Corrosion Properties of Boron- and Manganese-Alloyed Stainless Steels as a Material for the Bipolar Plates of PEM Fuel Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Model Solution
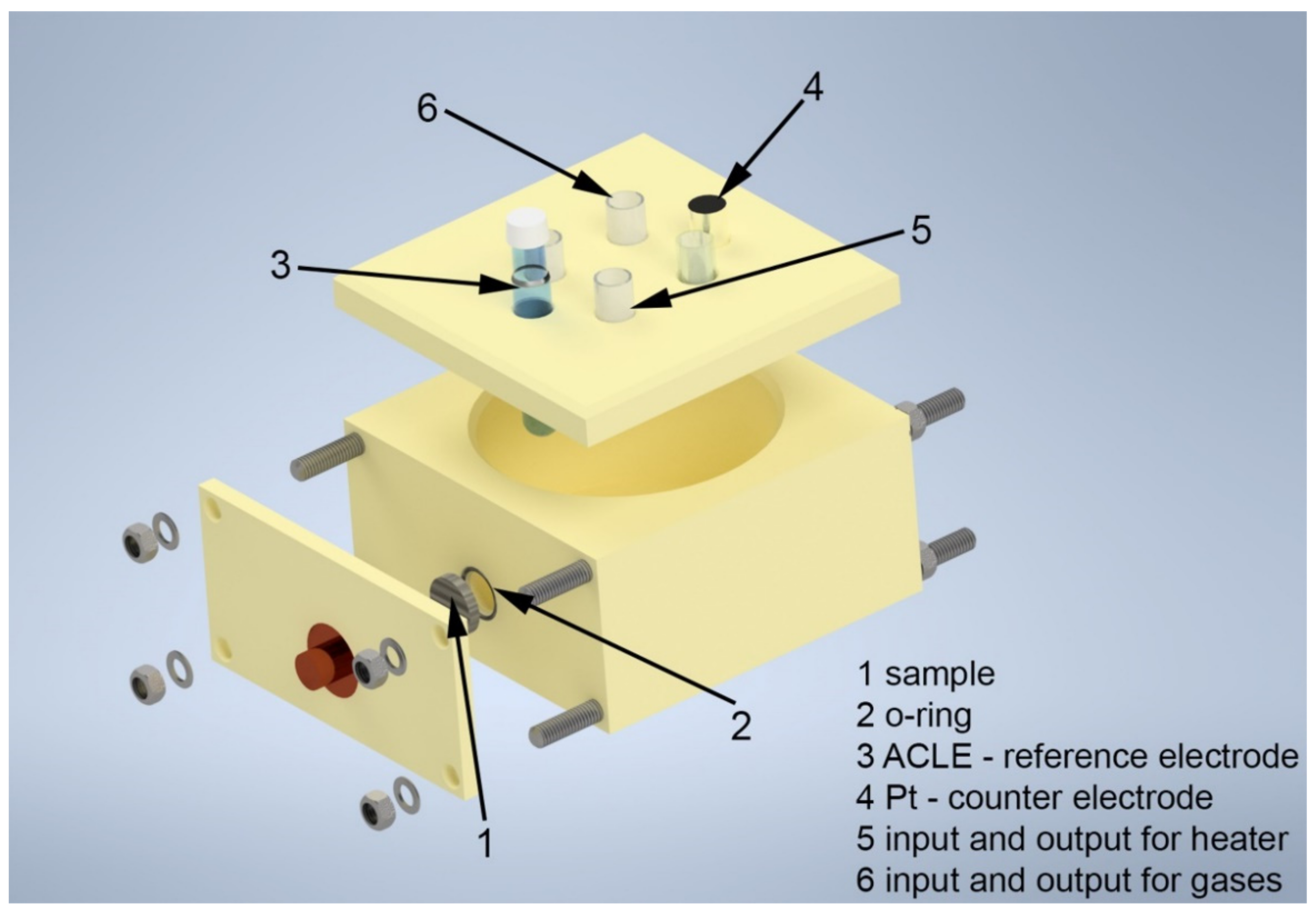
2.2. Electrochemical Measurements
2.3. Accelerated Corrosion Tests
2.4. Potentiodynamic Polarization According to DOE Requirements
2.5. Potentiostatic Polarization According to DOE Requirements
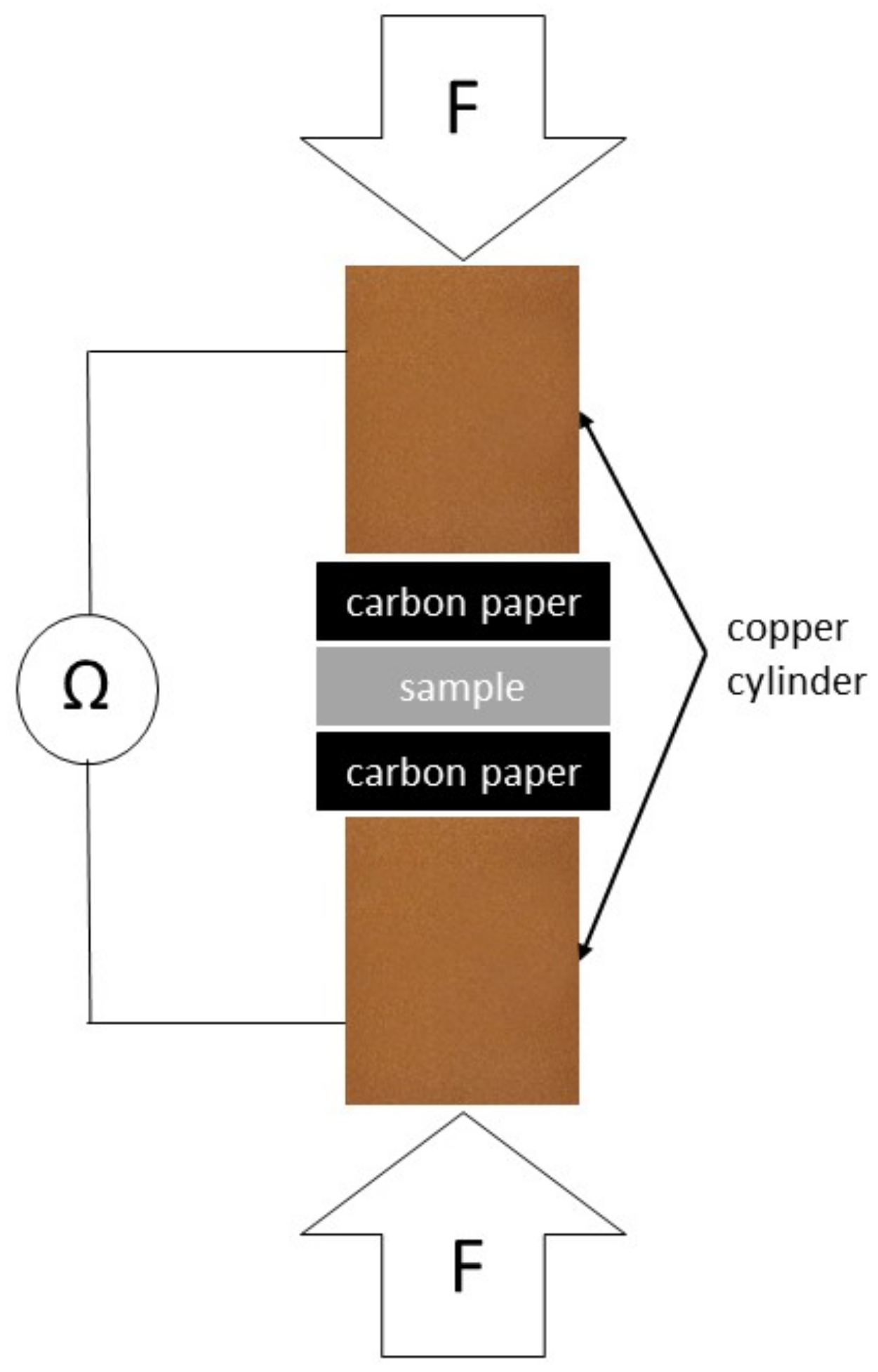
2.6. Interfacial Contact Resistance (ICR)
3. Results and Discussion
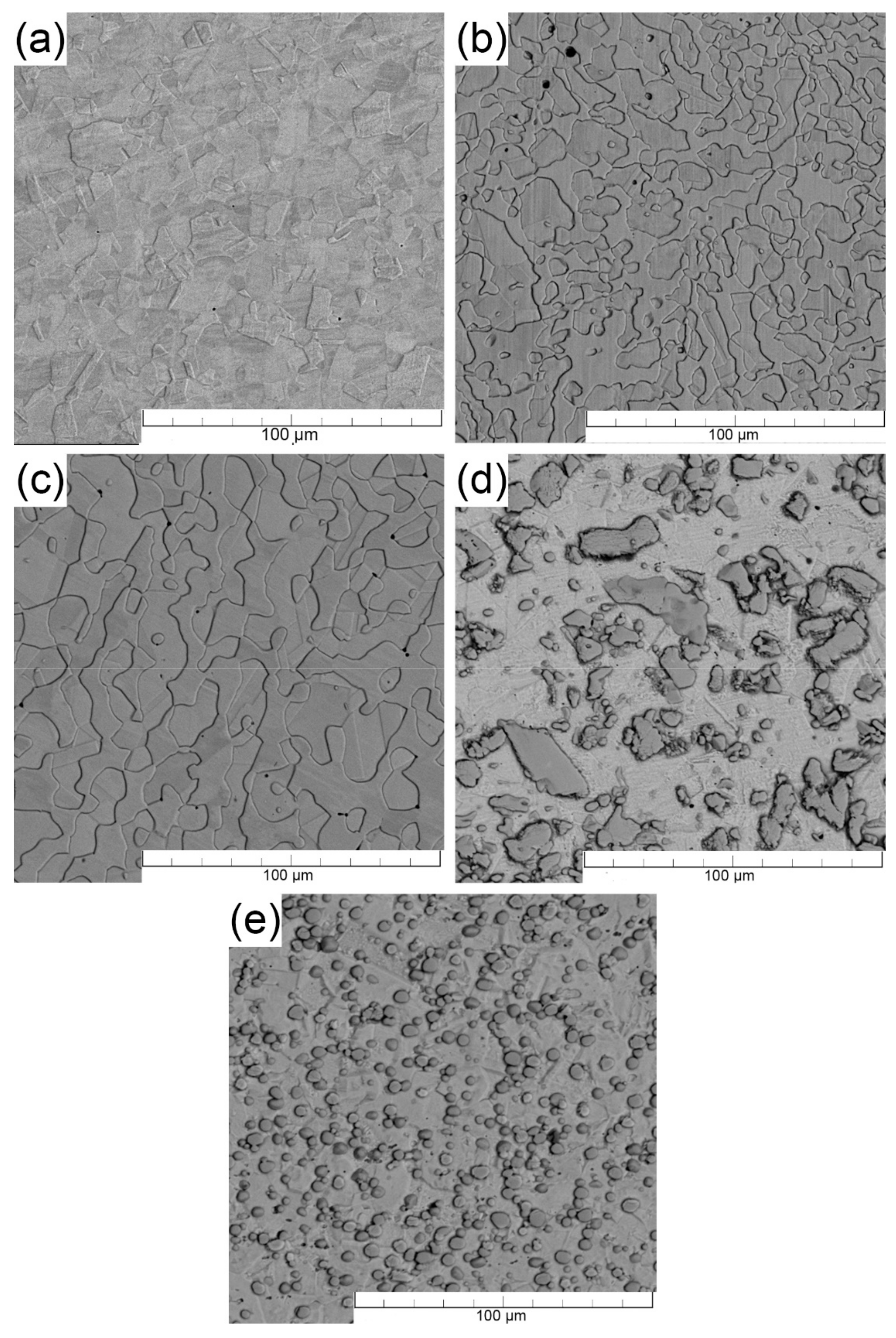
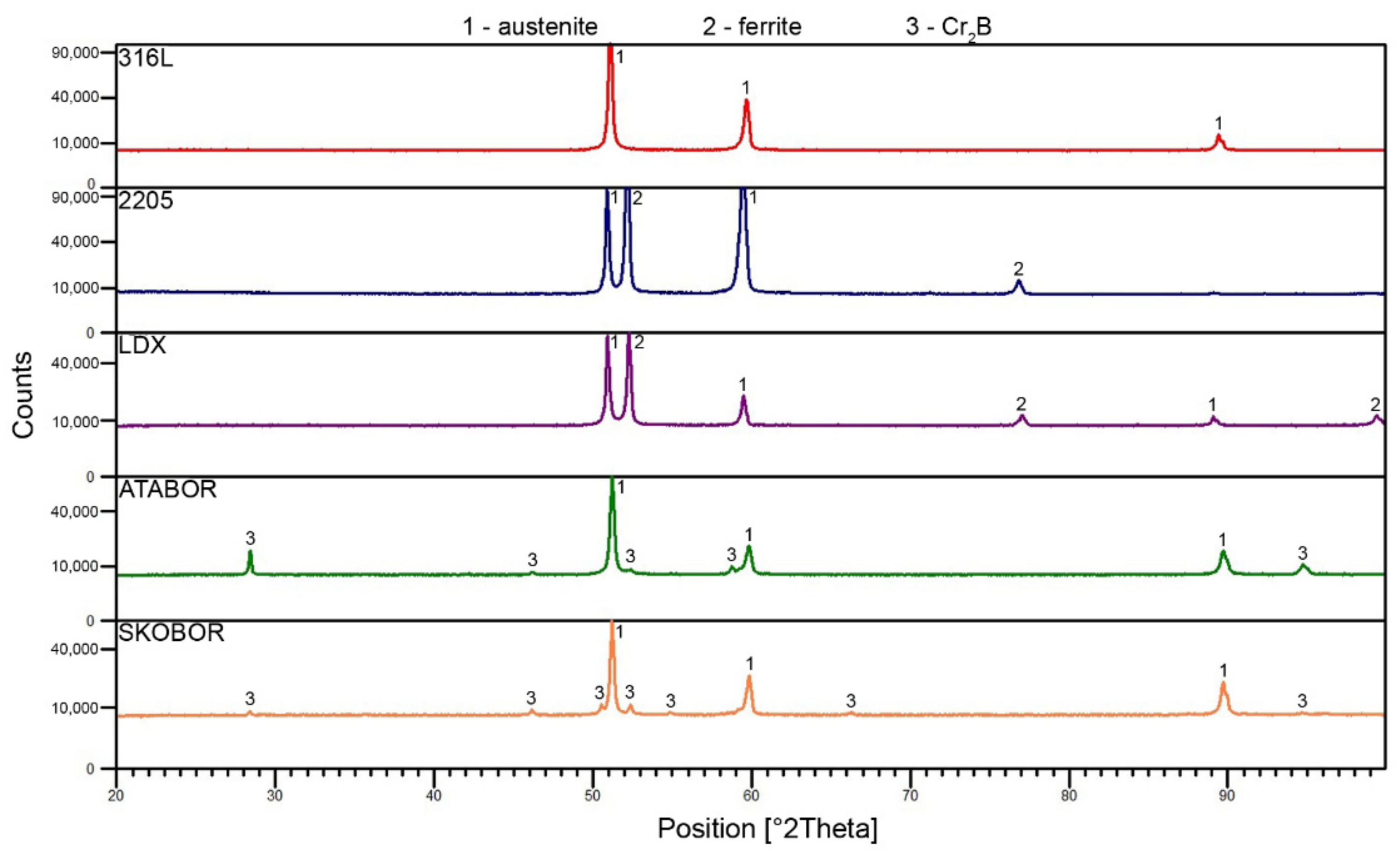
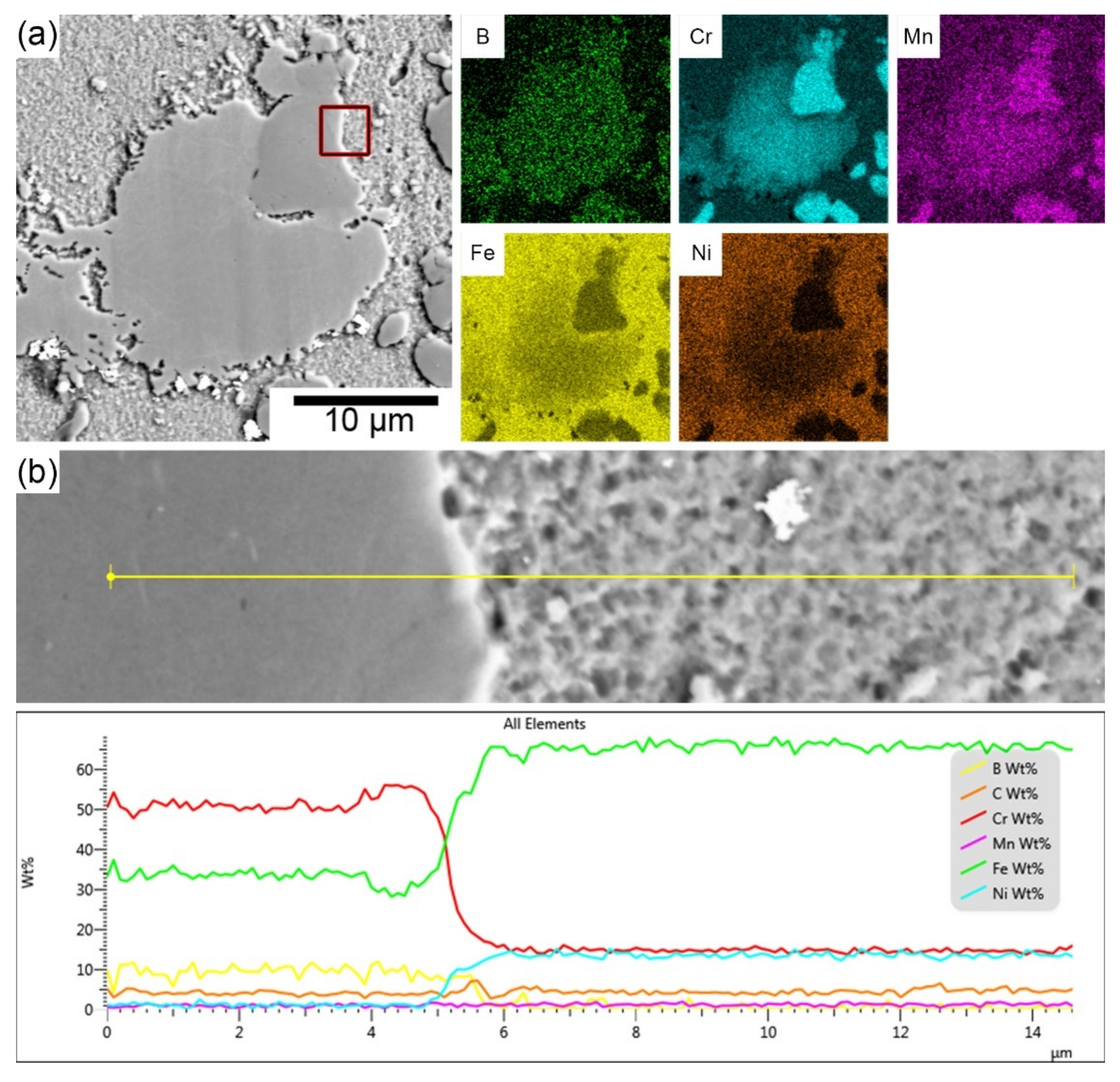
3.1. Microstructure of Tested Stainless Steels
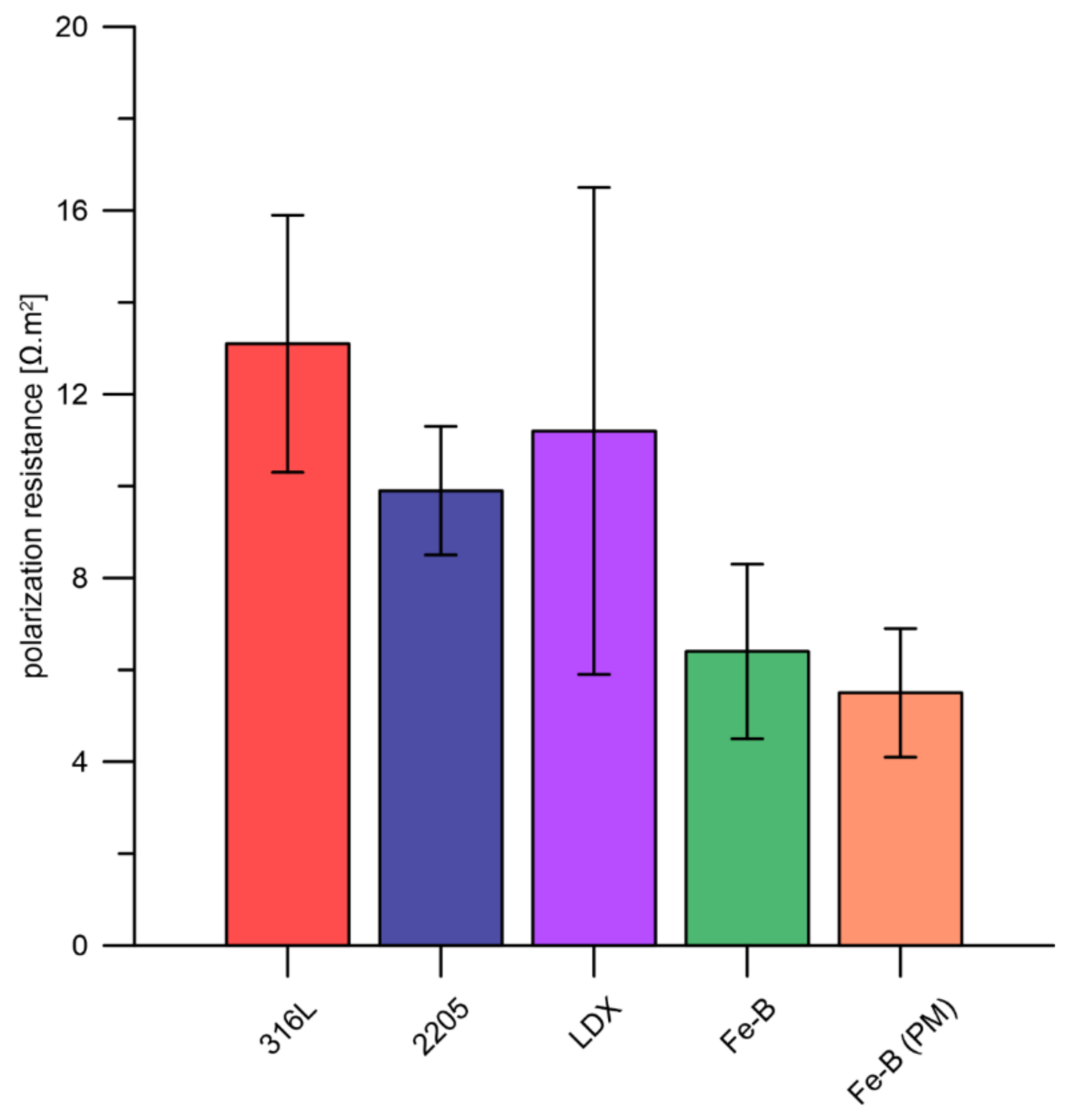
3.2. Accelerated Corrosion Tests
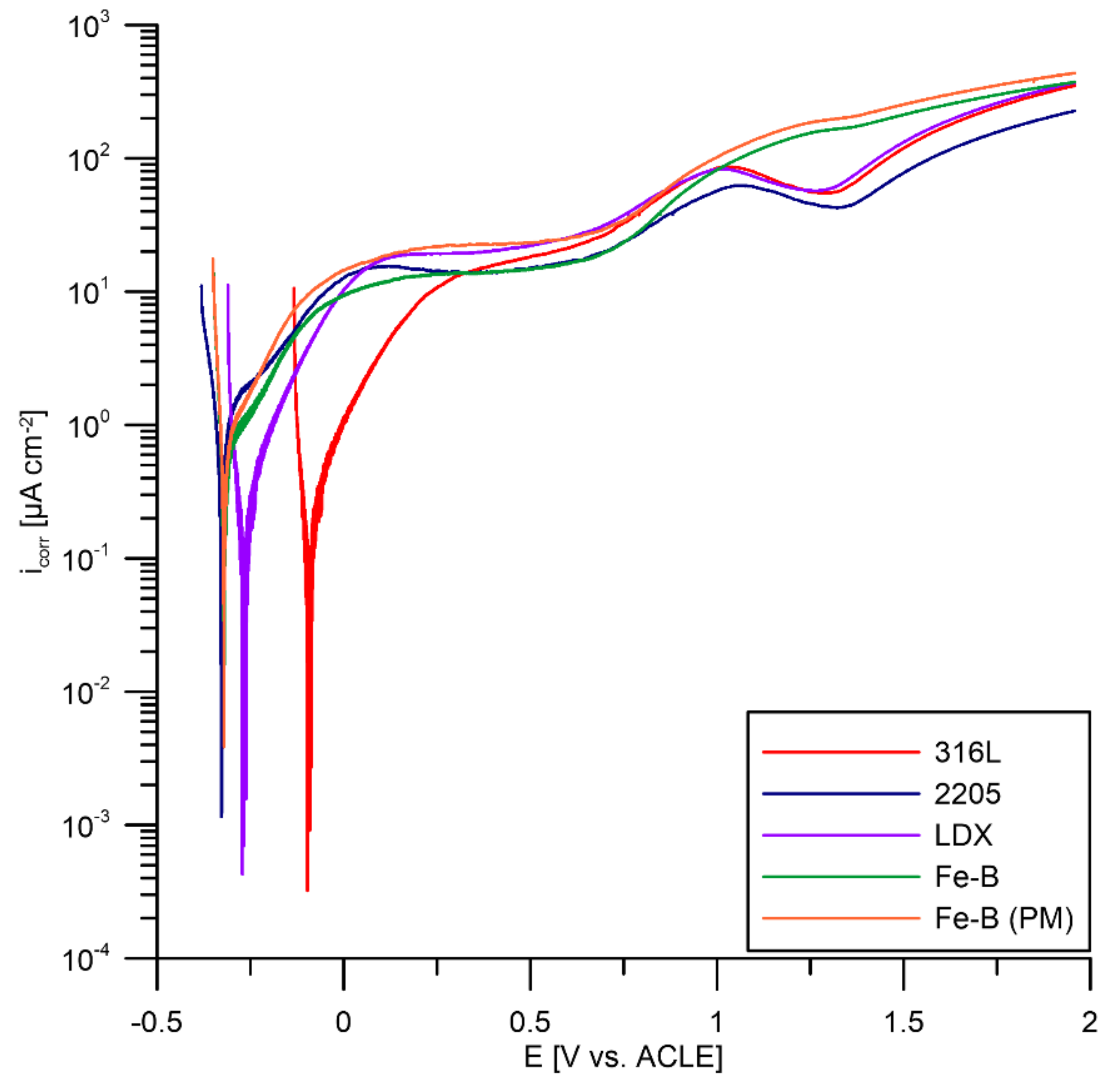
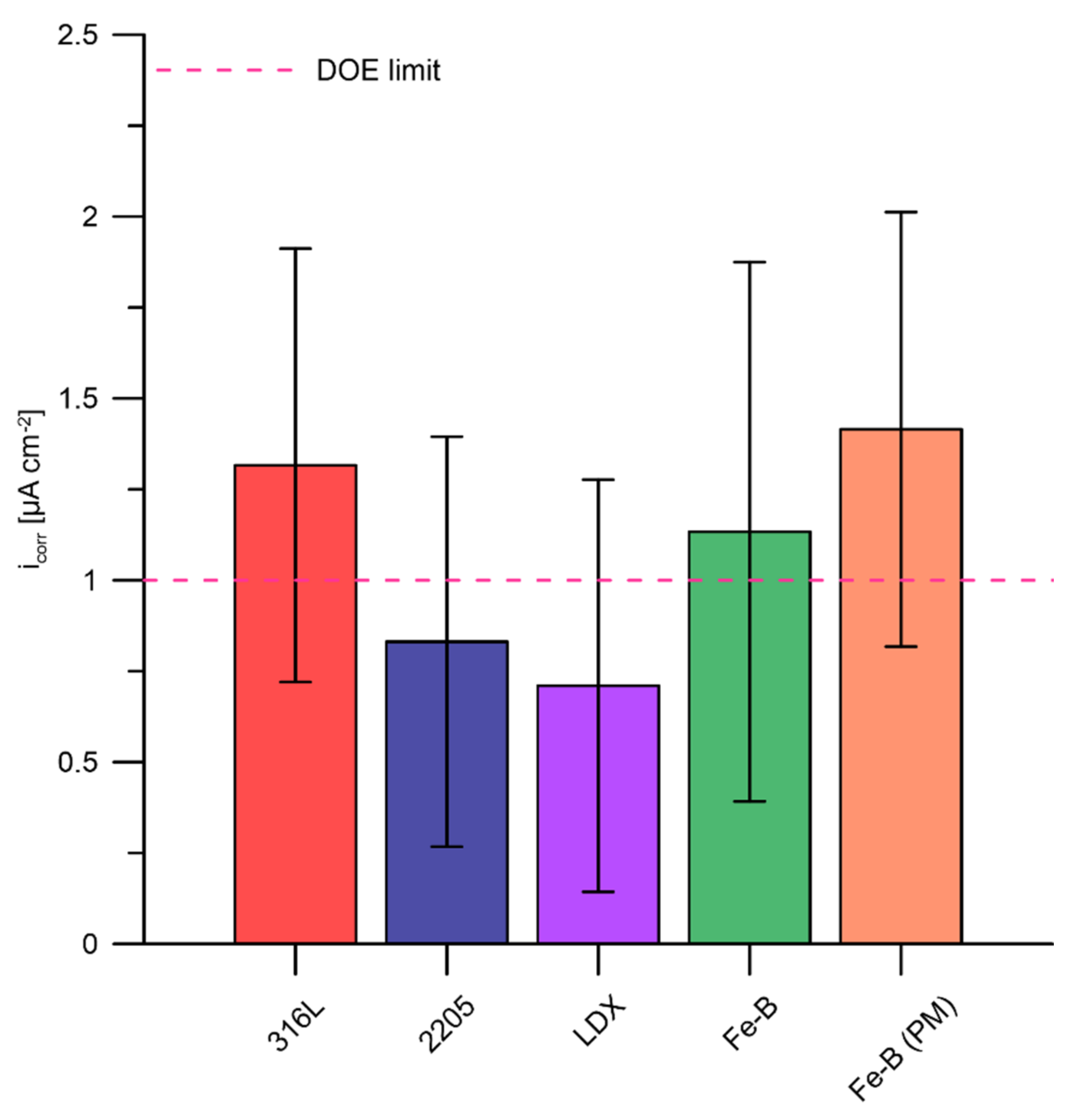
3.3. Potentiodynamic Polarization According to DOE
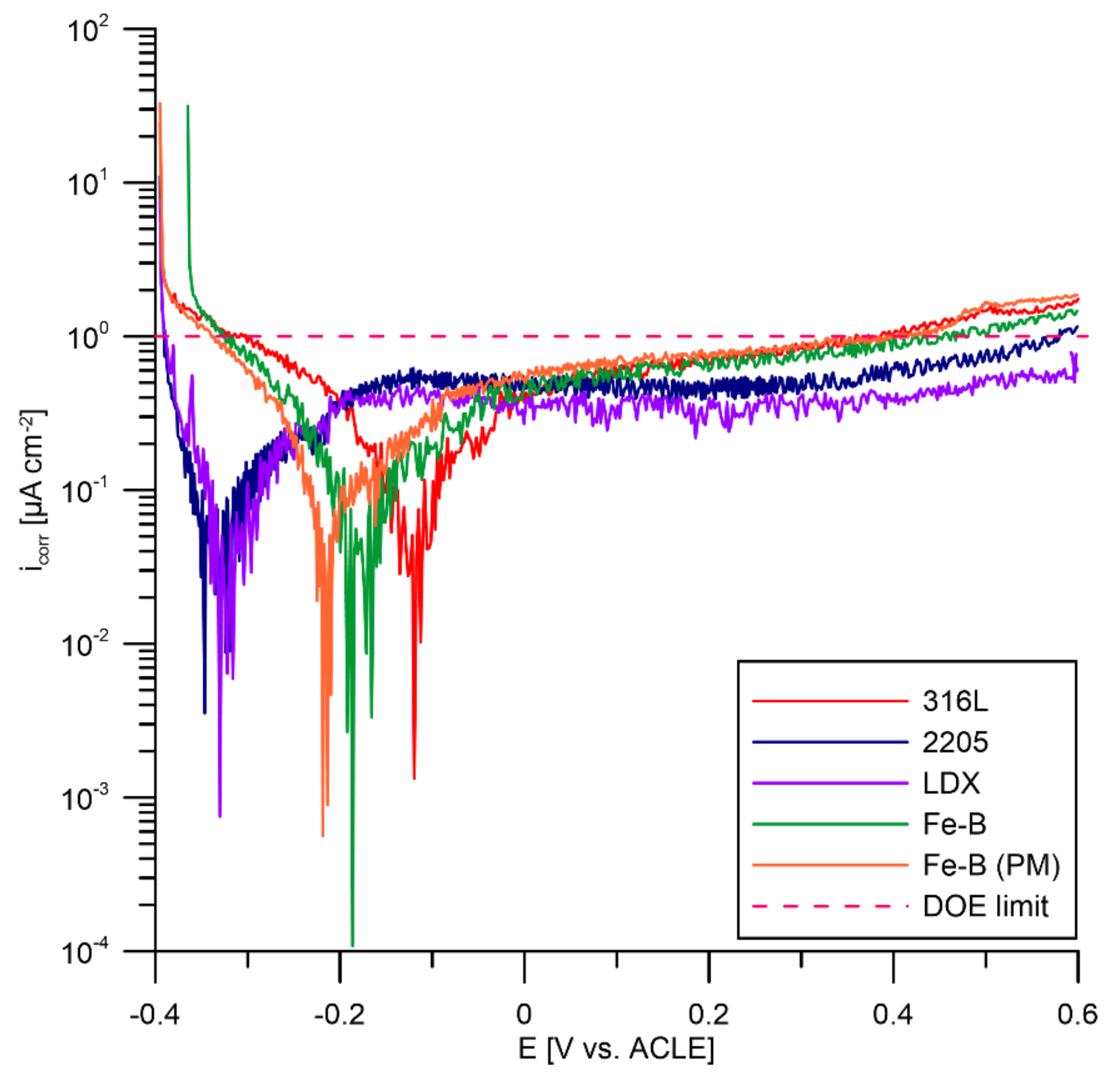
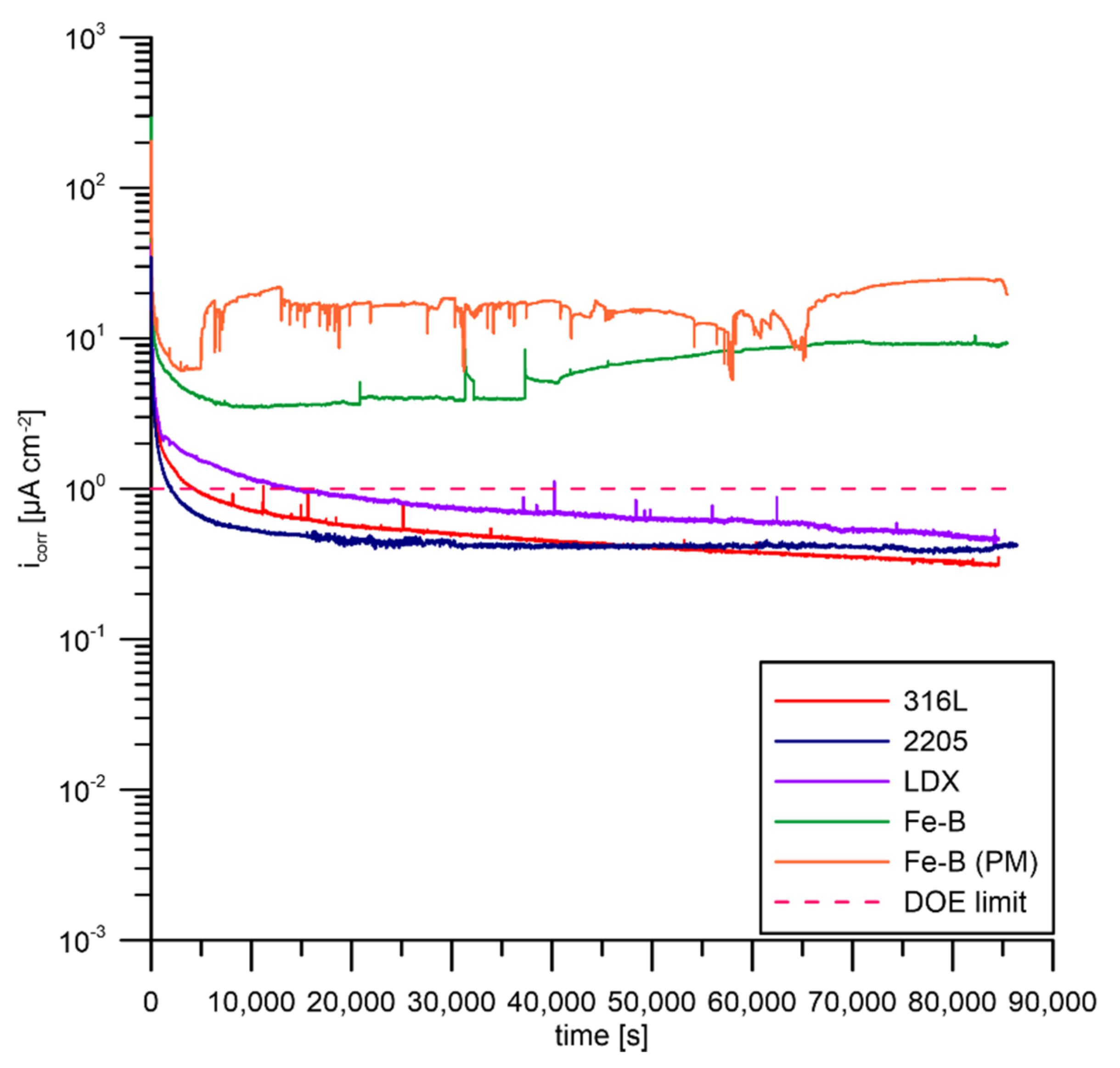
3.4. Potentiostatic Polarisation According to DOE
3.5. Interfacial Contact Resistance
4. Conclusions
- The best results were obtained for austenitic steel 316L and duplex steels 2205 and LDX.
- Fe-B and Fe-B (PM) steels contain significant amounts of chromium, which is bound in the microstructure in borides, and therefore the surrounding matrix is depleted of chromium and the steel itself loses its protective properties.
- The results of the accelerated corrosion tests served only for a quick comparison of the tested steels from the corrosion resistance point of view. No conclusions are drawn from them as to the overall suitability of any of the steels tested.
- The LDX and 2205 duplex steels achieved promising results.
- The 316L steel achieved approximately similar results to boron-alloyed steels.
- The results showed that all the materials are prone to crevice corrosion. It cannot be judged responsibly which of the boron-free stainless steels (316L, LDX, 2205) achieved the best results in this experiment.
- The boron-bearing steels, Fe-B and Fe-B (PM), exhibited much higher corrosion current values above the DOE limit as a result of the limited passivability and stability of passivation resulting from chromium depletion by chromium boride precipitates.
- Each of the tested steels exceeded the DOE limits after potentiostatic testing.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Chang, K.-Y. The optimal design for PEMFC modeling based on Taguchi method and genetic algorithm neural networks. Int. J. Hydrogen Energy 2011, 36, 13683–13694. [Google Scholar] [CrossRef]
- Yu, Y.; Shironita, S.; Mizukami, T.; Nakatsuyama, K.; Souma, K.; Umeda, M. Corrosion-resistant characteristics of nitrided Ni-free stainless steel for bipolar plate of polymer electrolyte fuel cell. Int. J. Hydrogen Energy 2017, 42, 6303–6309. [Google Scholar] [CrossRef]
- Yang, G.; Yu, S.; Mo, J.; Kang, Z.; Dohrmann, Y.; List, F.A.; Green, J.B.; Babu, S.S.; Zhang, F.-Y. Bipolar plate development with additive manufacturing and protective coating for durable and high-efficiency hydrogen production. J. Power Source 2018, 396, 590–598. [Google Scholar] [CrossRef]
- Wang, X.-Z.; Luo, H.; Luo, J.-L. Effects of hydrogen and stress on the electrochemical and passivation behaviour of 304 stainless steel in simulated PEMFC environment. Electrochim. Acta 2019, 293, 60–77. [Google Scholar] [CrossRef]
- Ozen, D.N.; Timurkutluk, B.; Altinisik, K. Effects of operation temperature and reactant gas humidity levels on performance of PEM fuel cells. Renew. Sustain. Energy Rev. 2016, 59, 1298–1306. [Google Scholar] [CrossRef]
- Ogungbemi, E.; Ijaodola, O.; Khatib, F.N.; Wilberforce, T.; El Hassan, Z.; Thompson, J.; Ramadan, M.; Olabi, A.G. Fuel cell membranes—Pros and cons. Energy 2019, 172, 155–172. [Google Scholar] [CrossRef]
- Antunes, R.A.; Oliveira, M.C.L.; Ett, G.; Ett, V. Corrosion of metal bipolar plates for PEM fuel cells: A review. Int. J. Hydrogen Energy 2010, 35, 3632–3647. [Google Scholar] [CrossRef]
- Boyacı San, F.G.; Okur, O. The effect of compression molding parameters on the electrical and physical properties of polymer composite bipolar plates. Int. J. Hydrogen Energy 2017, 42, 23054–23069. [Google Scholar] [CrossRef]
- Wang, J. System integration, durability and reliability of fuel cells: Challenges and solutions. Appl. Energy 2017, 189, 460–479. [Google Scholar] [CrossRef]
- Branislav, P.; Tomáš, B. Palivový článok—Zdroj energie. ATP J. 2007, 7, 83–84. [Google Scholar]
- Alo, O.A.; Otunniyi, I.O.; Pienaar, H.; Iyuke, S.E. Materials for Bipolar Plates in Polymer Electrolyte Membrane Fuel Cell: Performance Criteria and Current Benchmarks. Procedia Manuf. 2017, 7, 395–401. [Google Scholar] [CrossRef]
- Taherian, R. A review of composite and metallic bipolar plates in proton exchange membrane fuel cell: Materials, fabrication, and material selection. J. Power Source 2014, 265, 370–390. [Google Scholar] [CrossRef]
- Shimpalee, S.; Lilavivat, V.; McCrabb, H.; Khunatorn, Y.; Lee, H.K.; Lee, W.K.; Weidner, J.W. Investigation of bipolar plate materials for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2016, 41, 13688–13696. [Google Scholar] [CrossRef]
- Asri, N.F.; Husaini, T.; Sulong, A.B.; Majlan, E.H.; Daud, W.R.W. Coating of stainless steel and titanium bipolar plates for anticorrosion in PEMFC: A review. Int. J. Hydrogen Energy 2017, 42, 9135–9148. [Google Scholar] [CrossRef]
- Lee, K.H.; Jin, C.K.; Kang, C.G.; Seo, H.Y.; Kim, J.D. Fabrication of Titanium Bipolar Plates by Rubber Forming Process and Evaluation Characteristics of TiN coated Titanium Bipolar Plates. Fuel Cells 2015, 15, 170–177. [Google Scholar] [CrossRef]
- Zhang, D.; Duan, L.; Guo, L.; Wang, Z.; Zhao, J.; Tuan, W.-H.; Niihara, K. TiN-coated titanium as the bipolar plate for PEMFC by multi-arc ion plating. Int. J. Hydrogen Energy 2011, 36, 9155–9161. [Google Scholar] [CrossRef]
- Gou, Y.; Chen, H.; Li, R.; Geng, J.; Shao, Z. Nb–Cr–C coated titanium as bipolar plates for proton exchange membrane fuel cells. J. Power Source 2022, 520, 230797. [Google Scholar] [CrossRef]
- Dong, P.; Li, Z.; Feng, S.; Wu, Z.; Cao, Q.; Li, L.; Chen, Q.; Han, X. Fabrication of titanium bipolar plates for proton exchange membrane fuel cells by uniform pressure electromagnetic forming. Int. J. Hydrogen Energy 2021, 46, 38768–38781. [Google Scholar] [CrossRef]
- Wang, J.; Min, L.; Fang, F.; Zhang, W.; Wang, Y. Electrodeposition of graphene nano-thick coating for highly enhanced performance of titanium bipolar plates in fuel cells. Int. J. Hydrogen Energy 2019, 44, 16909–16917. [Google Scholar] [CrossRef]
- Karimi, S.; Fraser, N.; Roberts, B.; Foulkes, F.R. A Review of Metallic Bipolar Plates for Proton Exchange Membrane Fuel Cells: Materials and Fabrication Methods. Adv. Mater. Sci. Eng. 2012, 2012, 828070. [Google Scholar] [CrossRef]
- Sadeghian, Z.; Hadidi, M.R.; Salehzadeh, D.; Nemati, A. Hydrophobic octadecylamine-functionalized graphene/TiO2 hybrid coating for corrosion protection of copper bipolar plates in simulated proton exchange membrane fuel cell environment. Int. J. Hydrogen Energy 2020, 45, 15380–15389. [Google Scholar] [CrossRef]
- Hsieh, S.-S.; Huang, C.-F.; Feng, C.-L. A novel design and micro-fabrication for copper (Cu) electroforming bipolar plates. Micron 2008, 39, 263–268. [Google Scholar] [CrossRef]
- Nikam, V.V.; Reddy, R.G. Copper alloy bipolar plates for polymer electrolyte membrane fuel cell. Electrochim. Acta 2006, 51, 6338–6345. [Google Scholar] [CrossRef]
- Feng, K.; Guo, X.; Li, Z.; Yao, C.; Wu, Y. Investigation of multi-coating process treated magnesium alloy as bipolar plate in polymer electrolyte membrane fuel cell. Int. J. Hydrogen Energy 2016, 41, 6020–6028. [Google Scholar] [CrossRef]
- Hao, W.; Ma, H.; Sun, G.; Li, Z. Developing high performance magnesium phosphate cement composite bipolar plates for fuel cells. Energy Procedia 2019, 158, 1980–1985. [Google Scholar] [CrossRef]
- Barranco, J.; Barreras, F.; Lozano, A.; Lopez, A.M.; Roda, V.; Martin, J.; Maza, M.; Fuentes, G.G.; Almandoz, E. Cr and Zr/Cr nitride CAE-PVD coated aluminum bipolar plates for polymer electrolyte membrane fuel cells. Int. J. Hydrogen Energy 2010, 35, 11489–11498. [Google Scholar] [CrossRef]
- Bolouri, A.; Kang, C.G. Study on dimensional and corrosion properties of thixoformed A356 and AA7075 aluminum bipolar plates for proton exchange membrane fuel cells. Renew. Energy 2014, 71, 616–628. [Google Scholar] [CrossRef]
- Barranco, J.; Barreras, F.; Lozano, A.; Maza, M. Influence of CrN-coating thickness on the corrosion resistance behaviour of aluminium-based bipolar plates. J. Power Source 2011, 196, 4283–4289. [Google Scholar] [CrossRef]
- Hou, K.-H.; Lin, C.-H.; Ger, M.-D.; Shiah, S.-W.; Chou, H.-M. Analysis of the Corrosion Behavior of Al Alloy Bipolar Plate for Proton Exchange Membrane Fuel Cell (PEMFC) Under Operating Thermal Conditions. Int. J. Green Energy 2012, 9, 71–83. [Google Scholar] [CrossRef]
- Mele, C.; Bozzini, B. Localised corrosion processes of austenitic stainless steel bipolar plates for polymer electrolyte membrane fuel cells. J. Power Source 2010, 195, 3590–3596. [Google Scholar] [CrossRef]
- Yuan, X.Z.; Wang, H.; Zhang, J.; Wilkinson, D.P. Bipolar plates for PEM fuel cells—From materials to processing. J. New Mater. Electrochem. Syst. 2005, 8, 257–267. [Google Scholar]
- Lee, S.-J.; Huang, C.-H.; Lai, J.-J.; Chen, Y.-P. Corrosion-resistant component for PEM fuel cells. J. Power Source 2004, 131, 162–168. [Google Scholar] [CrossRef]
- Yi, P.; Zhang, D.; Qiu, D.; Peng, L.; Lai, X. Carbon-based coatings for metallic bipolar plates used in proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2019, 44, 6813–6843. [Google Scholar] [CrossRef]
- Lin, K.; Li, X.; Dong, H.; Du, S.; Lu, Y.; Ji, X.; Gu, D. Surface modification of 316 stainless steel with platinum for the application of bipolar plates in high performance proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2017, 42, 2338–2348. [Google Scholar] [CrossRef]
- Pugal Mani, S.; Srinivasan, A.; Rajendran, N. Effect of nitrides on the corrosion behaviour of 316L SS bipolar plates for Proton Exchange Membrane Fuel Cell (PEMFC). Int. J. Hydrogen Energy 2015, 40, 3359–3369. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Lu, Z.; Wang, L.; Li, W. Preparation and performances of electrically conductive Nb-doped TiO2 coatings for 316 stainless steel bipolar plates of proton-exchange membrane fuel cells. Corros. Sci. 2018, 142, 249–257. [Google Scholar] [CrossRef]
- Dundar, F.; Dur, E.; Mahabunphachai, S.; Koç, M. Corrosion resistance characteristics of stamped and hydroformed proton exchange membrane fuel cell metallic bipolar plates. J. Power Source 2010, 195, 3546–3552. [Google Scholar] [CrossRef]
- Huang, P.; Chen, Z.; Zhang, J.; Wu, M.; Liu, Y.; Zhang, F.; Chen, Y.; Chen, X. Stainless steel bipolar plate fuel cell with different flow field structures prepared by laser additive manufacturing. Int. J. Heat Mass Transf. 2022, 183, 122186. [Google Scholar] [CrossRef]
- Sánchez-Molina, M.; Amores, E.; Rojas, N.; Kunowsky, M. Additive manufacturing of bipolar plates for hydrogen production in proton exchange membrane water electrolysis cells. Int. J. Hydrogen Energy 2021, 46, 38983–38991. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical properties of biomedical titanium alloys. Mater. Sci. Eng. A 1998, 243, 231–236. [Google Scholar] [CrossRef]
- Mouritz, A.P. Introduction to Aerospace Materials. In Introduction to Aerospace Materials; Mouritz, A.P., Ed.; Woodhead Publishing: Sawston, UK, 2012; pp. 202–223. [Google Scholar] [CrossRef]
- Oyj, O. Handbook of Stainless Steel; Outokumpu Oyj: Helsinki, Finland, 2017; pp. 45–53. [Google Scholar]
- Feng, K.; Wu, G.; Li, Z.; Cai, X.; Chu, P.K. Corrosion behavior of SS316L in simulated and accelerated PEMFC environments. Int. J. Hydrogen Energy 2011, 36, 13032–13042. [Google Scholar] [CrossRef]
- Li, D.G.; Wang, J.D.; Chen, D.R.; Liang, P. Molybdenum addition enhancing the corrosion behaviors of 316 L stainless steel in the simulated cathodic environment of proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2015, 40, 5947–5957. [Google Scholar] [CrossRef]
- Miyazawa, A.; Tada, E.; Nishikata, A. Influence of corrosion of SS316L bipolar plate on PEFC performance. J. Power Source 2013, 231, 226–233. [Google Scholar] [CrossRef]
- Bozec, N.L.; Comepre, C.; L’Her, M.; Laouenan, A.; Costa, D.; Marcus, P. Infuence of stainless steel surface treatment on the oxygen reduction reaction in seawater. Corros. Sci. 2001, 43, 765–786. [Google Scholar] [CrossRef]
- Dadfar, M.; Salehi, M.; Golozar, M.A.; Trasatti, S.; Casaletto, M.P. Surface and corrosion properties of modified passive layer on 304 stainless steel as bipolar plates for PEMFCs. Int. J. Hydrogen Energy 2017, 42, 25869–25876. [Google Scholar] [CrossRef]
- Dadfar, M.; Salehi, M.; Golozar, M.A.; Trasatti, S. Surface modification of 304 stainless steels to improve corrosion behavior and interfacial contact resistance of bipolar plates. Int. J. Hydrogen Energy 2016, 41, 21375–21384. [Google Scholar] [CrossRef]
- Kumagai, M.; Myung, S.-T.; Katada, Y.; Yashiro, H. Stability of type 310S stainless steel bipolar plates tested at various current densities in proton exchange membrane fuel cells. Electrochim. Acta 2016, 211, 754–760. [Google Scholar] [CrossRef]
- Jinlong, L.; Zhuqing, W.; Tongxiang, L.; Ken, S.; Hideo, M. Enhancing the corrosion resistance of the 2205 duplex stainless steel bipolar plates in PEMFCs environment by surface enriched molybdenum. Results Phys. 2017, 7, 3459–3464. [Google Scholar] [CrossRef]
- Wang, J.; Sun, J.; Li, S.; Wen, Z.; Ji, S. Surface diffusion modification AISI 304SS stainless steel as bipolar plate material for proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2012, 37, 1140–1144. [Google Scholar] [CrossRef]
- Davies, D.P.; Adcock, P.L.; Turpin, M.; Rowen, S.J. Stainless steel as a bipolar plate material for solid polymer fuel cells. J. Power Source 2000, 86, 237–242. [Google Scholar] [CrossRef]
- Kumagai, M.; Myung, S.-T.; Kuwata, S.; Asaishi, R.; Yashiro, H. Corrosion behavior of austenitic stainless steels as a function of pH for use as bipolar plates in polymer electrolyte membrane fuel cells. Electrochim. Acta 2008, 53, 4205–4212. [Google Scholar] [CrossRef]
- Zhou, Y.; Engelberg, D.L. Application of bipolar electrochemistry to assess the ambient temperature corrosion resistance of solution annealed type 2205 duplex stainless steel. Mater. Chem. Phys. 2022, 275, 125183. [Google Scholar] [CrossRef]
- Weil, K.S.; Kim, J.Y.; Xia, G.; Coleman, J.; Yang, Z.G. Boronization of nickel and nickel clad materials for potential use in polymer electrolyte membrane fuel cells. Surf. Coat. Technol. 2006, 201, 4436–4441. [Google Scholar] [CrossRef]
- Williams, W.S. Transition-metal carbides. Prog. Solid State Chem. 1971, 6, 57–118. [Google Scholar] [CrossRef]
- Iversen, A.K. Stainless steels in bipolar plates—Surface resistive properties of corrosion resistant steel grades during current loads. Corros. Sci. 2006, 48, 1036–1058. [Google Scholar] [CrossRef]
- Hinds, G.; Brightman, E. Towards more representative test methods for corrosion resistance of PEMFC metallic bipolar plates. Int. J. Hydrogen Energy 2015, 40, 2785–2791. [Google Scholar] [CrossRef]
- Office of Energy Efficiency & Renewable Energy. DOE Technical Targets for Polymer Electrolyte Membrane Fuel Cell Components. 2019. Available online: https://www.energy.gov/eere/fuelcells/doe-technical-targets-polymer-electrolyte-membrane-fuel-cell-components (accessed on 20 March 2021).
- Yang, Y.; Guo, L.-j.; Liu, H. Corrosion characteristics of SS316L as bipolar plate material in PEMFC cathode environments with different acidities. Int. J. Hydrogen Energy 2011, 36, 1654–1663. [Google Scholar] [CrossRef]
- Yoon, W.; Huang, X.; Fazzino, P.; Reifsnider, K.L.; Akkaoui, M.A. Evaluation of coated metallic bipolar plates for polymer electrolyte membrane fuel cells. J. Power Source 2008, 179, 265–273. [Google Scholar] [CrossRef]
- Stoulil, J.; Hemmer, V.; Šefl, V.; Bystrianský, J. Corrosion resistance of new powder metallurgy boron-containing stainless steel in the nuclear repository environment. Mater. Corros. 2015, 66, 342–346. [Google Scholar] [CrossRef]
- Yamada, K.; Ohtani, H.; Hasebe, M. Thermodynamic Analysis of the Fe-Cr-B Ternary System. High Temp. Mater. Processes 2008, 27, 269–284. [Google Scholar] [CrossRef]
- Wang, H.; Wang, T. A comparative study of high boron alloys with 2.0 wt% B based on 304 and 316 stainless steels. Mater. Lett. 2021, 285, 129035. [Google Scholar] [CrossRef]
- Serafini, F.L.; Peruzzo, M.; Krindges, I.; Ordoñez, M.F.C.; Rodrigues, D.; Souza, R.M.; Farias, M.C.M. Microstructure and mechanical behavior of 316L liquid phase sintered stainless steel with boron addition. Mater. Charact. 2019, 152, 253–264. [Google Scholar] [CrossRef]
- Loria, E.A.; Isaacs, H.S. Type 304 Stainless Steel With 0.5% Boron for Storage of Spent Nuclear Fuel. JOM 1980, 32, 10–17. [Google Scholar] [CrossRef]
- Szewczyk-Nykiel, A. The effect of the addition of boron on the densification, microstructure and properties of sintered 17-4 ph stainless steel. Tech. Trans. 2014, 2-M, 85–96. [Google Scholar]
- Papadias, D.D.; Ahluwalia, R.K.; Thomson, J.K.; Meyer, H.M.; Brady, M.P.; Wang, H.; Turner, J.A.; Mukundan, R.; Borup, R. Degradation of SS316L bipolar plates in simulated fuel cell environment: Corrosion rate, barrier film formation kinetics and contact resistance. J. Power Source 2015, 273, 1237–1249. [Google Scholar] [CrossRef]
- Feng, K.; Shen, Y.; Mai, J.; Liu, D.; Cai, X. An investigation into nickel implanted 316L stainless steel as a bipolar plate for PEM fuel cell. J. Power Source 2008, 182, 145–152. [Google Scholar] [CrossRef]
- Feng, K.; Shen, Y.; Liu, D.; Chu, P.K.; Cai, X. Ni–Cr Co-implanted 316L stainless steel as bipolar plate in polymer electrolyte membrane fuel cells. Int. J. Hydrogen Energy 2010, 35, 690–700. [Google Scholar] [CrossRef]
- Jeon, W.S.; Kim, J.G.; Kim, Y.J.; Han, J.G. Electrochemical properties of TiN coatings on 316L stainless steel separator for polymer electrolyte membrane fuel cell. Thin Solid Film. 2008, 516, 3669–3672. [Google Scholar] [CrossRef]
- Wind, J.; Späh, R.; Kaiser, W.; Böhm, G. Metallic bipolar plates for PEM fuel cells. J. Power Source 2002, 105, 256–260. [Google Scholar] [CrossRef]
- Vladimír, Č. Korozivzdorné Oceli a Slitiny; Academia: Prague, Czech Republic, 1999. [Google Scholar]
- Fremunt, P.; Podrábksý, T. Konstrukční Oceli; Akademické Nakladatelství CERM: Brno, Czech Republic, 1996. [Google Scholar]
- Richards, J.; Cremers, C.; Fischer, P.; Schmidt, K. Corrosion Studies on Electro Polished Stainless Steels for the Use as Metallic Bipolar Plates in PEMFC Applications. Energy Procedia 2012, 20, 324–333. [Google Scholar] [CrossRef]
- Yun, Y.-H. Deposition of gold–titanium and gold–nickel coatings on electropolished 316L stainless steel bipolar plates for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2010, 35, 1713–1718. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, D. Effect of surface treatment on the interfacial contact resistance and corrosion resistance of Fe–Ni–Cr alloy as a bipolar plate for polymer electrolyte membrane fuel cells. Energy 2014, 64, 242–247. [Google Scholar] [CrossRef]
- Olsson, C.O.A.; Landolt, D. Film Growth during Anodic Polarization in the Passive Region on 304 Stainless Steels with Cr, Mo, or W Additions Studied with EQCM and XPS. J. Electrochem. Soc. 2001, 148, B438. [Google Scholar] [CrossRef]
- Kim, J.S.; Peelen, W.H.A.; Hemmes, K.; Makkus, R.C. Effect of alloying elements on the contact resistance and the passivation behaviour of stainless steels. Corros. Sci. 2002, 44, 635–655. [Google Scholar] [CrossRef]
- Schoeler, A.C.; Kaun, T.D.; Krumpelt, M. Corrosion behavior of coated steels and Mn- and Co-alloyed steels for separator materials on the cathode side in molten carbonate fuel cells. Mater. Corros. Werkstof. Korros. 2000, 51, 797–807. [Google Scholar] [CrossRef]



| 304 1.4301 | 316L 1.4404 | 904L 1.4539 | 254SMO 1.4547 | 2205 1.4462 | LDX 1.4162 | Pure Ti Grade 2 | DOE Requirement | |
|---|---|---|---|---|---|---|---|---|
| A (%) | ≈55 | ≈55 | ≈50 | ≈50 | ≈35 | ≈38 | ≈20 | 40 |
| Property | Target | Notes |
|---|---|---|
| Cost | <3 $ kW−1 | 2002 dollars, 500,000 stacks per year |
| Corrosion resistance (anode) | <1 µA cm−2 no active peak | pH 3, 0.1 ppm HF, 80 °C, Ar purge Potentiodynamic test −0.4 V to + 0.6 V (ACLE), 0.1 mV s−1 |
| Corrosion resistance (cathode) | <1 µA cm−2 | pH 3, 0.1 ppm HF, 80 °C, aerated Potentiostatic test (>24 h) 0.6 V (ACLE), ipassive < 1 µA cm−2 |
| Electrical conductivity | >100 S cm−1 | - |
| Areal specific resistance | <0.01 Ω cm2 | Including contact resistance at 138 N cm−2 |
| Hydrogen permeability | <1.3 × 10−14 cm3 | ASTM D1434, 80 °C, 3 atm, 100% RH |
| Flexural strength | >25 MPa | ASTM D790-10 |
| Forming elongation | 40% | ASTM E8M-01 |
| C | Cr | Ni | Mo | Cu | B | Mn | |
|---|---|---|---|---|---|---|---|
| FeCr18Ni13Mo3 (316L) | 0.03 | 17.4 | 14.4 | 2.8 | - | - | 1.8 |
| FeCr21Ni5Mo3Mn2 (2205) | 0.04 | 22.6 | 5.6 | 2.6 | - | - | 1.6 |
| FeCr21Ni2Mo0.5Cu0.5Mn4 (LDX) | 0.03 | 21.5 | 1.5 | 0.2 | 0.2 | - | 4.7 |
| FeCr18Ni13B1Mn2 (Fe-B) | 0.03 | 19.1 | 12.7 | - | - | 1.2 | 1.1 |
| FeCr19Ni13B1Mn2 (Fe-B-PM) | 0.04 | 19.2 | 13.3 | - | - | 1.2 | 1.5 |
| Material | 316L | LDX | 2205 | Fe-B | Fe-B (PM) |
|---|---|---|---|---|---|
| Ecorr (mV vs. ACLE) | −159 ± 66 | −301 ± 93 | −282 ± 49 | −313 ± 71 | −259 ± 28 |
| Specimen | 316L | 2205 | LDX | Fe-B | Fe-B (PM) |
|---|---|---|---|---|---|
| Ecorr (V vs. ACLE) | −0.022 | −0.135 | −0.148 | −0.228 | −0.173 |
| icorr (μA cm−2) | 0.245 | 0.177 | 0.371 | 0.815 | 0.800 |
| βc (V per decade) | 0.251 | 0.198 | 0.251 | 0.171 | 0.214 |
| Specimen | 316L | 2205 | LDX | Fe-B | Fe-B (PM) |
|---|---|---|---|---|---|
| Ecorr (V vs. ACLE) | −0.097 | −0.328 | −0.272 | −0.320 | −0.320 |
| icorr (μA cm−2) | 0.482 | 1.051 | 0.192 | 0.816 | 0.963 |
| βa (V per decade) | 0.237 | 0.261 | 0.192 | 0.306 | 0.205 |
| Specimen | 316L | 2205 | LDX | Fe-B | Fe-B (PM) |
|---|---|---|---|---|---|
| Exposed | 454 | 438 | 782 | 670 | 309 |
| Ground | 8.5 | 10.6 | 11.9 | 9.9 | 12.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lovaši, T.; Pečinka, V.; Ludvík, J.; Kubásek, J.; Průša, F.; Kouřil, M. Corrosion Properties of Boron- and Manganese-Alloyed Stainless Steels as a Material for the Bipolar Plates of PEM Fuel Cells. Materials 2022, 15, 6557. https://doi.org/10.3390/ma15196557
Lovaši T, Pečinka V, Ludvík J, Kubásek J, Průša F, Kouřil M. Corrosion Properties of Boron- and Manganese-Alloyed Stainless Steels as a Material for the Bipolar Plates of PEM Fuel Cells. Materials. 2022; 15(19):6557. https://doi.org/10.3390/ma15196557
Chicago/Turabian StyleLovaši, Tomáš, Vojtěch Pečinka, Jakub Ludvík, Jiří Kubásek, Filip Průša, and Milan Kouřil. 2022. "Corrosion Properties of Boron- and Manganese-Alloyed Stainless Steels as a Material for the Bipolar Plates of PEM Fuel Cells" Materials 15, no. 19: 6557. https://doi.org/10.3390/ma15196557






