Heparin-Loaded Alginate Hydrogels: Characterization and Molecular Mechanisms of Their Angiogenic and Anti-Microbial Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Loading of Heparin on Alginate Wound Dressings
2.3. FTIR (Fourier Transform Infrared Spectroscopy)
2.4. Swelling Studies
2.5. Antimicrobial Assay
2.6. Cell Culture
2.7. In-Vitro Biocompatibility Assay
2.8. Cell Viability Assay
2.9. RNA Extraction and Quantitative RT-PCR
2.10. Wound Scratch Assay
2.11. Statistical Analysis
3. Results
3.1. FTIR Analysis: OH Groups and the Homogeneity of Both Heparin and Alginate
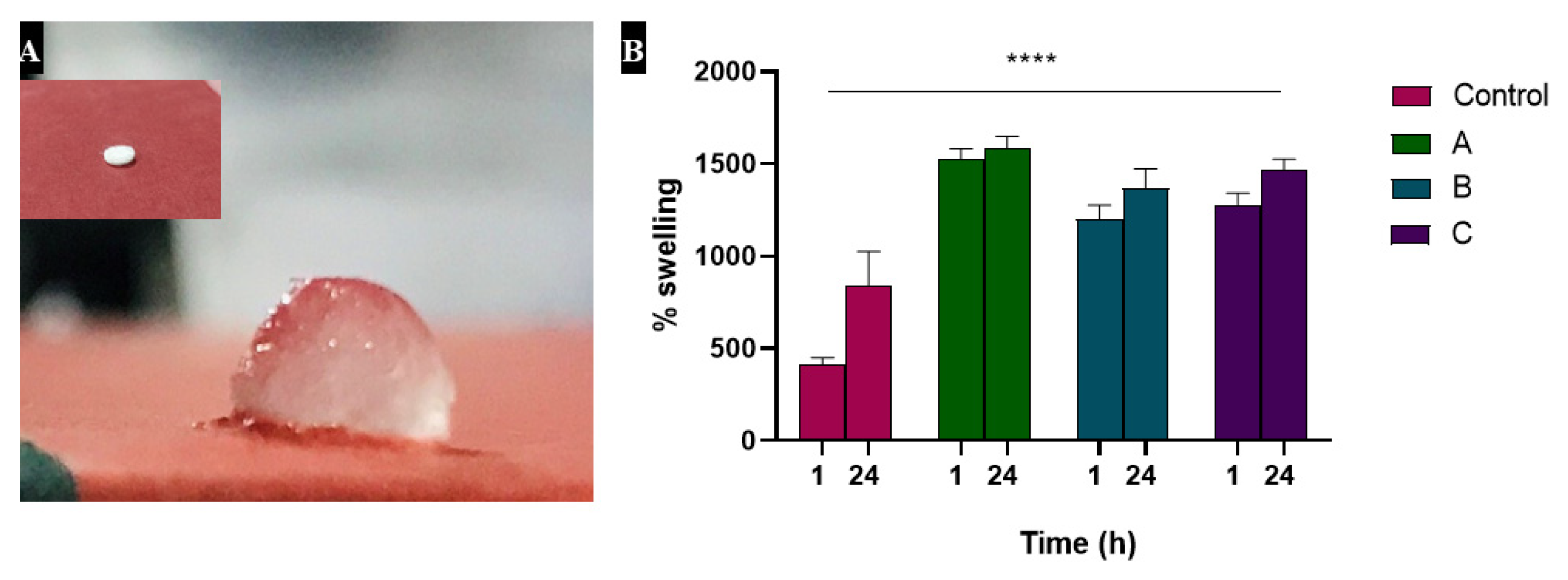
3.2. Swelling Studies Showed Increased Absorption by Heparin
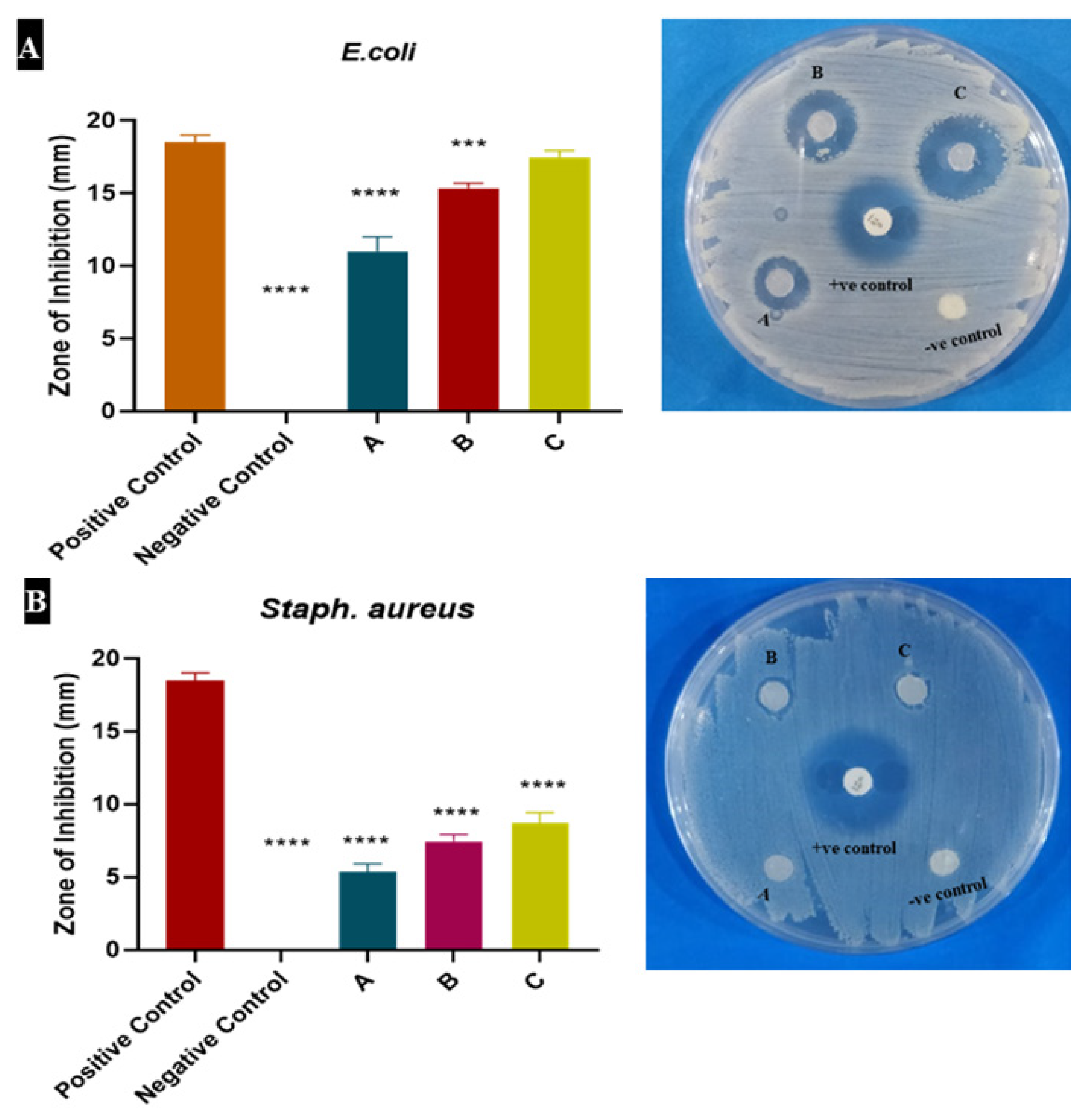
3.3. Heparin Increased the Antimicrobial Potential of Alginate Dressing
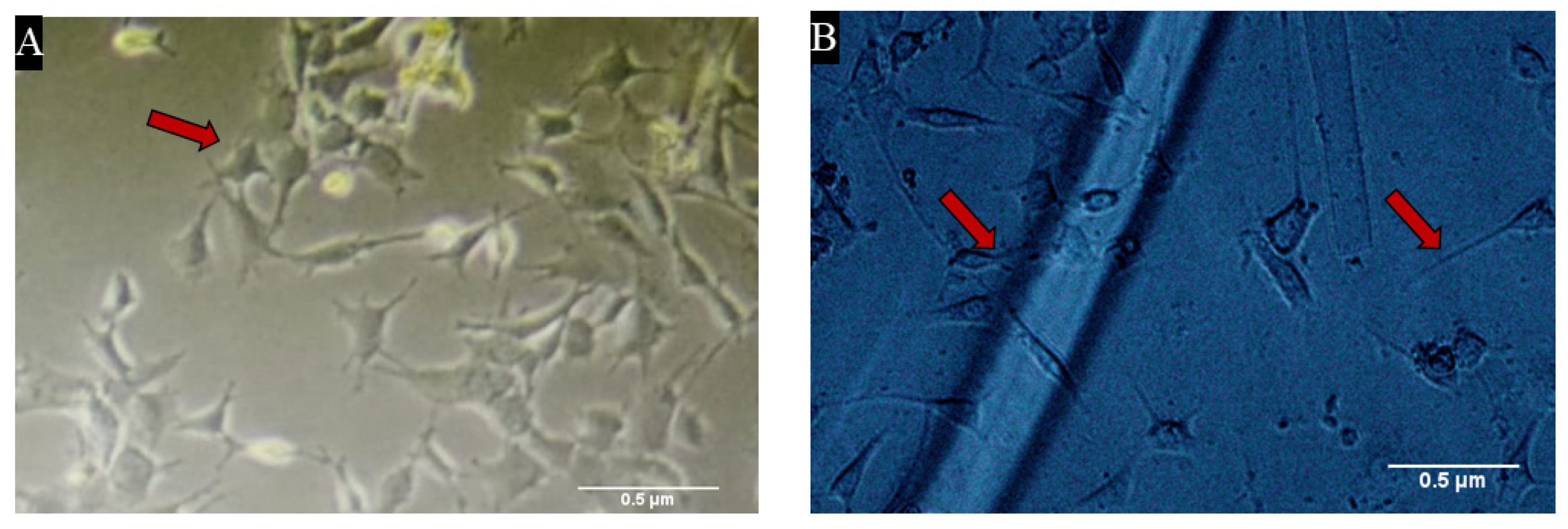
3.4. Heparin Loaded Alginate Dressings Were Highly Biocompatible
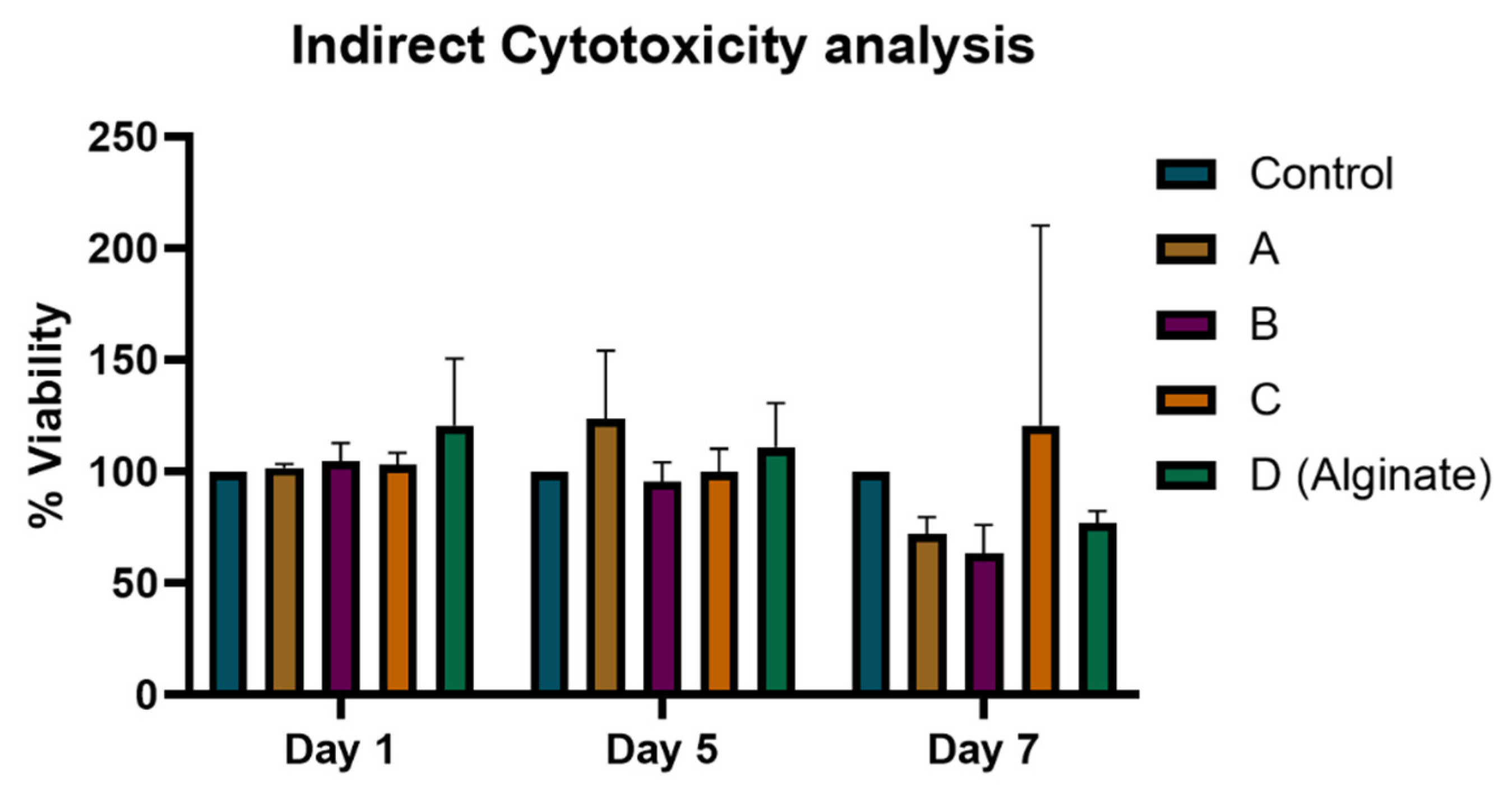
3.5. Heparin-Loaded Hydrogels Did Not Show Any Cytotoxic Effect on Cells
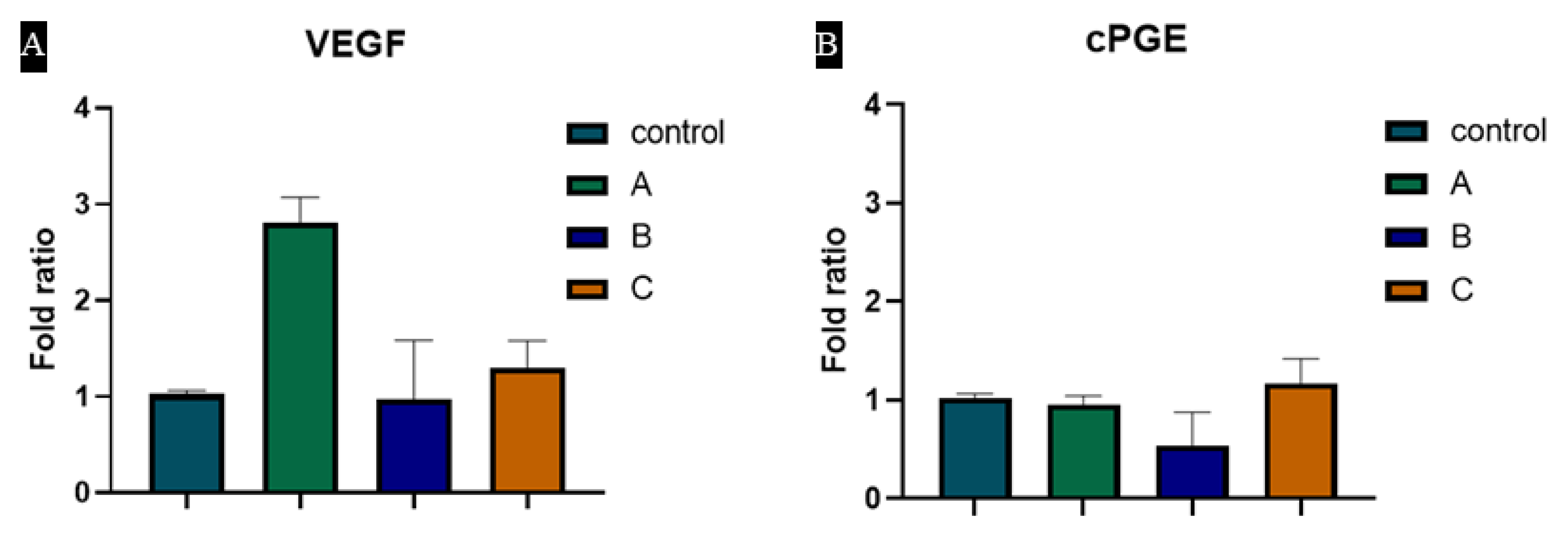
3.6. Hydrogels Induce VEGF and cPGE Synthase Expression
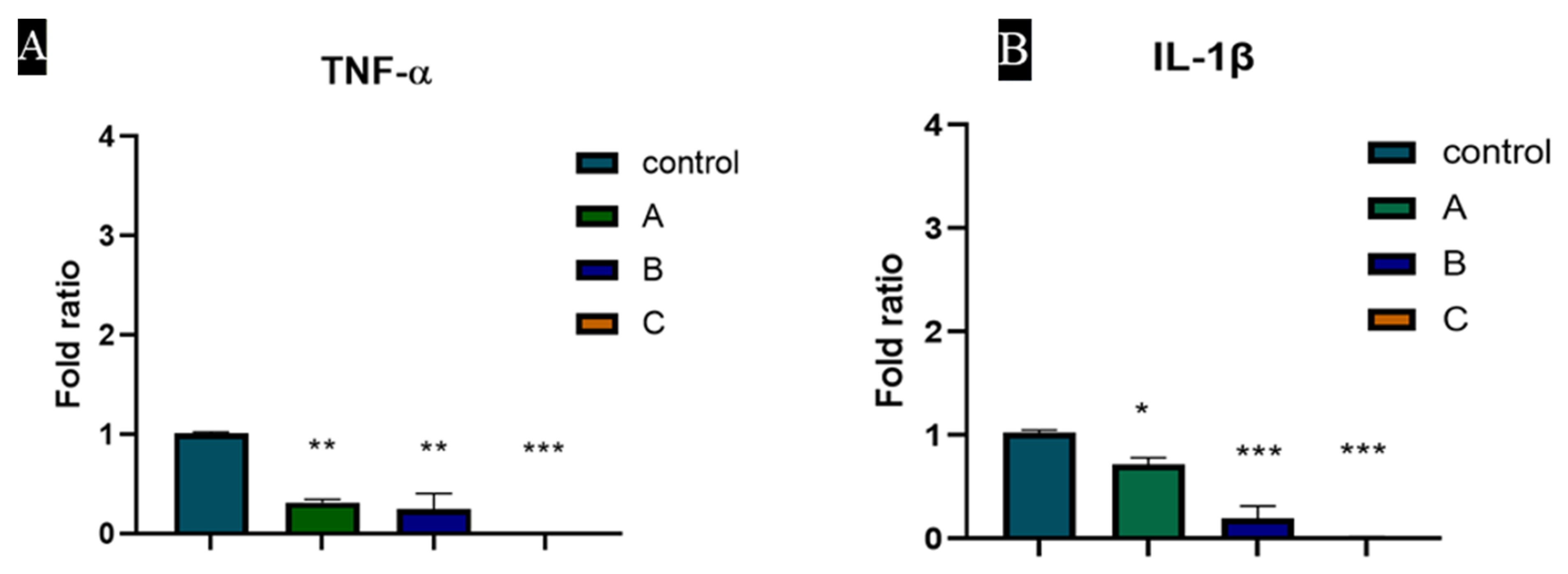
3.7. TNF-α and IL-1β Expression Was Downregulated by Heparin Coated Hydrogel
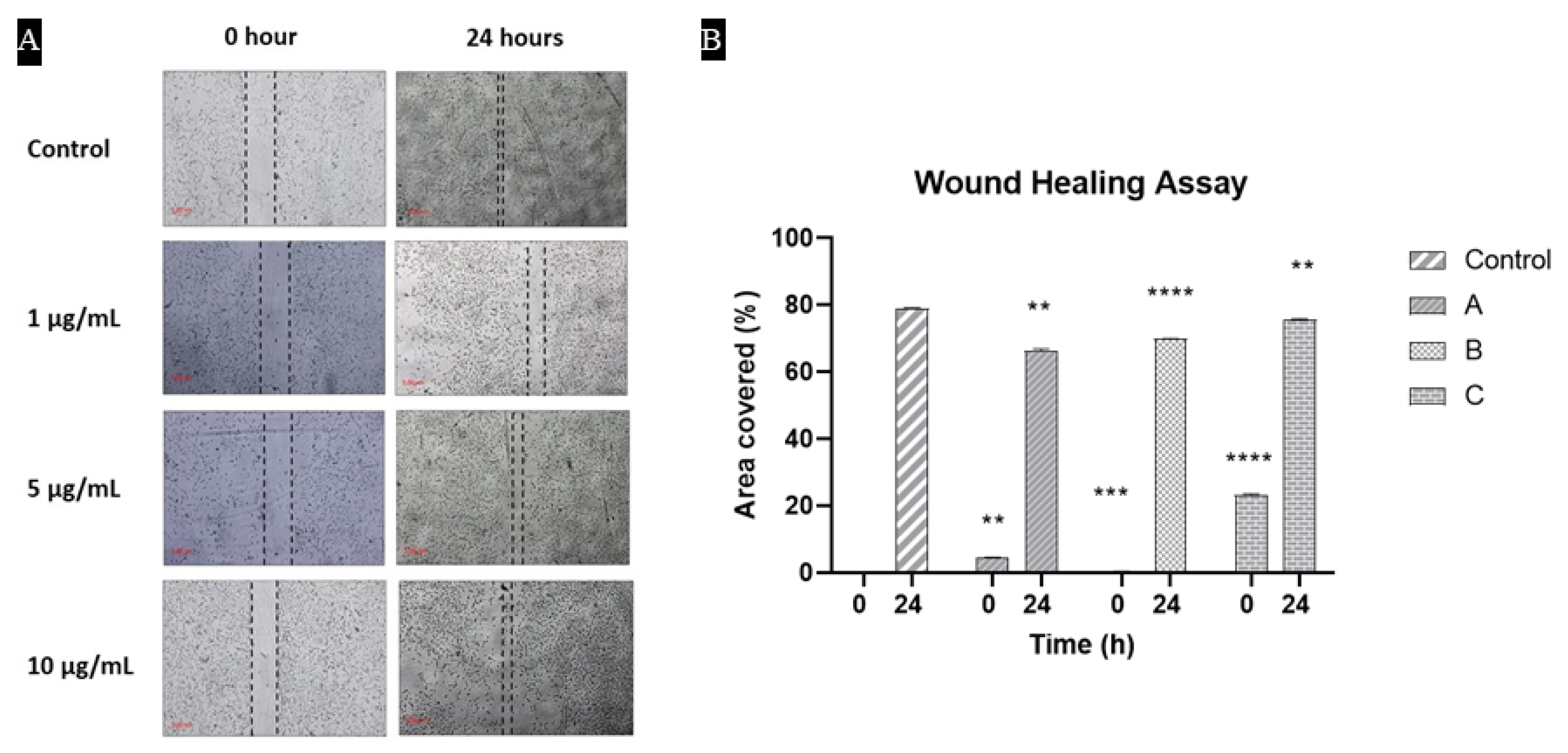
3.8. Cells Were Proliferated and Covered the Scratch in the Presence of Hydrogels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beldon, P. Basic science of wound healing. Surgery 2010, 28, 409–412. [Google Scholar] [CrossRef]
- Gauglitz, G.G.; Korting, H.C.; Pavicic, T.; Ruzicka, T.; Jeschke, M.G. Hypertrophic Scarring and Keloids: Pathomechanisms and Current and Emerging Treatment Strategies. Mol. Med. 2010, 17, 113–125. [Google Scholar] [CrossRef]
- Rahimi, M.; Noruzi, E.B.; Sheykhsaran, E.; Ebadi, B.; Kariminezhad, Z.; Molaparast, M.; Mehrabani, M.G.; Mehramouz, B.; Yousefi, M.; Ahmadi, R.; et al. Carbohydrate polymer-based silver nanocomposites: Recent progress in the antimicrobial wound dressings. Carbohydr. Polym. 2019, 231, 115696. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Baranoski, S.; Ayello, E.A. Wound Care Essentials: Practice Principles; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Cullum, N.; Buckley, H.; Dumville, J.; Hall, J.; Lamb, K.; Madden, M.; Morley, R.; O’Meara, S.; Goncalves, P.S.; Soares, M.; et al. Wounds Research for Patient Benefit: A 5-Year Programme of Research; NIHR Journals Library: Southampton, UK, 2016. [Google Scholar]
- Flanagan, M. Barriers to the implementation of best practice in wound care. Wounds UK 2005, 1, 74. [Google Scholar]
- Pornanong, A. Introduction to Biomaterials for Wound Healing. Wound Health Biomater. 2016, 2, 3–38. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The extracellular matrix in myocardial injury, repair, and remodeling. J. Clin. Investig. 2017, 127, 1600–1612. [Google Scholar] [CrossRef]
- Veith, A.P.; Henderson, K.; Spencer, A.; Sligar, A.D.; Baker, A.B. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv. Drug Deliv. Rev. 2018, 146, 97–125. [Google Scholar] [CrossRef]
- Ridiandries, A.; Tan, J.T.M.; Bursill, C.A. The Role of Chemokines in Wound Healing. Int. J. Mol. Sci. 2018, 19, 3217. [Google Scholar] [CrossRef]
- Peplow, P.V. Glycosaminoglycan: A candidate to stimulate the repair of chronic wounds. Thromb. Haemost. 2005, 94, 4–16. [Google Scholar] [CrossRef]
- Yadav, P.; Yadav, H.; Shah, V.G.; Shah, G.; Dhaka, G. Biomedical Biopolymers, their Origin and Evolution in Biomedical Sciences: A Systematic Review. J. Clin. Diagn. Res. 2015, 9, ZE21–ZE25. [Google Scholar] [CrossRef] [PubMed]
- Charron, P.N.; Garcia, L.M.; Tahir, I.; Floreani, R. Bio-inspired green light crosslinked alginate-heparin hydrogels support HUVEC tube formation. J. Mech. Behav. Biomed. Mater. 2022, 125, 104932. [Google Scholar] [CrossRef] [PubMed]
- Young, E. The anti-inflammatory effects of heparin and related compounds. Thromb. Res. 2008, 122, 743–752. [Google Scholar] [CrossRef]
- Liang, Y.; Kiick, K.L. Heparin-functionalized polymeric biomaterials in tissue engineering and drug delivery applications. Acta Biomater. 2013, 10, 1588–1600. [Google Scholar] [CrossRef] [PubMed]
- Frueh, F.S.; Menger, M.D.; Lindenblatt, N.; Giovanoli, P.; Laschke, M.W. Current and emerging vascularization strategies in skin tissue engineering. Crit. Rev. Biotechnol. 2016, 37, 613–625. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Cui, F.; Rankl, C.; Qin, J.; Guan, Y.; Guo, X.; Zhang, B.; Tang, J. Interaction of vascular endothelial growth factor and heparin quantified by single molecule force spectroscopy. Nanoscale 2020, 12, 11927–11935. [Google Scholar] [CrossRef]
- Xie, J.; Wan, J.; Tang, X.; Li, W.; Peng, B. Heparin modification improves the re-endothelialization and angiogenesis of decellularized kidney scaffolds through antithrombosis and anti-inflammation in vivo. Transl. Androl. Urol. 2021, 10, 3656–3668. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.-N.; Mao, Z.-X.; Wu, Y.; Liang, M.-X.; Wang, D.-D.; Chen, X.; Chang, P.-A.; Zhang, W.; Tang, J.-H. The anti-cancer properties of heparin and its derivatives: A review and prospect. Cell Adhes. Migr. 2020, 14, 118–128. [Google Scholar] [CrossRef]
- Oremus, M.; Hanson, M.; Whitlock, R.; Young, E.; Archer, C.; Cin, A.D.; Gupta, A.; Raina, P. A Systematic Review of Heparin to Treat Burn Injury. J. Burn Care Res. 2007, 28, 794–804. [Google Scholar] [CrossRef]
- Page, C. Heparin and Related Drugs: Beyond Anticoagulant Activity. ISRN Pharmacol. 2013, 2013, 910743. [Google Scholar] [CrossRef]
- Lin, B.; Fabbi, M.; Driver, V.R. The Role of Heparin in Wound Healing. Review Digest. 2011. Available online: https://www.liebertpub.com/doi/abs/10.1089/9781934854280.273 (accessed on 26 August 2021).
- Sen, S.; Meteoglu, I.; Ogurlu, M.; Sen, S.; Derinceoz, O.O.; Barutca, S. Topical heparin: A promising agent for the prevention of tracheal stenosis in airway surgery. J. Surg. Res. 2009, 157, e23–e29. [Google Scholar] [CrossRef] [PubMed]
- Leppänen, V.-M.; Tvorogov, D.; Kisko, K.; Prota, A.E.; Jeltsch, M.; Anisimov, A.; Markovic-Mueller, S.; Stuttfeld, E.; Goldie, K.N.; Ballmer-Hofer, K.; et al. Structural and mechanistic insights into VEGF receptor 3 ligand binding and activation. Proc. Natl. Acad. Sci. USA 2013, 110, 12960–12965. [Google Scholar] [CrossRef] [PubMed]
- Litvinova, L.S.; Yurova, K.A.; Khaziakhmatova, O.G.; Khlusova, M.Y.; Malashchenko, V.V.; Shunkin, E.O.; Todosenko, N.M.; Norkin, I.K.; Ivanov, P.A.; Khlusov, I.A. Osteogenic and Angiogenic Properties of Heparin as a System for Delivery of Biomolecules for Bone Bioengineering: A Brief Critical Review. Biochem. Suppl. Ser. B Biomed. Chem. 2021, 15, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Kocak, F.Z.; Yar, M.; Rehman, I.U. Hydroxyapatite-Integrated, Heparin- and Glycerol-Functionalized Chitosan-Based Injectable Hydrogels with Improved Mechanical and Proangiogenic Performance. Int. J. Mol. Sci. 2022, 23, 5370. [Google Scholar] [CrossRef] [PubMed]
- Zubairi, W.; Zehra, M.; Mehmood, A.; Iqbal, F.; Badar, R.; Hasan, A.; Yar, M. Evaluation of angiogenic potential of heparin and thyroxine releasing wound dressings. Int. J. Polym. Mater. Polym. Biomater. 2021, 71, 1164–1175. [Google Scholar] [CrossRef]
- El-Kased, R.; Amer, R.I.; Attia, D.; Elmazar, M.M. Honey-based hydrogel: In vitro and comparative In vivo evaluation for burn wound healing. Sci. Rep. 2017, 7, 9692. [Google Scholar] [CrossRef]
- Straccia, M.C.; D’Ayala, G.G.; Romano, I.; Oliva, A.; Laurienzo, P. Alginate Hydrogels Coated with Chitosan for Wound Dressing. Mar. Drugs 2015, 13, 2890–2908. [Google Scholar] [CrossRef]
- Bernardez, L.; Lima, L.D.A. Improved method for enumerating sulfate-reducing bacteria using optical density. MethodsX 2015, 2, 249–255. [Google Scholar] [CrossRef]
- Webster, T.; Bhardwaj, G. Increased NIH 3T3 fibroblast functions on cell culture dishes which mimic the nanometer fibers of natural tissues. Int. J. Nanomed. 2015, 10, 5293–5299. [Google Scholar] [CrossRef][Green Version]
- Tayarani-Najaran, Z.; Yazdian-Robati, R.; Amini, E.; Salek, F.; Arasteh, F.; Emami, S.A. The mechanism of neuroprotective effect of Viola odorata against serum/glucose deprivation-induced PC12 cell death. Avicenna J. Phytomed. 2019, 9, 491–498. [Google Scholar]
- Jiang, J.; Ma, J.; Liu, B.; Wang, Y. Combining a Simple Method for DNA/RNA/Protein Co-Purification and Arabidopsis Protoplast Assay to Facilitate Viroid Research. Viruses 2019, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, S.; Ranzato, E. Scratch Wound Healing Assay. Methods Mol. Biol. 2020, 2109, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Jonkman, J.; Cathcart, J.A.; Xu, F.; Bartolini, M.E.; Amon, J.E.; Stevens, K.M.; Colarusso, P. An introduction to the wound healing assay using live-cell microscopy. Cell Adhes. Migr. 2014, 8, 440–451. [Google Scholar] [CrossRef]
- Daemi, H.; Barikani, M. Synthesis and characterization of calcium alginate nanoparticles, sodium homopolymannuronate salt and its calcium nanoparticles. Sci. Iran. 2012, 19, 2023–2028. [Google Scholar] [CrossRef]
- Devlin, A.; Mauri, L.; Guerrini, M.; Yates, E.A.; Skidmore, M.A. The use of ATR-FTIR spectroscopy to characterise crude heparin samples by composition and structural features. bioRxiv 2019. [Google Scholar] [CrossRef]
- Pilbeam, C.C.; Choudhary, S.; Blackwell, K.; Raisz, L.G. Chapter 58—Prostaglandins and Bone Metabolism. In Principles of Bone Biology, 3rd ed.; Bilezikian, J.P., Raisz, L.G., Martin, T.J., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 1235–1271. [Google Scholar]
- Zelová, H.; Hošek, J. TNF-α signalling and inflammation: Interactions between old acquaintances. Inflamm. Res. 2013, 62, 641–651. [Google Scholar] [CrossRef]
- Yar, M.; Gigliobianco, G.; Shahzadi, L.; Dew, L.; Siddiqi, S.A.; Khan, A.F.; Chaudhry, A.A.; Rehman, I.u.; MacNeil, S. Production of chitosan PVA PCL hydrogels to bind heparin and induce angiogenesis. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 466–476. [Google Scholar] [CrossRef]
- Pacini, S.; Gulisano, M.; Vannucchi, S.; Ruggiero, M. Poly-L-lysine/Heparin Stimulates Angiogenesis in Chick Embryo Chorioallantoic Membrane. Biochem. Biophys. Res. Commun. 2002, 290, 820–823. [Google Scholar] [CrossRef]
- Rema, R.; Rajendran, K.; Ragunathan, M. Angiogenic efficacy of Heparin on chick chorioallantoic membrane. Vasc. Cell 2012, 4, 8. [Google Scholar] [CrossRef]
- Norrby, K.; Nordenhem, A. Dalteparin, a low-molecular-weight heparin, promotes angiogenesis mediated by heparin-binding VEGF-A in vivo. APMIS 2010, 118, 949–957. [Google Scholar] [CrossRef]
- Munarin, F.; Kabelac, C.; Coulombe, K.L. Heparin-modified alginate microspheres enhance neovessel formation in hiPSC -derived endothelial cells and heterocellular in vitro models by controlled release of vascular endothelial growth factor. J. Biomed. Mater. Res. Part A 2021, 109, 1726–1736. [Google Scholar] [CrossRef] [PubMed]
- Fazil, S.; Shah, H.; Noreen, M.; Yar, M.; Farooq Khan, A.; Zaman Safi, S.; Yousef Alomar, S.; Fahad Alkhuriji, A.; Mualla Alharbi, H.; Sohail Afzal, M. Evaluation of molecular mechanisms of heparin-induced angiogenesis, in human umbilical vein endothelial cells. J. King Saud Univ.—Sci. 2021, 33, 101534. [Google Scholar] [CrossRef]
- Sweeney, I.R.; Miraftab, M.; Collyer, G. A critical review of modern and emerging absorbent dressings used to treat exuding wounds. Int. Wound J. 2012, 9, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, C.; Hipler, U.-C.; Elsner, P.; Tittelbach, J. Clinical efficacy of dressings for treatment of heavily exuding chronic wounds. Chronic Wound Care Manag. Res. 2015, 2, 101–111. [Google Scholar] [CrossRef]
- Malik, M.H.; Shahzadi, L.; Batool, R.; Safi, S.Z.; Khan, A.S.; Khan, A.F.; Chaudhry, A.A.; Rehman, I.U.; Yar, M. Thyroxine-loaded chitosan/carboxymethyl cellulose/hydroxyapatite hydrogels enhance angiogenesis in in-ovo experiments. Int. J. Biol. Macromol. 2020, 145, 1162–1170. [Google Scholar] [CrossRef]
- Seib, F.P.; Herklotz, M.; Burke, K.A.; Maitz, M.F.; Werner, C.; Kaplan, D.L. Multifunctional silk–heparin biomaterials for vascular tissue engineering applications. Biomaterials 2014, 35, 83–91. [Google Scholar] [CrossRef]
- Newland, B.; Ehret, F.; Hoppe, F.; Eigel, D.; Pette, D.; Newland, H.; Welzel, P.B.; Kempermann, G.; Werner, C. Macroporous heparin-based microcarriers allow long-term 3D culture and differentiation of neural precursor cells. Biomaterials 2020, 230, 119540. [Google Scholar] [CrossRef]
- Rabenstein, D.L. Heparin and heparan sulfate: Structure and function. Nat. Prod. Rep. 2002, 19, 312–331. [Google Scholar] [CrossRef]
- Kharkar, P.M.; Kiick, K.L.; Kloxin, A.M. Designing degradable hydrogels for orthogonal control of cell microenvironments. Chem. Soc. Rev. 2013, 42, 7335–7372. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawaz, A.; Zaman Safi, S.; Sikandar, S.; Zeeshan, R.; Zulfiqar, S.; Mehmood, N.; Alobaid, H.M.; Rehman, F.; Imran, M.; Tariq, M.; et al. Heparin-Loaded Alginate Hydrogels: Characterization and Molecular Mechanisms of Their Angiogenic and Anti-Microbial Potential. Materials 2022, 15, 6683. https://doi.org/10.3390/ma15196683
Nawaz A, Zaman Safi S, Sikandar S, Zeeshan R, Zulfiqar S, Mehmood N, Alobaid HM, Rehman F, Imran M, Tariq M, et al. Heparin-Loaded Alginate Hydrogels: Characterization and Molecular Mechanisms of Their Angiogenic and Anti-Microbial Potential. Materials. 2022; 15(19):6683. https://doi.org/10.3390/ma15196683
Chicago/Turabian StyleNawaz, Ayesha, Sher Zaman Safi, Shomaila Sikandar, Rabia Zeeshan, Saima Zulfiqar, Nadia Mehmood, Hussah M. Alobaid, Fozia Rehman, Muhammad Imran, Muhammad Tariq, and et al. 2022. "Heparin-Loaded Alginate Hydrogels: Characterization and Molecular Mechanisms of Their Angiogenic and Anti-Microbial Potential" Materials 15, no. 19: 6683. https://doi.org/10.3390/ma15196683
APA StyleNawaz, A., Zaman Safi, S., Sikandar, S., Zeeshan, R., Zulfiqar, S., Mehmood, N., Alobaid, H. M., Rehman, F., Imran, M., Tariq, M., Ali, A., Emran, T. B., & Yar, M. (2022). Heparin-Loaded Alginate Hydrogels: Characterization and Molecular Mechanisms of Their Angiogenic and Anti-Microbial Potential. Materials, 15(19), 6683. https://doi.org/10.3390/ma15196683






