Synthesis and Corrosion Inhibition Potential of Cerium/Tetraethylenepentamine Dithiocarbamate Complex on AA2024-T3 in 3.5% NaCl
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis Procedures
2.2.1. Preparation of the Dithiocarbamate Ligand—Sodium Tetraethylenepentamine Dithiocarbamate (TEPA/CSSNa)
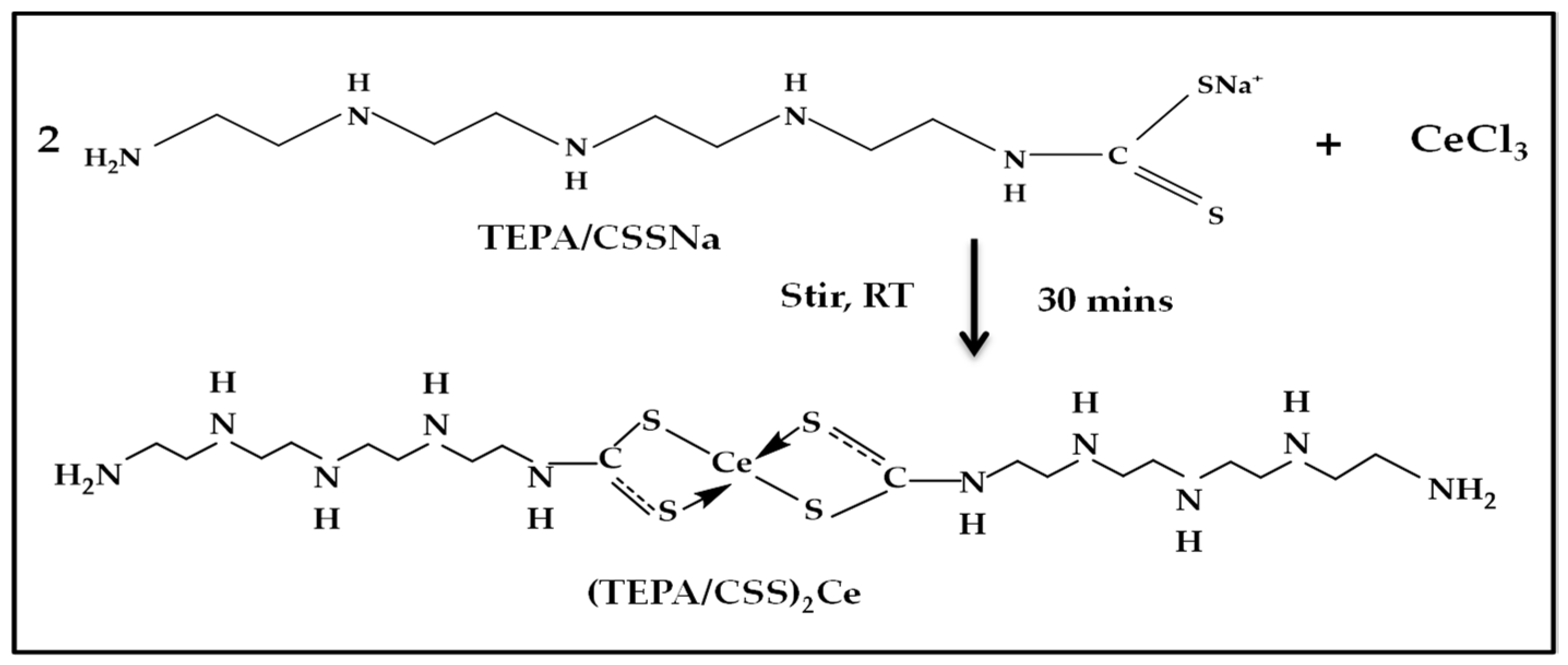
2.2.2. Synthesis of the Metal–Dithiocarbamate Coordinate Compound—Cerium/Ditetraethylenepentamine Dithiocarbamate ((TEPA/CSS)2Ce) Complex
2.3. Characterization of the Synthesized Compounds
2.4. Corrosion Media
2.5. Electrochemical Tests
2.6. Surface Characterization
3. Results
3.1. Characterization of the Synthesized Compounds
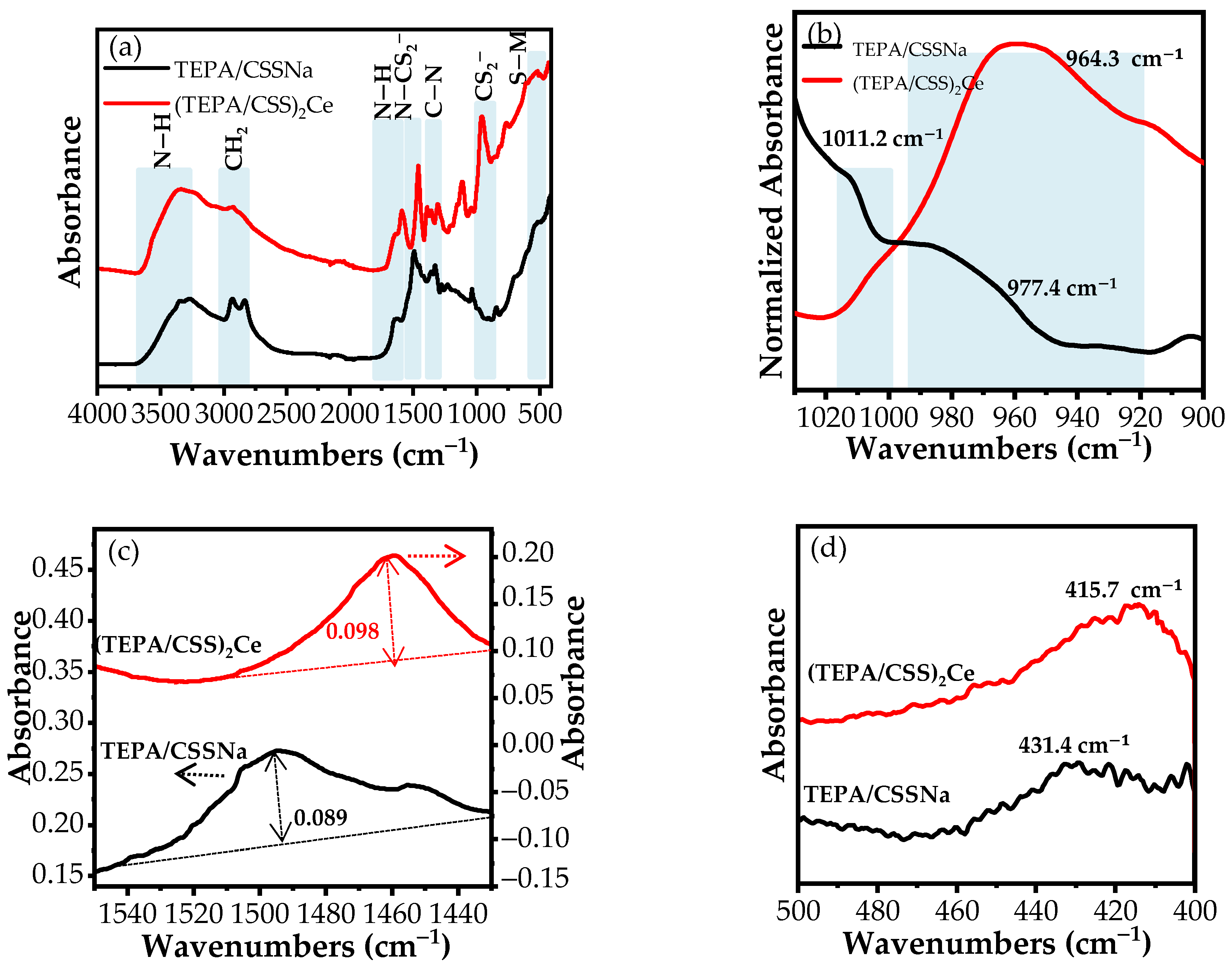
3.1.1. FT-IR
TEPA/CSSNa
(TEPA/CSS)2Ce
3.1.2. XRD
3.2. Electrochemical Studies
3.2.1. EIS Measurements
3.2.2. Potentiodynamic Polarization Measurements
3.3. Surface Characterization
3.3.1. SEM-EDS Analysis
3.3.2. XPS Analysis
3.4. Corrosion Inhibition Mechanism
- (a)
- Interaction between CS2− groups and intermetallic compounds.
- (b)
- Interaction between CS2− groups and intermediate Ce compounds.
- (c)
- The direct deposition of the (TEPA/CSS)2Ce complex on the Al surface.
- (d)
- The chemical interactions between the lone electron pairs and unoccupied d-orbitals of the metal.
4. Comparison with Other Corrosion Inhibitors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prakashaiah, B.G.; Vinaya Kumara, D.; Anup Pandith, A.; Nityananda Shetty, A.; Amitha Rani, B.E. Corrosion Inhibition of 2024-T3 Aluminum Alloy in 3.5% NaCl by Thiosemicarbazone Derivatives. Corros. Sci. 2018, 136, 326–338. [Google Scholar] [CrossRef]
- Curioni, M.; Saenz de Miera, M.; Skeldon, P.; Thompson, G.E.; Ferguson, J. Macroscopic and Local Filming Behavior of AA2024 T3 Aluminum Alloy During Anodizing in Sulfuric Acid Electrolyte. J. Electrochem. Soc. 2008, 155, C387. [Google Scholar] [CrossRef]
- Hu, T.; Shi, H.; Wei, T.; Liu, F.; Fan, S.; Han, E.H. Cerium Tartrate as a Corrosion Inhibitor for AA 2024-T3. Corros. Sci. 2015, 95, 152–161. [Google Scholar] [CrossRef]
- Rodič, P.; Milošev, I.; Lekka, M.; Andreatta, F.; Fedrizzi, L. Study of the Synergistic Effect of Cerium Acetate and Sodium Sulphate on the Corrosion Inhibition of AA2024-T3. Electrochim. Acta 2019, 308, 337–349. [Google Scholar] [CrossRef]
- Olgiati, M.; Denissen, P.J.; Garcia, S.J. When All Intermetallics Dealloy in AA2024-T3: Quantifying Early Stage Intermetallic Corrosion Kinetics under Immersion. Corros. Sci. 2021, 192, 109836. [Google Scholar] [CrossRef]
- Sano, T.; Klob, D.; Sano, Y.; Grum, J.; Roman, Š. Improvement of Corrosion Resistance of AA2024-T3 Using Femtosecond Laser Peening without Protective and Con Fi Ning Medium. Corros. Sci. 2018, 143, 46–55. [Google Scholar] [CrossRef]
- Li, Y.; Wang, D.; Lei, Z. Experimental and Theoretical Research on a New Corrosion Inhibitor for Effective Oil and Gas Acidification. RSC Adv. 2019, 9, 26464. [Google Scholar] [CrossRef]
- Fernine, Y.; Ech-chihbi, E.; Arrousse, N.; El Hajjaji, F.; Bousraf, F.; Ebn Touhami, M.; Rais, Z.; Taleb, M. Ocimum Basilicium Seeds Extract as an Environmentally Friendly Antioxidant and Corrosion Inhibitor for Aluminium Alloy 2024-T3 Corrosion in 3 Wt% NaCl Medium. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127232. [Google Scholar] [CrossRef]
- Peter, R.; Ingrid, M. Corrosion Inhibition of Pure Aluminum and Alloys AA2024-T3 and AA7075-T6 by Cerium (III) and Cerium (IV) Salts. J. Electrochem. Soc. 2016, 163, C85–C93. [Google Scholar]
- Williams, G.; Coleman, A.J.; McMurray, H.N. Inhibition of Aluminium Alloy AA2024-T3 Pitting Corrosion by Copper Complexing Compounds. Electrochim. Acta 2010, 55, 5947–5958. [Google Scholar] [CrossRef]
- Mohammadi, I.; Shahrabi, T.; Mahdavian, M.; Izadi, M. Sodium Diethyldithiocarbamate as a Novel Corrosion Inhibitor to Mitigate Corrosion of 2024-T3 Aluminum Alloy in 3.5 Wt% NaCl Solution. J. Mol. Liq. 2020, 307, 112965. [Google Scholar] [CrossRef]
- Lopez-Garrity, O.; Frankel, G.S. Corrosion Inhibition of Aa2024-T3 by Sodium Silicate. Electrochim. Acta 2014, 130, 9–21. [Google Scholar] [CrossRef]
- Ilman, M.N. Chromate Inhibition of Environmentally Assisted Fatigue Crack Propagation of Aluminium Alloy AA 2024-T3 in 3.5% NaCl Solution. Int. J. Fatigue 2014, 62, 228–235. [Google Scholar] [CrossRef]
- Zhao, J.; Xia, L.; Sehgal, A.; Lu, D.; McCreery, R.L.; Frankel, G.S. Effects of Chromate and Chromate Conversion Coatings on Corrosion of Aluminum Alloy 2024-T3. Surf. Coat. Technol. 2001, 140, 51–57. [Google Scholar] [CrossRef]
- Clark, W.J.; Ramsey, J.D.; McCreery, R.L.; Frankel, G.S. A Galvanic Corrosion Approach to Investigating Chromate Effects on Aluminum Alloy 2024-T3. J. Electrochem. Soc. 2002, 149, B179. [Google Scholar] [CrossRef]
- Frankel, G.S.; McCreery, R.L. Inhibition of Al Alloy Corrosion by Chromates. Electrochem. Soc. Interface 2001, 10, 34–38. [Google Scholar] [CrossRef]
- Izadi, M.; Rad, A.R.; Shahrabi, T.; Mohammadi, I. The Combined Action of L -Cysteine and L -Histidine as a Significant Eco-Friendly Protective System to Enhance the Corrosion Protection Performance of AA2024-T3 Alloy in 0.1 M NaCl Solution: Electrochemical and Surface Studies. Mater. Chem. Phys. 2020, 250, 122997. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, Y.; Zuo, Y. Applied Surface Science Evolution of the Corrosion Process of AA 2024-T3 in an Alkaline NaCl Solution with Sodium Dodecylbenzenesulfonate and Lanthanum Chloride Inhibitors. Appl. Surf. Sci. 2015, 357, 735–744. [Google Scholar] [CrossRef]
- Leary, S.G.; Deacon, G.B.; Junk, P.C.; Forsyth, M. The Nature of the Surface Film on Steel Treated with Cerium and Lanthanum Cinnamate Based Corrosion Inhibitors. Corros. Sci. 2006, 48, 404–419. [Google Scholar] [CrossRef]
- Somers, A.E.; Hinton, B.R.W.; De Bruin-dickason, C.; Deacon, G.B.; Junk, P.C.; Forsyth, M. New, Environmentally Friendly, Rare Earth Carboxylate Corrosion Inhibitors for Mild Steel. Corros. Sci. 2018, 139, 430–437. [Google Scholar] [CrossRef]
- Ho, D.; Soc, J.E.; Ho, D.; Brack, N.; Scully, J.; Markley, T.; Forsyth, M.; Hinton, B. Cerium Dibutylphosphate as a Corrosion Inhibitor for AA2024-T3 Aluminum Alloys Cerium Dibutylphosphate as a Corrosion Inhibitor for AA2024-T3 Aluminum Alloys. J. Electrochem. Soc. 2006, 153, B392. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, I.; Shahrabi, T.; Mahdavian, M.; Izadi, M. Cerium / Diethyldithiocarbamate Complex as a Novel Corrosion Inhibitive Pigment for AA2024-T3. Sci. Rep. 2020, 10, 5043. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; hossein Mostafatabar, A.; Ramezanzadeh, M. Estimating the Synergistic Corrosion Inhibition Potency of (2-(3,4-)-3,5,7-Trihydroxy-4H-Chromen-4-One) and Trivalent-Cerium Ions on Mild Steel in NaCl Solution. Constr. Build. Mater. 2020, 261, 119923. [Google Scholar] [CrossRef]
- Mohammadi, I.; Shahrabi, T.; Mahdavian, M.; Izadi, M. Improving the Protection Performance of AA2024-T3 in 3.5 Wt% NaCl Solution Using the Synergistic Effect of Cerium Cations and Diethyldithiocarbamate Molecules. J. Electrochem. Soc. 2020, 167, 131506. [Google Scholar] [CrossRef]
- Deyab, M.A. Effect of Nonionic Surfactant as an Electrolyte Additive on the Performance of Aluminum-Air Battery. J. Power Sources 2019, 412, 520–526. [Google Scholar] [CrossRef]
- Zhou, S.; Zhao, H.; Zhang, B.; Fang, P. Stabilization of Cadmium- and Lead-Contaminated Sites Using Sodium Tetraethylenepentamine-Multi Dithiocarbamate. Water Air Soil Pollut. 2017, 228, 1–12. [Google Scholar] [CrossRef]
- Oladipo, S.D.; Omondi, B.; Mocktar, C. Synthesis and Structural Studies of Nickel(II)- and Copper(II)-N,N′-Diarylformamidine Dithiocarbamate Complexes as Antimicrobial and Antioxidant Agents. Polyhedron 2019, 170, 712–722. [Google Scholar] [CrossRef]
- Nyamen, L.D.; Rajasekhar, S. Heterocyclic Dithiocarbamates: Precursors for Shape Controlled Growth of CdS Nanoparticles. N. J. Chem. 2011, 35, 1133–1139. [Google Scholar] [CrossRef]
- Goyal, M.; Vashisht, H.; Kumar, A.; Kumar, S.; Bahadur, I. Isopentyltriphenylphosphonium Bromideionic Liquid as a Newly Effective Corrosion Inhibitor on Metal-Electrolyte Interface in Acidic Medium: Experimental, Surface Morphological (SEM-EDX & AFM) and Computational Analysis. J. Mol. Liq. 2020, 316, 113838. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. Potential of Borage Fl Ower Aqueous Extract as an Environmentally Sustainable Corrosion Inhibitor for Acid Corrosion of Mild Steel: Electrochemical and Theoretical Studies. J. Mol. Liq. 2019, 277, 895–911. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Mouaden, K.E.L.; Quraishi, M.A.; Bazzi, L. International Journal of Biological Macromolecules Aminotriazolethiol-Functionalized Chitosan as a Macromolecule-Based Bioinspired Corrosion Inhibitor for Surface Protection of Stainless Steel In. Int. J. Biol. Macromol. 2020, 152, 234–241. [Google Scholar] [CrossRef]
- Hemapriya, V.; Prabakaran, M.; Chitra, S.; Swathika, M.; Kim, S.; Chung, I. Utilization of Biowaste as an Eco-Friendly Biodegradable Corrosion Inhibitor for Mild Steel in 1 Mol/L HCl Solution. Arab. J. Chem. 2020, 13, 8684–8696. [Google Scholar] [CrossRef]
- Fakhry, H.; El Faydy, M.; Benhiba, F.; Laabaissi, T.; Bouassiria, M.; Allali, M.; Lakhrissi, B.; Oudda, H.; Guenbour, A.; Warad, I.; et al. A Newly Synthesized Quinoline Derivative as Corrosion Inhibitor for Mild Steel in Molar Acid Medium: Characterization (SEM/EDS), Experimental and Theoretical Approach. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125746. [Google Scholar] [CrossRef]
- Shargh, S.F.; Saadat, A.; Najafi, A.; Gharehshiran, M.R.K.; Khalaj, G. Investigating the Effect of Post Weld Heat Treatment on Corrosion Properties of Explosive Bonded Interface of AA5083/AA1050/SS 321 Tubes. Mater. Res. Express 2020, 7, 036529. [Google Scholar] [CrossRef]
- Pham, T.H.; Lee, W.; Kim, J. Chrysanthemum Coronarium Leaves Extract as an Eco-Friendly Corrosion Inhibitor for Aluminum Anode in Aluminum-Air Battery. J. Mol. Liq. 2022, 347, 118269. [Google Scholar] [CrossRef]
- Odularu, A.T.; Ajibade, P.A. Dithiocarbamates: Challenges, Control, and Approaches to Excellent Yield, Characterization, and Their Biological Applications. Bioinorg. Chem. Appl. 2019, 2019, 8260496. [Google Scholar] [CrossRef]
- Bobinihi, F.F.; Osuntokun, J.; Onwudiwe, D.C. Syntheses and Characterization of Nickel(II) Dithiocarbamate Complexes Containing NiS4 and NiS2PN Moieties: Nickel Sulphide Nanoparticles from a Single Source Precursor. J. Saudi Chem. Soc. 2018, 22, 381–395. [Google Scholar] [CrossRef]
- Bond, A.M.; Martin, R.L. Electrochemistry and Redox Behaviour of Transition Metal Dithiocarbamates. Coord. Chem. Rev. 1984, 54, 23–98. [Google Scholar] [CrossRef]
- Akintola, O.S.; Saleh, T.A.; Khaled, M.M.; Charles, O.; Hamouz, S. Al Journal of the Taiwan Institute of Chemical Engineers Removal of Mercury (II) via a Novel Series of Cross-Linked Polydithiocarbamates. J. Taiwan Inst. Chem. Eng. 2016, 60, 602–616. [Google Scholar] [CrossRef]
- Saiyed, T.A.; Adeyemi, J.O.; Onwudiwe, D.C. The Structural Chemistry of Zinc(II) and Nickel(II) Dithiocarbamate Complexes. Open Chem. 2021, 19, 974–986. [Google Scholar] [CrossRef]
- Jayakumar, G.; Irudayaraj, A.A.; Raj, A.D. Particle Size Effect on the Properties of Cerium Oxide (CeO2) Nanoparticles Synthesized by Hydrothermal Method To Cite This Version: HAL Id: Hal-01499374. Mech. Mater. Sci. Eng. J. 2017, 9, 2–7. [Google Scholar]
- Indu, V.; Sunil, R. Structural Characterization and Rietveld Refinement of CeO2 /CoFe2O4 Nanocomposites Prepared via Coprecipitation Method. IOP Conf. Ser. Mater. Sci. Eng. 2020, 872, 012170. [Google Scholar] [CrossRef]
- Xuechao, H.; Junhui, D. Regeneration Mechanism of Sulfur Absorption Via Samarium-Doped Cerium Adsorbents in the Gas Atmosphere of O2/N2. Materials. 2020, 13, 1225. [Google Scholar] [CrossRef]
- Liu, W.; Duan, H.; Wei, D.; Cui, B.; Wang, X. Stability of Diethyl Dithiocarbamate Chelates with Cu(II), Zn(II) and Mn(II). J. Mol. Struct. 2019, 1184, 375–381. [Google Scholar] [CrossRef]
- Kumari, P.D.R.; Nayak, J.; Shetty, A.N. 3-Methyl-4-Amino-5-Mercapto-1,2,4-Triazole as Corrosion Inhibitor for 6061 Al Alloy in 0.5 M Sodium Hydroxide Solution. J. Coat. Technol. Res. 2011, 8, 685–695. [Google Scholar] [CrossRef]
- Rosliza, R.; Wan Nik, W.B.; Senin, H.B. The Effect of Inhibitor on the Corrosion of Aluminum Alloys in Acidic Solutions. Mater. Chem. Phys. 2008, 107, 281–288. [Google Scholar] [CrossRef]
- Shen, C.B.; Wang, S.G.; Yang, H.Y. Corrosion and Corrosion Inhibition by Thiourea of Bulk Nanocrystallized Industrial Pure Iron in Dilute HCl Solution. Corros. Sci. 2006, 48, 1655–1665. [Google Scholar] [CrossRef]
- Njoku, D.I.; Li, Y.; Lgaz, H.; Oguzie, E.E. Dispersive Adsorption of Xylopia Aethiopica Constituents on Carbon Steel in Acid-Chloride Medium: A Combined Experimental and Theoretical Approach. J. Mol. Liq. 2018, 249, 371–388. [Google Scholar] [CrossRef]
- Zhang, B.; He, C.; Chen, X.; Tian, Z.; Li, F. The Synergistic Effect of Polyamidoamine Dendrimers and Sodium Silicate on the Corrosion of Carbon Steel in Soft Water. Corros. Sci. 2015, 90, 585–596. [Google Scholar] [CrossRef]
- Jandaghi, M.R.; Saboori, A.; Khalaj, G.; Shiran, M.K.G. Microstructural Evolutions and Its Impact on the Corrosion Behaviour of Explosively Welded Al/Cu Bimetal. Metals 2020, 10, 634. [Google Scholar] [CrossRef]
- Avci, G. Corrosion Inhibition of Indole-3-Acetic Acid on Mild Steel in 0.5 M HCl. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 730–736. [Google Scholar] [CrossRef]
- Zhang, D.; Yuan, T.; Wei, G.; Wang, H.; Gao, L.; Lin, T. Preparation of Self-Healing Hydrophobic Coating on AA6061 Alloy Surface and Its Anti-Corrosion Property. J. Alloy. Compd. 2019, 774, 495–501. [Google Scholar] [CrossRef]
- Faustin, M.; Maciuk, A.; Salvin, P.; Roos, C.; Lebrini, M. Corrosion Inhibition of C38 Steel by Alkaloids Extract of Geissospermum Laeve in 1M Hydrochloric Acid: Electrochemical and Phytochemical Studies. Corros. Sci. 2015, 92, 287–300. [Google Scholar] [CrossRef]
- El Kacimi, Y.; Azaroual, M.A.; Touir, R.; Galai, M.; Alaoui, K.; Sfaira, M.; Ebn Touhami, M.; Kaya, S. Corrosion Inhibition Studies for Mild Steel in 5.0 M HCl by Substituted Phenyltetrazole. Euro-Mediterr. J. Environ. Integr. 2017, 2, 1–11. [Google Scholar] [CrossRef]
- Jafari, H.; Akbarzade, K.; Danaee, I. Corrosion Inhibition of Carbon Steel Immersed in a 1 M HCl Solution Using Benzothiazole Derivatives. Arab. J. Chem. 2019, 12, 1387–1394. [Google Scholar] [CrossRef]
- Gerengi, H.; Solomon, M.M.; Bagci, F.E.; Abai, E.J. An Evaluation of the Anticorrosion e Ff Ect of Ethylene Glycol for AA7075-T6 Alloy in 3. 5 % NaCl Solution. 2018, 116, 264–272. [Google Scholar] [CrossRef]
- Tian, H.; Li, W.; Hou, B. Novel Application of a Hormone Biosynthetic Inhibitor for the Corrosion Resistance Enhancement of Copper in Synthetic Seawater. Corros. Sci. 2011, 53, 3435–3445. [Google Scholar] [CrossRef]
- Avci, G. Inhibitor Effect of N,N′-Methylenediacrylamide on Corrosion Behavior of Mild Steel in 0.5 M HCl. Mater. Chem. Phys. 2008, 112, 234–238. [Google Scholar] [CrossRef]
- Andreatta, F.; Fedrizzi, L. Corrosion Inhibitors. In Active Protective Coatings; Hughes, A.E., Mol, J.M.C., Zheludkevich, M.L., Buchheit, E.G., Eds.; Springer Publishing: New York, NY, USA, 2016; Volume 233, pp. 59–84. [Google Scholar] [CrossRef]
- Park, I.; Choi, S.; Kim, J. Aluminum Anode for Aluminum-Air Battery e Part II: In Fl Uence of In Addition on the Electrochemical Characteristics of Al-Zn Alloy in Alkaline Solution. J. Power Sources 2017, 357, 47–55. [Google Scholar] [CrossRef]
- Chanyathunyaroj, K.; Phetchcrai, S.; Laungsopapun, G. Fatigue Characteristics of 6061 Aluminum Alloy Subject to 3.5 % NaCl Environment. Int. J. Fatigue 2020, 133, 105420. [Google Scholar] [CrossRef]
- Shao, M.; Fu, Y.; Hu, R.; Lin, C. A Study on Pitting Corrosion of Aluminum Alloy 2024-T3 by Scanning Microreference Electrode Technique. Mater. Sci. Eng. A. 2003, 344, 323–327. [Google Scholar] [CrossRef]
- Boag, A.; Taylor, R.J.; Muster, T.H.; Goodman, N.; Mcculloch, D.; Ryan, C.; Rout, B.; Jamieson, D.; Hughes, A.E. Stable Pit Formation on AA2024-T3 in a NaCl Environment. Corros. Sci. 2010, 52, 90–103. [Google Scholar] [CrossRef]
- Bethencourt, M.; Botana, F.J.; Cano, M.J.; Marcos, M. Behaviour of the Alloy AA2017 in Aqueous Solutions of NaCl. Part I: Corrosion Mechanisms. Corros. Sci. 2009, 51, 518–524. [Google Scholar] [CrossRef]
- Zhang, Z.; Ba, H.; Wu, Z. Sustainable Corrosion Inhibitor for Steel in Simulated Concrete Pore Solution by Maize Gluten Meal Extract: Electrochemical and Adsorption Behavior Studies. Constr. Build. Mater. 2019, 227, 117080. [Google Scholar] [CrossRef]
- Verma, C.; Singh, P.; Bahadur, I.; Ebenso, E.E.; Quraishi, M.A. Electrochemical, Thermodynamic, Surface and Theoretical Investigation of 2-Aminobenzene-1,3-Dicarbonitriles as Green Corrosion Inhibitor for Aluminum in 0.5 M NaOH. J. Mol. Liq. 2015, 209, 767–778. [Google Scholar] [CrossRef]
- Flores, E.A.; Olivares, O.; Likhanova, N.V.; Domínguez-aguilar, M.A.; Nava, N.; Guzman-lucero, D.; Corrales, M. Sodium Phthalamates as Corrosion Inhibitors for Carbon Steel in Aqueous Hydrochloric Acid Solution. Corros. Sci. 2011, 53, 3899–3913. [Google Scholar] [CrossRef]
- Bokati, K.S.; Dehghanian, C. Journal of Environmental Chemical Engineering Adsorption Behavior of 1H-Benzotriazole Corrosion Inhibitor on Aluminum Alloy 1050, Mild Steel and Copper in Arti Fi Cial Seawater. J. Environ. Chem. Eng. 2018, 6, 1613–1624. [Google Scholar] [CrossRef]
- Corrales-luna, M.; Le, T.; Romero-romo, M.; Palomar-pardavé, M.; Arce-estrada, E.M. 1-Ethyl 3-Methylimidazolium Thiocyanate Ionic Liquid as Corrosion Inhibitor of API 5L X52 Steel in H2SO4 and HCl Media. Corros. Sci. 2019, 153, 85–99. [Google Scholar] [CrossRef]
- Kharitonov, D.S.; Dobryden, I.; Sefer, B.; Ryl, J.; Wrzesi, A.; Makarova, I.V.; Bobowska, I.; Kurilo, I.I.; Claesson, P.M. Surface and Corrosion Properties of AA6063-T5 Aluminum Alloy in Molybdate-Containing Sodium Chloride Solutions. Corros. Sci. 2020, 171, 108658. [Google Scholar] [CrossRef]
- Bortamuly, R.; Konwar, G.; Boruah, P.K.; Das, M.R.; Mahanta, D. CeO2-PANI-HCl and CeO2-PANI-PTSA Composites: Synthesis, Characterization, and Utilization as Supercapacitor Electrode Materials. Ionics 2020, 26, 5747–5756. [Google Scholar] [CrossRef]
- Xue, Y.X.; Zuo, Z.; Li, Y.; Liu, H.; Li, Y. Graphdiyne-Supported NiCo2S4 Nanowires: A Highly Active and Stable 3D Bifunctional Electrode Material. Small 2017, 13, 1700936. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Jia, D.; Al-enizi, A.M.; Elzatahry, A.A.; Zheng, G. From Water Oxidation to Reduction: Homologous Ni–Co Based Nanowires as Complementary Water Splitting Electrocatalysts. Adv. Energy Mater. 2015, 5, 1402031. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, Y.; Xie, Y.; Tian, G.; Guo, S.; Han, T.; Fu, H. Hydrogenated CeO2-xSx Mesoporous Hollow. Chem. Commun. 2016, 52, 2521–2524. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Hu, E.; Gaskell, K.J.; Fan, X.; Gao, T.; Cui, C.; Ghose, S.; Yang, X. A Chemically Stabilized Sulfur Cathode for Lean Electrolyte Lithium Sulfur Batteries. Proc. Natl. Acad. Sci. USA 2020, 117, 14712–14720. [Google Scholar] [CrossRef]
- Eloirdi, R.; Cakir, P.; Huber, F.; Seibert, A.; Konings, R.; Gouder, T. Applied Surface Science X-Ray Photoelectron Spectroscopy Study of the Reduction and Oxidation of Uranium and Cerium Single Oxide Compared to (U-Ce) Mixed Oxide Fi Lms. Appl. Surf. Sci. 2018, 457, 566–571. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, B.; Yun, S.; Zhang, W.; Xia, C.; Afzal, M.; Cai, Y.; Liu, Y.; Wang, Y.; Wang, H. Fast Ionic Conduction in Semiconductor CeO 2- δ Electrolyte Fuel Cells. NPG Asia Mater. 2019, 11, 51. [Google Scholar] [CrossRef]
- Kumar, N.; Biswas, K. Ultra Refined Pure Aluminium Nanoparticles Cryomilling: An Environment Friendly Approach of Preparation Large Quantity Ultra Refined Pure. Integr. Med. Res. 2017, 8, 63–74. [Google Scholar] [CrossRef]
- Delgado, D.; Minakshi, M.; Kim, D.J.; Kyeong, W.C. Influence of the Oxide Content in the Catalytic Power of Raney Nickel in Influence of the Oxide Content in the Catalytic Power of Raney Nickel in Hydrogen Generation. Anal. Lett. 2017, 50, 2386–2401. [Google Scholar] [CrossRef]
- Song, C.; Li, X.; Wang, S.; Meng, Q. RSC Advances Enhanced Conversion and Stability of Biosynthetic Selenium Nanoparticles Using Fetal Bovine Serum. RSC Adv. 2016, 6, 103948–103954. [Google Scholar] [CrossRef]
- Awaja, F.; Gilbert, M.; Kelly, G.; Fox, B.; Brynolf, R.; Pigram, P.J. Surface Properties of Polypropylene Following a Novel Industrial Surface-Treatment Process. Surf. Interface Anal. Int. J. Devoted Dev. Appl. Tech. Anal. Surf. Interfaces Thin Film. 2008, 40, 1454–1462. [Google Scholar] [CrossRef]
- Abdul, A.; Yao, Y.; Shah, R.; Qi, P.; Miao, L. High-Performance Lithium Sulfur Batteries Enabled by a Synergy between Sulfur and Carbon Nanotubes. Energy Storage Mater. 2019, 16, 194–202. [Google Scholar] [CrossRef]
- Du, F.; Cao, N.; Zhang, Y.; Fu, P.; Wu, Y.; Lin, Z. PEDOT: PSS/Graphene Quantum Dots Films with Enhanced Thermoelectric Properties via Strong Interfacial Interaction and Phase Separation. Sci. Rep. 2018, 8, 6441. [Google Scholar] [CrossRef]
- Galai, M.; Rbaa, M.; Ouakki, M.; Abousalem, A.S.; Ech-chihbi, E.; Dahmani, K. Chemically Functionalized of 8-Hydroxyquinoline Derivatives as Efficient Corrosion Inhibition for Steel in 1.0 M HCl Solution: Experimental and Theoretical Studies. Surf. Interfaces 2020, 21, 100695. [Google Scholar] [CrossRef]
- Jagannath, T.P.; Nityananda, N.A. Inhibitor for Aged 18 Ni 250 Grade Maraging Steel in 0.5 M Sulfuric Acid. J. Appl. Electrochem. 2011, 41, 223–233. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Z.; Zhang, B.; Wang, H. Performance and Mechanism of Copper Removal from Wastewater by Sodium Tetraethylenepentamine-N,N′,N′′,N′′′,N′′′′-Pentadithiocarboxylic Acid. J. Mol. Struct. 2021, 1242, 130727. [Google Scholar] [CrossRef]
- Arnott, D.R.; Ryan, N.E.; Hinton, B.R.W.; Sexton, B.A.; Hughes, A.E. Auger and XPS Studies of Cerium Corrosion Inhibition on 7075 Aluminum Alloy. Appl. Surf. Sci. 1985, 22–23, 236–251. [Google Scholar] [CrossRef]
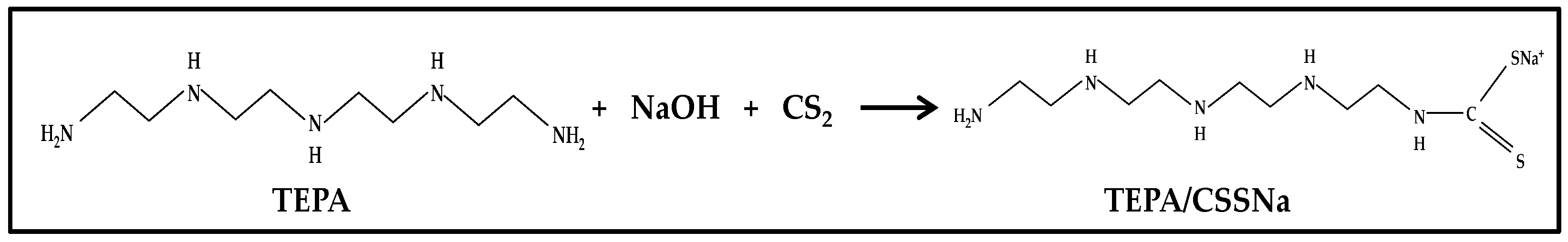

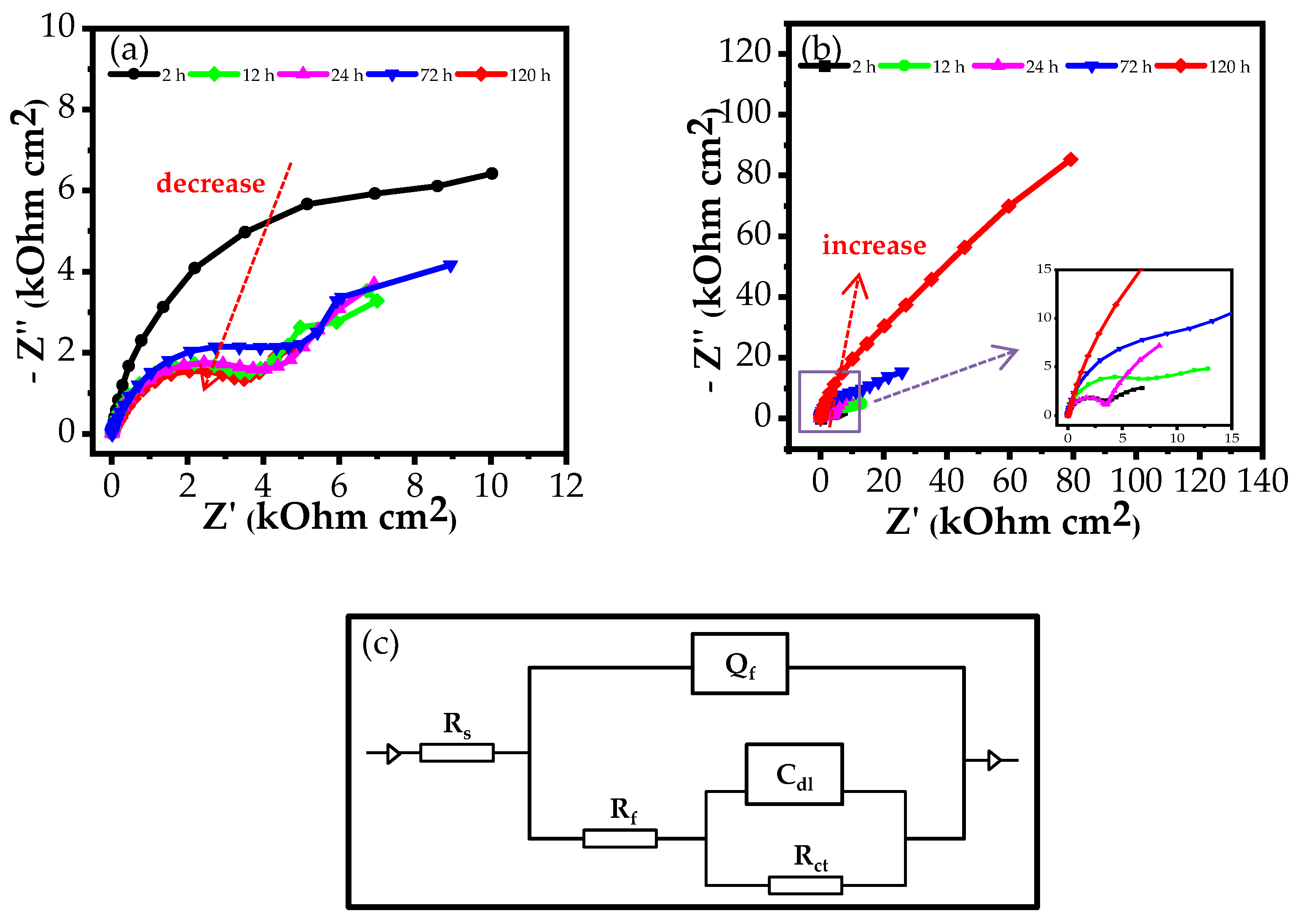
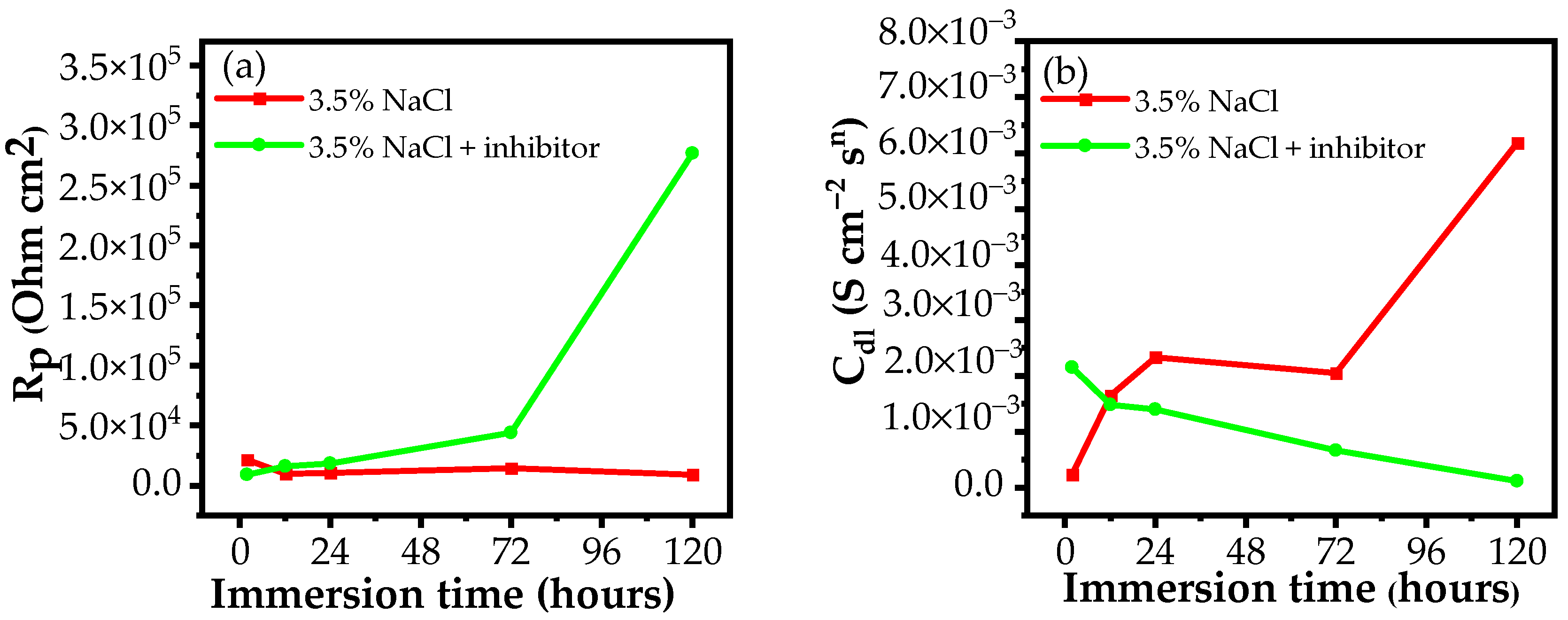
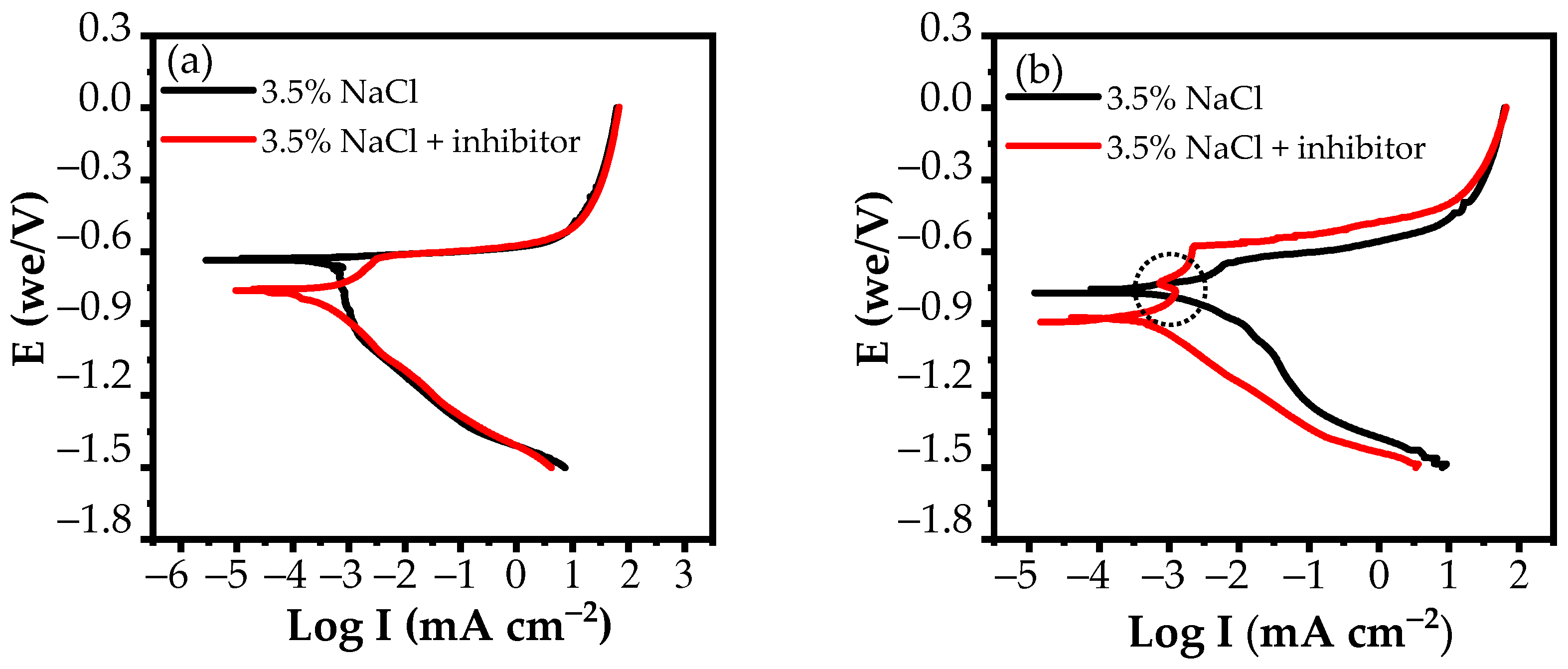
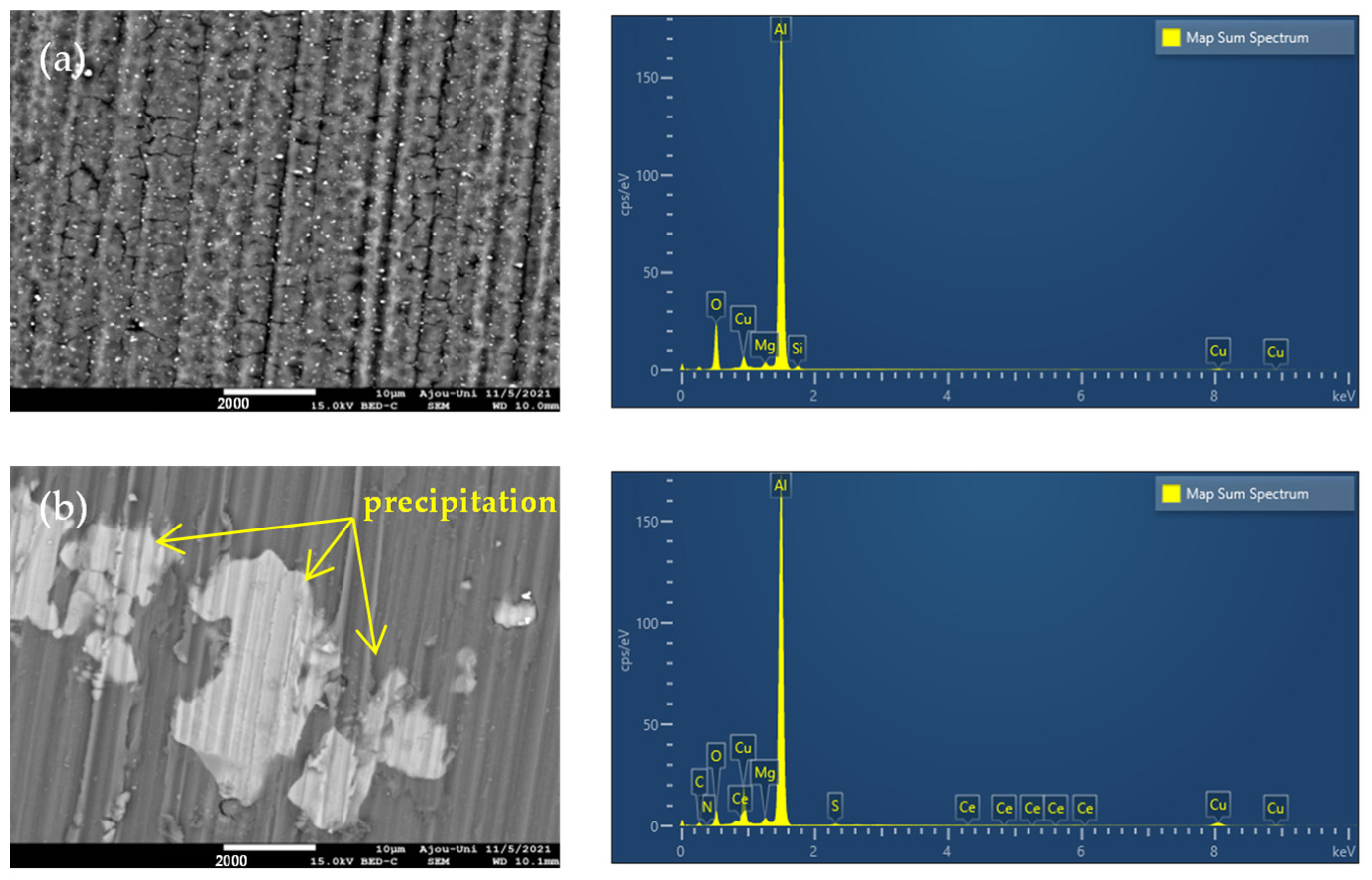

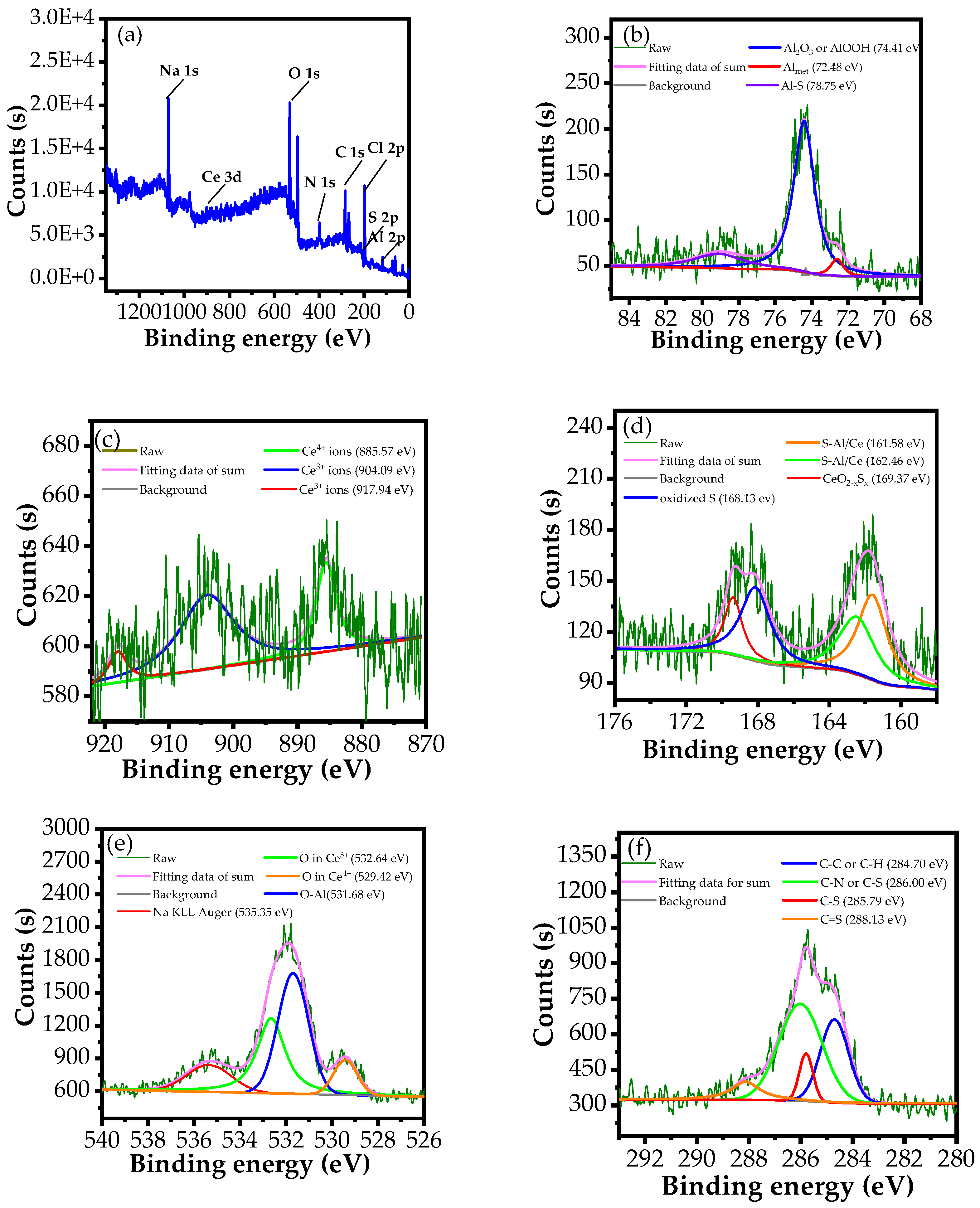

| Medium | Immersion Time (h) | Rs (Ω cm2) | Qf (S cm−2sn) | n | Rf (Ω cm2) | Cdl (S cm−2sn) | Rct (Ω cm2) | Rp (Ω cm2) | E (%) |
|---|---|---|---|---|---|---|---|---|---|
| 3.5% NaCl | 2 | 10.33 | 2.5510−5 | 0.91 | 13.1103 | 0.2410−3 | 8.22103 | 21.32103 | - |
| 12 | 9.43 | 5.3910−5 | 0.89 | 4.19103 | 1.6610−3 | 5.94103 | 10.13103 | - | |
| 24 | 9.29 | 6.5810−5 | 0.86 | 4.52103 | 2.3410−3 | 6.36103 | 10.88103 | - | |
| 72 | 9.19 | 9.1810−5 | 0.83 | 5.58103 | 2.0510−3 | 9.00103 | 14.58103 | - | |
| 120 | 9.05 | 18.1210−5 | 0.77 | 4.32103 | 6.1810−3 | 4.47103 | 8.79103 | - | |
| 3.5% NaCl + inhibitor | 2 | 10.34 | 8.0010−5 | 0.94 | 4.13103 | 2.1610−3 | 4.85103 | 8.97103 | - |
| 12 | 10.39 | 7.9910−5 | 0.93 | 9.01103 | 1.4810−3 | 7.37103 | 16.38103 | 33.55 | |
| 24 | 9.75 | 3.1910−5 | 0.95 | 3.91103 | 1.3910−3 | 14.2103 | 18.11103 | 39.91 | |
| 72 | 10.41 | 7.2610−5 | 0.90 | 19.78103 | 0.66 10−3 | 24.03103 | 43.81103 | 66.72 | |
| 120 | 9.35 | 4.4210−5 | 0.82 | 118.70103 | 0.1210−3 | 158.40103 | 277.10103 | 96.80 |
| (a) 24 h | |||||
|---|---|---|---|---|---|
| Solution | Ecorr (V) | Ebr (V) | EpitEcorr (V) | Icorr (μA cm−2) | E (%) |
| 3.5% NaCl | −0.63 | - | - | 489 | - |
| 3.5% NaCl + inhibitor | −0.77 | −0.62 | 0.15 | 331 | 32.31 |
| (b) 120 h | |||||
| Solution | Ecorr (V) | Ebr (V) | EpitEcorr (V) | Icorr (μA cm−2) | E (%) |
| 3.5% NaCl | −0.76 | −0.66 | 0.1 | 2390 | - |
| 3.5% NaCl + inhibitor | −0.89 | −0.57 | 0.32 | 437 | 81.72 |
| Solution | Al | O | Cu | Mg | Si | C | S | N | Ce |
|---|---|---|---|---|---|---|---|---|---|
| 3.5% NaCl | 71.1 | 21.6 | 5.3 | 0.9 | 1.1 | - | - | - | - |
| 3.5% NaCl + inhibitor | 69.4 | 7.2 | 14.1 | 0.7 | - | 7.8 | 0.4 | 0.3 | 0.1 |
| Elements | Cl 2p | Na 1s | O 1s | Al 2p | C 1s | N 1s | S 2p | Ce 3d |
|---|---|---|---|---|---|---|---|---|
| Atomic (%) | 13.2 | 9.1 | 23.15 | 10.00 | 31.71 | 9.53 | 2.61 | 0.70 |
| Solutions | Immersion Time (hours) | Efficiency (%) | Year | References |
|---|---|---|---|---|
| 3.5% NaCl + (E)-2-(2,4-dihydroxybenzylidene) Hydrazinecarbothioamide | 120 | 90 | 2018 | [1] |
| 3.5% NaCl + (E)-2-(2-hydroxybenzylidence) hydrazinecarbothioamide | 120 | 74.9 | 2018 | [1] |
| 3.5% NaCl + (TEPA/CSS)2Ce | 120 | 96.8 | 2022 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, T.H.; Lee, W.-H.; Son, G.-H.; Tran, T.T.; Kim, J.-G. Synthesis and Corrosion Inhibition Potential of Cerium/Tetraethylenepentamine Dithiocarbamate Complex on AA2024-T3 in 3.5% NaCl. Materials 2022, 15, 6631. https://doi.org/10.3390/ma15196631
Pham TH, Lee W-H, Son G-H, Tran TT, Kim J-G. Synthesis and Corrosion Inhibition Potential of Cerium/Tetraethylenepentamine Dithiocarbamate Complex on AA2024-T3 in 3.5% NaCl. Materials. 2022; 15(19):6631. https://doi.org/10.3390/ma15196631
Chicago/Turabian StylePham, Thi Huong, Woo-Hyuk Lee, Gyeong-Ho Son, Trang Thu Tran, and Jung-Gu Kim. 2022. "Synthesis and Corrosion Inhibition Potential of Cerium/Tetraethylenepentamine Dithiocarbamate Complex on AA2024-T3 in 3.5% NaCl" Materials 15, no. 19: 6631. https://doi.org/10.3390/ma15196631





