Effect of Alloying Elements and Low Temperature Plasma Nitriding on Corrosion Resistance of Stainless Steel
Abstract
:1. Introduction
2. Experimental and Numerical Simulation Methods
2.1. Experimental Methods
2.1.1. Experimental Materials and Sample Preparation
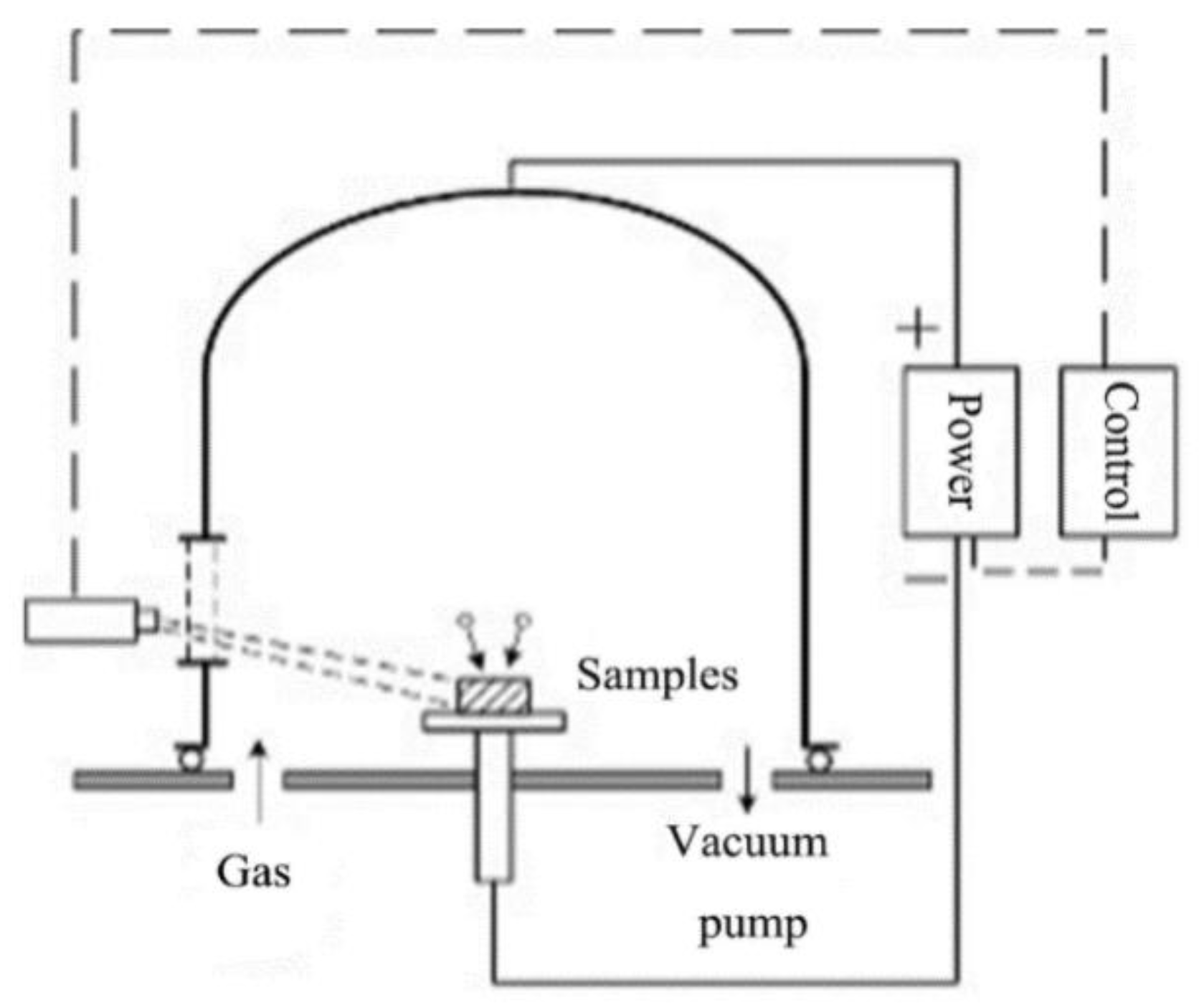
2.1.2. Low Temperature Plasma Nitriding Treatment
2.1.3. Characterization
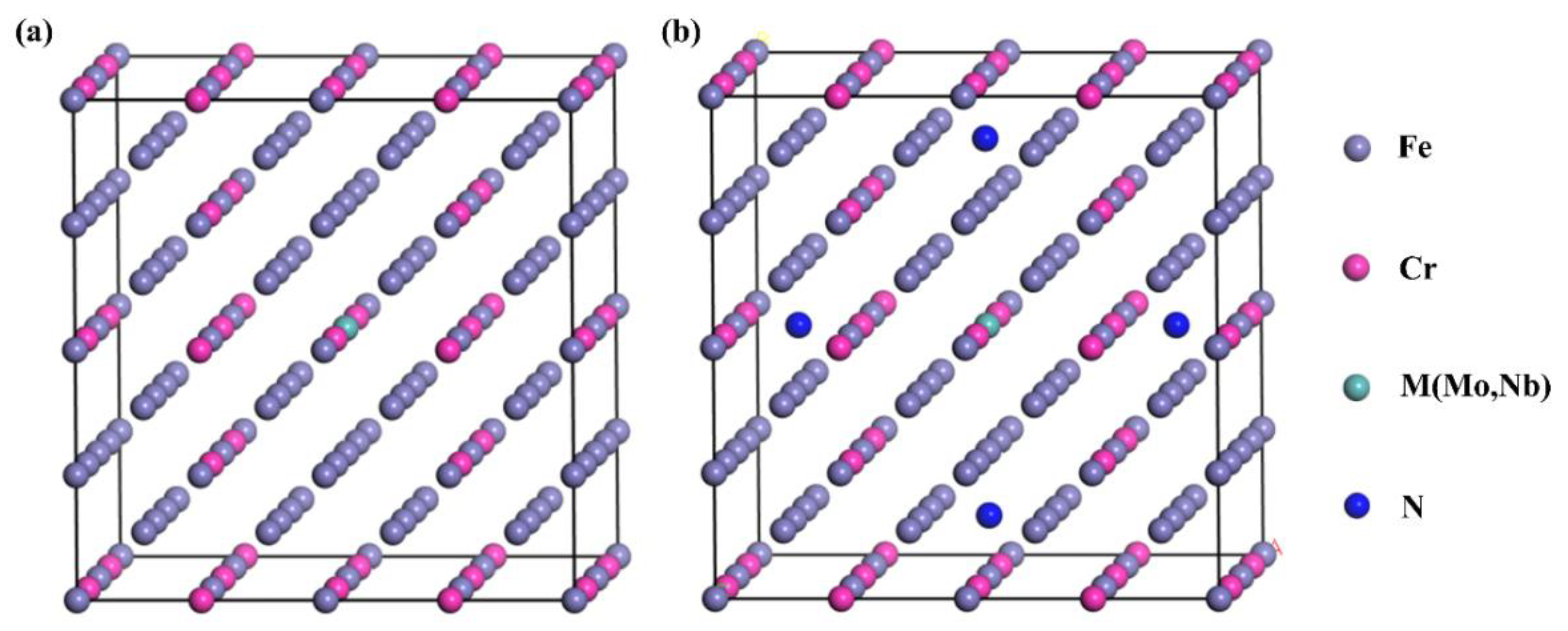
2.2. First-Principles Calculations
3. Results and Discussion
3.1. DFT Results
3.2. Experimental Results
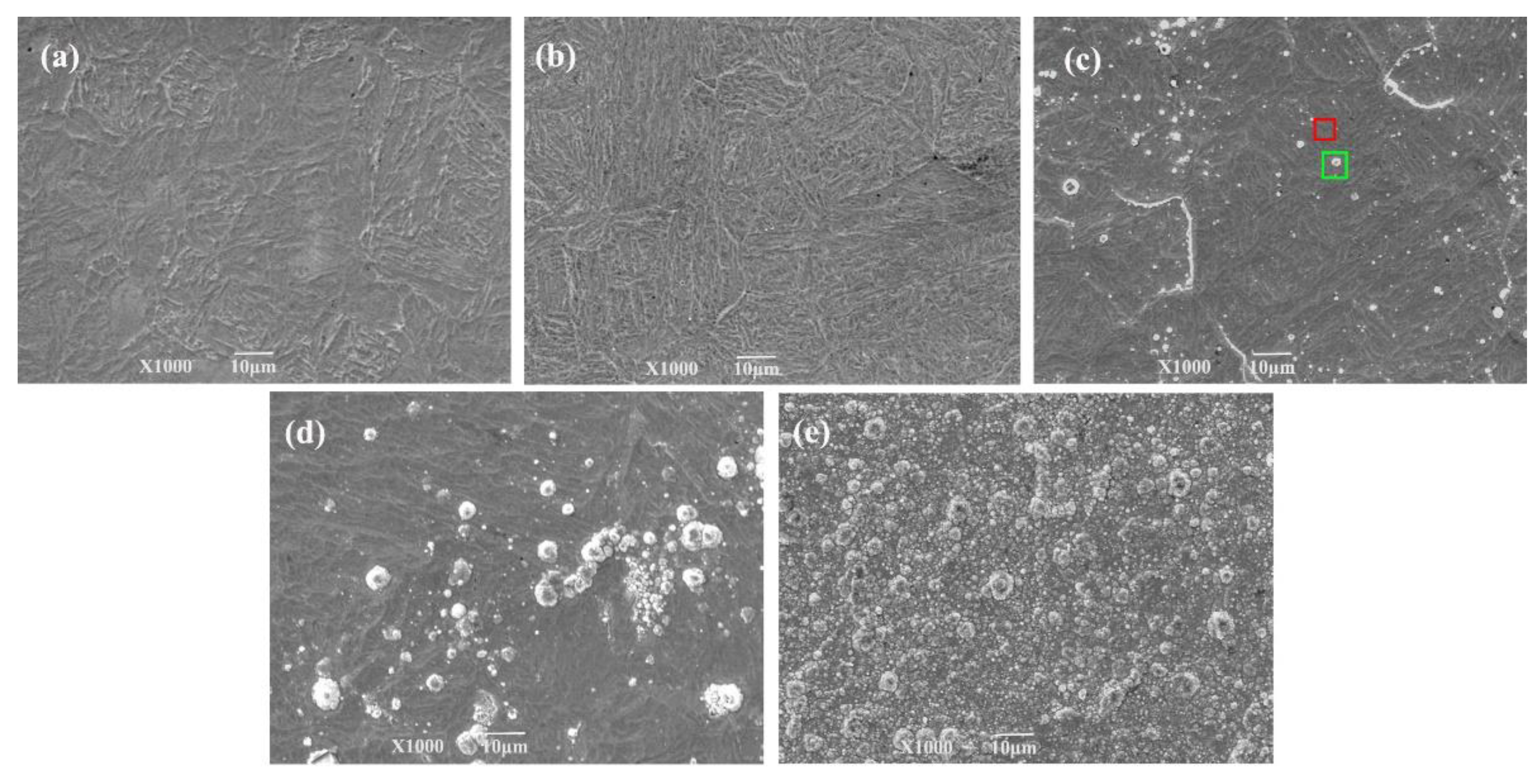
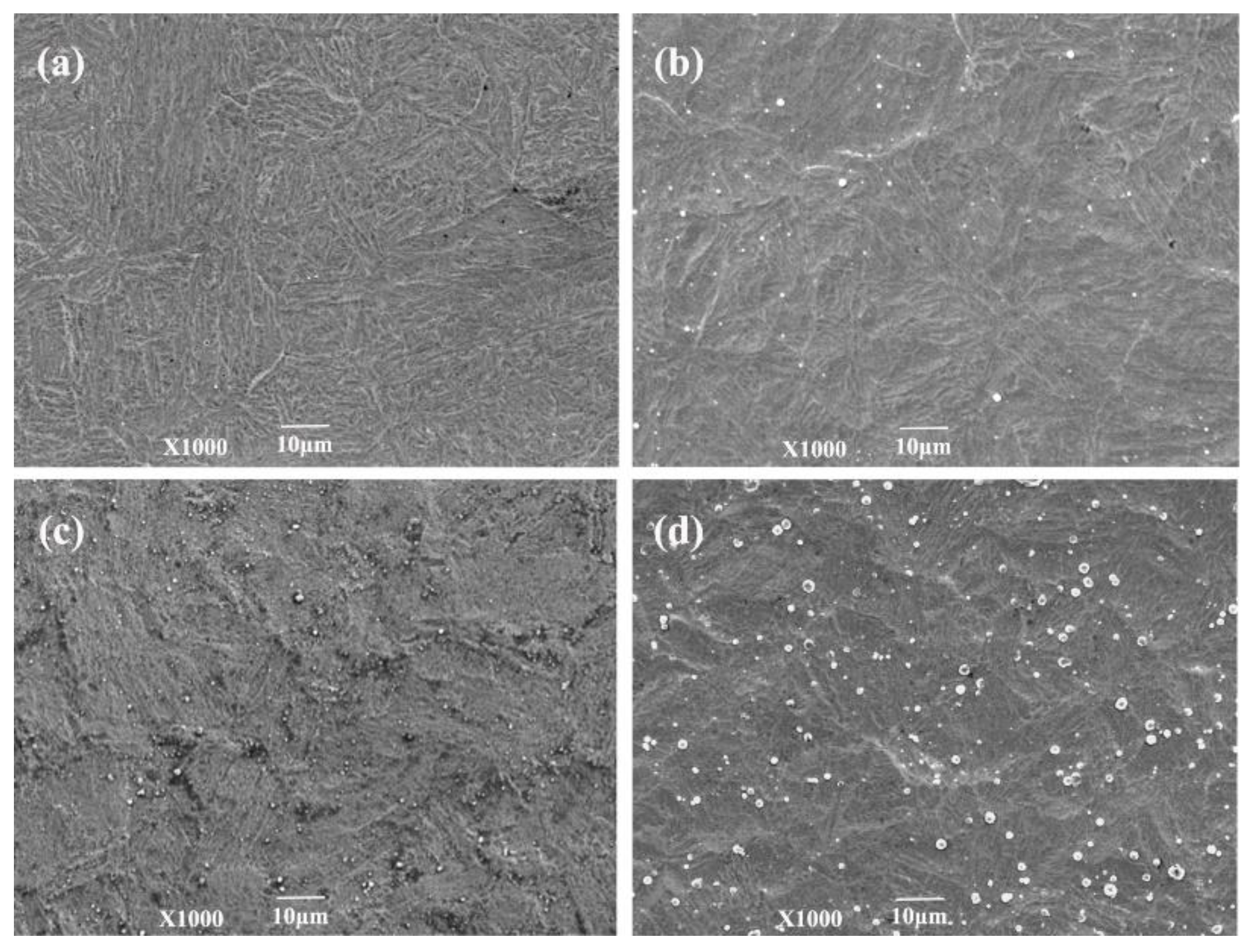
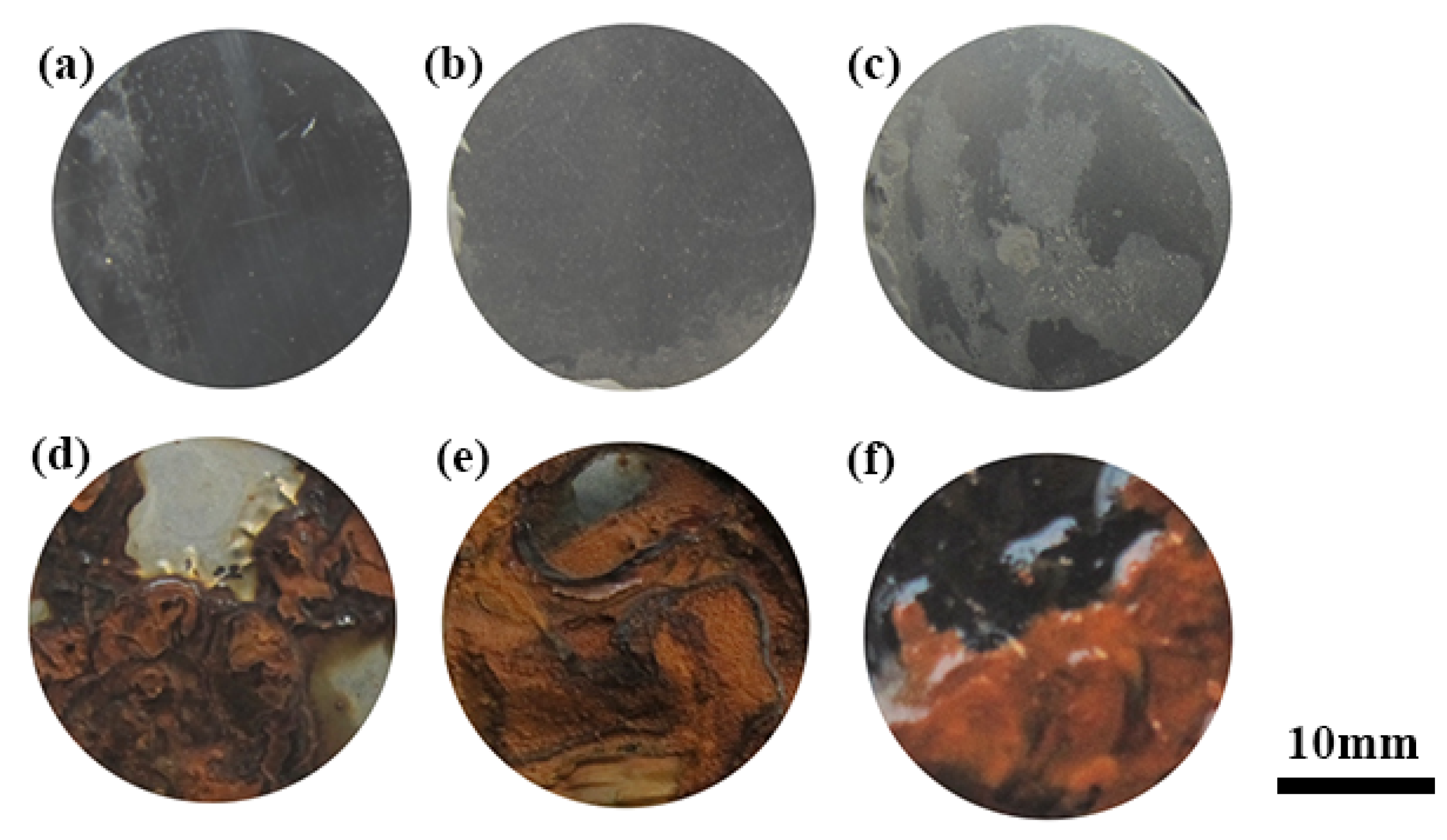
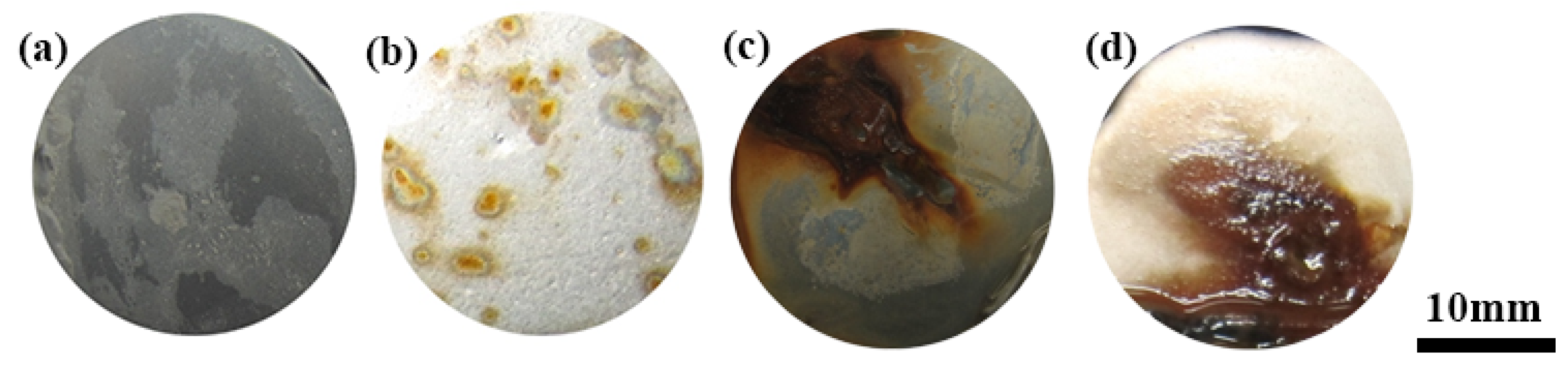
3.2.1. Surface Morphology and Microstructure
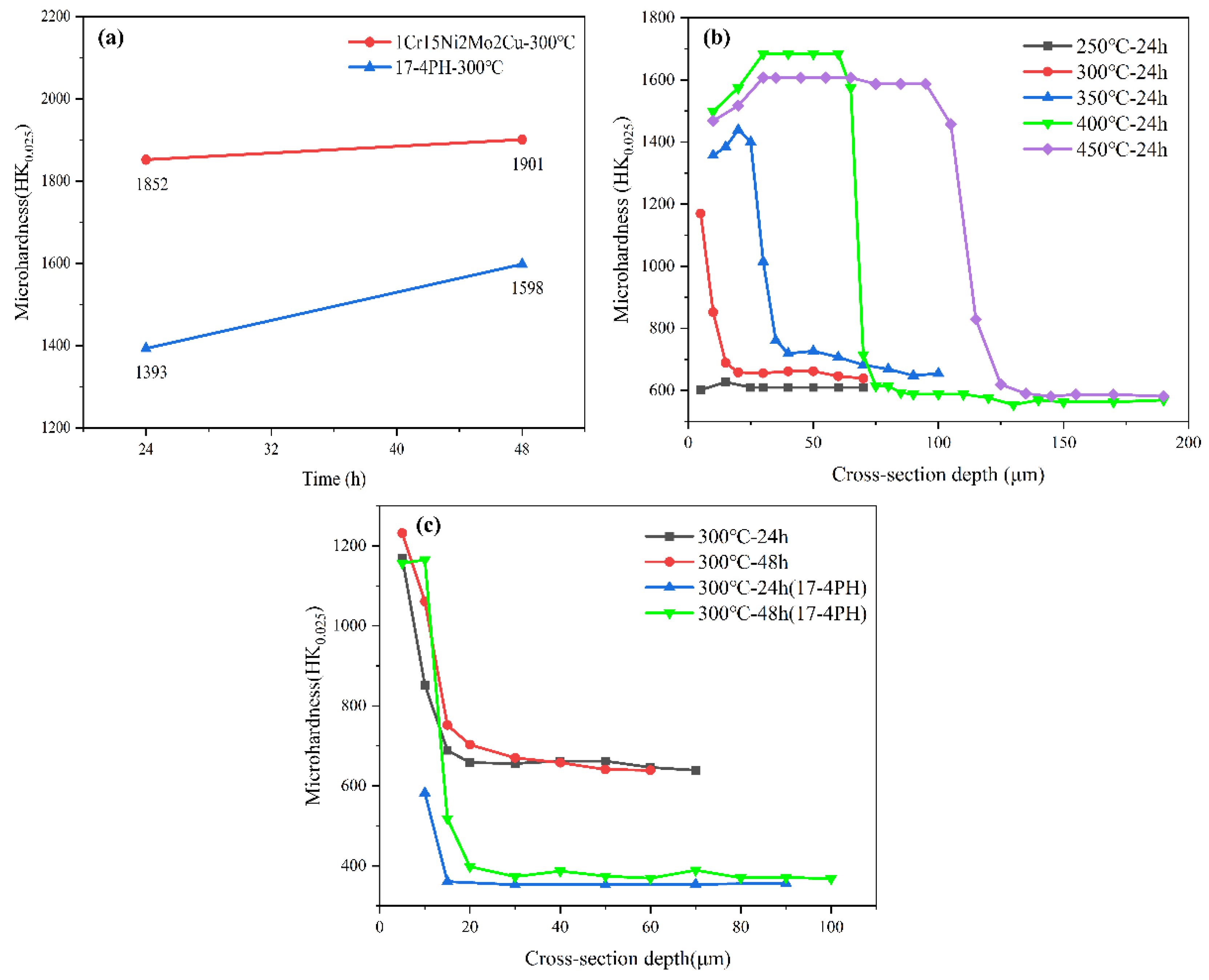
3.2.2. Microhardness Analysis
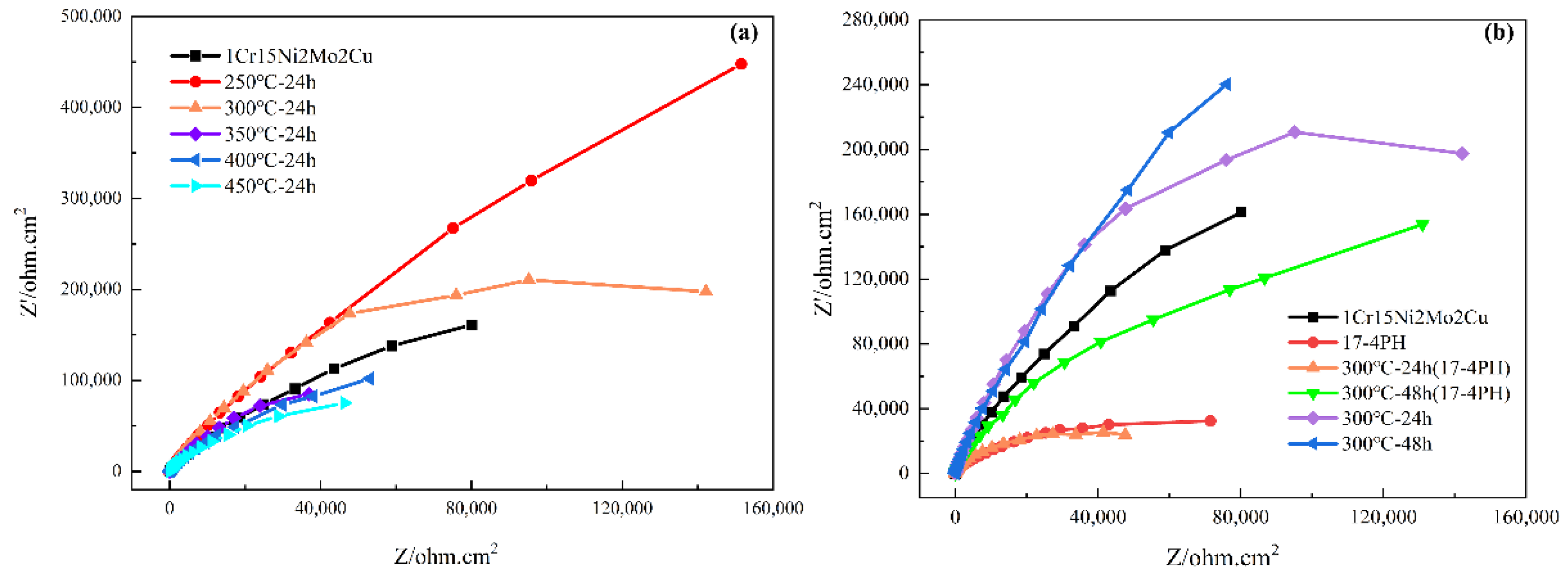
3.2.3. Corrosion Resistance Behavior
4. Conclusions
- (1)
- 1Cr15Ni2Mo2Cu precipitation hardening steel containing Mo had better corrosion resistance than 17-4PH precipitation hardening stainless steel containing Nb. The former still had better corrosion resistance after low temperature nitriding treatment. This was attributed to the higher stability, lower Fermi level, and lower electron conduction concentration and electrochemical activity of stainless steel containing molybdenum than stainless steel containing niobium.
- (2)
- At the same ion nitriding temperature, the surface hardness of 1Cr15Ni2Mo2Cu steel increased linearly, and the corrosion resistance decreased when the nitriding time was prolonged. Under the same nitriding time (24 h), the nitriding temperature increased from 300 °C to 450 °C, and the surface hardness and nitriding layer depth of nitriding steel increased gradually, but the corrosion resistance decreased gradually. This was attributed to the Cr-poor phenomenon caused by the formation of CrN in the nitride layer.
- (3)
- The surface hardness of 1Cr15Ni2Mo2Cu steel could be significantly improved and good corrosion resistance could be maintained by ion nitriding treatment at 300 °C for 24 h.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xi, Y.; Liu, D.; Han, D. Improvement of Corrosion and Wear Resistances of AISI 420 Martensitic Stainless Steel Using Plasma Nitriding at Low Temperature. Surf. Coat. Technol. 2008, 202, 2577–2583. [Google Scholar] [CrossRef]
- Schönbauer, B.M.; Mayer, H. Effect of Small Defects on the Fatigue Strength of Martensitic Stainless Steels. Int. J. Fatigue 2019, 127, 362–375. [Google Scholar] [CrossRef]
- Isfahany, A.N.; Saghafian, H.; Borhani, G. The Effect of Heat Treatment on Mechanical Properties and Corrosion Behavior of AISI420 Martensitic Stainless Steel. J. Alloys Compd. 2011, 509, 3931–3936. [Google Scholar] [CrossRef]
- Bekmurzayeva, A.; Duncanson, W.J.; Azevedo, H.S.; Kanayeva, D. Surface Modification of Stainless Steel for Biomedical Applications: Revisiting a Century-Old Material. Mater. Sci. Eng. C 2018, 93, 1073–1089. [Google Scholar] [CrossRef] [PubMed]
- Espitia, L.A.; Dong, H.; Li, X.-Y.; Pinedo, C.E.; Tschiptschin, A.P. Scratch Test of Active Screen Low Temperature Plasma Nitrided AISI 410 Martensitic Stainless Steel. Wear 2017, 376, 30–36. [Google Scholar] [CrossRef]
- Sun, Y.; Li, X.; Bell, T. Low Temperature Plasma Carburising of Austenitic Stainless Steels for Improved Wear and Corrosion Resistance. Surf. Eng. 2002, 19, 49–54. [Google Scholar] [CrossRef]
- Dong, H.; Esfandiari, M.; Li, X.Y. On the Microstructure and Phase Identification of Plasma Nitrided 17-4PH Precipitation Hardening Stainless Steel. Surf. Coat. Technol. 2008, 202, 2969–2975. [Google Scholar] [CrossRef]
- Li, G.; Wang, J.; Li, C.; Peng, Q.; Gao, J.; Shen, B. Microstructure and Dry-Sliding Wear Properties of DC Plasma Nitrided 17-4 PH Stainless Steel. Nucl Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2008, 266, 1964–1970. [Google Scholar] [CrossRef]
- Li, C.X.; Bell, T. Corrosion Properties of Plasma Nitrided AISI 410 Martensitic Stainless Steel in 3.5% NaCl and 1% HCl Aqueous Solutions. Corros. Sci. 2006, 48, 2036–2049. [Google Scholar] [CrossRef]
- Lo, K.H.; Shek, C.H.; Lai, J.K.L. Recent Developments in Stainless Steels. Mater. Sci. Eng. R Rep. 2009, 65, 39–104. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Bell, T. Structure and Corrosion Resistance of Plasma Nitrided Stainless Steel. Surf. Eng. 2013, 1, 131–136. [Google Scholar] [CrossRef]
- Li, C.X.; Bell, T. Corrosion Properties of Active Screen Plasma Nitrided 316 Austenitic Stainless Steel. Corros. Sci. 2004, 46, 1527–1547. [Google Scholar] [CrossRef]
- Fossati, A.; Borgioli, F.; Galvanetto, E.; Bacci, T. Corrosion Resistance Properties of Glow-Discharge Nitrided AISI 316L Austenitic Stainless Steel in NaCl Solutions. Corros. Sci. 2006, 48, 1513–1527. [Google Scholar] [CrossRef]
- Mingolo, N.; Tschiptschin, A.P.; Pinedo, C.E. On the Formation of Expanded Austenite during Plasma Nitriding of an AISI 316L Austenitic Stainless Steel. Surf. Coat. Technol. 2006, 201, 4215–4218. [Google Scholar] [CrossRef]
- Wu, K.; Liu, G.Q.; Wang, L.; Xu, B.F. Research on New Rapid and Deep Plasma Nitriding Techniques of AISI 420 Martensitic Stainless Steel. Vacuum 2010, 84, 870–875. [Google Scholar] [CrossRef]
- Toro, A.; Misiolek, W.Z.; Tschiptschin, A.P. Correlations between Microstructure and Surface Properties in a High Nitrogen Martensitic Stainless Steel. Acta Mater. 2003, 51, 3363–3374. [Google Scholar] [CrossRef]
- Kim, S.K.; Yoo, J.S.; Priest, J.M.; Fewell, M.P. Characteristics of Martensitic Stainless Steel Nitrided in a Low-Pressure RF Plasma. Surf. Coat. Technol. 2003, 163, 380–385. [Google Scholar] [CrossRef]
- Rovani, A.C.; Breganon, R.; de Souza, G.S.; Brunatto, S.F.; Pintaúde, G. Scratch Resistance of Low-Temperature Plasma Nitrided and Carburized Martensitic Stainless Steel. Wear 2017, 376, 70–76. [Google Scholar] [CrossRef]
- Scheuer, C.J.; Possoli, F.A.A.; Borges, P.C.; Cardoso, R.P.; Brunatto, S.F. AISI 420 Martensitic Stainless Steel Corrosion Resistance Enhancement by Low-Temperature Plasma Carburizing. Electrochim. Acta 2019, 317, 70–82. [Google Scholar] [CrossRef]
- Lippold, J.C.; Kotecki, D.J. Welding Metallurgy and Weldability of Stainless Steels; Wiley: New York, NY, USA, 2005. [Google Scholar]
- Dalibon, E.; Charadia, R.; Cabo, A.; Brühl, S.P. Short Time Ion Nitriding of AISI 420 Martensitic Stainless Steel to Improve Wear and Corrosion Resistance. Mater. Res. 2019, 22, e20190415. [Google Scholar] [CrossRef]
- Sun, Y.; Bell, T. Low Temperature Plasma Nitriding Characteristics of Precipitation Hardening Stainless Steel. Surf. Eng. 2013, 19, 331–336. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab Initio Molecular Dynamics for Liquid Metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B 1998, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Yue, W. Accurate and Simple Density Functional for the Electronic Exchange Energy: Generalized Gradient Approximation. Phys. Rev. B 1986, 33, 8800–8802. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Blöchl, P.E.; Jepsen, O.; Andersen, O.K. Improved Tetrahedron Method for Brillouin-Zone Integrations. Phys. Rev. B 1994, 49, 16223–16233. [Google Scholar] [CrossRef]
- Sato, N. Anodic Breakdown of Passive Films on Metals. J. Electrochem. Soc. 1982, 129, 255–260. [Google Scholar] [CrossRef]
- Li, H.; Yu, H.; Zhou, T.; Yin, B.; Yin, S.; Zhang, Y. Effect of Tin on the Corrosion Behavior of Sea-Water Corrosion-Resisting Steel. Mater. Des. 2015, 84, 1–9. [Google Scholar] [CrossRef]
- Gavriljuk, V.G.; Shanina, B.D.; Berns, H. Ab Initio Development of a High-Strength Corrosion-Resistant Austenitic Steel. Acta Mater. 2008, 56, 5071–5082. [Google Scholar] [CrossRef]
- Sun, Y.-T.; Tan, X.; Lei, L.-L.; Li, J.; Jiang, Y.-M. Revisiting the Effect of Molybdenum on Pitting Resistance of Stainless Steels. Tungsten 2021, 3, 329–337. [Google Scholar] [CrossRef]
- Lim, Y.S.; Kim, J.S.; Ahn, S.J.; Kwon, H.S.; Katada, Y. The Influences of Microstructure and Nitrogen Alloying on Pitting Corrosion of Type 316L and 20 Wt.% Mn-Substituted Type 316L Stainless Steels. Corros. Sci. 2001, 43, 53–68. [Google Scholar] [CrossRef]
- Baba, H.; Kodama, T.; Katada, Y. Role of Nitrogen on the Corrosion Behavior of Austenitic Stainless Steels. Corros. Sci. 2002, 44, 2393–2407. [Google Scholar] [CrossRef]
- Chen, W.; Wang, J.; Li, J.; Zheng, Y.; Li, H.; Liu, Y.; Han, P. Effect of the Rotation Speed during Friction Stir Welding on the Microstructure and Corrosion Resistance of SAF 2707 Hyper Duplex Stainless Steel. Steel Res. Int. 2018, 89, 1700425. [Google Scholar] [CrossRef]
- Borgioli, F.; Galvanetto, E.; Bacci, T. Corrosion Behaviour of Low Temperature Nitrided Nickel-Free, AISI 200 and AISI 300 Series Austenitic Stainless Steels in NaCl Solution. Corros. Sci. 2018, 136, 352–365. [Google Scholar] [CrossRef]





| Steels | C | Si | Mn | Mo | Nb | Ni | Cr | Cu | S | P | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1Cr15 | 0.14 | 1.0 | 1.0 | 2 | — | 2 | 16.5 | 0.75 | 0.03 | 0.02 | Bal. |
| 17-4PH | 0.07 | 0.4 | 0.74 | — | 0.5 | 4.23 | 16.92 | 3.44 | 0.03 | 0.03 | Bal. |
| Material | /% | Hardness/HRC | |||
|---|---|---|---|---|---|
| 1Cr15 | 1030 | 1500 | 15.3 | 50.2 | 55 |
| 17-4PH | 970 | 1012 | 17.4 | 66.3 | 37 |
| System | Etot/eV | Ecoh/eV | Eform/eV |
|---|---|---|---|
| Fe105Cr22Mo | −1067.451 | −4.672 | −0.250 |
| Fe105Cr22MoN | −1076.635 | −4.683 | −0.254 |
| Fe105Cr22MoN2 | −1084.695 | −4.689 | −0.254 |
| Fe105Cr22MoN4 | −1107.624 | −4.741 | −0.294 |
| Fe105Cr22Nb | −1059.471 | −4.621 | −0.193 |
| Fe105Cr22NbN | −1075.693 | −4.687 | −0.253 |
| Fe105Cr22NbN2 | −1084.790 | −4.697 | −0.257 |
| Fe105Cr22NbN4 | −1105.607 | −4.736 | −0.284 |
| System | Ef/eV |
|---|---|
| Fe105Cr22Mo | 7.599 |
| Fe105Cr22MoN | 7.628 |
| Fe105Cr22MoN2 | 7.633 |
| Fe105Cr22MoN4 | 7.644 |
| Fe105Cr22Nb | 7.632 |
| Fe105Cr22NbN | 7.626 |
| Fe105Cr22NbN2 | 7.635 |
| Fe105Cr22NbN4 | 7.668 |
| Sign | N | Cr | Fe | Ni | Mo |
|---|---|---|---|---|---|
| Precipitate area (green rectangle) | 9.67 | 16.02 | 72.91 | 3.63 | 1.60 |
| Smooth area (red rectangle) | 6.22 | 16.13 | 69.02 | 3.37 | 1.37 |
| Nitriding Temperature and Times | Ecorr (V vs. SCE) | Icorr (μA/ cm2) |
|---|---|---|
| 1Cr15 | −0.061 | 0.0463 |
| 17-4PH | −0.104 | 0.218 |
| 250 °C–24 h | −0.0468 | 0.128 |
| 300 °C–24 h | −0.0409 | 0.103 |
| 350 °C–24 h | −0.0974 | 0.188 |
| 400 °C–24 h | −0.135 | 0.233 |
| 450 °C–24 h | −0.139 | 0.204 |
| 300 °C–24 h | −0.0409 | 0.103 |
| 300 °C–48 h | −0.060 | 0.0389 |
| 300 °C–24 h (17-4PH) | −0.111 | 0.267 |
| 300 °C–48 h (17-4PH) | −0.099 | 0.0617 |
| Steels | Rs/(Ω·cm2) | CPE | Rct/(Ω·cm2) | |
|---|---|---|---|---|
| Y0/(S·secn·cm−2) | n | |||
| 1Cr15 | 4.508 | 2.569 × 10−5 | 0.9093 | 7.127 × 105 |
| 17-4PH | 5.088 | 5.324 × 10−5 | 0.8749 | 4.551 × 105 |
| 250 °C–24 h | 11.30 | 2.441 × 10−5 | 0.9133 | 2.156 × 106 |
| 300 °C–24 h | 4.506 | 3.004 × 10−5 | 0.9145 | 7.500 × 105 |
| 350 °C–24 h | 8.847 | 1.074 × 10−4 | 0.8593 | 1.546 × 105 |
| 400 °C–24 h | 6.111 | 8.972 × 10−5 | 0.8658 | 3.180 × 105 |
| 450 °C–24 h | 33.63 | 1.298 × 10−4 | 0.8663 | 1.038 × 105 |
| 300 °C–24 h | 4.506 | 3.004 × 10−5 | 0.9145 | 7.500 × 105 |
| 300 °C–48 h | 4.194 | 4.188 × 10−5 | 0.9135 | 1.130 × 106 |
| 300 °C–24 h (17-4PH) | 4.162 | 5.061 × 10−5 | 0.8904 | 1.074 × 105 |
| 300 °C–48 h (17-4PH) | 4.579 | 4.156 × 10−5 | 0.8917 | 5.242 × 105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, D.; Zhang, X.; Li, W.; Ma, A.; Fan, K.; Xing, W. Effect of Alloying Elements and Low Temperature Plasma Nitriding on Corrosion Resistance of Stainless Steel. Materials 2022, 15, 6575. https://doi.org/10.3390/ma15196575
Liu Y, Liu D, Zhang X, Li W, Ma A, Fan K, Xing W. Effect of Alloying Elements and Low Temperature Plasma Nitriding on Corrosion Resistance of Stainless Steel. Materials. 2022; 15(19):6575. https://doi.org/10.3390/ma15196575
Chicago/Turabian StyleLiu, Yanjie, Daoxin Liu, Xiaohua Zhang, Wenfeng Li, Amin Ma, Kaifa Fan, and Wanzi Xing. 2022. "Effect of Alloying Elements and Low Temperature Plasma Nitriding on Corrosion Resistance of Stainless Steel" Materials 15, no. 19: 6575. https://doi.org/10.3390/ma15196575
APA StyleLiu, Y., Liu, D., Zhang, X., Li, W., Ma, A., Fan, K., & Xing, W. (2022). Effect of Alloying Elements and Low Temperature Plasma Nitriding on Corrosion Resistance of Stainless Steel. Materials, 15(19), 6575. https://doi.org/10.3390/ma15196575






