Biodiesel Production Using Lithium Metasilicate Synthesized from Non-Conventional Sources
Abstract
:1. Introduction
2. Materials and methods
3. Results and Discussion
3.1. Synthesis of Lithium Metasilicate (Li2SiO3)
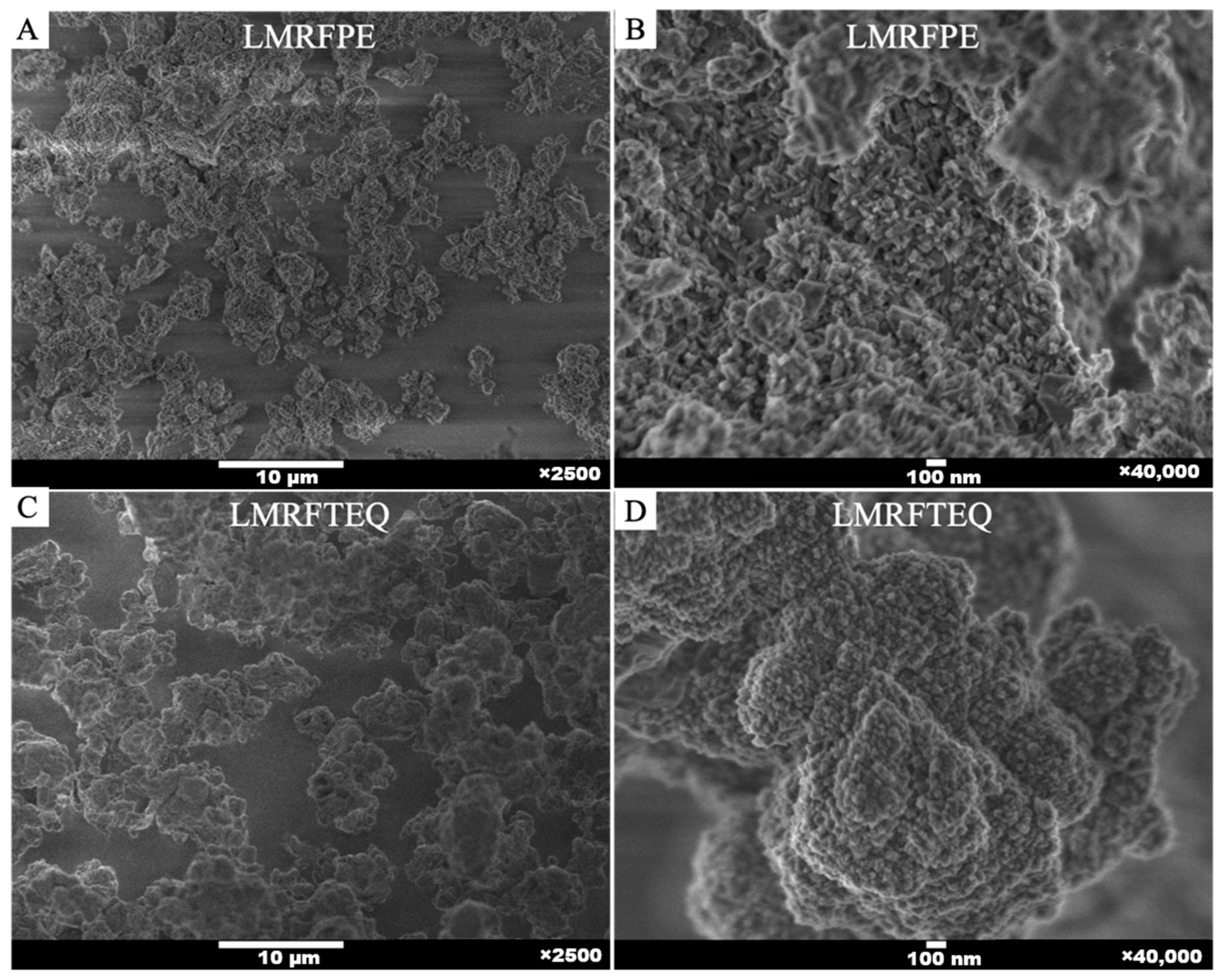
3.2. Lithium Metasilicate Characterization
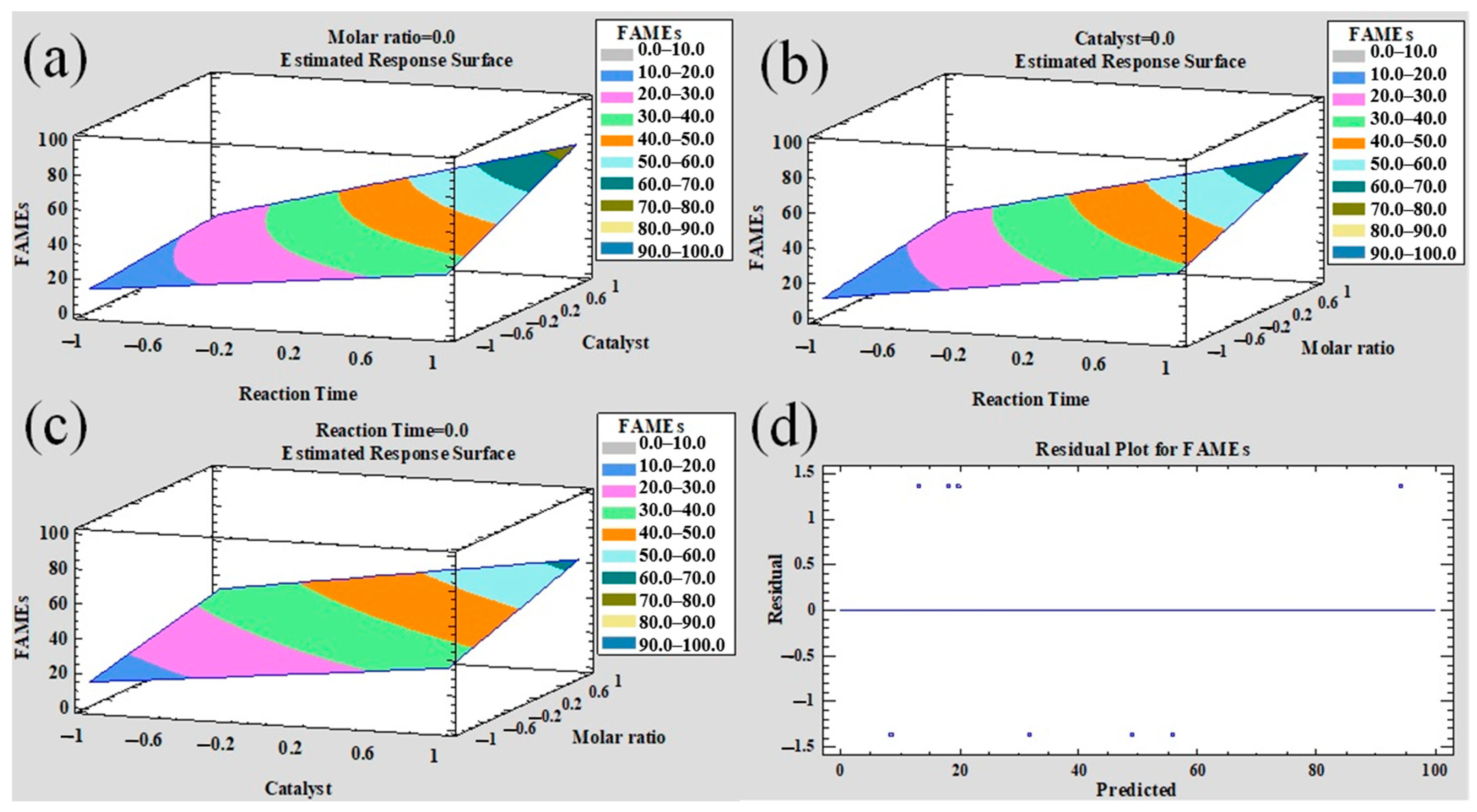
3.3. Biodiesel Production (Transesterification Reaction)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ayoub, M.; Yusoff, M.H.M.; Nazir, M.H.; Zahid, I.; Ameen, M.; Sher, F.; Floresyona, D.; Budi Nursanto, E. A comprehensive review on oil extraction and biodiesel production technologies. Sustainability 2021, 13, 788. [Google Scholar]
- Dincer, K. Lower emissions from biodiesel combustion. Energy Sources Part A Recover. Util. Environ. Eff. 2008, 30, 963–968. [Google Scholar] [CrossRef]
- Balat, M. An overview of biofuels and policies in the European union. Energy Sources Part B Econ. Plan. Policy 2007, 2, 167–181. [Google Scholar] [CrossRef]
- Demirbas, A. Biofuels sources, biofuel policy, biofuel economy and global biofuel projections. Energy Convers. Manag. 2008, 49, 2106–2116. [Google Scholar] [CrossRef]
- Nizami, A.S.; Rehan, M.; Waqas, M.; Naqvi, M.; Ouda, O.K.; Shahzad, K.; Miandad, R.; Khan, M.Z.; Syamsiro, M.; Ismail, I.M.I.; et al. Waste biorefineries: Enabling circular economies in developing countries. Bioresour. Technol. 2017, 241, 1101–1117. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: A review. Biotechnol. Adv. 2010, 28, 500–518. [Google Scholar] [CrossRef]
- Demirbas, A. Political, economic and environmental impacts of biofuels: A review. Appl. Energy 2009, 86, S108–S117. [Google Scholar] [CrossRef]
- Shan, R.; Lu, L.; Shi, Y.; Yuan, H.; Shi, J. Catalysts from renewable resources for biodiesel production. Energy Convers. Manag. 2018, 178, 277–289. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Wu, X.; Leung, M.K.H. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Liu, X.; He, H.; Wang, Y.; Zhu, S. Transesterification of soybean oil to biodiesel using CaO as a solid base catalyst. Fuel 2008, 87, 216–221. [Google Scholar] [CrossRef]
- Kouzu, M.; Kasuno, T.; Tajika, M.; Sugimoto, Y.; Yamanaka, S.; Hidaka, J. Calcium oxide as a solid base catalyst for transesterification of soybean oil and its application to biodiesel production. Fuel 2008, 87, 2798–2806. [Google Scholar] [CrossRef]
- López Granados, M.; Martín Alonso, D.; Alba-Rubio, A.C.; Mariscal, R.; Ojeda, M.; Brettes, P. Transesterification of triglycerides by CaO: Increase of the reaction rate by biodiesel addition. Energy Fuels 2009, 23, 2259–2263. [Google Scholar] [CrossRef]
- Buasri, A.; Chaiyut, N.; Loryuenyong, V.; Wongweang, C.; Trongyong, S. Application of eggshell wastes as a heterogeneous catalyst for biodiesel production. Sustain. Energy 2013, 1, 7–13. [Google Scholar]
- Buasri, A.; Chaiyut, N.; Loryuenyong, V.; Worawanitchaphong, P.; Trongyong, S. Calcium oxide derived from waste shells of mussel, cockle, and scallop as the heterogeneous catalyst for biodiesel production. Sci. World J. 2013, 2013, 460923. [Google Scholar] [CrossRef] [PubMed]
- Foroutan, R.; Peighambardoust, S.J.; Mohammadi, R.; Peighambardoust, S.H.; Ramavandi, B. Application of waste chalk/CoFe2O4/K2CO3 composite as a reclaimable catalyst for biodiesel generation from sunflower oil. Chemosphere 2022, 289, 133226. [Google Scholar] [CrossRef] [PubMed]
- Laudthong, P.K.C.; Nualpaeng, W.; Changsuwan, P.; Tongprem, P. Industrial eggshell wastes as the heterogeneous catalysts for microwave-assisted biodiesel production. Catal. Today 2012, 190, 112–116. [Google Scholar]
- Machorro, J.J.; Lazaro, A.L.; Espejel-Ayala, F.; Coutino-Gonzalez, E.; Chavarria-Hernandez, J.C.; Godínez, L.A.; Rodríguez-Velazquez, F.J. The roles of the structure and basic sites of sodium titanates on transesterificacion reactions to obtain biodiesel. Catalysis 2019, 9, 989. [Google Scholar]
- Wang, J.; Chen, K.; Chen, C. Biodiesel production from soybean oil catalyzed by K2SiO3/C. Chin. J. Catal. 2011, 32, 1592–1596. [Google Scholar] [CrossRef]
- Wang, J.X.; Chen, K.T.; Huang, S.T.; Chen, C.C. Application of Li2SiO3 as a heterogeneous catalyst in the production of biodiesel from soybean oil. Chin. Chem. Lett. 2011, 22, 1363–1366. [Google Scholar] [CrossRef]
- Guo, F.; Wei, N.; Xiu, Z.-L.; Fang, Z. Transesterification mechanism of soybean oil to biodiesel catalyzed by calcined sodium silicate. Fuel 2012, 93, 468–472. [Google Scholar] [CrossRef]
- Chen, K.-T.; Wang, J.-X.; Dai, Y.-M.; Wang, P.-H.; Liou, C.-Y.; Nien, C.-W.; Wu, J.-S.; Chen, C.-C. Rice husk ash as a catalyst precursor for biodiesel production. J. Taiwan Inst. Chem. Eng. 2013, 44, 622–629. [Google Scholar] [CrossRef]
- Wang, J.-X.; Chen, K.-T.; Wu, J.-S.; Wang, P.-H.; Huang, S.-T.; Chen, C.-C. Production of biodiesel through transesterification of soybean oil using lithium orthosilicate solid catalyst. Fuel Process. Technol. 2012, 104, 167–173. [Google Scholar] [CrossRef]
- Dai, Y.-M.; Chen, K.-T.; Wang, Y.-J.; Chen, C.-C. Application of Peanut Husk Ash as a L.-C. Solid Catalyst for Biodiesel Production. Int. J. Chem. Eng. Appl. 2014, 5, 276–280. [Google Scholar] [CrossRef]
- Dai, Y.M.; Chen, K.T.; Wang, P.H.; Chen, C.C. Solid-base catalysts for biodiesel production by using silica in agricultural wastes and lithium carbonate. Adv. Powder Technol. 2016, 27, 2432–2438. [Google Scholar] [CrossRef]
- Obernberger, I.; Brunner, T.; Bärnthaler, G. Chemical properties of solid biofuels—Significance and impact. Biomas Bioenergy 2006, 30, 973–982. [Google Scholar] [CrossRef]
- Bejarano-Peña, W.D.; Alcántar-Vázquez, B.; Ramírez-Zamora, R.M. Synthesis and evaluation in the CO2 capture process of potassium-modified lithium silicates produced from steel metallurgical slags. Mater. Res. Bull. 2021, 141, 111353. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Yang, J. Hydrothermal synthesis and methylene blue adsorption performance of novel 3D hierarchical Li2SiO5 hydrate particles. Sci. Rep. 2020, 10, 5545. [Google Scholar] [CrossRef]
- Alemi, A.; Khademinia, S.; Woo Joo, S.; Dolatyari, M.; Bakhtiari, A. Hydrothermal synthesis, characterization, optical properties of lithium meta- and disilicate nanomaterials and theoretical calculations. Int. J. Bio-Inorg. Hybrid Nanomater. 2012, 1, 137–149. [Google Scholar]
- Bennington, K.O.; Ferrante, M.J.; Stuve, J.M. Thermodynamic Properties of Two Lithium Silicates (Li2SiO3 and Li2Si2O5); Report of Investigations; Bureau of Mines: Washington, DC, USA, 1976; Volume 8187. [Google Scholar]
- Ortiz-Landeros, J.; Contreras-García, M.E.; Gómez-Yáñes, C.; Pfeiffer, H. Surfactant-assisted hydrothermal crystallization of nanostructured lithium metasilicate (Li2SiO3) hollow spheres: (I) Synthesis, structural and microstructural characterization. J. Solid State Chem. 2011, 184, 1304–1311. [Google Scholar] [CrossRef]
- Lai, Y.; Tang, X.; Zhang, H.; Hung, X.; Li, J.; Su, H. Relationship between the structure and microwave dielectric properties of non-stoichiometric Li2+xSiO3 ceramics. Ceram. Int. 2017, 43, 2664–2669. [Google Scholar] [CrossRef]
- Richet, P.; Mysen, B.O.; Andrault, D. Melting and premelting of silicates: Raman spectroscopy and X-ray diffraction of Li2SiO3 and Na2SiO3. Phys Chem Miner. 1996, 23, 157–172. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Oliver, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquérol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalyst. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Lin, D.-Y.; Chen, B.-H. Metasilicate-based catalyst prepared from natural diatomaceous earth for biodiesel production. Renew. Energy 2019, 138, 1042–1050. [Google Scholar] [CrossRef]
- Cherikkallinmel, S.K.; Sugunan, S.; Njarakkattuvalappil Narayanan, B.N.; Abdul Faisal, P.; Benjamin, S. Statistical optimization for lithium silicate catalyzed production of biodiesel from waste cooking oil. Korean J. Chem. Eng. 2017, 34, 2840–2851. [Google Scholar] [CrossRef]
- Najeeb, J.; Akram, S.; Mumtaz, M.W.; Danish, M.; Irfan, A.; Touqueer, T.; Rashid, U.; Ghani, W.A.A.K.; Choong, T.S.Y. Nanobiocatalysts for biodiesel synthesis through transesterification–A review. Catalyst 2021, 11, 171. [Google Scholar] [CrossRef]
- Alemi, A.; Khademinia, S. Part I: (Li2SiO3)–mild condition hydrothermal synthesis, characterization, and optical properties. Int. Nano Lett. 2015, 5, 15–20. [Google Scholar] [CrossRef] [Green Version]





| Hammet Titration | ||||
|---|---|---|---|---|
| Air Exposure Time (h) | ||||
| T0 = 0 | T1 = 24 | T2 = 48 | T3 = 72 | |
| LMRFPE | 13.2 < H < 15 | 9.9 < H < 14.7 | 9.0 < H < 14.7 | 9.5 < H < 12 |
| LMRFTEQ | 10 < H < 14.3 | 9.8 < H < 14.3 | 8.7 < H < 13.2 | 8.5 < H < 12 |
| LMConv | 13.8 < H < 15 | 9.7 < H < 14.5 | 8.5 < H < 15 | 9.5 < H < 12.5 |
| CaO | 15 < H < 18 | 7.3 < H < 10.1 | 7.0 < H < 9.9 | 7.0 < H < 9.8 |
| Reaction Time (min) | Catalyst (wt.%) | Molar Ratio Methanol/Soybean Oil (mol/mol) | |
|---|---|---|---|
| 6:1 | 18:1 | ||
| 60 | 1 | 7.1 | 19.4 |
| 5 | 14.5 | 30.4 | |
| 180 | 1 | 21.1 | 47.6 |
| 5 | 54.5 | 95.5 | |
| Source | Sum of Squares | p-Value |
|---|---|---|
| Reaction Time (A) | 2712.16 | 0.0470 |
| Catalyst (B) | 1242.51 | 0.0693 |
| Methanol-oil (C) | 1144.81 | 0.0722 |
| Reaction Time:Catalyst (AB) | 494.551 | 0.1092 |
| Reaction Time:Methanol-oil (AC) | 193.061 | 0.1722 |
| Catalyst:Methanol-oil (BC) | 40.9513 | 0.3451 |
| Error | 14.8512 | |
| Total | 5842.9 | |
| R2 = 99.74% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coutino-Gonzalez, E.; Ávila-Gutiérrez, M.; Hernández-Palomares, A.; Olvera, L.I.; Rodríguez-Valadez, F.J.; Espejel-Ayala, F. Biodiesel Production Using Lithium Metasilicate Synthesized from Non-Conventional Sources. Materials 2022, 15, 6753. https://doi.org/10.3390/ma15196753
Coutino-Gonzalez E, Ávila-Gutiérrez M, Hernández-Palomares A, Olvera LI, Rodríguez-Valadez FJ, Espejel-Ayala F. Biodiesel Production Using Lithium Metasilicate Synthesized from Non-Conventional Sources. Materials. 2022; 15(19):6753. https://doi.org/10.3390/ma15196753
Chicago/Turabian StyleCoutino-Gonzalez, Eduardo, Mario Ávila-Gutiérrez, Arnold Hernández-Palomares, Lilian I. Olvera, Francisco J. Rodríguez-Valadez, and Fabricio Espejel-Ayala. 2022. "Biodiesel Production Using Lithium Metasilicate Synthesized from Non-Conventional Sources" Materials 15, no. 19: 6753. https://doi.org/10.3390/ma15196753






