Recycling of Cement Industry Waste for Alkali-Activated Materials Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mix Design
2.3. Methods
3. Results and Discussion
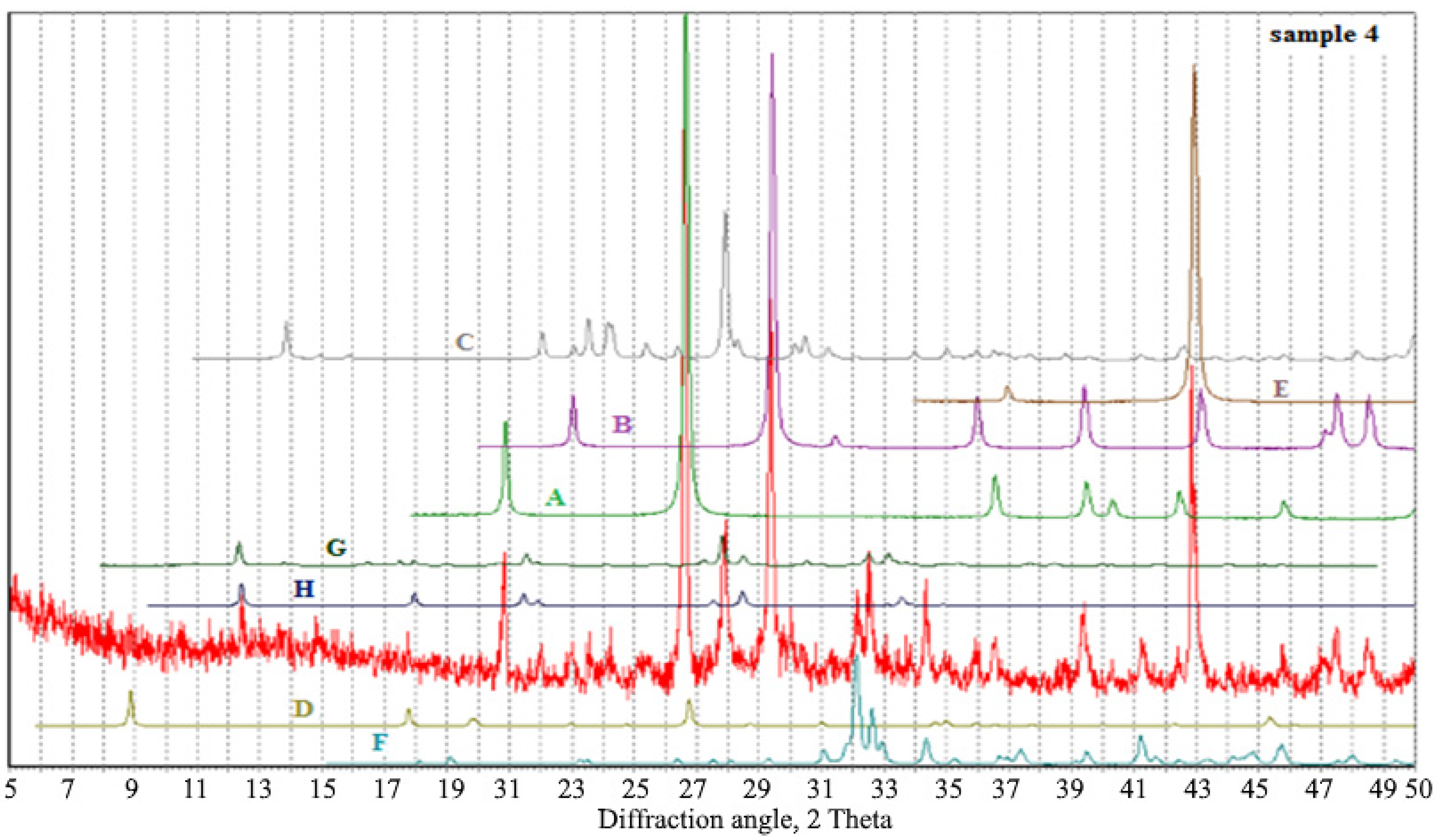
3.1. Characterization of the Dusts
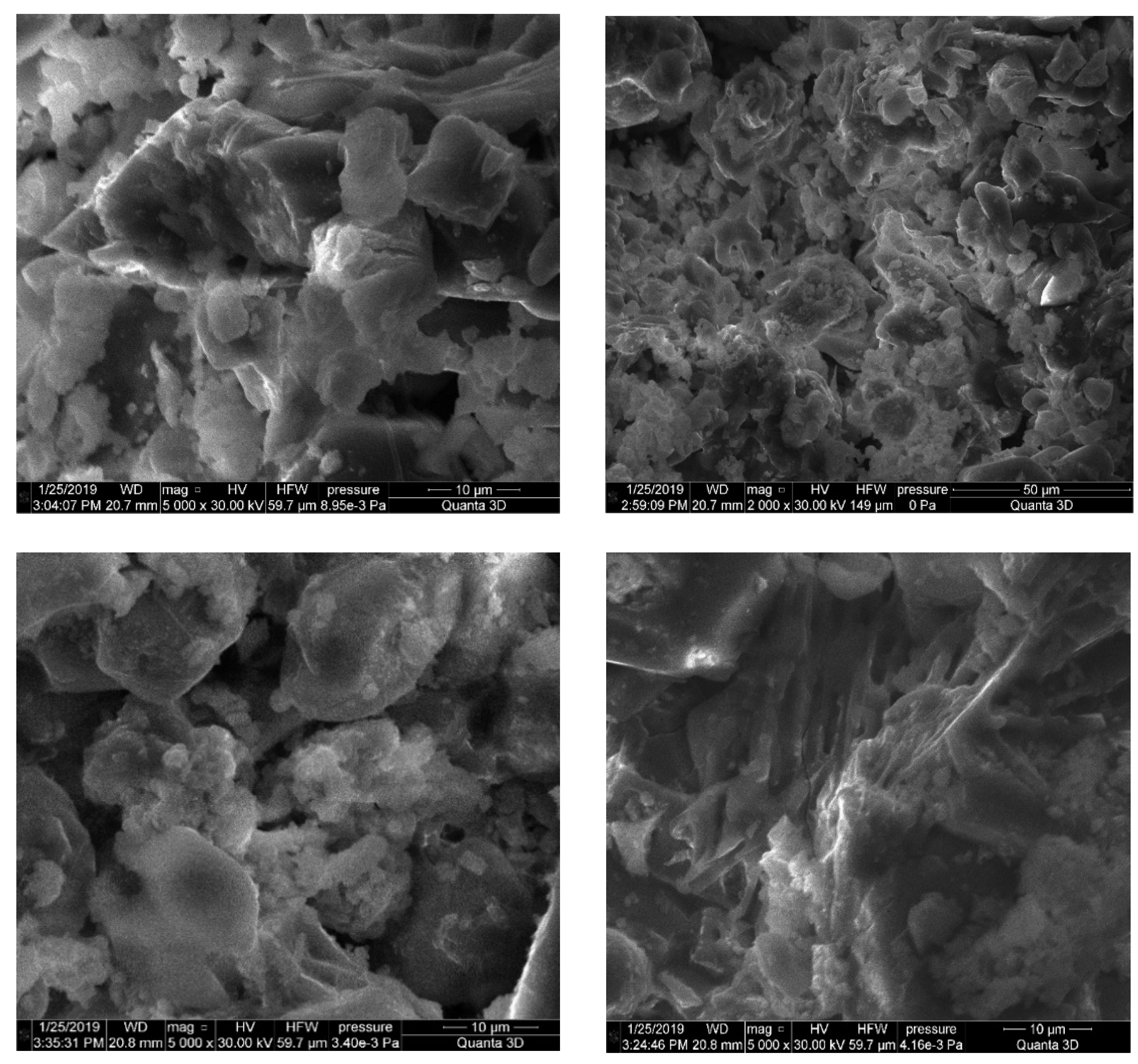
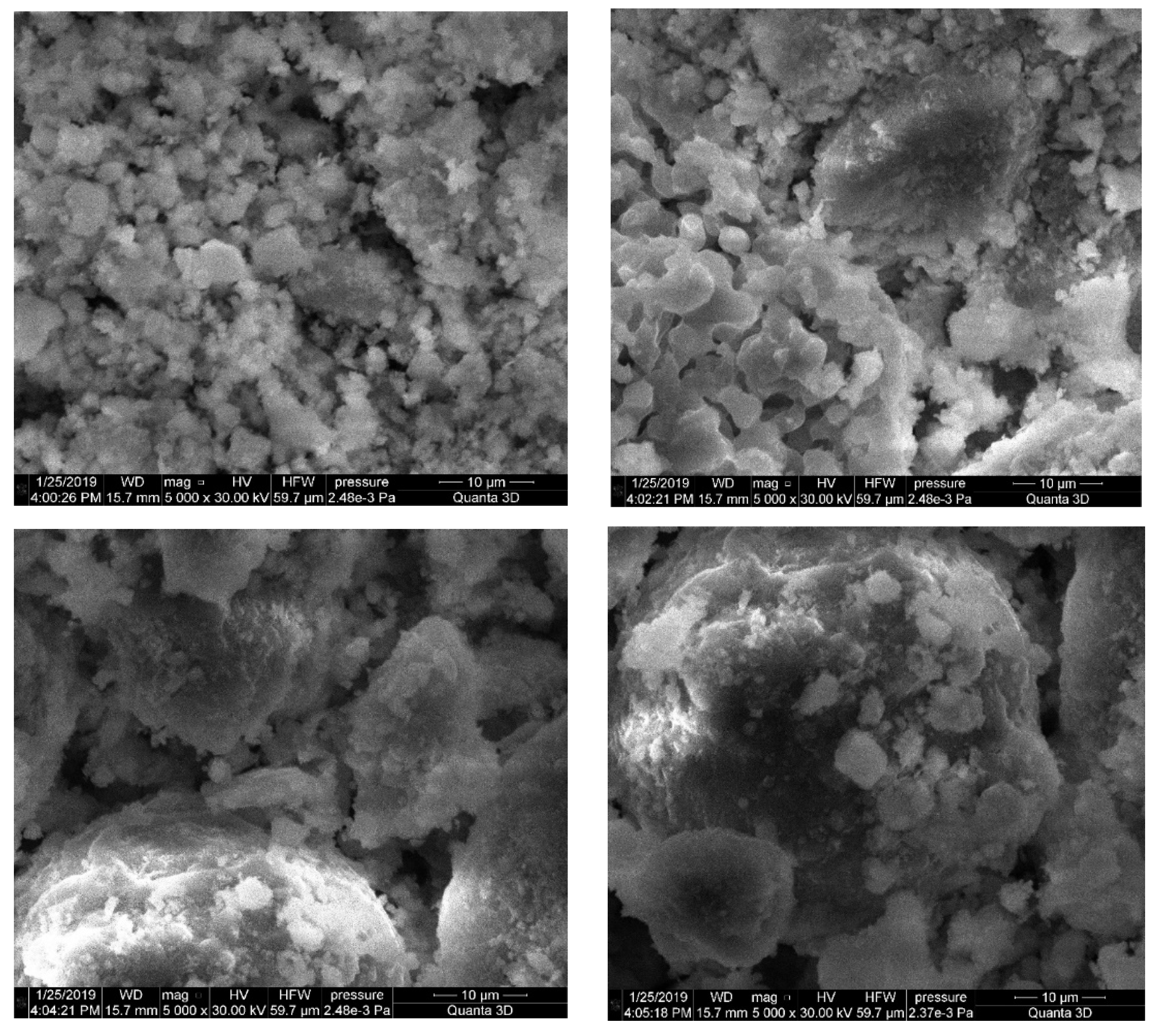
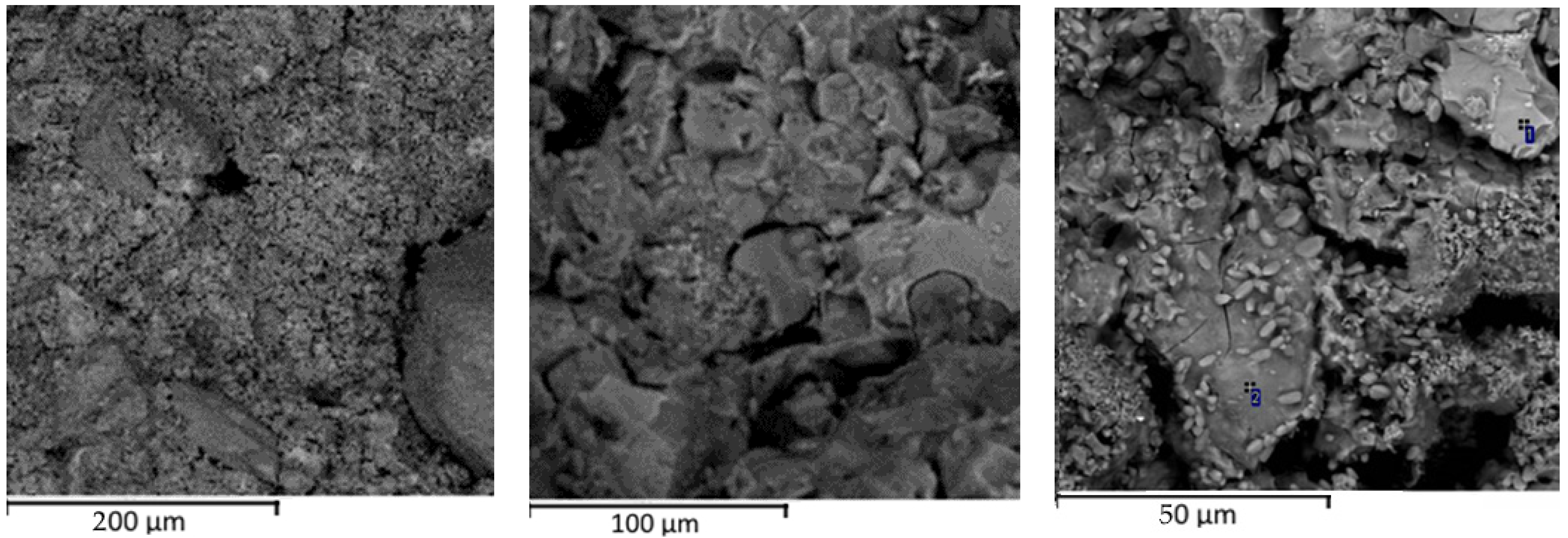
3.2. Microstructure of Alkali-Activated Material
3.3. Fresh, Physical, and Mechanical Properties of the Alkali-Activated Material
4. Conclusions
- Grains of cement production waste are represented by coarse volumetric particles with pronounced cleavage, small leaves, and the clear presence of minerals. The mineral composition of cement production waste is characterized by calcium silicates, which guarantee good binding properties.
- The results of the X-ray diffraction analysis of the samples based on the cement-free binder of alkaline activation using clinker dust and aspiration dust confirmed the presence of calcite, quartz, feldspar close to albite, micas, and zeolites. The obtained products of the chemical interaction of the components of the binder confirm the effectiveness of the newly developed AAM.
- As a result of comparing several binders, it was found that the binder based on aspiration dust with Na2SiO3 and Na2SiF6 is the most effective since its specimens achieved a density of 1.8 g/cm3, maximum compressive strength of 50.7 MPa, flexural strength of 5.6 MPa, and minimum setting time (starting at 24 min and ending at 36 min), with a water absorption of 12.8 wt. %.
- Compared with traditional concrete on cement CEM I 42.5 N, it was noted that with nearly equivalent strength properties achieved, the standard consistency is 2–3 times higher, which is not a negative factor due to the absence of water, and, hence, cracks. Reducing the density by 30% allows for the use of alkali-activated materials for lightweight and high-strength structures. Its several-times-faster setting start and end times indicate a potential acceleration of construction production. The water absorption by weight of the developed materials and traditional concretes is nearly the same.
- The research results will be of interest to specialists in the construction industry since the proposed recipes for eco-friendly alkali-activated materials are an alternative to expensive and energy-intensive Portland cement, and they enable the creation of strong and durable concrete and reinforced concrete composites.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Her, S.; Park, J.; Li, P.; Bae, S. Feasibility Study on Utilization of Pulverized Eggshell Waste as an Alternative to Limestone in Raw Materials for Portland Cement Clinker Production. Constr. Build. Mater. 2022, 324, 126589. [Google Scholar] [CrossRef]
- Pavlíková, M.; Zemanová, L.; Záleská, M.; Pokorný, J.; Lojka, M.; Jankovský, O.; Pavlík, Z. Ternary Blended Binder for Production of a Novel Type of Lightweight Repair Mortar. Materials 2019, 12, 996. [Google Scholar] [CrossRef] [PubMed]
- Tolstoy, A.; Lesovik, V.; Fediuk, R.; Amran, M.; Gunasekaran, M.; Vatin, N.; Vasilev, Y. Production of Greener High-Strength Concrete Using Russian Quartz Sandstone Mine Waste Aggregates. Materials 2020, 13, 5575. [Google Scholar] [CrossRef]
- Zhao, J.; Tong, L.; Li, B.; Chen, T.; Wang, C.; Yang, G.; Zheng, Y. Eco-Friendly Geopolymer Materials: A Review of Performance Improvement, Potential Application and Sustainability Assessment. J. Clean. Prod. 2021, 307, 127085. [Google Scholar] [CrossRef]
- López, F.J.; Sugita, S.; Tagaya, M.; Kobayashi, T. Metakaolin-Based Geopolymers for Targeted Adsorbents to Heavy Metal Ion Separation. J. Mater. Sci. Chem. Eng. 2014, 02, 16–27. [Google Scholar] [CrossRef]
- Salamanova, M.S.; Murtazaev, S.-A.Y.; Nakhaev, M.R. Possible Alternative Solutions to Problems in the Cement Industry. Stroit. Mater. 2020, 778, 73–77. [Google Scholar] [CrossRef]
- Aliev, S.A.; Murtazayeva, R.S.-A.; Salamanova, M.S. Structure and properties of connecting alkaline activation using cement dust. Her. Dagestan State Tech. Univ. Tech. Sci. 2019, 46, 148–157. [Google Scholar] [CrossRef]
- Burduhos Nergis, D.D.; Abdullah, M.M.A.B.; Vizureanu, P.; Tahir, M.F.M. Geopolymers and Their Uses: Review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 374, 012019. [Google Scholar] [CrossRef]
- Yang, N.; Das, C.S.; Xue, X.; Li, W.; Dai, J.-G. Geopolymer Coating Modified with Reduced Graphene Oxide for Improving Steel Corrosion Resistance. Constr. Build. Mater. 2022, 342, 127942. [Google Scholar] [CrossRef]
- Mohseni, E. Assessment of Na2SiO3 to NaOH Ratio Impact on the Performance of Polypropylene Fiber-Reinforced Geopolymer Composites. Constr. Build. Mater. 2018, 186, 904–911. [Google Scholar] [CrossRef]
- Öztürk, O. Comparison of Frost Resistance for the Fiber Reinforced Geopolymer and Cementitious Composites. Mater. Today Proc. 2022, 65, 1504–1510. [Google Scholar] [CrossRef]
- Mahdi, S.N.; Babu R, D.V.; Hossiney, N.; Abdullah, M.M.A.B. Strength and Durability Properties of Geopolymer Paver Blocks Made with Fly Ash and Brick Kiln Rice Husk Ash. Case Stud. Constr. Mater. 2022, 16, e00800. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Z.; Wang, Y.; Feng, J. Preparation and Properties of Alkali Activated Metakaolin-Based Geopolymer. Materials 2016, 9, 767. [Google Scholar] [CrossRef] [PubMed]
- Assi, L.N.; Deaver, E.; Elbatanouny, M.K.; Ziehl, P. Investigation of Early Compressive Strength of Fly Ash-Based Geopolymer Concrete. Constr. Build. Mater. 2016, 112, 807–815. [Google Scholar] [CrossRef]
- Makul, N.; Fediuk, R.; Amran, M.; Zeyad, A.M.; Murali, G.; Vatin, N.; Klyuev, S.; Ozbakkaloglu, T.; Vasilev, Y. Use of Recycled Concrete Aggregates in Production of Green Cement-Based Concrete Composites: A Review. Crystals 2021, 11, 232. [Google Scholar] [CrossRef]
- Zhu, X.; Lu, C.; Li, W.; Zhou, S.; Li, F.; Xiao, J.; Shah, S.P. Effects of Carbon Nanofibers on Hydration and Geopolymerization of Low and High-Calcium Geopolymers. Cem. Concr. Compos. 2022, 133, 104695. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Zou, J.; Reid, A.; Wang, H. Toward an Indexing Approach to Evaluate Fly Ashes for Geopolymer Manufacture. Cem. Concr. Res. 2016, 85, 163–173. [Google Scholar] [CrossRef]
- Sturm, P.; Gluth, G.J.G.; Brouwers, H.J.H.; Kühne, H.-C. Synthesizing One-Part Geopolymers from Rice Husk Ash. Constr. Build. Mater. 2016, 124, 961–966. [Google Scholar] [CrossRef]
- Fediuk, R.; Timokhin, R.; Mochalov, A.; Otsokov, K.; Lashina, I. Performance Properties of High-Density Impermeable Cementitious Paste. J. Mater. Civ. Eng. 2019, 31, 04019013. [Google Scholar] [CrossRef]
- Loganina, V.; Fediuk, R.; Usanova, K.; Timokhin, R. Regularities of Change in the Properties of Paint Coatings on Cement Concretes at Moistening. In Energy, Environmental and Construction Engineering; Lecture Notes in Civil Engineering; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Fediuk, R.; Smoliakov, A.; Stoyushko, N. Increase in Composite Binder Activity. IOP Conf. Ser. Mater. Sci. Eng. 2016, 156, 012042. [Google Scholar] [CrossRef]
- Yavuz, E.; Kul Gul, N.I.; Kockal, N.U. Characterization of Class C and F Fly Ashes Based Geopolymers Incorporating Silica Fume. Ceram. Int. 2022, in press. [Google Scholar] [CrossRef]
- Shoaei, P.; Ameri, F.; Reza Musaeei, H.; Ghasemi, T.; Cheah, C.B. Glass Powder as a Partial Precursor in Portland Cement and Alkali-Activated Slag Mortar: A Comprehensive Comparative Study. Constr. Build. Mater. 2020, 251, 118991. [Google Scholar] [CrossRef]
- Fediuk, R.S.; Yushin, A.M. The Use of Fly Ash the Thermal Power Plants in the Construction. IOP Conf. Ser. Mater. Sci. Eng. 2015, 93, 012070. [Google Scholar] [CrossRef]
- Volodchenko, A.A.; Lesovik, V.S.; Volodchenko, A.N.; Glagolev, E.S.; Zagorodnjuk, L.H.; Pukharenko, Y.V. Composite Performance Improvement Based on Non-Conventional Natural and Technogenic Raw Materials. Int. J. Pharm. Technol. 2016, 8, 18856–18867. [Google Scholar]
- Payá, J.; Agrela, F.; Rosales, J.; Morales, M.M.; Borrachero, M.V. Application of Alkali-Activated Industrial Waste. In New Trends in Eco-Efficient and Recycled Concrete; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Gao, X.X.; Michaud, P.; Joussein, E.; Rossignol, S. Behavior of Metakaolin-Based Potassium Geopolymers in Acidic Solutions. J. Non. Cryst. Solids 2013, 380, 95–102. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L. Durability of Alkali-Activated Materials: Progress and Perspectives. J. Am. Ceram. Soc. 2014, 97, 997–1008. [Google Scholar] [CrossRef]
- Kadhim, A.; Sadique, M.; Al-Mufti, R.; Hashim, K. Long-Term Performance of Novel High-Calcium One-Part Alkali-Activated Cement Developed from Thermally Activated Lime Kiln Dust. J. Build. Eng. 2020, 32, 101766. [Google Scholar] [CrossRef]
- Abid, S.R.; Murali, G.; Amran, M.; Vatin, N.; Fediuk, R.; Karelina, M. Evaluation of Mode II Fracture Toughness of Hybrid Fibrous Geopolymer Composites. Materials 2021, 14, 349. [Google Scholar] [CrossRef]
- Brooks, R.; Bahadory, M.; Tovia, F.; Rostami, H. Properties of Alkali-Activated Fly Ash: High Performance to Lightweight. Int. J. Sustain. Eng. 2010, 3, 211–218. [Google Scholar] [CrossRef]
- Othman, R.; Jaya, R.P.; Muthusamy, K.; Sulaiman, M.; Duraisamy, Y.; Abdullah, M.M.A.B.; Przybył, A.; Sochacki, W.; Skrzypczak, T.; Vizureanu, P.; et al. Relation between Density and Compressive Strength of Foamed Concrete. Materials 2021, 14, 2967. [Google Scholar] [CrossRef]
- Danish, A.; Mosaberpanah, M.A.; Salim, M.U. Past and Present Techniques of Self-Healing in Cementitious Materials: A Critical Review on Efficiency of Implemented Treatments. J. Mater. Res. Technol. 2020, 9, 6883–6899. [Google Scholar] [CrossRef]
- Danish, A.; Mosaberpanah, M.A. Formation Mechanism and Applications of Cenospheres: A Review. J. Mater. Sci. 2020, 55, 4539–4557. [Google Scholar] [CrossRef]
- Cano, M.; Reina, T.R.; Portillo, E.; Gallego Fernández, L.M.; Navarrete, B. Characterization of Emissions of Condensable Particulate Matter under Real Operation Conditions in Cement Clinker Kilns Using Complementary Experimental Techniques. Sci. Total Environ. 2021, 786, 147472. [Google Scholar] [CrossRef] [PubMed]
- Pieper, C.; Wirtz, S.; Schaefer, S.; Scherer, V. Numerical Investigation of the Impact of Coating Layers on RDF Combustion and Clinker Properties in Rotary Cement Kilns. Fuel 2021, 283, 118951. [Google Scholar] [CrossRef]
- Hanein, T.; Hayashi, Y.; Utton, C.; Nyberg, M.; Martinez, J.-C.; Quintero-Mora, N.-I.; Kinoshita, H. Pyro Processing Cement Kiln Bypass Dust: Enhancing Clinker Phase Formation. Constr. Build. Mater. 2020, 259, 120420. [Google Scholar] [CrossRef]
- Pieper, C.; Liedmann, B.; Wirtz, S.; Scherer, V.; Bodendiek, N.; Schaefer, S. Interaction of the Combustion of Refuse Derived Fuel with the Clinker Bed in Rotary Cement Kilns: A Numerical Study. Fuel 2020, 266, 117048. [Google Scholar] [CrossRef]
- Yamashita, M.; Tanaka, H.; Sakai, E.; Tsuchiya, K. Mineralogical Study of High SO3 Clinker Produced Using Waste Gypsum Board in a Cement Kiln. Constr. Build. Mater. 2019, 217, 507–517. [Google Scholar] [CrossRef]
- Wu, W.-N.; Liu, X.-Y.; Hu, Z.; Zhang, R.; Lu, X.-Y. Improving the Sustainability of Cement Clinker Calcination Process by Assessing the Heat Loss through Kiln Shell and Its Influencing Factors: A Case Study in China. J. Clean. Prod. 2019, 224, 132–141. [Google Scholar] [CrossRef]
- N’ZI, Y.G.; Tarasiewicz, S. Phenomenological Approach to Model a Clinker Rotary Kiln. IFAC Proc. Vol. 2013, 46, 197–202. [Google Scholar] [CrossRef]
- Rivera-Austrui, J.; Martinez, K.; Marco-Almagro, L.; Abalos, M.; Abad, E. Long-Term Sampling of Dioxin-like Substances from a Clinker Kiln Stack Using Alternative Fuels. Sci. Total Environ. 2014, 485–486, 528–533. [Google Scholar] [CrossRef]





| Electrostatic Precipitator Dust | True Density, kg/m3 | Bulk Density, kg/m3 | Specific Surface Area, m2/kg |
|---|---|---|---|
| Aspiration | 2.59 | 1.13 | 280 |
| Clinker | 3.12 | 1.24 | 210 |
| Electrostatic Precipitator Dust | CaO | SiO2 | Al2O3 | Fe2O3 | K2O | MgO |
|---|---|---|---|---|---|---|
| Aspiration | 64.14 | 20.31 | 4.68 | 3.47 | 6.43 | 0.97 |
| Clinker | 71.64 | 16.89 | 4.11 | 4.30 | 1.57 | 1.49 |
| Mix ID | Components, kg per 1 m3 | ||||||
|---|---|---|---|---|---|---|---|
| Clinker Dust | Aspiration Dust | Na2SiO3 | H2O | Na2SiF6 | NaOH | Sand | |
| K1 | 690 | - | 270 | - | - | 69 | 1035 |
| K2 | 690 | - | 270 | - | 69 | 1035 | |
| A1 | - | 690 | 270 | - | - | 69 | 1035 |
| A2 | - | 690 | - | 270 | - | 69 | 1035 |
| A3 | - | 690 | 270 | - | 16.8 | 69 | 1035 |
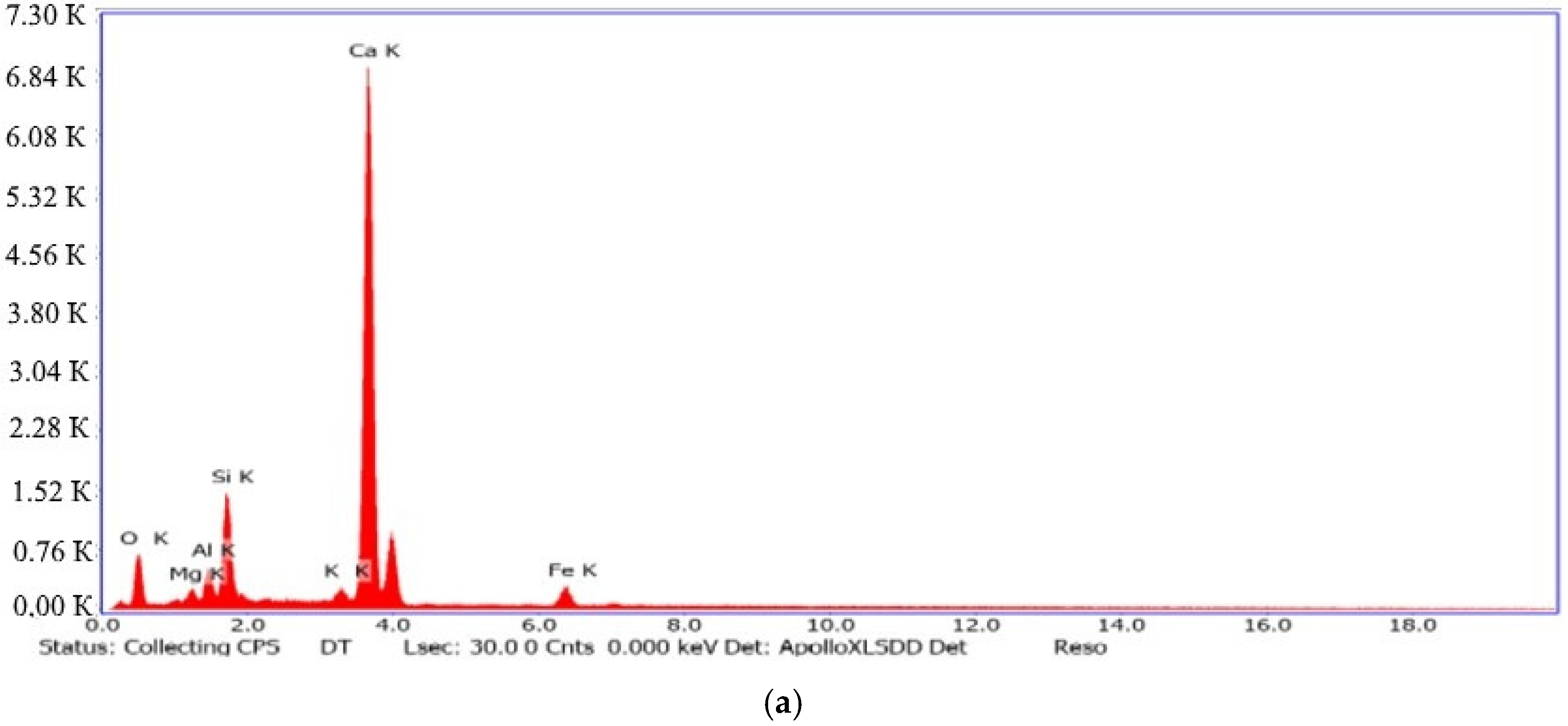
| Spectra | Na2O | MgO | Al2O3 | SiO2 | K2O | CaO | Fe2O3 | Total |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.00 | 0.81 | 5.34 | 41.40 | 0.00 | 36.64 | 0.68 | 85.36 |
| 2 | 0.25 | 1.72 | 6.04 | 36.98 | 0.00 | 35.43 | 1.02 | 81.44 |
| 3 | 0.33 | 1.14 | 0,96 | 28.78 | 0.18 | 64.20 | 0.85 | 96.44 |
| 4 | 0.00 | 0.00 | 2,94 | 29.77 | 0.00 | 46.01 | 0.00 | 78.72 |
| 5 | 0.17 | 0.39 | 4,70 | 34.97 | 0.29 | 36.16 | 0.70 | 77.39 |
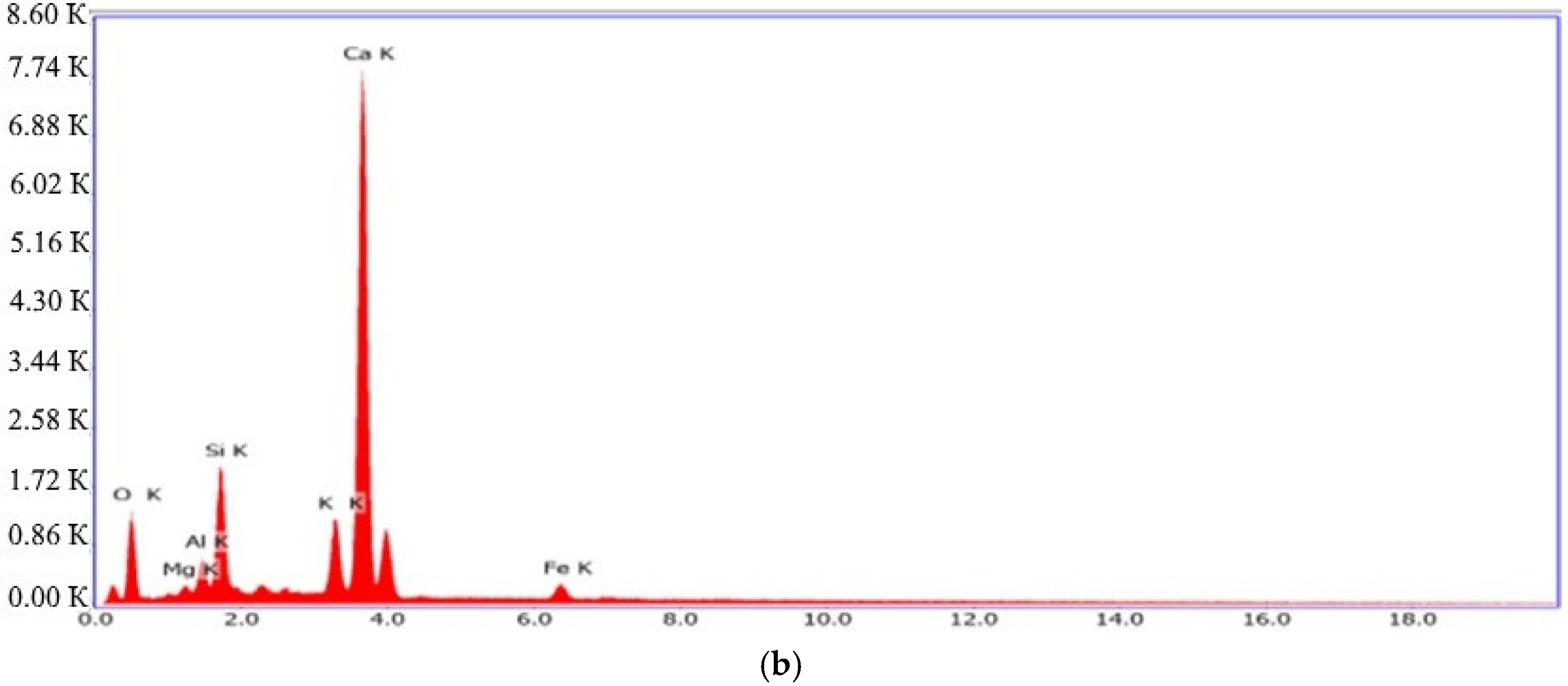
| Spectra | Na2O | MgO | Al2O3 | SiO2 | K2O | CaO | MnO | Fe2O3 | Total |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.02 | 0.75 | 0.61 | 25.96 | 0.08 | 67.81 | 0.00 | 0.48 | 95.70 |
| 2 | 0.15 | 0.60 | 4.75 | 43.95 | 0.03 | 38.60 | 0.26 | 0.44 | 88.78 |
| Properties | Clinker Dust, S sp = 210 m2/kg | Aspiration Dust, Ssp = 280 m2/kg | |||
|---|---|---|---|---|---|
| Type of Temperer | |||||
| Na2SiO3 | H2O | Na2SiO3 | H2O | Na2SiO3 + Na2SiF6 | |
| Standard consistency, % | 50.0 | 30.0 | 72.5 | 42.0 | 70.0 |
| Setting time start/end, hours-min | 00–40 01–20 | 00–54 02–03 | 00–16 00–31 | 06–08 07–16 | 00–24 00–36 |
| Average density, g/cm3 | 1.80 | 1.70 | 1.70 | 1.70 | 1.80 |
| Water absorption, wt. % | 11.3 | 12.5 | 12.0 | 12.5 | 12.8 |
| Strength, MPa: flexural compressive | 4.8 43.7 | 0.3 7.8 | 5.1 50.1 | 0.3 7.9 | 5.2 50.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salamanova, M.; Murtazaev, S.-A.; Saidumov, M.; Alaskhanov, A.; Murtazaeva, T.; Fediuk, R. Recycling of Cement Industry Waste for Alkali-Activated Materials Production. Materials 2022, 15, 6660. https://doi.org/10.3390/ma15196660
Salamanova M, Murtazaev S-A, Saidumov M, Alaskhanov A, Murtazaeva T, Fediuk R. Recycling of Cement Industry Waste for Alkali-Activated Materials Production. Materials. 2022; 15(19):6660. https://doi.org/10.3390/ma15196660
Chicago/Turabian StyleSalamanova, Madina, Sayd-Alvi Murtazaev, Magomed Saidumov, Arbi Alaskhanov, Tamara Murtazaeva, and Roman Fediuk. 2022. "Recycling of Cement Industry Waste for Alkali-Activated Materials Production" Materials 15, no. 19: 6660. https://doi.org/10.3390/ma15196660
APA StyleSalamanova, M., Murtazaev, S.-A., Saidumov, M., Alaskhanov, A., Murtazaeva, T., & Fediuk, R. (2022). Recycling of Cement Industry Waste for Alkali-Activated Materials Production. Materials, 15(19), 6660. https://doi.org/10.3390/ma15196660






