Bacterial Carbonate Precipitation Using Active Metabolic Pathway to Repair Mortar Cracks
Abstract
:1. Introduction
2. Materials and Methods
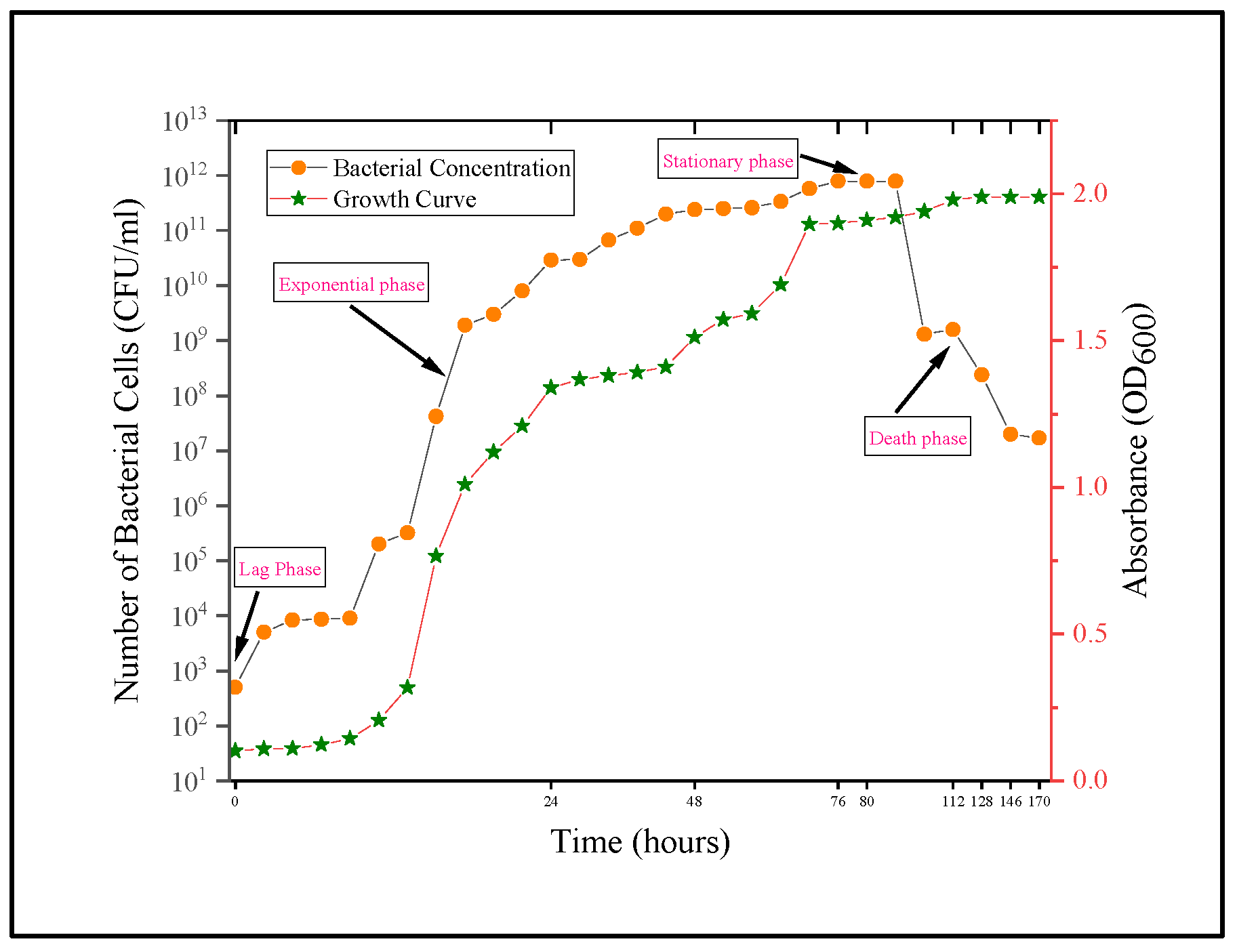
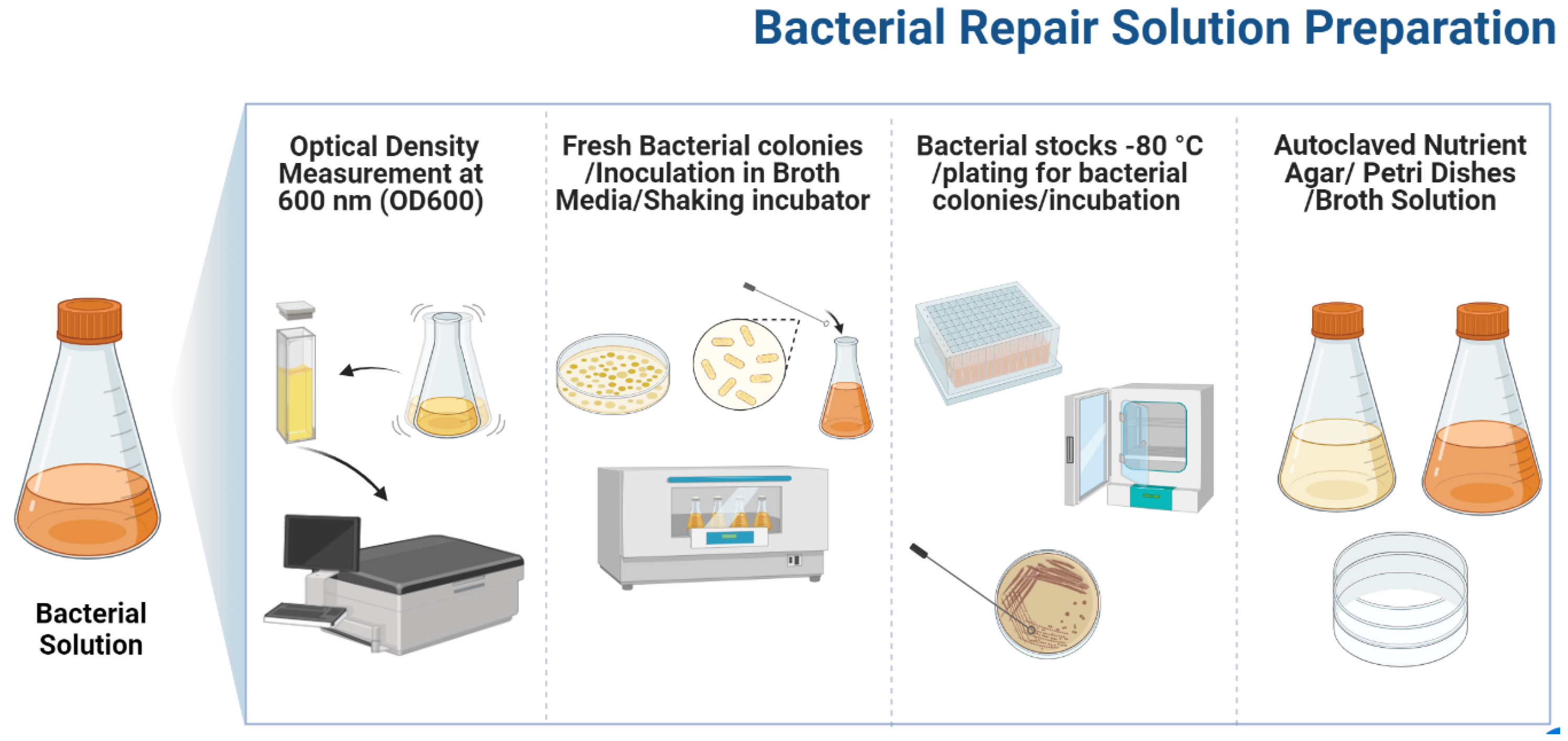
2.1. Microbial Solution
2.2. Calcium Lactate Solution
2.3. Mortar Samples
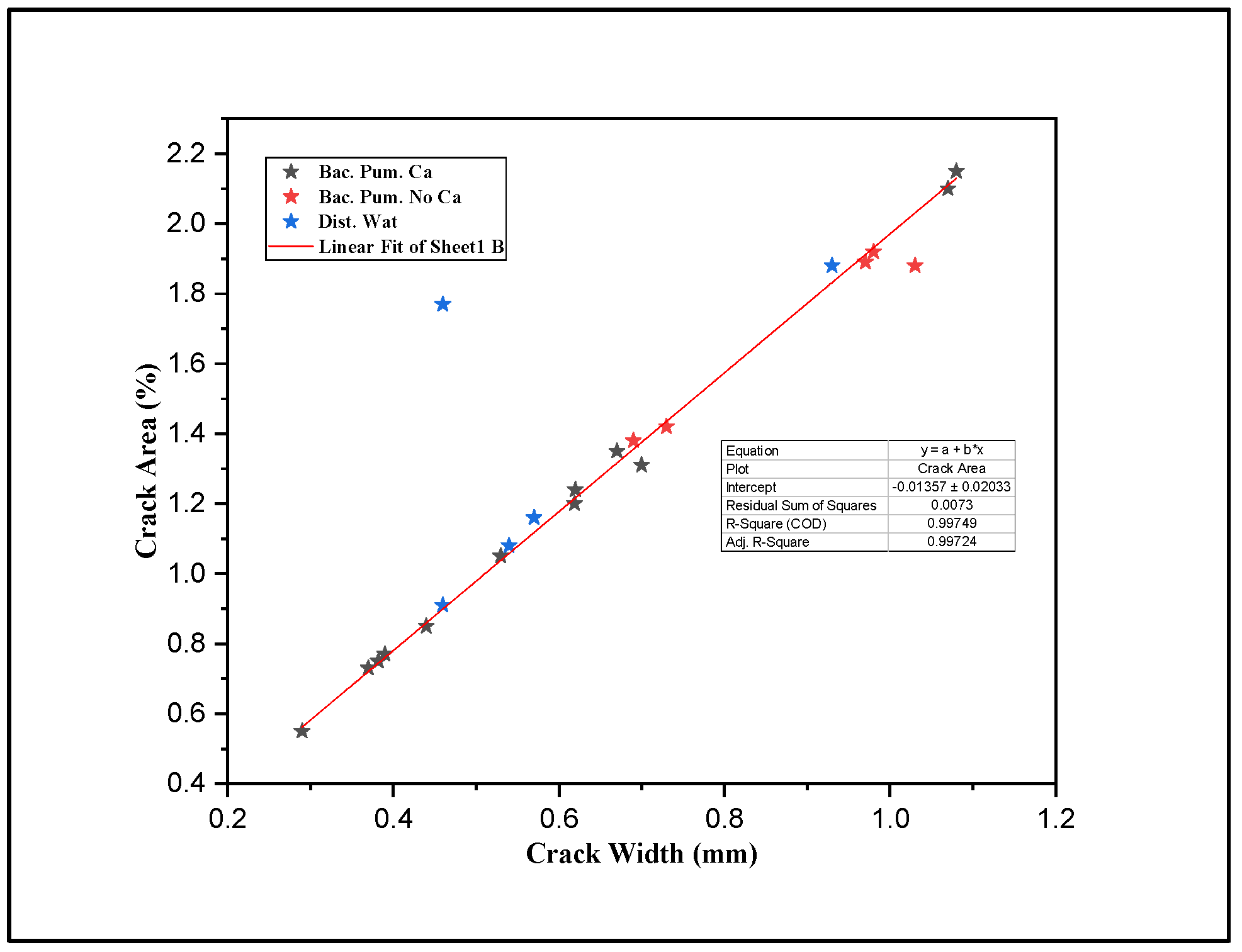
2.4. Crack Formation and Image Analysis
ACAavg (mm2) = Average Crack Area.
ACLavg (mm) = Average Crack Length.
CA (mm2) = Crack Area.
SCA (mm2) = Sample Cross-Section Area.
2.5. Microbial Crack Repair
- The mortar crack sample was submerged in 100 mL bacterium solution in a sterilized plastic cup for 4 h.
- The plastic cup was covered using cling film to avoid any external contamination.
- On completion after 4 h, the samples were removed from the plastic cup and left to dry for 24 h.
- The protocol was repeated from step (1) to step (3) for the next cycle.
- The cracked mortar samples were immersed in 100 mL bacterium solution in a sterilized plastic cup for 4 h. The plastic cup was covered with cling film
- On completion after 4 h, the samples were placed on the table for 10 min and the surface was wiped using a paper towel.
- All the repair samples were soaked in the container of Ca-lactate solution for 24 h.
- The samples were removed from the food source and left to dry on the table for 15 min.
- The protocol was repeated from step (1) to step (4) for the next cycle.
2.6. Crack Repair Investigation
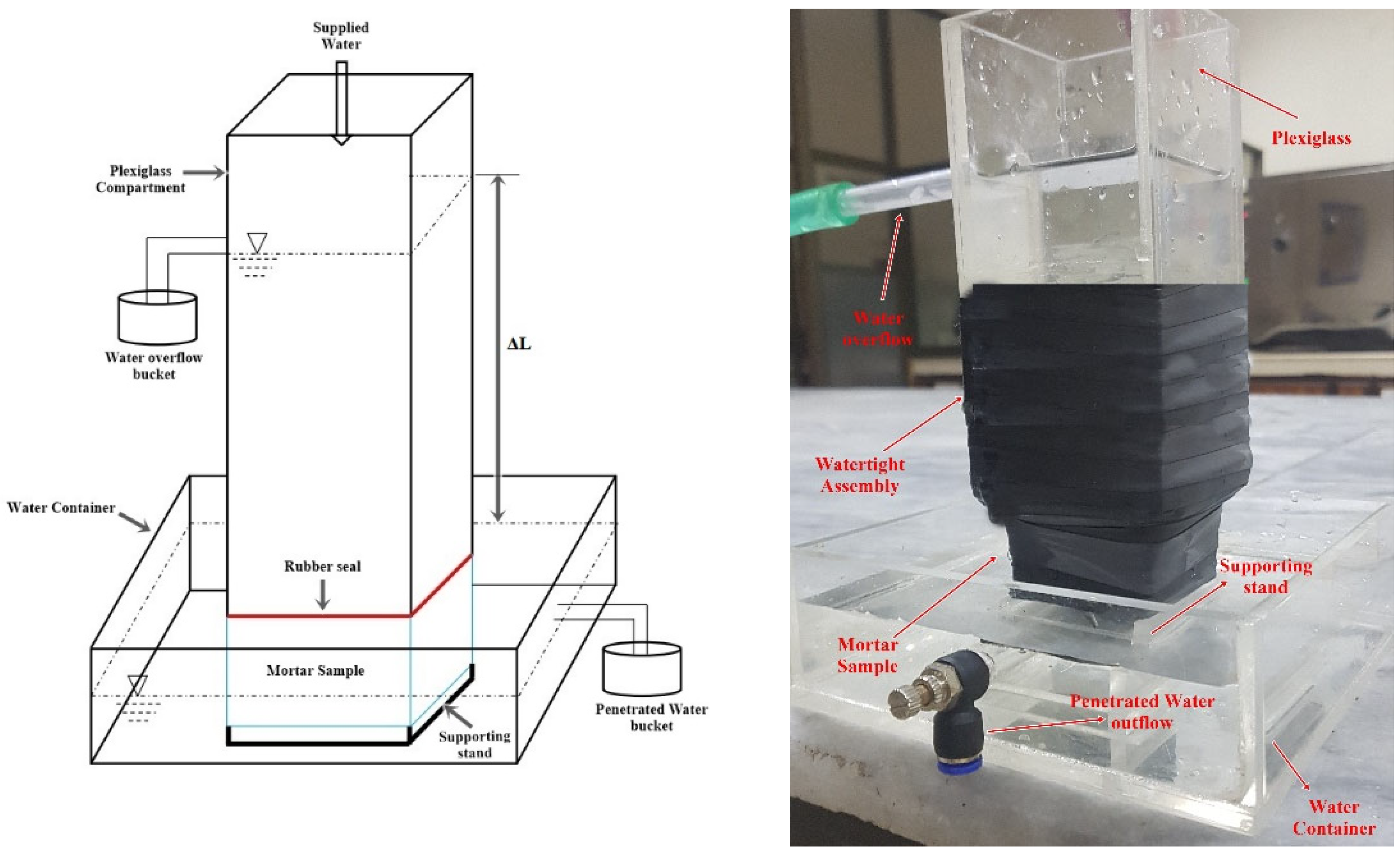
2.7. Water Permeability
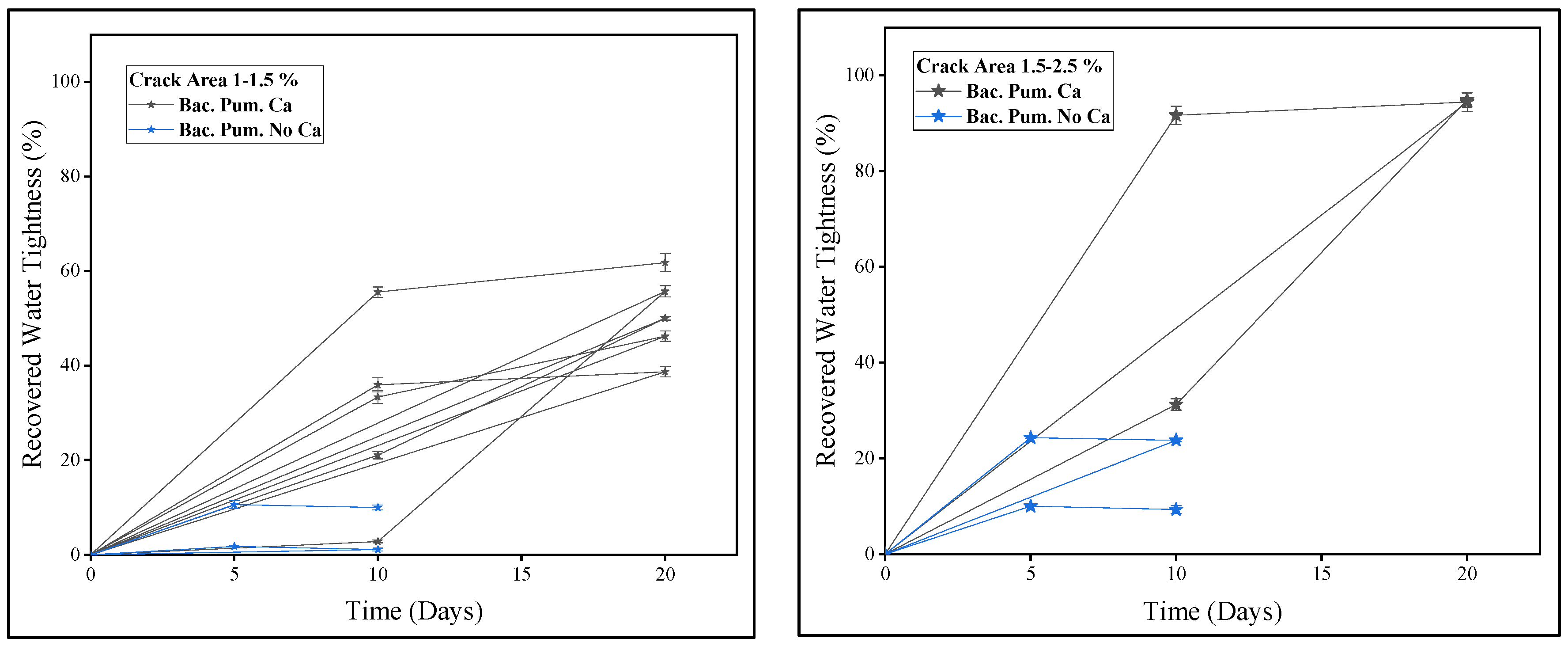
2.8. Healing and Watertightness Evaluation
2.9. Ultra-Sonic Pulse Velocity (UPV) Evaluation
2.10. Recovered Compressive Strength
2.11. Microstructural Confirmation of Calcite
3. Results and Discussion
3.1. Healing Assessment
3.1.1. Digital Image Processing
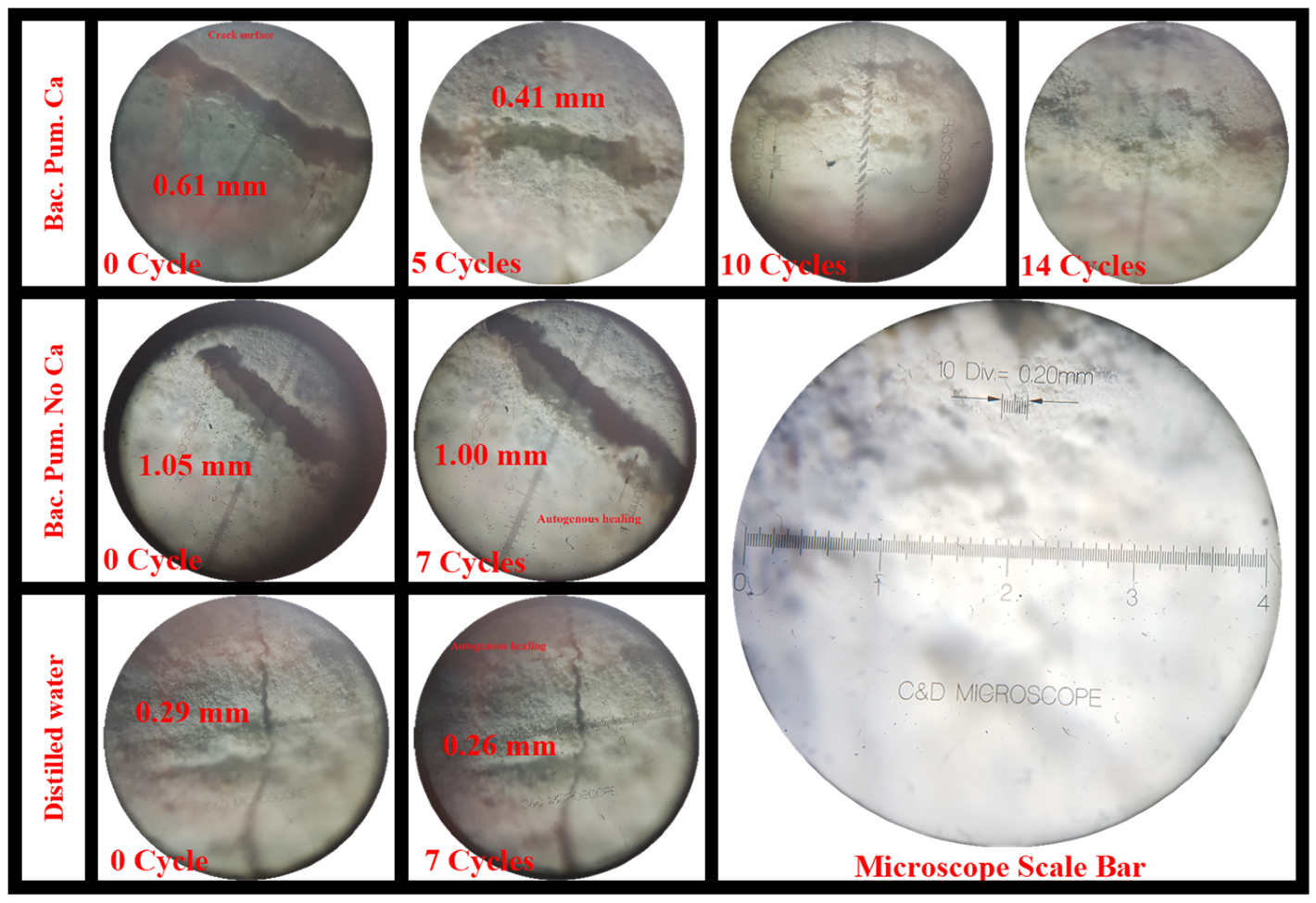
3.1.2. Optical Microscope
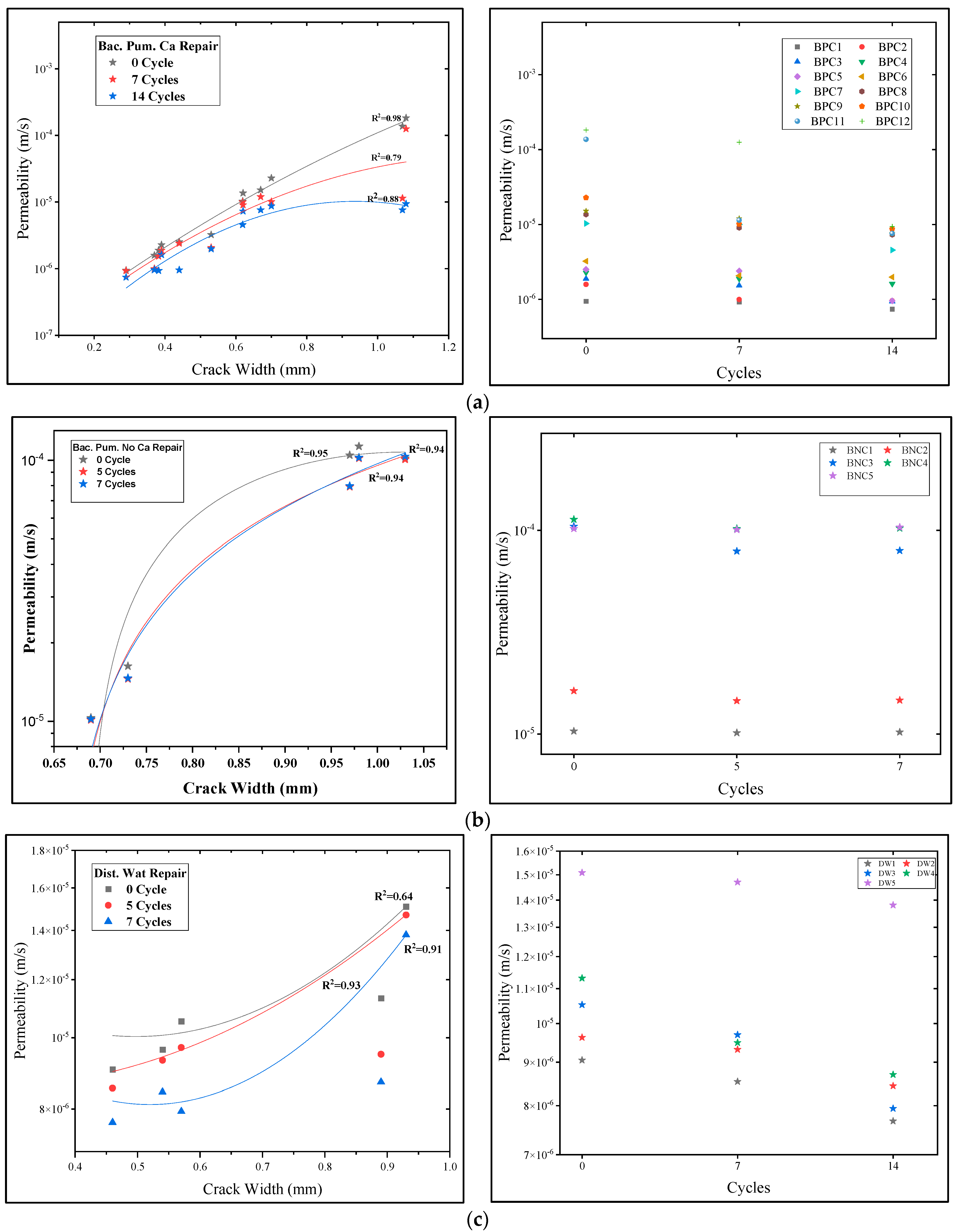
3.2. Permeability Assessment
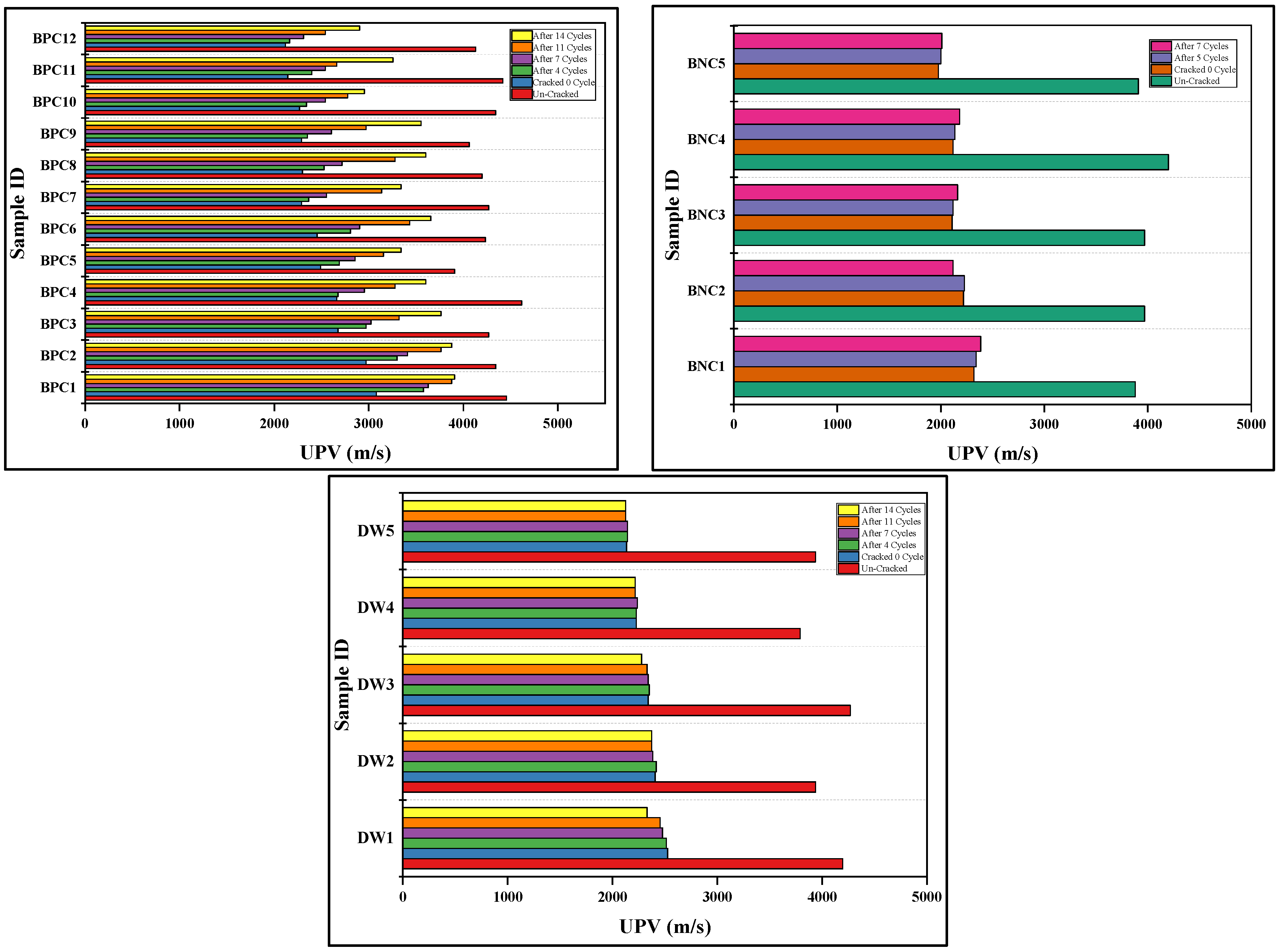
3.3. Ultra-Sonic Assessment
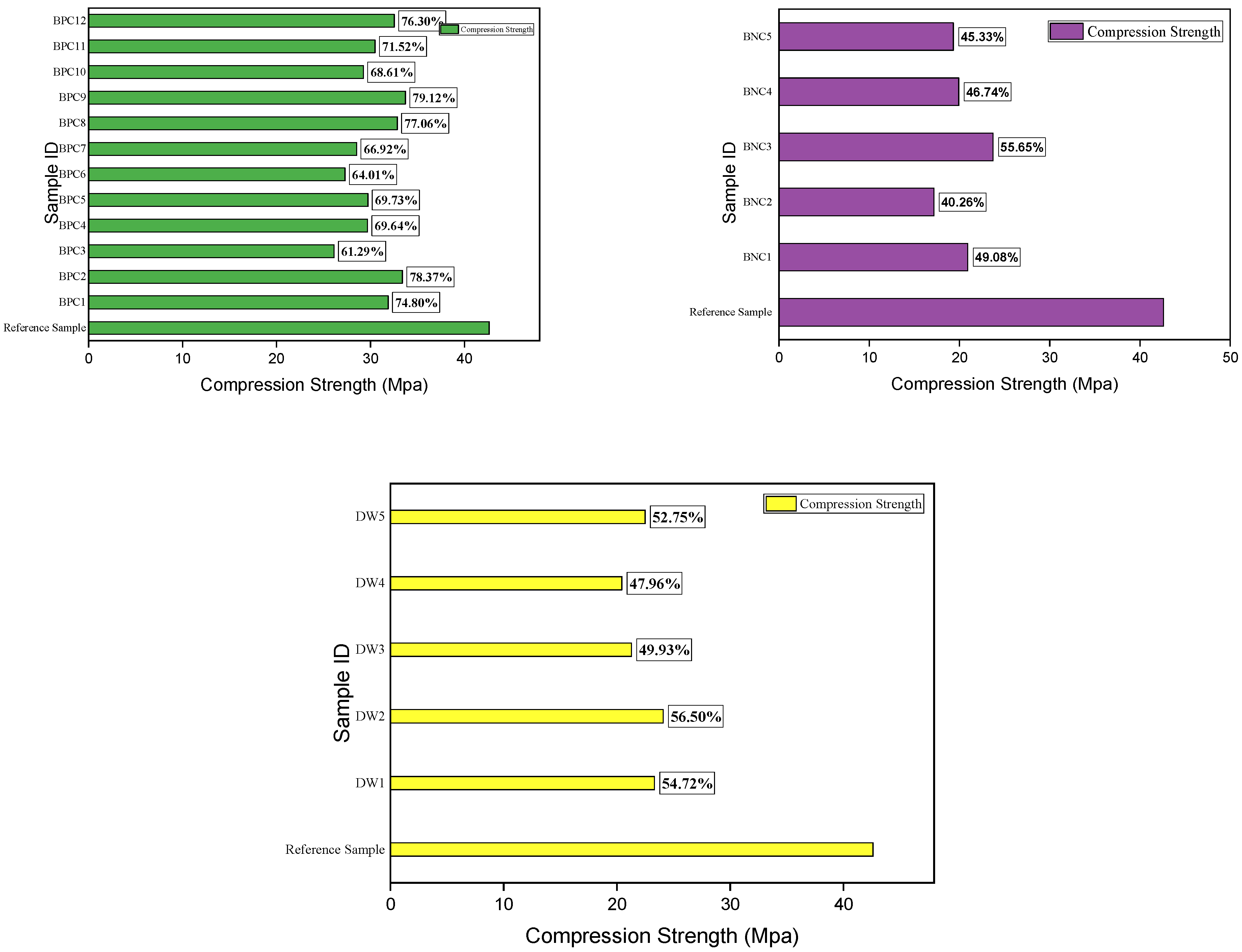
3.4. Recovered Compressive Strength
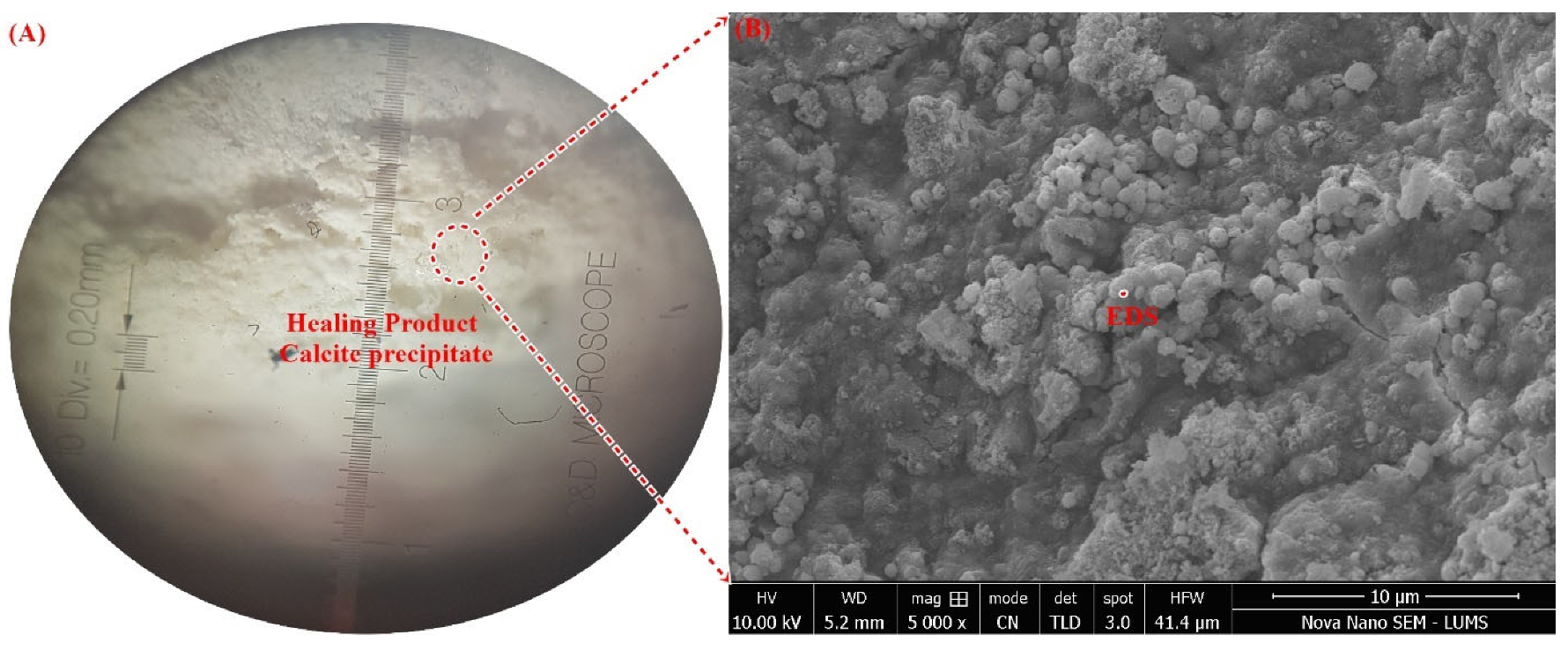
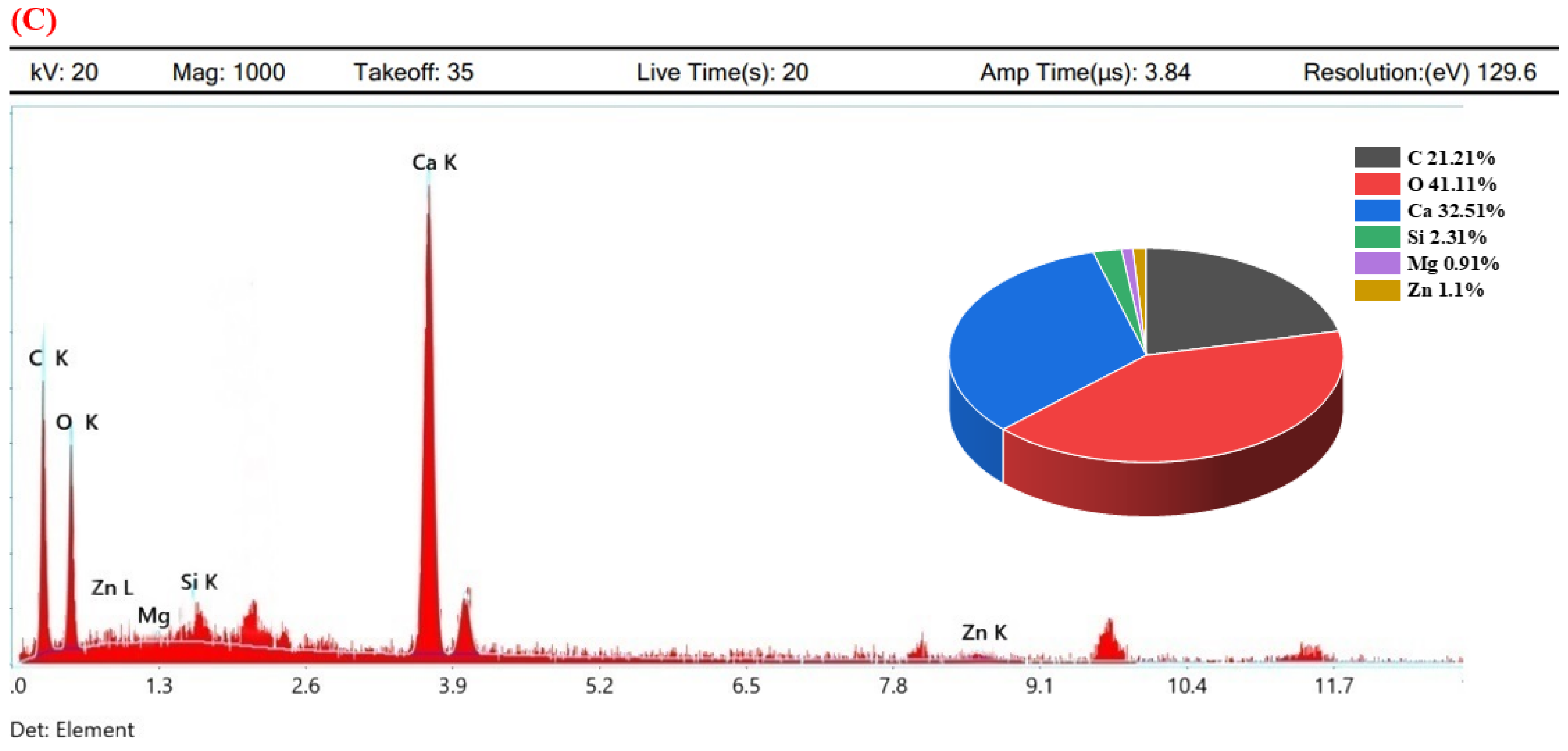
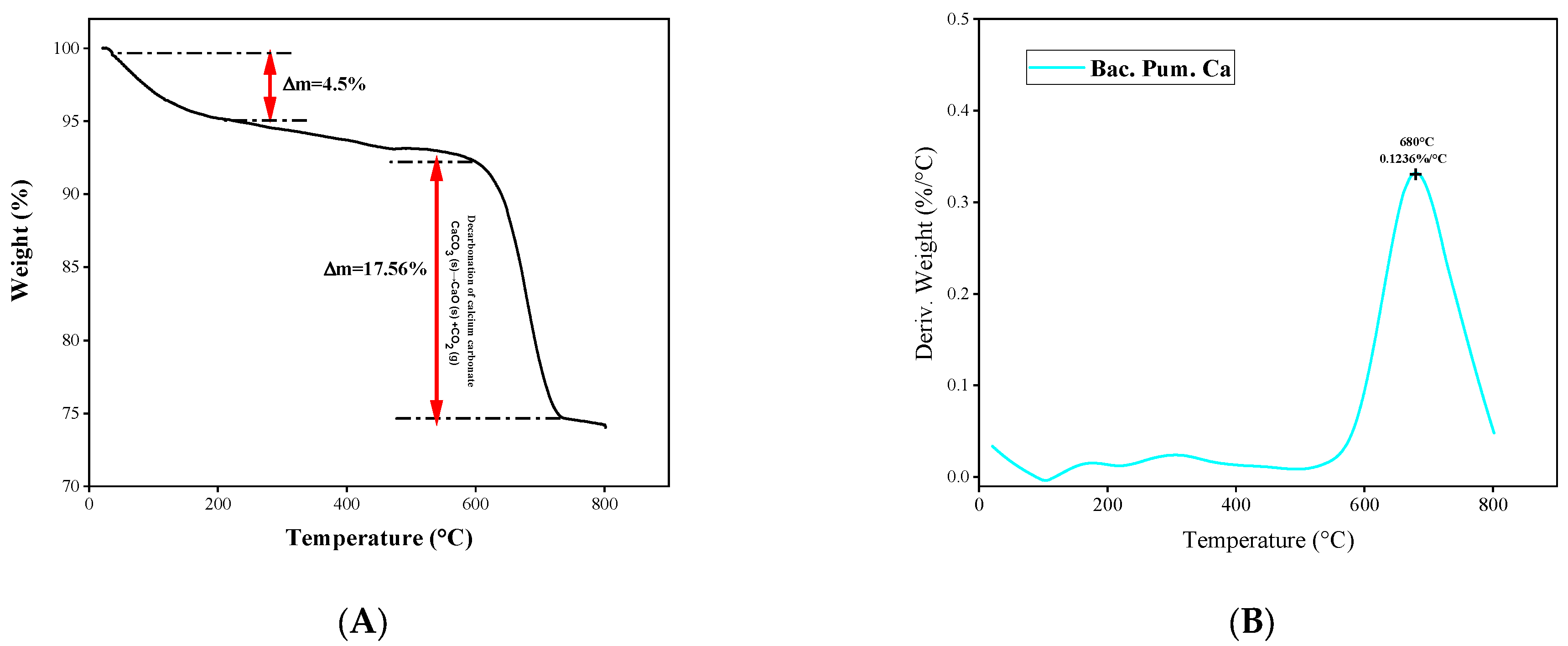
3.5. Microstructural Confirmation of Calcite Formation
4. Conclusions
- As the number of cycles increases, mortar fractures treated with biodeposition with a Ca-lactate food source showed progressive crack healing. The majority of the samples had a healed area of more than 50% after seven cycles of repair. After 14 cycles, all the fractures were sealed with a calcite coating on the top surface. Samples treated with a bacterial solution and pure water, however, failed to exhibit any discernible crack healing.
- The biodeposition treatment significantly decreased the permeability of samples of cracked mortar. Before repair, the permeability of cracked mortar samples with average fracture widths of 0.29 to 1.08 mm ranged from 9.4603 × 10−7 to 1.8227 × 10−4 m/s. A reduction in permeability between 7.4396 × 10−7 and 9.3953 × 10−6 m/s was observed in mortar samples after 14 cycles of biodeposition repair. However, treatment with bacterial solution and distilled water alone did not significantly reduce sample permeability. This is due to the inefficiency of autogenous crack healing caused by cement hydration for larger crack widths.
- The ultra-sonic investigation of samples repaired by biodeposition with Ca-lactate ranged from 2116.67 to 3078.79 m/s before repair. After the application of 14 cycles of biodeposition repair, the elapsed ultra-sonic time reduced significantly due to crack closure. The sonic values of the repaired samples ranged from 2902.86 to 3907.69 m/s, showing a substantial increase in ultra-sonic value after repair, whereas samples after the application of seven cycles of bacterial solution repair and distilled water repair did not show any substantial change in ultra-sonic value.
- The recovered compressive strength of samples repaired by biodeposition with Ca-lactate ranged from 26.12 to 33.72 N/mm2. This shows recovered compressive strength of up to 79% of the uncracked mortar sample. However, the sample treated with a bacterial solution and distilled water resulted in only up to 55% and 56% compressive strength recovery, respectively.
- The forensic analysis of the healing product from biodeposition treatment with Ca-lactate confirmed the presence of calcites. The obtained RAMAN spectra were compared with the trends of pure calcite from the RRUFF database. The obtained vibrational modes, such as internal-mode Ag, due to v1 stretching, had lower intensity Eg due to in-plane bending mode v4; transitional and rotational lattice modes; lattice mode peaks at 1087 cm−1 and 710 cm−1, and below 300 cm−1, 282 cm−1, and 151 cm−1, respectively. These resultant modes and respective peaks resemble calcite from the published literature.
- The obtained XRD spectrum diffraction peaks of the healing material matched perfectly with the pure calcite diffraction peaks of calcite. Further, the XRD pattern “Joint Committee of Powder Diffraction Standards (JCPDS)” card number 5-586 resulted in Miller indices and planes belonging to calcite.
- The obtained SEM micrograph showed the dense spherical crystals of calcite, which was further confirmed by elemental mapping using spot-EDS.
- The thermogravimetric analysis (TGA) revealed that the thermal degradation of the healing product closely matched the documented calcite patterns.
5. Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Tittelboom, K.; De Belie, N.; De Muynck, W.; Verstraete, W. Use of bacteria to repair cracks in concrete. Cem. Concr. Res. 2010, 40, 157–166. [Google Scholar] [CrossRef]
- Kanwal, M.; Khushnood, R.A.; Khaliq, W.; Wattoo, A.G.; Shahid, T. Synthesis of pyrolytic carbonized bagasse to immobilize Bacillus subtilis; application in healing micro-cracks and fracture properties of concrete. Cem. Concr. Compos. 2022, 126, 104334. [Google Scholar] [CrossRef]
- De Muynck, W.; Debrouwer, D.; De Belie, N.; Verstraete, W. Bacterial carbonate precipitation improves the durability of cementitious materials. Cem. Concr. Res. 2008, 38, 1005–1014. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Wang, Z.; Yao, W. Use of recycled concrete aggregates as carriers for self-healing of concrete cracks by bacteria with high urease activity. Constr. Build. Mater. 2022, 337, 127581. [Google Scholar] [CrossRef]
- Shaheen, N.; Jalil, A.; Adnan, F.; Arsalan Khushnood, R. Isolation of alkaliphilic calcifying bacteria and their feasibility for enhanced CaCO3 precipitation in bio-based cementitious composites. Microb. Biotechnol. 2021, 14, 1044–1059. [Google Scholar] [CrossRef]
- Minnebo, P.; Thierens, G.; De Valck, G.; Van Tittelboom, K.; De Belie, N.; Van Hemelrijck, D.; Tsangouri, E. A novel design of autonomously healed concrete: Towards a vascular healing network. Materials 2017, 10, 49. [Google Scholar] [CrossRef]
- Khushnood, R.A.; Arif, A.; Shaheen, N.; Zafar, A.G.; Hassan, T.; Akif, M. Bio-inspired self-healing and self-sensing cementitious mortar using Bacillus subtilis immobilized on graphitic platelets. Constr. Build. Mater. 2022, 316, 125818. [Google Scholar] [CrossRef]
- Castanier, S.; Le Métayer-Levrel, G.; Perthuisot, J.-P. Bacterial Roles in the Precipitation of Carbonate Minerals. In Microbial Sediments; Springer: Berlin/Heidelberg, Germany, 2000; pp. 32–39. [Google Scholar] [CrossRef]
- Ramakrishnan, V.; Bang, S.S.; Deo, K.S. A novel technique for repairing cracks in high performance concrete using bacteria. In Proceedings of the International Conference on HPHSC; Elsevier: Amsterdam, The Netherlands, 1998; pp. 597–618. [Google Scholar]
- Shaheen, N.; Khushnood, R.A.; Khaliq, W.; Murtaza, H.; Iqbal, R.; Khan, M.H. Synthesis and characterization of bio-immobilized nano/micro inert and reactive additives for feasibility investigation in self-healing concrete. Constr. Build. Mater. 2019, 226, 492–506. [Google Scholar] [CrossRef]
- Xu, J.; Tang, Y.; Wang, X.; Wang, Z.; Yao, W. Application of ureolysis-based microbial CaCO3 precipitation in self-healing of concrete and inhibition of reinforcement corrosion. Constr. Build. Mater. 2020, 265, 120364. [Google Scholar] [CrossRef]
- Jongvivatsakul, P.; Janprasit, K.; Nuaklong, P.; Pungrasmi, W.; Likitlersuang, S. Investigation of the crack healing performance in mortar using microbially induced calcium carbonate precipitation (MICP) method. Constr. Build. Mater. 2019, 212, 737–744. [Google Scholar] [CrossRef]
- Wang, J.; Van Tittelboom, K.; De Belie, N.; Verstraete, W. Use of silica gel or polyurethane immobilized bacteria for self-healing concrete. Constr. Build. Mater. 2012, 26, 532–540. [Google Scholar] [CrossRef]
- Intarasoontron, J.; Pungrasmi, W.; Nuaklong, P.; Jongvivatsakul, P.; Likitlersuang, S. Comparing performances of MICP bacterial vegetative cell and microencapsulated bacterial spore methods on concrete crack healing. Constr. Build. Mater. 2021, 302, 124227. [Google Scholar] [CrossRef]
- Bang, S.S.; Galinat, J.K.; Ramakrishnan, V. Calcite precipitation induced by polyurethane-immobilized Bacillus pasteurii. Enzyme Microb. Technol. 2001, 28, 404–409. [Google Scholar] [CrossRef]
- Rong, H.; Wei, G.; Ma, G.; Zhang, Y.; Zheng, X.; Zhang, L.; Xu, R. Influence of bacterial concentration on crack self-healing of cement-based materials. Constr. Build. Mater. 2020, 244, 118372. [Google Scholar] [CrossRef]
- Jonkers, H.M.; Mors, R.M.; Sierra-Beltran, M.G.; Wiktor, V. Biotech solutions for concrete repair with enhanced durability. In Biopolymers and Biotech Admixtures for Eco-Efficient Construction Materials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 253–271. [Google Scholar] [CrossRef]
- Jonkers, H.M. Self Healing Concrete: A Biological Approach; Springer Series in Materials Science; Springer: Dordrecht, The Netherlands, 2007; Volume 100, pp. 195–204. [Google Scholar]
- Anbu, P.; Kang, C.H.; Shin, Y.J.; So, J.S. Formations of calcium carbonate minerals by bacteria and its multiple applications. Springerplus 2016, 5, 250. [Google Scholar] [CrossRef]
- Jonkers, H.M.; Thijssen, A.; Muyzer, G.; Copuroglu, O.; Schlangen, E. Application of bacteria as self-healing agent for the development of sustainable concrete. Ecol. Eng. 2010, 36, 230–235. [Google Scholar] [CrossRef]
- Ayobami Adebola, B.; Williams Kehinde, K.; Tolulope Roland, L.; Rotimi Emmanuel, S.; Snyman, J.; Julius, N. Use of Sustainable Materials in Self-Healing Concrete. In Strength of Materials; IntechOpen: Vienna, Austria, 2020. [Google Scholar] [CrossRef]
- Choi, S.G.; Wang, K.; Wen, Z.; Chu, J. Mortar crack repair using microbial induced calcite precipitation method. Cem. Concr. Compos. 2017, 83, 209–221. [Google Scholar] [CrossRef]
- Abo-El-Enein, S.A.; Ali, A.H.; Talkhan, F.N.; Abdel-Gawwad, H.A. Utilization of microbial induced calcite precipitation for sand consolidation and mortar crack remediation. HBRC J. 2012, 8, 185–192. [Google Scholar] [CrossRef]
- Menon, R.R.; Luo, J.; Chen, X.; Zhou, H.; Liu, Z.; Zhou, G.; Zhang, N.; Jin, C. Screening of Fungi for Potential Application of Self-Healing Concrete. Sci. Rep. 2019, 9, 2075. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, J. Application of microbial precipitation in self-healing concrete: A review on the protection strategies for bacteria. Constr. Build. Mater. 2021, 306, 124950. [Google Scholar] [CrossRef]
- Shaheen, N.; Khushnood, R.A.; Musarat, M.A.; Alaloul, W.S. Self-Healing Nano-Concrete for Futuristic Infrastructures: A Review. Arab. J. Sci. Eng. 2022, 47, 5365–5375. [Google Scholar] [CrossRef]
- Sheoran, R.; Gupta, A.K. The Influence of Environmental Factors on Growth of Penicillium sp. Indo Glob. J. Pharm. Sci. 2014, 4, 123–132. [Google Scholar] [CrossRef]
- Daskalakis, M.I.; Rigas, F.; Bakolas, A.; Magoulas, A.; Kotoulas, G.; Katsikis, I.; Karageorgis, A.P.; Mavridou, A. Vaterite bio-precipitation induced by Bacillus pumilus isolated from a solutional cave in Paiania, Athens, Greece. Int. Biodeterior. Biodegrad. 2015, 99, 73–84. [Google Scholar] [CrossRef]
- C 150-84; (Reaffirmed 2012) Standard Specification for Portland Cement. ASTM Standard: West Conshohocken, PA, USA, 2012; pp. 1–8.
- C109/C109M-05; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars. ASTM International: West Conshohocken, PA, USA, 2005.
- Hoang, N.D. Detection of Surface Crack in Building Structures Using Image Processing Technique with an Improved Otsu Method for Image Thresholding. Adv. Civ. Eng. 2018, 2018, 3924120. [Google Scholar] [CrossRef]
- Khan, M.B.E.; Shen, L.; Dias-da-Costa, D. Self-healing behaviour of bio-concrete in submerged and tidal marine environments. Constr. Build. Mater. 2021, 277, 122332. [Google Scholar] [CrossRef]
- Wei, W.; Ding, L.; Luo, H.; Li, C.; Li, G. Automated bughole detection and quality performance assessment of concrete using image processing and deep convolutional neural networks. Constr. Build. Mater. 2021, 281, 122576. [Google Scholar] [CrossRef]
- Guo, P.; Meng, W.; Bao, Y. Automatic identification and quantification of dense microcracks in high-performance fiber-reinforced cementitious composites through deep learning-based computer vision. Cem. Concr. Res. 2021, 148, 106532. [Google Scholar] [CrossRef]
- Wan, T.; Wang, H.; Feng, P.; Diab, A. Concave distribution characterization of asphalt pavement surface segregation using smartphone and image processing based techniques. Constr. Build. Mater. 2021, 301, 124111. [Google Scholar] [CrossRef]
- Zhang, Z.; Weng, Y.; Ding, Y.; Qian, S. Use of genetically modified bacteria to repair cracks in concrete. Materials 2019, 12, 3912. [Google Scholar] [CrossRef]
- Paksoy, N.G. Evaluation of Cement Mortars by Ultrasound. In Proceedings of the 4th Middle East NDT Conference and Exhibition, Manama, Bahrain, 2–5 December 2007. [Google Scholar]
- ASTM C597; Standard Test Method for Pulse Velocity Through Concrete. ASTM International: West Conshohocken, PA, USA, 2006; pp. 1–4.
- Tlili, M.M.; Ben Amor, M.; Gabrielli, C.; Joiret, S.; Maurin, G.; Rousseau, P. Characterization of CACO3 hydrates by micro-Raman spectroscopy. J. Raman Spectrosc. 2002, 33, 10–16. [Google Scholar] [CrossRef]
- De La Pierre, M.; Carteret, C.; Maschio, L.; André, E.; Orlando, R.; Dovesi, R. The Raman spectrum of CaCO3 polymorphs calcite and aragonite: A combined experimental and computational study. J. Chem. Phys. 2014, 140, 164509. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, F.C.; Purcell-Milton, F.; Framont, V.; Cleary, O.; Dunne, P.W.; Gun’ko, Y.K. Synthesis of CaCO3 nano- and micro-particles by dry ice carbonation. Chem. Commun. 2017, 53, 6657–6660. [Google Scholar] [CrossRef]
- RRUFF. Calcite Database (Calcite R040070). Available online: https://rruff.info/ (accessed on 2 June 2022).
- Kalhori, H.; Bagherpour, R. Application of carbonate precipitating bacteria for improving properties and repairing cracks of shotcrete. Constr. Build. Mater. 2017, 148, 249–260. [Google Scholar] [CrossRef]
- Lors, C.; Ducasse-Lapeyrusse, J.; Gagné, R.; Damidot, D. Microbiologically induced calcium carbonate precipitation to repair microcracks remaining after autogenous healing of mortars. Constr. Build. Mater. 2017, 141, 461–469. [Google Scholar] [CrossRef]
- Wang, K.; Jansen, D.C.; Shah, S.P.; Karr, A.F. Permeability study of cracked concrete. Cem. Concr. Res. 1997, 27, 381–393. [Google Scholar] [CrossRef]
- Zhang, L.V.; Nehdi, M.L.; Suleiman, A.R.; Allaf, M.M.; Gan, M.; Marani, A.; Tuyan, M. Crack self-healing in bio-green concrete. Compos. Part B Eng. 2021, 227, 109397. [Google Scholar] [CrossRef]
- Benzerara, K.; Bolzoni, R.; Monteil, C.; Beyssac, O.; Forni, O.; Alonso, B.; Asta, M.P.; Lefevre, C. The gammaproteobacterium Achromatium forms intracellular amorphous calcium carbonate and not (crystalline) calcite. Geobiology 2021, 19, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Guo, X.J.; Li, Y.Z.; Huang, T. Experimental and visual research on the microbial induced carbonate precipitation by Pseudomonas aeruginosa. AMB Express 2017, 7, 57. [Google Scholar] [CrossRef]
- Al-Jaroudi, S.S.; Ul-Hamid, A.; Mohammed, A.R.I.; Saner, S. Use of X-ray powder diffraction for quantitative analysis of carbonate rock reservoir samples. Powder Technol. 2007, 175, 115–121. [Google Scholar] [CrossRef]
- Kim, H.J.; Eom, H.J.; Park, C.; Jung, J.; Shin, B.; Kim, W.; Chung, N.; Choi, I.G.; Park, W. Calcium carbonate precipitation by Bacillus and sporosarcina strains isolated from concrete and analysis of the bacterial community of concrete. J. Microbiol. Biotechnol. 2015, 26, 540–548. [Google Scholar] [CrossRef]
- Kawaai, K.; Nishida, T.; Saito, A.; Hayashi, T. Application of bio-based materials to crack and patch repair methods in concrete. Constr. Build. Mater. 2022, 340, 127718. [Google Scholar] [CrossRef]







| SiO2 | MgO | CaO | Fe2O3 | Na2O | ZnO | K2O | SO3 | Al2O3 | P2O5 |
|---|---|---|---|---|---|---|---|---|---|
| 18.3 | 1.5 | 61.29 | 3.20 | 0.76 | 2.1 | 0.97 | 2.51 | 7.31 | 0.089 |
| Average Particle Size (µm) | Specific Gravity | Blain Fineness (cm2/gm) | Loss on Ignition |
|---|---|---|---|
| 9.5 | 3.14 | 1720 | 1.03 |
| Fineness Modulus (FM) | Absorption (%) | Specific Gravity | Specific Gravity (OD) | Specific Gravity (SSD) |
|---|---|---|---|---|
| 2.6 | 2.02 | 2.64 | 2.51 | 2.58 |
| Sr. | Type | Treatment | Composition | Denotation | Sample IDs |
|---|---|---|---|---|---|
| Realistic Crack | |||||
| 1 | Bio-deposition a | Bacillus pumilusb | No calcium source | Bac. Pum. No Ca d | BNC |
| Calcium lactate c | Bac. Pum. Ca | BPC | |||
| 2 | Soaking | Distilled water | -- | Dist. Wat | DW |
| a | MICP | ||||
| b | The following nutrients were included in the broth solution for bacterial germination: 17 g/L pancreatic digest of casein, 3 g/L papaic digest of soya bean meal, 5 g/L sodium chloride, 2.5 g/L dipotassium hydrogen phosphate, and 2.5 g/L dextrose/glucose. The bacterium solution had an OD600 of 1.3 with 2.9 × 1010 cells/mL. | ||||
| c | Firstly, treatment with the bacterium solution of 100 mL for 4 h was carried out and we removed a sample from the bacterium solution. Secondly, we soaked the sample in calcium lactate solution for 24 h. | ||||
| d | The bacterial solution only. | ||||
| Sample ID | Crack Width Determined Using Optical Microscope | Digital Image Processing (Average of Pixels) |
|---|---|---|
| (mm) | (mm) | |
| DW5 | 1.81 | 1.80 |
| DW3 | 1.1 | 1.12 |
| BPC7 | 1.12 | 1.11 |
| BPC9 | 1.4 | 1.4 |
| BNC3 | 1.31 | 1.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raza, A.; Khushnood, R.A. Bacterial Carbonate Precipitation Using Active Metabolic Pathway to Repair Mortar Cracks. Materials 2022, 15, 6616. https://doi.org/10.3390/ma15196616
Raza A, Khushnood RA. Bacterial Carbonate Precipitation Using Active Metabolic Pathway to Repair Mortar Cracks. Materials. 2022; 15(19):6616. https://doi.org/10.3390/ma15196616
Chicago/Turabian StyleRaza, Ali, and Rao Arsalan Khushnood. 2022. "Bacterial Carbonate Precipitation Using Active Metabolic Pathway to Repair Mortar Cracks" Materials 15, no. 19: 6616. https://doi.org/10.3390/ma15196616






