N.A.; Shariff, A.M.; Mohd Pauzi, M.M.I.; Khidzir, M.S.; Surmi, A. Insights on Cryogenic Distillation Technology for Simultaneous CO2 and H2S Removal for Sour Gas Fields. Molecules 2022, 27, 1424. [Google Scholar] [CrossRef]
- Lin, R.B.; Li, L.; Zhou, H.L.; Wu, H.; He, C.; Li, S.; Krishna, R.; Li, J.; Zhou, W.; Chen, B. Molecular sieving of ethylene from ethane using a rigid metal–organic framework. Nat. Mater. 2018, 17, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Kotoh, K.; Takashima, S.; Nakamura, Y. Molecular-sieving effect of zeolite 3A on adsorption of H2, HD and D2. Fusion Eng. Des. 2009, 84, 1108–1112. [Google Scholar] [CrossRef]
- Marzouk, S.A.; Al-Marzouqi, M.H.; El-Naas, M.H.; Abdullatif, N.; Ismail, Z.M. Removal of carbon dioxide from pressurized CO2–CH4 gas mixture using hollow fiber membrane contactors. J. Membr. Sci. 2010, 351, 21–27. [Google Scholar] [CrossRef]
- Jackiewicz-Zagórska, A.; Szwast, M.; Gac, J.; Werner, Ł.; Zalewski, M.; Jakubiak, S. New methods of natural gas adjusting for technological purposes based on modern filtration materials. Ecol. Chem. Eng. 2018, 25, 61–72. [Google Scholar] [CrossRef]
- Szwast, M.; Polak, D.; Zalewski, M. Novel gas separation membrane for energy. Desal. Water Treat. 2017, 64, 255–259. [Google Scholar] [CrossRef]
- Li, G.; Kujawski, W.; Válek, R.; Koter, S. A review–The development of hollow fibre membranes for gas separation processes. Int. J. Greenh. Gas Control. 2021, 104, 103195. [Google Scholar] [CrossRef]
- Basu, A.; Akhtar, J.; Rahman, M.H.; Islam, M.R. A review of separation of gases using membrane systems. Pet. Sci. Technol. 2004, 22, 1343–1368. [Google Scholar] [CrossRef]
- Sridhar, S.; Bee, S.; Bhargava, S. Membrane-based gas separation: Principle, applications and future potential. Chem. Eng. Dig. 2014, 1, 1–25. [Google Scholar]
- Baker, R.W.; Low, B.T. Gas separation membrane materials: A perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Freeman, B.D. Basis of permeability/selectivity tradeoff relations in polymeric gas separation membranes. Macromolecules 1999, 32, 375–380. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Koros, W.J.; Mahajan, R. Pushing the limits on possibilities for large scale gas separation: Which strategies? J. Membr. Sci. 2000, 175, 181–196. [Google Scholar] [CrossRef]
- Süer, M.G.; Baç, N.; Yilmaz, L. Gas permeation characteristics of polymer-zeolite mixed matrix membranes. J. Membr. Sci. 1994, 91, 77–86. [Google Scholar] [CrossRef]
- Joly, C.; Goizet, S.; Schrotter, J.C.; Sanchez, J.; Escoubes, M. Sol-gel polyimide-silica composite membrane: Gas transport properties. J. Membr. Sci. 1997, 130, 63–74. [Google Scholar] [CrossRef]
- Ismail, A.F.; Rahim, N.H.; Mustafa, A.; Matsuura, T.; Ng, B.C.; Abdullah, S.; Hashemifard, S.A. Gas separation performance of polyethersulfone/multi-walled carbon nanotubes mixed matrix membranes. Sep. Purif. Technol. 2011, 80, 20–31. [Google Scholar] [CrossRef]
- Nejad, M.N.; Asghari, M.; Afsari, M. Investigation of carbon nanotubes in mixed matrix membranes for gas separation: A review. ChemBioEng Rev. 2016, 3, 276–298. [Google Scholar] [CrossRef]
- Sze Lai, L.; Fong Yeong, Y.; Keong Lau, K.; Shariff Az, M. Zeolite imidazole frameworks membranes for CO2/CH4 separation from natural gas: A review. J. Appl. Sci. 2014, 14, 1161–1167. [Google Scholar] [CrossRef]
- Hwang, S.; Chi, W.S.; Lee, S.J.; Im, S.H.; Kim, J.H.; Kim, J. Hollow ZIF-8 nanoparticles improve the permeability of mixed matrix membranes for CO2/CH4 gas separation. J. Membr. Sci. 2015, 480, 11–19. [Google Scholar] [CrossRef]
- Li, Y.; Chung, T.S. Molecular-level mixed matrix membranes comprising Pebax® and POSS for hydrogen purification via preferential CO2 removal. Int. J. Hydrogen Energy 2010, 35, 10560–10568. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Amooghin, A.E.; Montazer-Rahmati, M.M.; Ismail, A.F.; Matsuura, T. State-of-the-art membrane based CO2 separation using mixed matrix membranes (MMMs): An overview on current status and future directions. Prog. Polym. Sci. 2014, 39, 817–861. [Google Scholar] [CrossRef]
- Azizi, N.; Mohammadi, T.; Behbahani, R.M. Comparison of permeability performance of PEBAX-1074/TiO2, PEBAX-1074/SiO2 and PEBAX-1074/Al2O3 nanocomposite membranes for CO2/CH4 separation. Chem. Eng. Res. Des. 2017, 117, 177–189. [Google Scholar] [CrossRef]
- Li, X.; Ma, L.; Zhang, H.; Wang, S.; Jiang, Z.; Guo, R.; Wu, H.; Cao, X.Z.; Yang, J.; Wang, B. Synergistic effect of combining carbon nanotubes and graphene oxide in mixed matrix membranes for efficient CO2 separation. J. Membr. Sci. 2015, 479, 1–10. [Google Scholar] [CrossRef]
- Mahajan, R.; Burns, R.; Schaeffer, M.; Koros, W.J. Challenges in forming successful mixed matrix membranes with rigid polymeric materials. J. Appl. Polym. Sci. 2002, 86, 881–890. [Google Scholar] [CrossRef]
- Dechnik, J.; Gascon, J.; Doonan, C.J.; Janiak, C.; Sumby, C.J. Mixed-matrix membranes. Angew. Chem. Int. Ed. 2017, 56, 9292–9310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Han, E.; Wu, Y.; Wang, X.; Wu, D. Surface decoration of short-cut polyimide fibers with multi-walled carbon nanotubes and their application for reinforcement of lightweight PC/ABS composites. Appl. Surf. Sci. 2018, 442, 124–137. [Google Scholar] [CrossRef]
- Wang, X.L.; Qu, Z.G.; Lai, T.; Ren, G.F.; Wang, W.K. Enhancing water transport performance of gas diffusion layers through coupling manipulation of pore structure and hydrophobicity. J. Power Sources 2022, 525, 231121. [Google Scholar] [CrossRef]
- Wijmans, J.G.; Baker, R.W. The solution-diffusion model: A review. J. Membr. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
- Moghadam, F.; Omidkhah, M.R.; Vasheghani-Farahani, E.; Pedram, M.Z.; Dorosti, F. The effect of TiO2 nanoparticles on gas transport properties of Matrimid 5218-based mixed matrix membranes. Sep. Purif. Technol. 2011, 77, 128–136. [Google Scholar] [CrossRef]
- Chung, T.S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Aroon, M.A.; Ismail, A.F.; Matsuura, T.; Montazer-Rahmati, M.M. Performance studies of mixed matrix membranes for gas separation: A review. Sep. Purif. Technol. 2010, 75, 229–242. [Google Scholar] [CrossRef]
- Nafisi, V.; Hägg, M.B. Development of dual layer of ZIF-8/PEBAX-2533 mixed matrix membrane for CO2 capture. J. Membr. Sci. 2014, 459, 244–255. [Google Scholar] [CrossRef]
- Maleh, M.S.; Raisi, A. Preparation of high performance mixed matrix membranes by one-pot synthesis of ZIF-8 nanoparticles into Pebax-2533 for CO2 separation. Chem. Eng. Res. Des. 2022, 186, 266–275. [Google Scholar] [CrossRef]
- Szwast, M.; Polak, D.; Marcjaniak, B. The influence of temperature and pressure of the feed on physical and chemical parameters of the membrane made of PEBA copolymer. Desal. Water Treat. 2018, 128, 193–198. [Google Scholar] [CrossRef]
- Wahab, M.A.; Sunarti, A.R. Development of PEBAX based membrane for gas separation: A review. Int. J. Membr. Sci. Technol. 2015, 2, 78–84. [Google Scholar]
- Halim, A.; Fu, Q.; Yong, Q.; Gurr, P.A.; Kentish, S.E.; Qiao, G.G. Soft polymeric nanoparticle additives for next generation gas separation membranes. J. Mater. Chem. A 2014, 2, 4999–5009. [Google Scholar] [CrossRef]
- Merey, Ş.; Longinos, S.N. The role of natural gas hydrate during natural gas transportation. OHU J. Eng. Sci. 2018, 7, 937–953. [Google Scholar] [CrossRef]
- Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O'Keeffe, M.; Yaghi, O.M. High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science 2008, 319, 939–943. [Google Scholar] [CrossRef]
- Pu, H.; Liu, L.; Chang, Z.; Yuan, J. Organic/inorganic composite membranes based on polybenzimidazole and nano-SiO2. Electrochim. Acta 2009, 54, 7536–7541. [Google Scholar] [CrossRef]
- Bolln, C.; Tsuchida, A.; Frey, H.; Mülhaupt, R. Thermal properties of the homologous series of 8-fold alkyl-substituted octasilsesquioxanes. Chem. Mater. 1997, 9, 1475–1479. [Google Scholar] [CrossRef]
- Macchione, M.; Jansen, J.C.; Drioli, E. The dry phase inversion technique as a tool to produce highly efficient asymmetric gas separation membranes of modified PEEK. Influence of temperature and air circulation. Desalination 2006, 192, 132–141. [Google Scholar] [CrossRef]
- Frisch, H.L. The time lag in diffusion. J. Phys. Chem. 1957, 61, 93–95. [Google Scholar] [CrossRef]
- Van Goethem, C.; Verbeke, R.; Pfanmöller, M.; Koschine, T.; Dickmann, M.; Timpel-Lindner, T.; Egger, W.; Bals, S.; Vankelecom, I.F.J. The role of MOFs in thin-film nanocomposite (TFN) membranes. J. Membr. Sci. 2018, 563, 938–948. [Google Scholar] [CrossRef]
- Mozia, S.; Grylewicz, A.; Zgrzebnicki, M.; Darowna, D.; Czyżewski, A. Investigations on the properties and performance of mixed-matrix polyethersulfone membranes modified with halloysite nanotubes. Polymers 2019, 11, 671. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Sun, J.; Wang, J.; Liu, M.; Yan, Z.; Zhu, B.; Li, Y.; Cao, X. MXene versus graphene oxide: Investigation on the effects of 2D nanosheets in mixed matrix membranes for CO2 separation. J. Membr. Sci. 2021, 620, 118850. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Zhang, Z.; Ouyang, G.; Hao, Z. Effects of mesoporous silica particle size and pore structure on the performance of polymer-mesoporous silica mixed matrix membranes. RSC Adv. 2021, 11, 36577–36586. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, Y.M. Gas permeation properties of poly (amide-6-b-ethylene oxide)–silica hybrid membranes. J. Membr. Sci. 2001, 193, 209–225. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, Y.; Chen, W.; Peng, J.; Dong, Y.; Jiang, Z.; Liu, H. Appearance of poly (ethylene oxide) segments in the polyamide layer for antifouling nanofiltration membranes. J. Membr. Sci. 2011, 382, 300–307. [Google Scholar] [CrossRef]
- Murali, R.S.; Kumar, K.P.; Ismail, A.F.; Sridhar, S. Nanosilica and H-Mordenite incorporated Poly (ether-block-amide)-1657 membranes for gaseous separations. Micropor. Mesopor. Mat. 2014, 197, 291–298. [Google Scholar] [CrossRef]
- Hassanajili, S.; Khademi, M.; Keshavarz, P. Influence of various types of silica nanoparticles on permeation properties of polyurethane/silica mixed matrix membranes. J. Membr. Sci. 2014, 453, 369–383. [Google Scholar] [CrossRef]
- Shieh, Y.T.; Liu, K.H. Solubility of CO2 in glassy PMMA and PS over a wide pressure range: The effect of carbonyl groups. J. Polym. Res. 2002, 9, 107–113. [Google Scholar] [CrossRef]

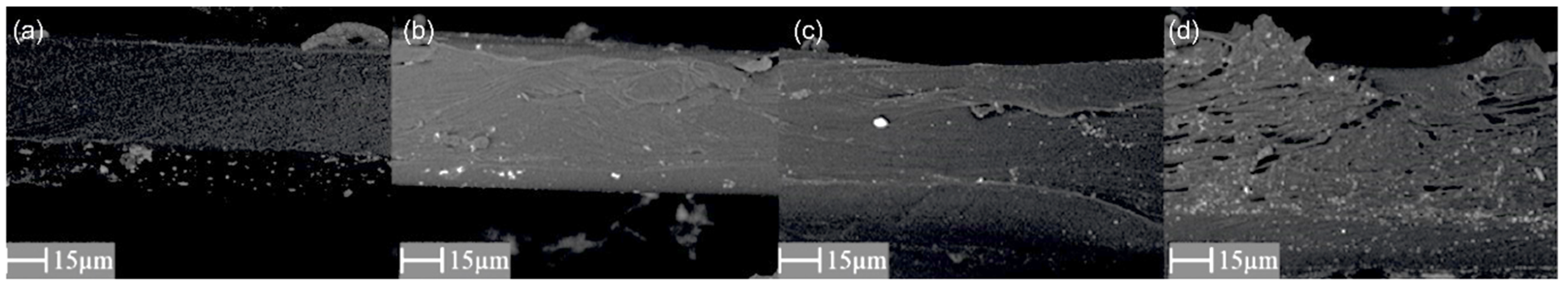
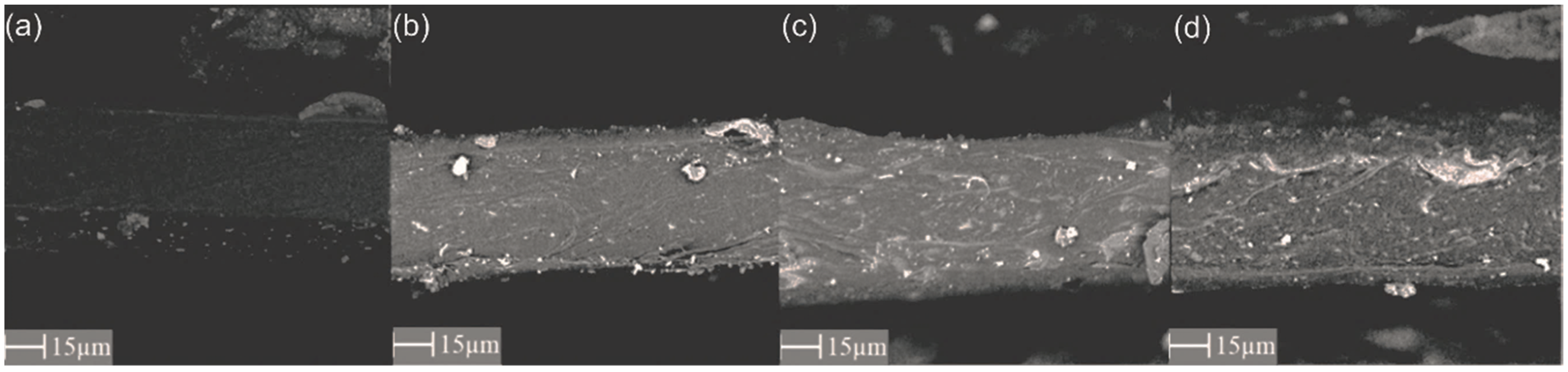
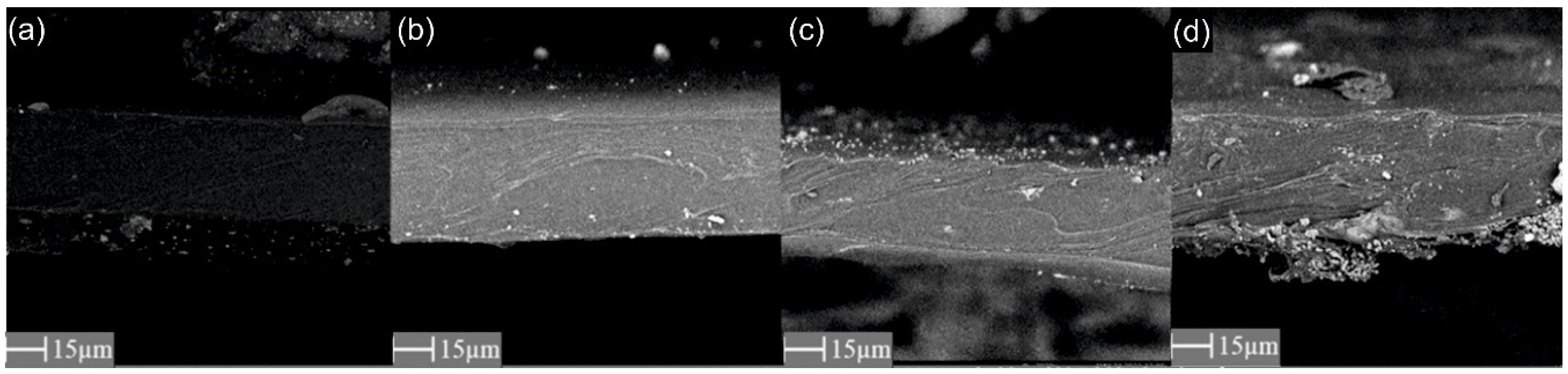
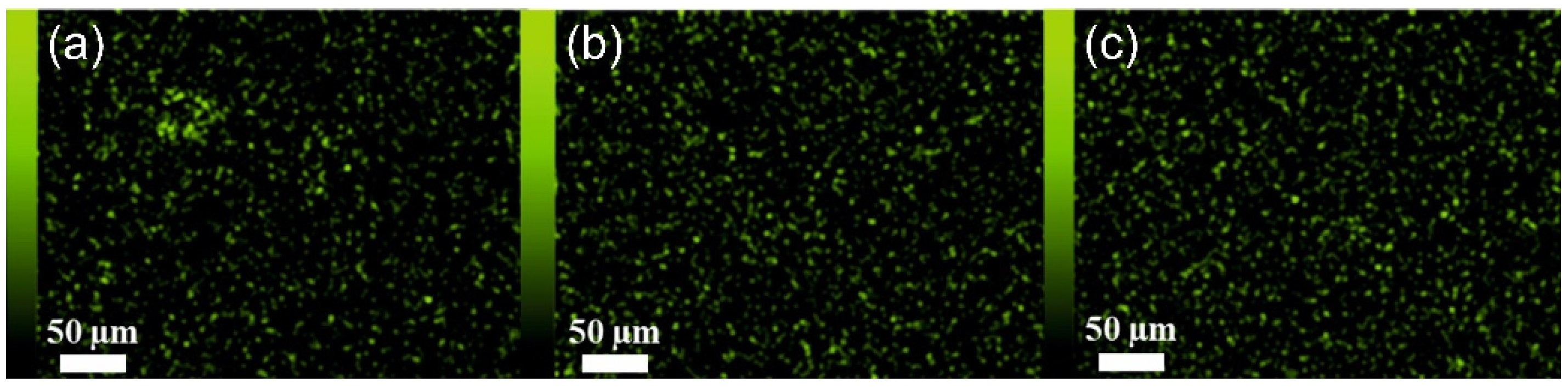




| Parameter | PEBAX 2533 | PEBAX 2533 + 2 wt% ZIF-8 | PEBAX 2533 + 5 wt% ZIF-8 | PEBAX 2533 + 10 wt% ZIF-8 |
|---|---|---|---|---|
| TmPE [°C] | 16.4 | 16.1 | 14.6 | 15.0 |
| HmPE [J/g] | 32.6 | 31.2 | 29.4 | 28.0 |
| XCPE [%] | 16.3 | 15.6 | 14.7 | 14.0 |
| TmPA [°C] | 141.0 | 140.7 | 137.9 | 138.3 |
| HmPA [J/g] | 7.5 | 6.4 | 6.5 | 7.0 |
| XCPA [%] | 3.3 | 2.8 | 2.8 | 3.0 |
| Xc [%] | 13.7 | 13.0 | 12.3 | 11.8 |
| Parameter | PEBAX 2533 | PEBAX 2533 + 2 wt% SiO2 | PEBAX 2533 + 5 wt% SiO2 | PEBAX 2533 + 10 wt% SiO2 |
|---|---|---|---|---|
| TmPE [°C] | 16.4 | 16.0 | 15.7 | 15.2 |
| HmPE [J/g] | 32.6 | 31.6 | 30.7 | 29.9 |
| XCPE [%] | 16.3 | 15.8 | 15.3 | 14.9 |
| TmPA [°C] | 141.0 | 140.8 | 140.5 | 140.2 |
| HmPA [J/g] | 7.5 | 8.1 | 8.9 | 8.2 |
| XCPA [%] | 3.3 | 3.5 | 3.9 | 3.6 |
| Xc [%] | 13.7 | 13.3 | 13.0 | 12.7 |
| Parameter | PEBAX 2533 | PEBAX 2533 + 0.75 wt% POSS-Ph | PEBAX 2533 + 2 wt% POSS-Ph | PEBAX 2533 + 8 wt% POSS-Ph |
|---|---|---|---|---|
| TmPE [°C] | 16.4 | 16.6 | 15.4 | 15.9 |
| HmPE [J/g] | 32.6 | 32.1 | 32.4 | 29.5 |
| XCPE [%] | 16.3 | 16.0 | 16.2 | 14.7 |
| TmPA [°C] | 141.0 | 140.0 | 140.3 | 139.7 |
| HmPA [J/g] | 7.5 | 7.5 | 8.1 | 7.4 |
| XCPA [%] | 3.3 | 3.3 | 3.5 | 3.2 |
| Xc [%] | 13.7 | 13.5 | 13.7 | 12.4 |
| CmZIF-8 [wt%] | 0 | 2 | 5 | 10 |
|---|---|---|---|---|
| PCO2 [barrer] | 210.4 ± 1.7 | 272.5 ± 2.2 | 298.4 ± 2.4 | 437.2 ± 3.5 |
| PCH4 [barrer] | 37.2 ± 0.6 | 47.0 ± 0.8 | 55.0 ± 0.9 | 88.1 ± 1.4 |
| PN2 [barrer] | 11.7 ± 0.3 | 14.8 ± 0.4 | 18.0 ± 0.5 | 36.1 ± 0.9 |
| CmZIF-8 [wt%] | 0 | 2 | 5 | 10 |
|---|---|---|---|---|
| αCO2/CH4 [-] | 5.66 ± 0.12 | 5.80 ± 0.12 | 5.43 ± 0.11 | 4.96 ± 0.10 |
| αCO2/N2 [-] | 17.98 ± 0.36 | 18.41 ± 0.37 | 16.58 ± 0.33 | 12.11 ± 0.24 |
| CmSiO2 [wt%] | 0 | 2 | 5 | 10 |
|---|---|---|---|---|
| PCO2 [barrer] | 210.4 ± 1.7 | 236.2 ± 1.9 | 258.6 ± 2.1 | 249.9 ± 2.0 |
| PCH4 [barrer] | 37.2 ± 0.6 | 38.0 ± 0.6 | 39.8 ± 0.6 | 40.7 ± 0.7 |
| PN2 [barrer] | 11.7 ± 0.3 | 12.2 ± 0.3 | 12.4 ± 0.3 | 12.8 ± 0.3 |
| CmSiO2 [wt%] | 0 | 2 | 5 | 10 |
|---|---|---|---|---|
| αCO2/CH4 [-] | 5.66 ± 0.12 | 6.27 ± 0.13 | 6.51 ± 0.13 | 6.14 ± 0.12 |
| αCO2/N2 [-] | 17.98 ± 0.36 | 19.45 ± 0.39 | 20.92 ± 0.42 | 19.52 ± 0.39 |
| CmPOSS-Ph [wt%] | 0 | 0.75 | 2 | 8 |
|---|---|---|---|---|
| PCO2 [barrer] | 210.4 ± 1.7 | 236.1 ± 1.9 | 240.2 ± 1.9 | 173.9 ± 1.4 |
| PCH4 [barrer] | 37.2 ± 0.6 | 40.2 ± 0.6 | 39.5 ± 0.6 | 36.3 ± 0.6 |
| PN2 [barrer] | 11.7 ± 0.3 | 11.6 ± 0.3 | 11.0 ± 0.3 | 10.7 ± 0.3 |
| CmPOSS-Ph [wt%] | 0 | 0.75 | 2 | 8 |
|---|---|---|---|---|
| αCO2/CH4 [-] | 5.66 ± 0.12 | 5.92 ± 0.11 | 6.08 ± 0.12 | 4.79 ± 0.10 |
| αCO2/N2 [-] | 17.98 ± 0.36 | 20.35 ± 0.41 | 21.82 ± 0.44 | 16.25 ± 0.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polak, D.; Szwast, M. Material and Process Tests of Heterogeneous Membranes Containing ZIF-8, SiO2 and POSS-Ph. Materials 2022, 15, 6455. https://doi.org/10.3390/ma15186455
Polak D, Szwast M. Material and Process Tests of Heterogeneous Membranes Containing ZIF-8, SiO2 and POSS-Ph. Materials. 2022; 15(18):6455. https://doi.org/10.3390/ma15186455
Chicago/Turabian StylePolak, Daniel, and Maciej Szwast. 2022. "Material and Process Tests of Heterogeneous Membranes Containing ZIF-8, SiO2 and POSS-Ph" Materials 15, no. 18: 6455. https://doi.org/10.3390/ma15186455





