Study on Mechanical Properties and Weakening Mechanism of Acid Corrosion Lamprophyre
Abstract
:1. Introduction
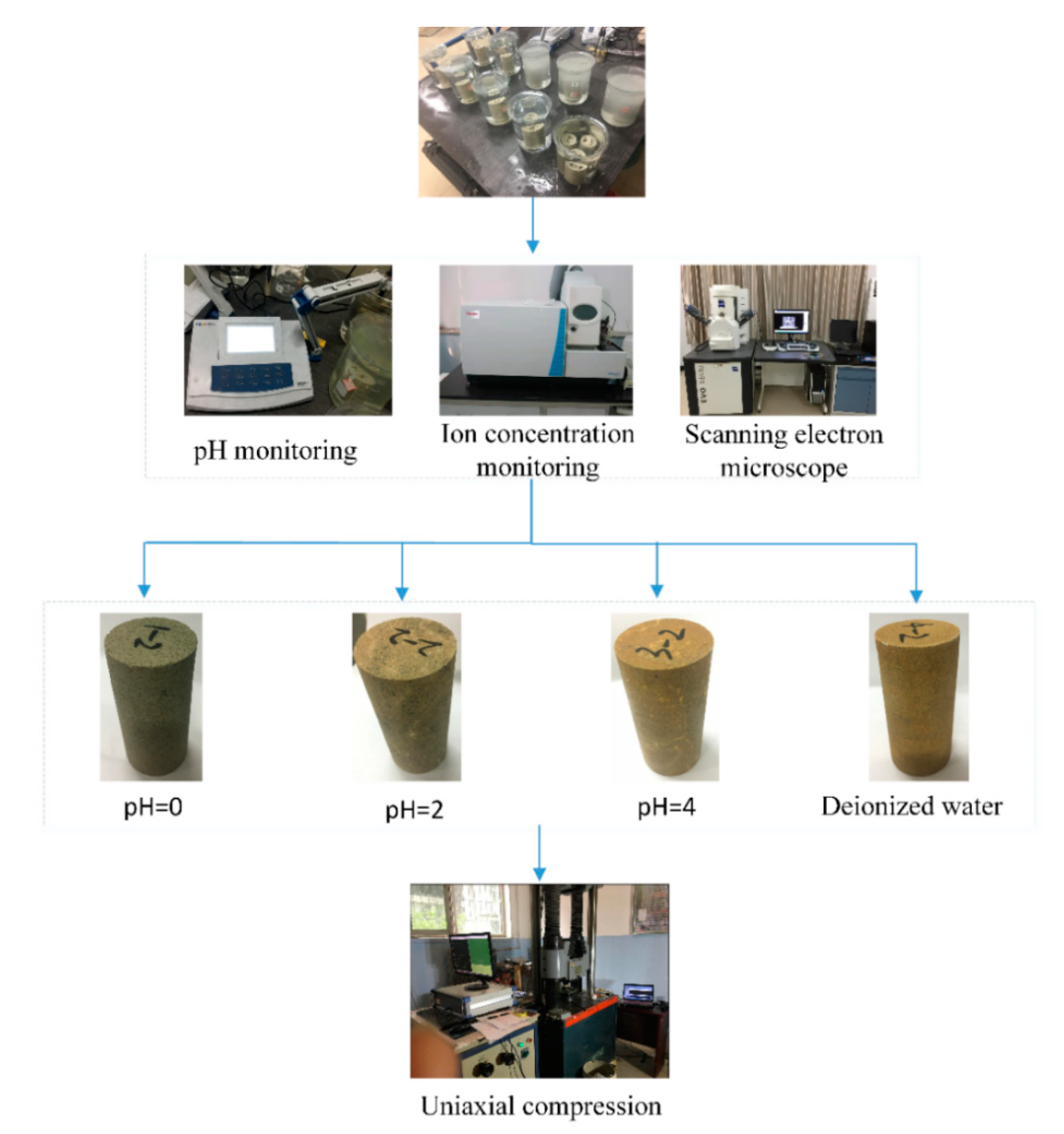
2. Materials and Methods
2.1. Preparation of Samples
2.2. Test Procedures
3. Mechanical Properties Analysis
3.1. Stress–Strain Relation
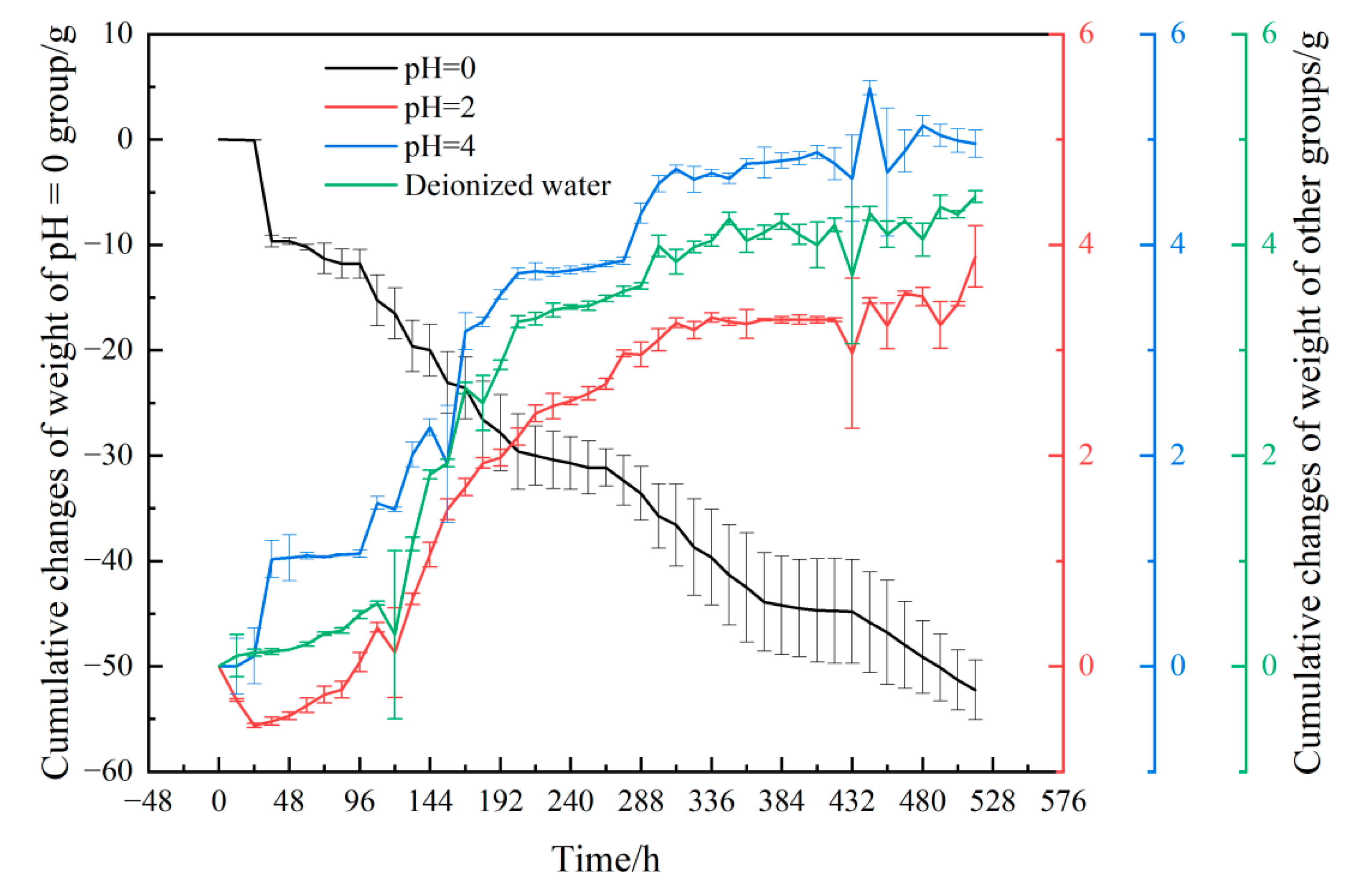
3.2. Weight Changes of Samples
3.3. Failure Characteristics of Uniaxial Compression
4. Characteristics and Mechanism of Acid Erosion
4.1. Reaction Process and Phenomena
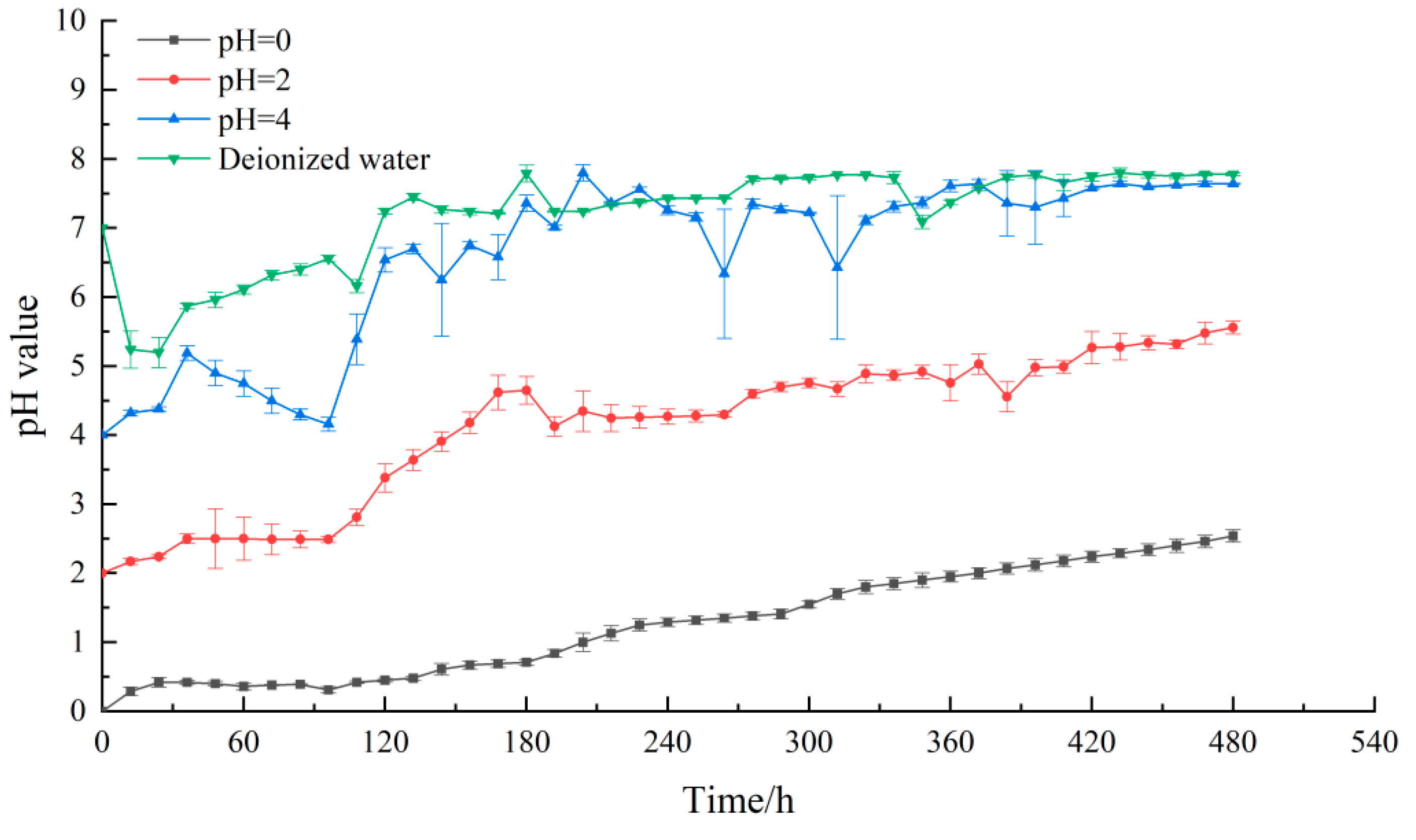
4.2. The Changes of pH Value
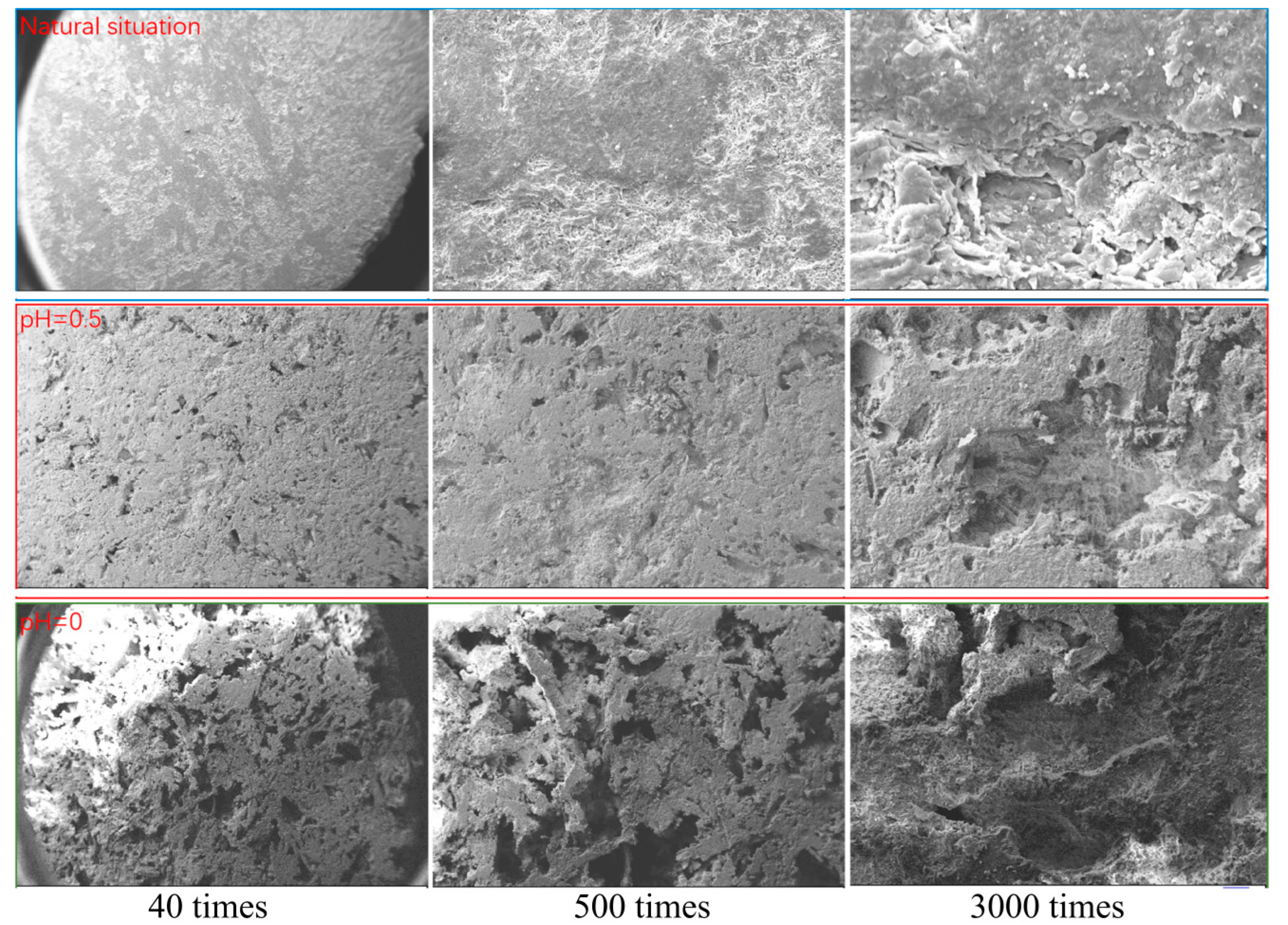
4.3. Microstructure Characteristics of Scanning Electron Microscopy
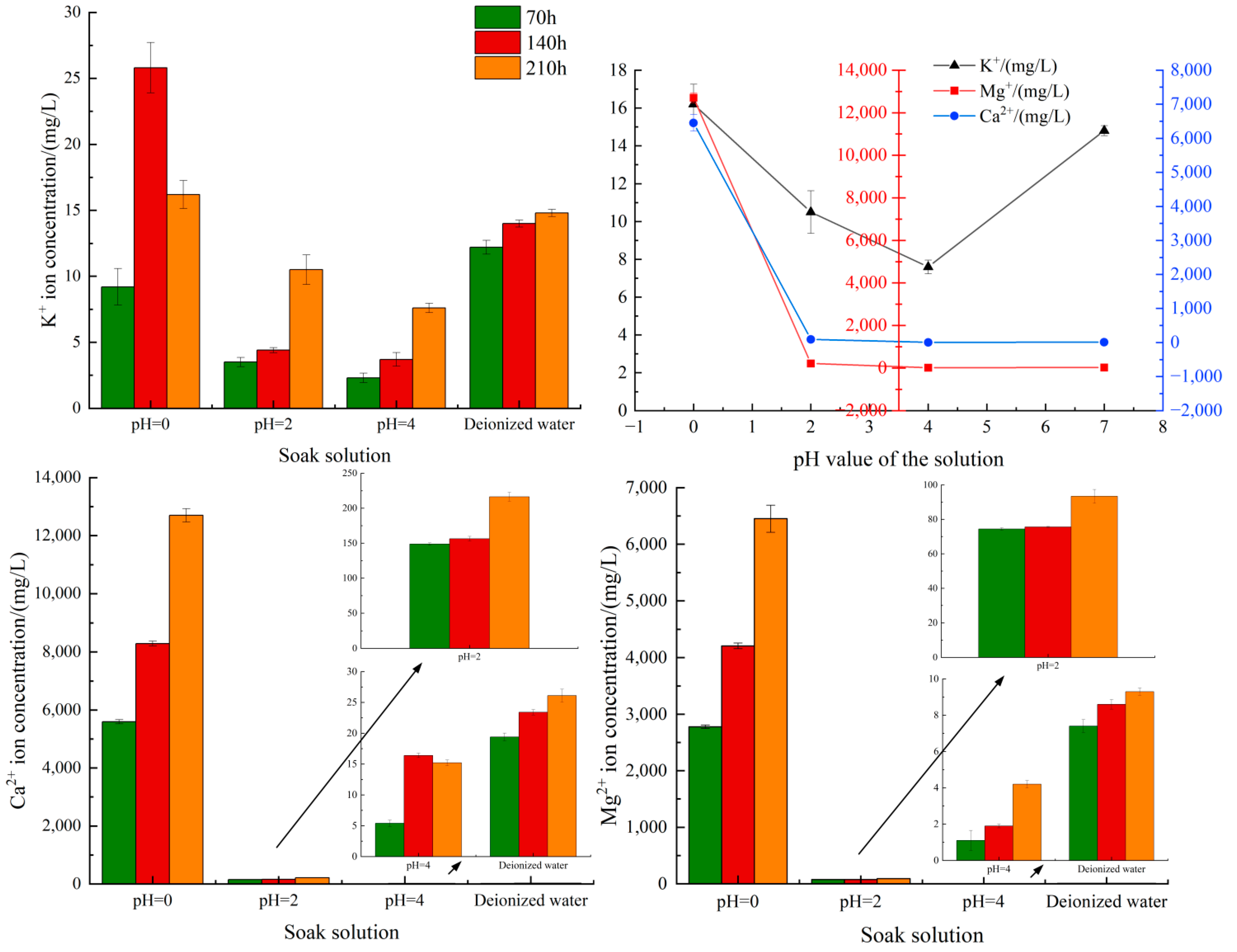
4.4. Variation Analysis of K+, Ca2+ and Mg2+ Concentrations
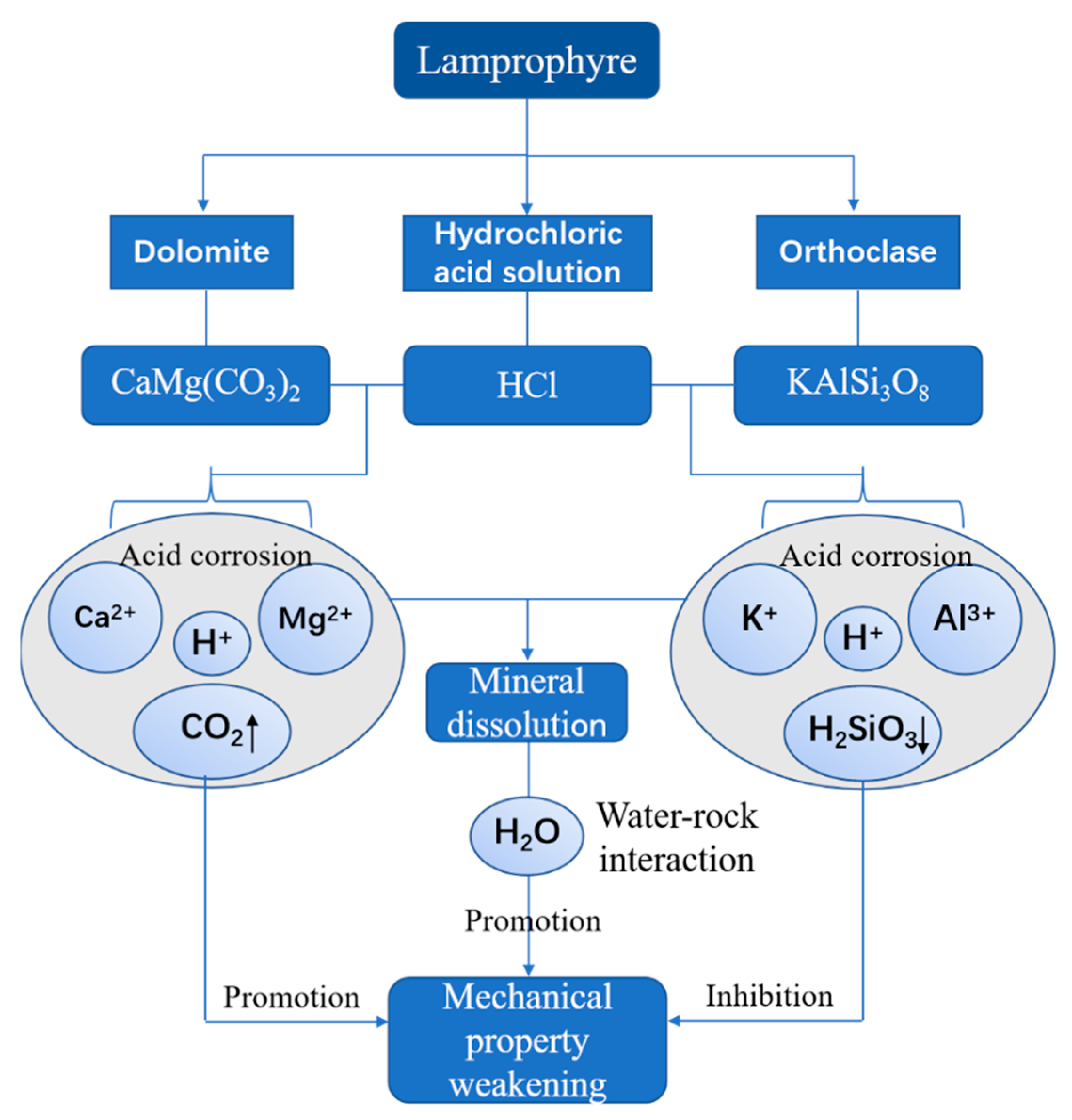
4.5. Strength Weakening Mechanism
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, J.; Feng, G.R. Roof Strata Behavior and Support Resistance Determination for Ultra-Thick Longwall Top Coal Caving Panel: A Case Study of the Tashan Coal Mine. Energies 2018, 11, 1041. [Google Scholar] [CrossRef]
- Guo, J.; Feng, G.R. Dynamic Mechanical Behavior of Dry and Water Saturated Igneous Rock with Acoustic Emission Monitoring. Shock. Vib. 2018, 4, 1–14. [Google Scholar] [CrossRef]
- Feng, G.R.; Sun, Q.; Guo, J. Numerical Simulation Study on Effect of Lamprophyre in Water on Overburden Migration in Ultrathick Coal Seam. Saf. Coal Mines 2019, 50, 48–53. [Google Scholar]
- Yang, Z.W. Research on the distribution law of the mining supporting pressure 4# coal seam in Yongdingzhuang Mine of Datong Mine Zone. China Coal 2013, 39, 60–63. [Google Scholar]
- Guo, J.G. Study on control of surrounding rock of tunnel with very thick coal seam intruded by igneous rock. J. Henan Polytech. Univ. Nat. Sci. 2017, 36, 15–20. [Google Scholar]
- Ling, S.X.; Wu, X.Y.; Sun, C.W.; Liao, X.; Ren, Y.; Li, X.N. Experimental Study of Chemical Damage and Mechanical Deterioration of Black Shale Due Water-Rock Chemical Action. J. Exp. Mech. 2016, 31, 511–524. [Google Scholar]
- Deng, G.Z.; Liu, H. Fracture mechanism of acid fracturing of sandstones gangue layer in fully mechanized mining face. Saf. Coal Mines 2021, 52, 75–83. [Google Scholar]
- Huo, R.K.; Han, F.; Li, S.G.; Wang, G.J. Experimental study on physicochemical and mechanical properties of acid-corroded sandstone. J. Xi’an Univ. Archit. Technol. Nat. Sci. Ed. 2019, 51, 21–26. [Google Scholar]
- Shu, G.L.; Huo, R.K. Experimental Study on Physicomechanical Properties of Sandstone under Acidic Environment. Adv. Civ. Eng. 2018, 2018, 1–15. [Google Scholar]
- Huo, R.K.; Li, S.G. CT Analysis on Mesoscopic Structure of Sandstone under Acidic Environment. Indian J. Geo-Mar. Sci. 2018, 47, 962–971. [Google Scholar]
- Yao, H.Y.; Feng, X.T.; Cui, Q.; Shen, L.F.; Zhou, H.; Cheng, C.B. Experimental study of effect of chemical corrosion on strength and deformation of hard brittle limestone. Rock Soil Mech. 2009, 30, 338–344. [Google Scholar]
- Ding, W.X.; Feng, X.T. Testing Study on Mechanical Effect for Limestone under chemical Erosion. Chin. J. Rock Mech. Eng. 2004, 23, 3571–3576. [Google Scholar]
- Chen, S.L.; Feng, X.T.; Li, S.J. The fracturing behaviors of Three Gorges granite under chemical erosion. Rock Soil Mech. 2003, 24, 817–821. [Google Scholar]
- Feng, X.T.; Wang, C.Y. Testing Study and Real-Time Observation of Rock Meso-Cracking Process under Chemical Erosion. Chin. J. Rock Mech. Eng. 2002, 21, 935–939. [Google Scholar]
- Chen, S.L.; Feng, X.T. The effect of chemical erosion on mechanical behaviors of Xiaolangdi sandstone. Rock Soil Mech. 2002, 284–287, 296. [Google Scholar] [CrossRef]
- He, C.M.; Guo, J.C. Mechanism Study of Acid on Mechanical Properties of Limestone. Chin. J. Rock Mech. Eng. 2013, 32, 3016–3021. [Google Scholar]
- Liu, X.R.; Li, D.L.; Wang, Z.; Zhang, L. The effect of dry-wet cycles with acidic wetting fluid on strength deterioration of shaly sandstone. Chin. J. Rock Mech. Eng. 2016, 35, 1543–1554. [Google Scholar]
- Deng, H.F.; Zhi, Y.Y. Mechanical properties of sandstone and damage evolution of microstructure under water-rock interact. Rock Soil Mech. 2019, 40, 3447–3456. [Google Scholar]
- Feng, X.W.; Wang, W. A rheological damage model of sandstone under water-rock chemical interaction. Rock Soil Mech. 2018, 39, 3340–3346, 3354. [Google Scholar]
- Wang, W.; Li, X.H. Experimental study of mechanical characteristics of sandy slate under chemical corrosion. Rock Soil Mech. 2017, 38, 2559–2566, 2573. [Google Scholar]
- Yu, J.; Zhang, X. Meso-damage and mechanical properties degradation of sandstone under combined effect of water chemical corrosion and freeze-thaw cycles. Rock Soil Mech. 2019, 40, 455–464. [Google Scholar]
- Liu, H.B.; Cui, S. Study on the Microstructure and Mechanical Properties of Carbonate Rock before and after Acidification. Chin. J. Undergr. Space Eng. 2020, 16, 1321–1327, 1434. [Google Scholar]
- Cui, B.; Feng, P.Y. Study on Microscopic Characteristics of Carbonate Acid Corrosion of M Oilfield in Middle East. J. Guangdong Univ. Petrochem. Technol. 2021, 31, 29–32, 36. [Google Scholar]
- Li, X.Y.; Wu, H.C. Influences of Microstructural Differences on Acid Corrosive Damage to Carbonate Rocks. Xinjiang Pet. Geol. 2021, 42, 188–193. [Google Scholar]
- Yang, H.; Xue, X.J. Establishment of igneous rock mechanical parameters model based on electric logging data inversion and its engineering application. Nat. Gas Ind. 2021, 41, 101–109. [Google Scholar]
- Shen, C.; Feng, G.R. The Influence of and Mechanism Study Tensile Mechanical Properties of Lamprophyre in Acid Condition. J. Taiyuan Univ. Technol. 2017, 48, 407–412. [Google Scholar]
- Guo, J.; Feng, G.R. Mechanical property variation under dynamic uniaxial compression and micro-mechanism of lamprophyre in saturated state. J. China Coal Soc. 2015, 40, 323–330. [Google Scholar]
- Chen, W.; Wu, L. Research on Uniaxial Compression Mechanics of Diorite under Flowing Acidic Solution Scouring. Minerals 2022, 12, 770. [Google Scholar] [CrossRef]
- Luo, X.Y.; Cao, P. Mechanical Behaviour of Anchored Rock Containing Weak Interlayer under Uniaxial Compression: Laboratory Test and Coupled DEM–FEM Simulation. Minerals 2022, 12, 492. [Google Scholar] [CrossRef]
- Zhang, P.F.; Zhang, Y.B. Experimental Research on Deformation Characteristics of Waste-Rock Material in Underground Backfill Mining. Minerals 2019, 9, 102. [Google Scholar] [CrossRef]
- Liu, G.S.; Li, L. An Investigation of the Uniaxial Compressive Strength of a Cemented Hydraulic Backfill Made of Alluvial Sand. Minerals 2017, 7, 4. [Google Scholar] [CrossRef]
- Liu, T.Y.; Cui, M.Y. Fracture and Damage Evolution of Multiple-Fractured Rock-like Material Subjected to Compression. Materials 2022, 15, 4326. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Qin, G.P. Deformation, Failure, and Acoustic Emission Characteristics under Different Lithological Confining Pressures. Materials 2022, 15, 4257. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.Q.; Huang, R.Q. Evolution of Mineral under Interaction of Water and Crystalline Rock. Mineral. Petrol. 2004, 43–47. [Google Scholar] [CrossRef]
- Liu, Z.X.; Wang, W. Method of energy evolution of rock under uniaxial compression test. J. China Coal Soc. 2020, 45, 3131–3139. [Google Scholar] [CrossRef]
- Xue, J.C.; Dong, L.J. Effect of Chemical Corrosion and Axial Compression on the Dynamic Strength Degradation Characteristics of White Sandstone under Cyclic Impact. Minerals 2022, 12, 429. [Google Scholar] [CrossRef]
- Yu, H.J.; Liu, H.L. Deformation and Failure Mechanism of Weakly Cemented Mudstone under Tri-Axial Compression: From Laboratory Tests to Numerical Simulation. Minerals 2022, 12, 153. [Google Scholar] [CrossRef]
- Chen, L.; Jia, B.X. Study on Mechanical Behavior and Energy Mechanism of Sandstone under Chemical Corrosion. Materials 2022, 15, 1613. [Google Scholar] [CrossRef]
- Luo, X.J.; Yang, W.D. Effects of pH on the Solubility of the Feldspar and the Development of Secondary Porosity. Bull. Mineral. Petrol. Geochem. 2001, 20, 103–107. [Google Scholar]




| Mineral Component | Dolomite | Orthoclase | Chlorite | Loweite | Montmorillonite | Illite | Pyrophyllite | Quartz | Pyroxmangite |
|---|---|---|---|---|---|---|---|---|---|
| Content/% | 19.63 | 31.4 | 1.46 | 13.16 | 3.11 | 1.67 | 6.70 | 9.32 | 13.64 |
| Peak Stress/MPa | Peak Stress Damage/% | |
|---|---|---|
| Drying | 132 ± 4 | 0 |
| Deionized water | 111 ± 7 | 15.91 |
| pH = 4 solution | 82 ± 5 | 37.88 |
| pH = 2 solution | 65 ± 3 | 50.76 |
| pH = 0 solution | 39 ± 3 | 70.45 |
| Soaking Solution | K+ Ion Concentration (mg/L) | Ca2+ Ion Concentration (mg/L) | Mg2+ Ion Concentration (mg/L) |
|---|---|---|---|
| Deionized water (70 h) | 12.2 ± 0.6 | 19.4 ± 0.7 | 7.4 ± 0.4 |
| Deionized water (140 h) | 14.0 ± 0.4 | 23.4 ± 0.5 | 8.6 ± 0.3 |
| Deionized water (210 h) | 14.8 ± 0.3 | 26.1 ± 1.2 | 9.3 ± 0.2 |
| pH = 0 (70 h) | 9.2 ± 1.6 | 5594.4 ± 81.7 | 2778.2 ± 34.2 |
| pH = 0 (140 h) | 25.8 ± 2.2 | 8290.3 ± 96.3 | 4209.1 ± 56.1 |
| pH = 0 (210 h) | 16.2 ± 1.2 | 12,700.2 ± 245.3 | 6451.9 ± 275.3 |
| pH = 2 (70 h) | 3.5 ± 0.4 | 148.8 ± 2.1 | 74.4 ± 0.8 |
| pH = 2 (140 h) | 4.4 ± 0.2 | 156.4 ± 3.6 | 75.6 ± 0.3 |
| pH = 2 (210 h) | 10.5 ± 1.3 | 216.3 ± 7.3 | 93.4 ± 4.6 |
| pH = 4 (70 h) | 2.3 ± 0.4 | 5.4 ± 0.5 | 1.1 ± 0.6 |
| pH = 4 (140 h) | 3.7 ± 0.6 | 16.4 ± 0.4 | 1.9 ± 0.1 |
| pH = 4 (210 h) | 7.6 ± 0.4 | 15.2 ± 0.5 | 4.2 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Mi, X.; Feng, G.; Qi, T.; Bai, J.; Wen, X.; Qian, R.; Zhu, L.; Guo, X.; Yu, L. Study on Mechanical Properties and Weakening Mechanism of Acid Corrosion Lamprophyre. Materials 2022, 15, 6634. https://doi.org/10.3390/ma15196634
Guo J, Mi X, Feng G, Qi T, Bai J, Wen X, Qian R, Zhu L, Guo X, Yu L. Study on Mechanical Properties and Weakening Mechanism of Acid Corrosion Lamprophyre. Materials. 2022; 15(19):6634. https://doi.org/10.3390/ma15196634
Chicago/Turabian StyleGuo, Jun, Xincheng Mi, Guorui Feng, Tingye Qi, Jinwen Bai, Xiaoze Wen, Ruipeng Qian, Linjun Zhu, Xingchen Guo, and Luyang Yu. 2022. "Study on Mechanical Properties and Weakening Mechanism of Acid Corrosion Lamprophyre" Materials 15, no. 19: 6634. https://doi.org/10.3390/ma15196634
APA StyleGuo, J., Mi, X., Feng, G., Qi, T., Bai, J., Wen, X., Qian, R., Zhu, L., Guo, X., & Yu, L. (2022). Study on Mechanical Properties and Weakening Mechanism of Acid Corrosion Lamprophyre. Materials, 15(19), 6634. https://doi.org/10.3390/ma15196634






