Geopolymer: A Systematic Review of Methodologies
Abstract
1. Introduction
2. Research Significance
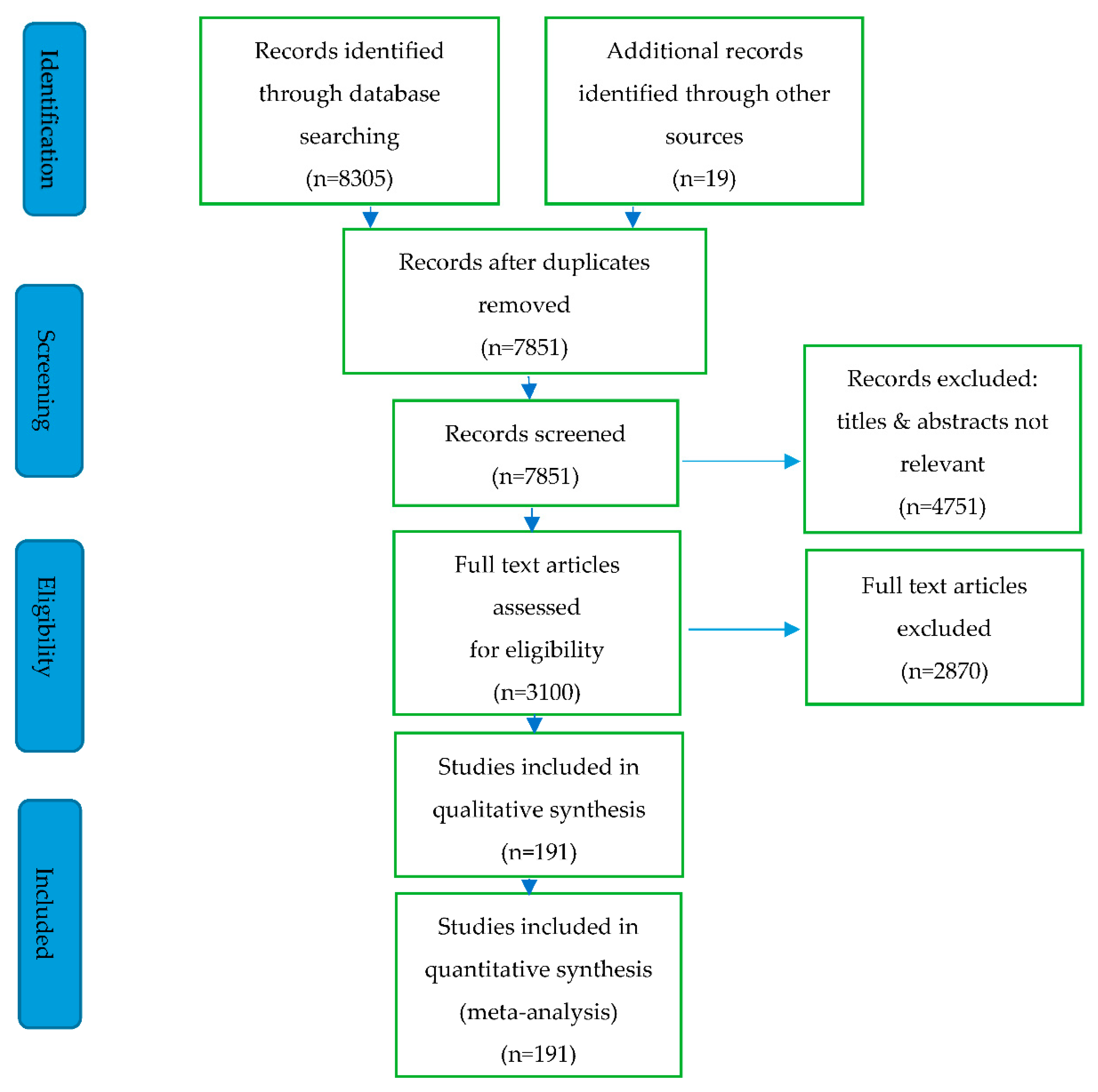
3. Methodology
4. Results and Discussion
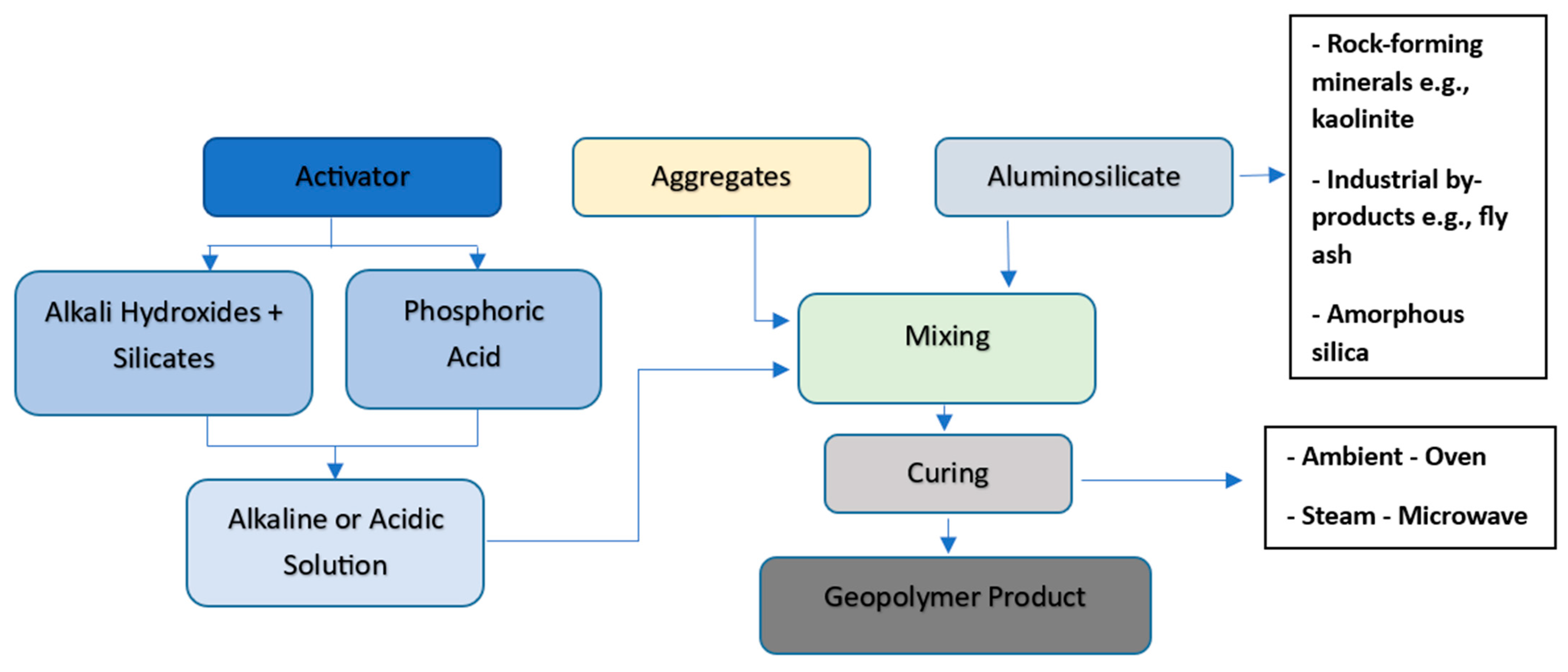
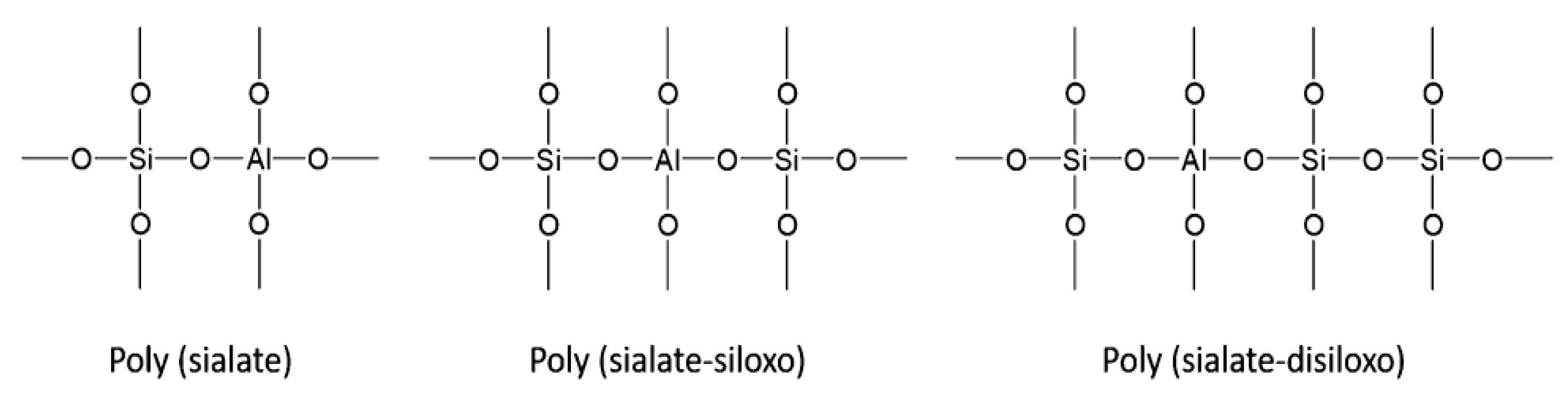
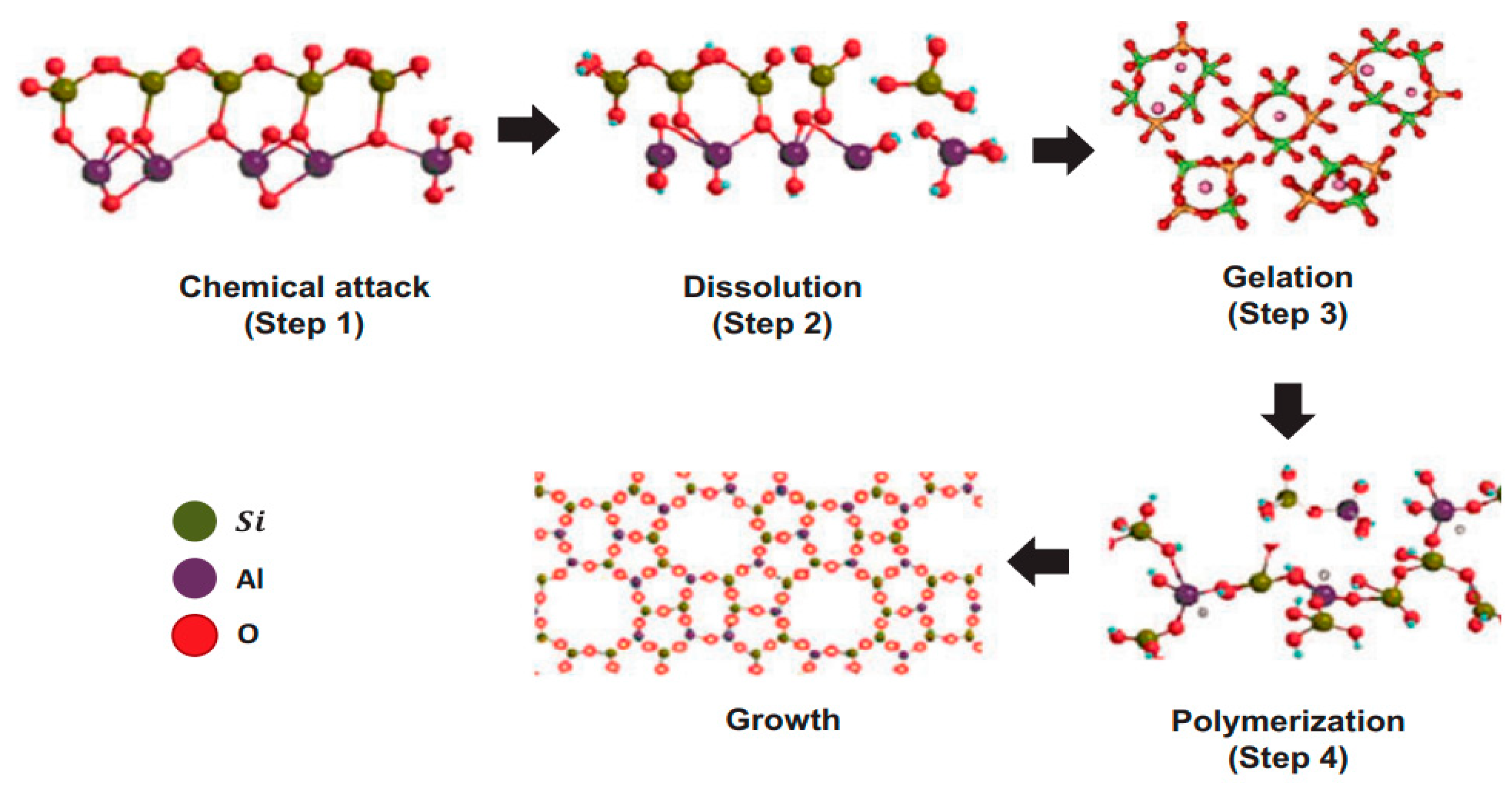
4.1. Definition and Chemistry
4.2. Raw Materials
4.2.1. Fly Ash
4.2.2. Phosphogypsum
4.2.3. Bottom Ash
4.2.4. Ground Granulated Blast Furnace Slag
4.2.5. Basic Oxygen Furnace Slag
4.2.6. Silica Fume
4.2.7. Flue gas desulphurization gypsum
4.2.8. Red Mud
4.2.9. Mine Tailings
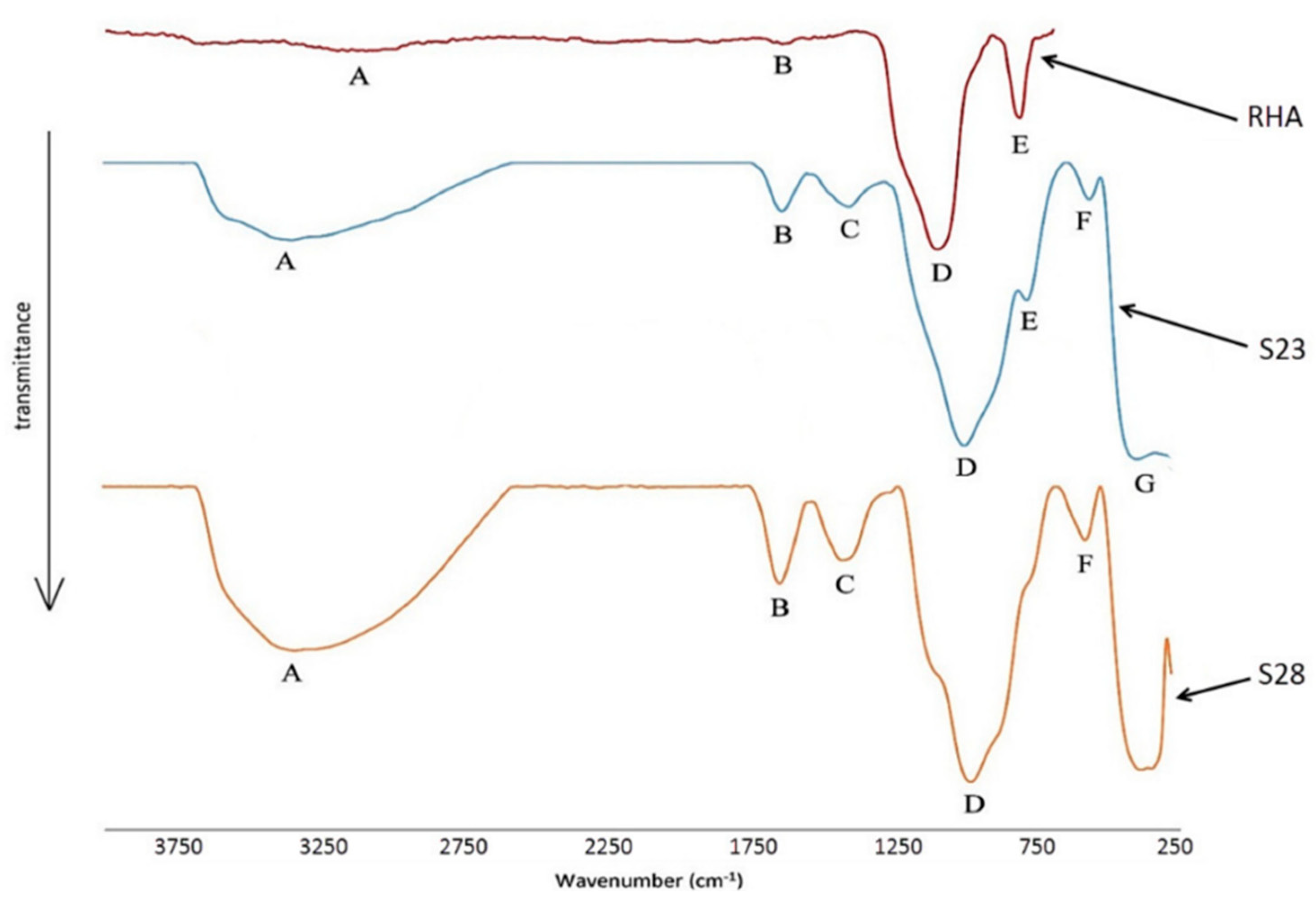
4.2.10. Rice Husk Ash
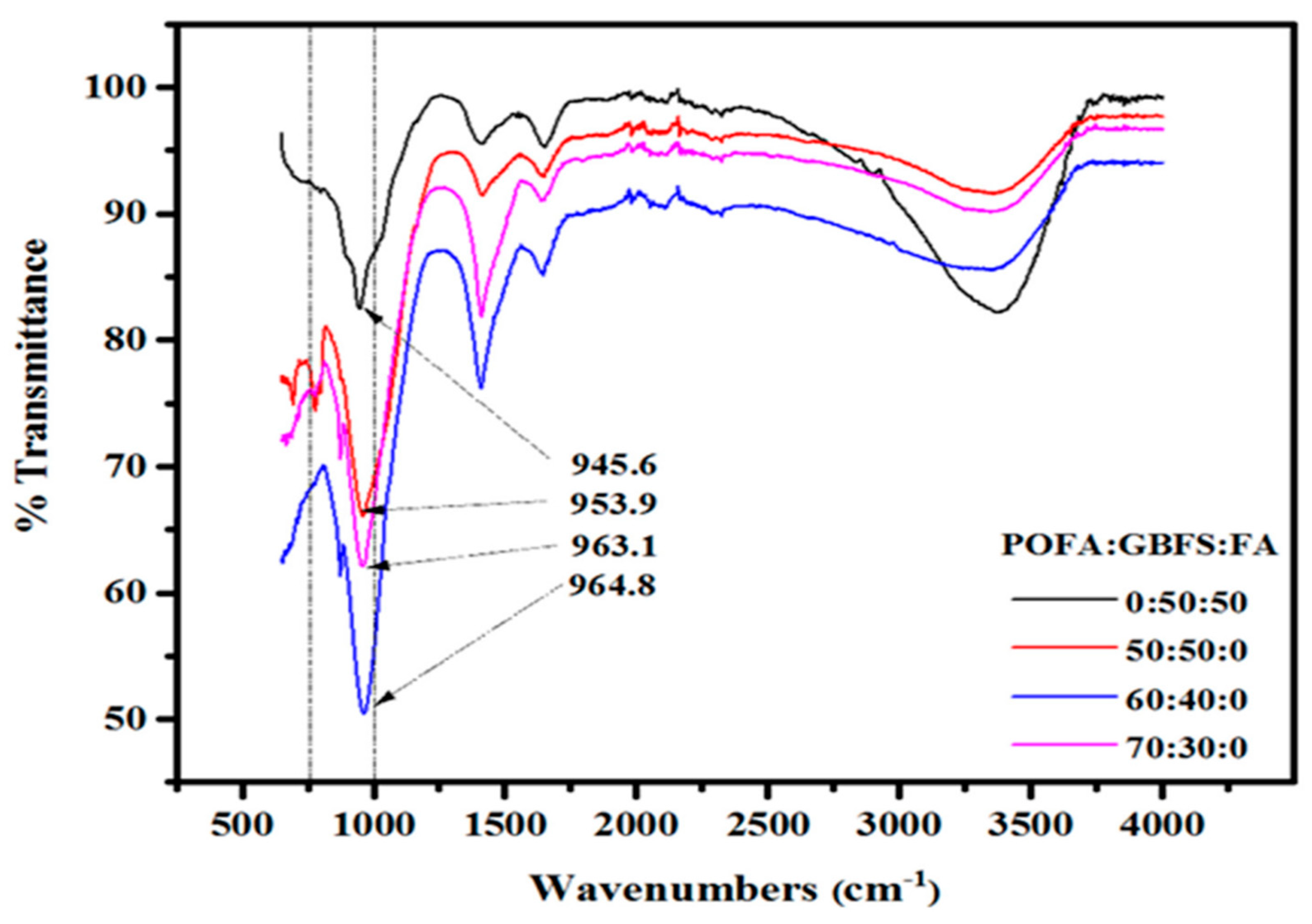
4.2.11. Palm Oil Fuel Ash
4.2.12. Waste Glass
5. Alkaline Activators and Their Properties
5.1. Alkaline Type
5.2. Alkaline Concentration
6. Impact of Curing
7. Admixture/Additive
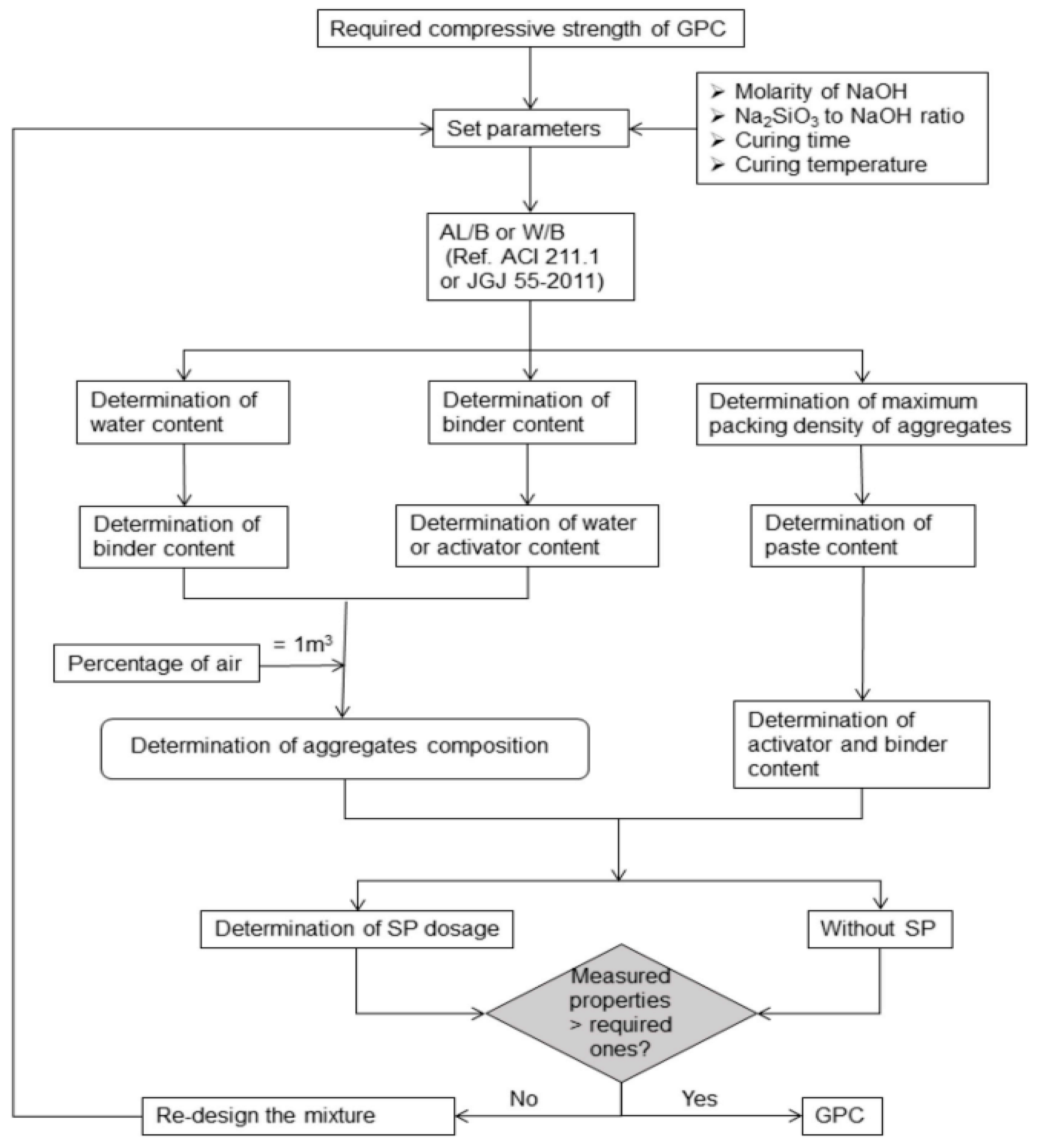
8. Mix Design
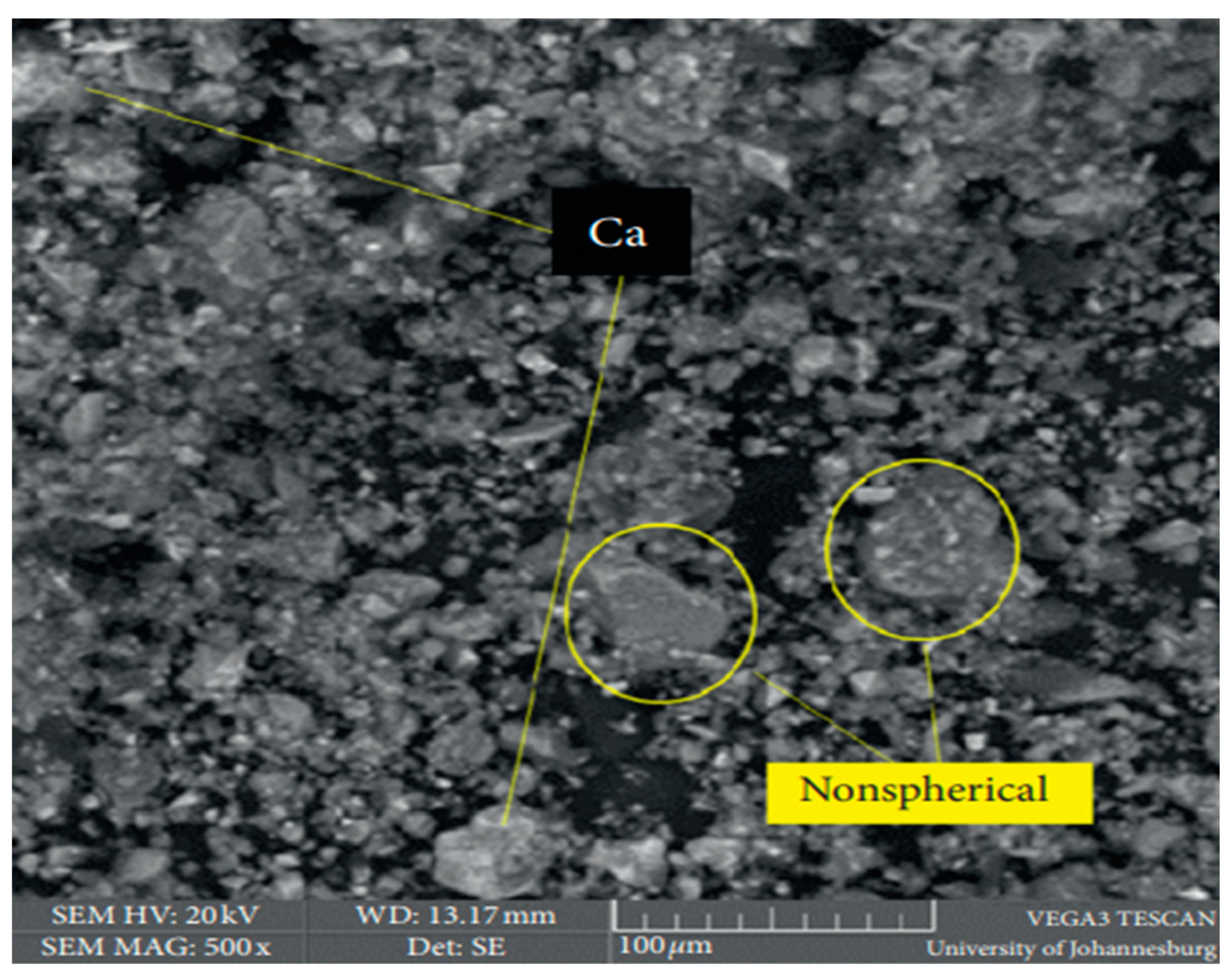
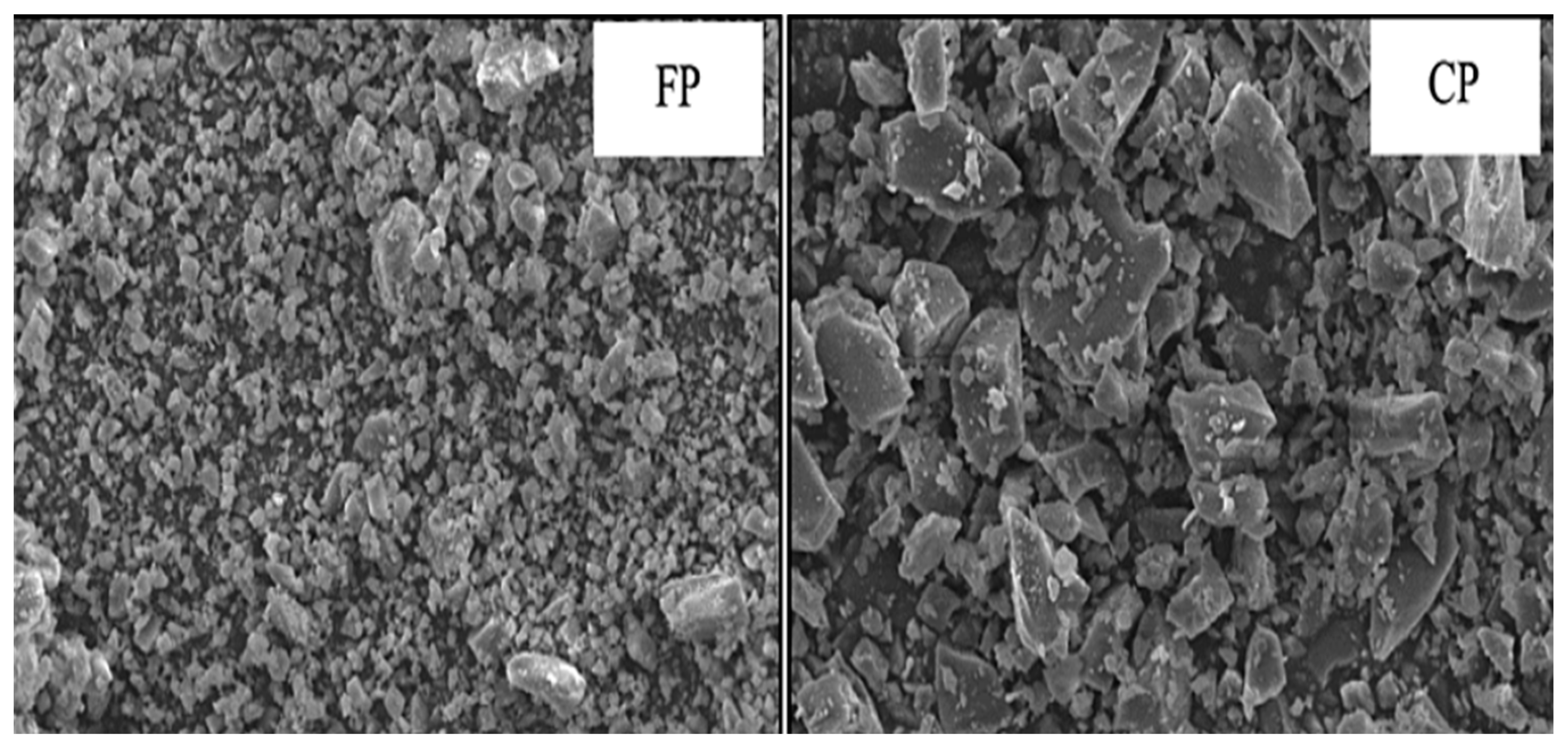
9. Microstructure
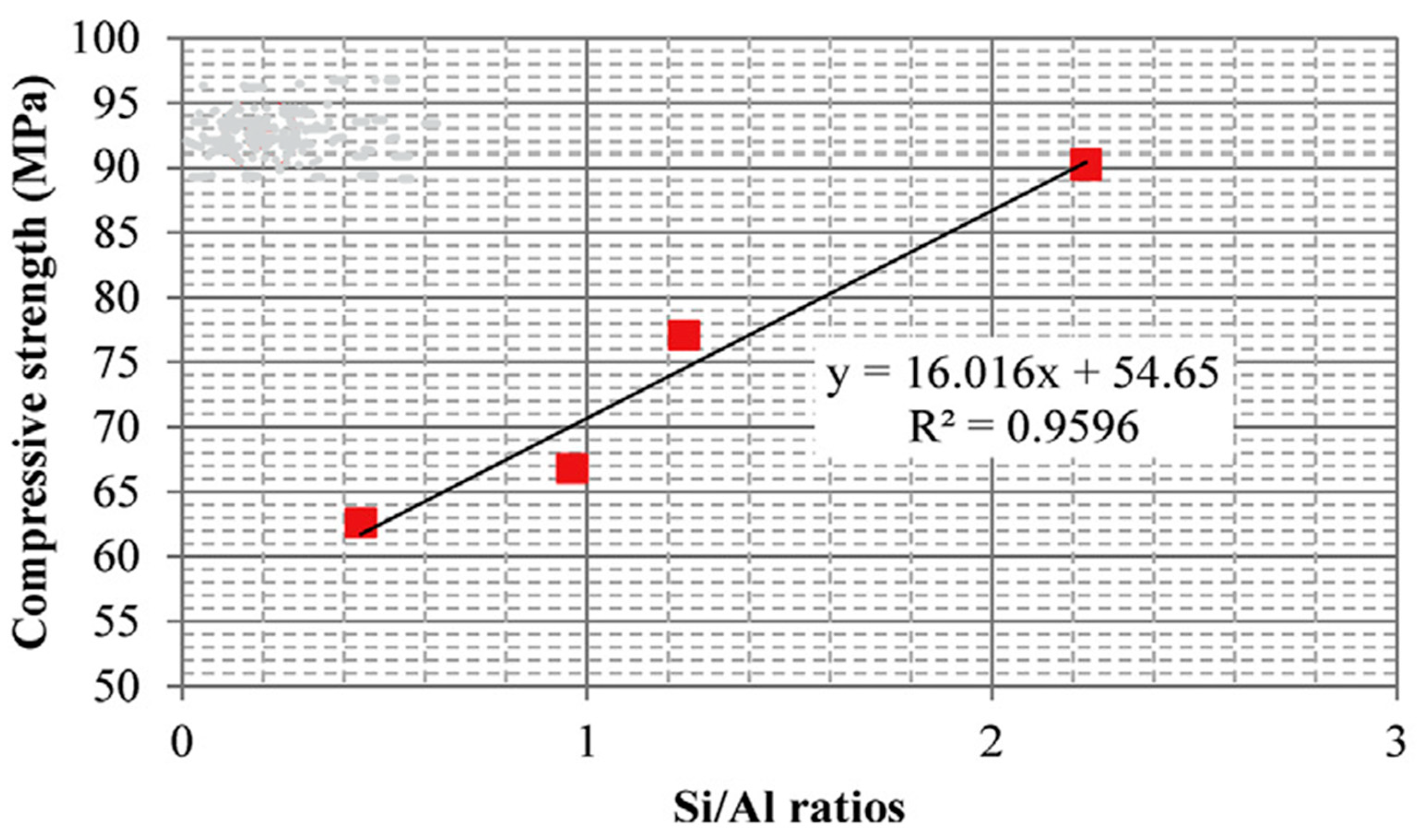
10. Mechanical Properties
11. Durability
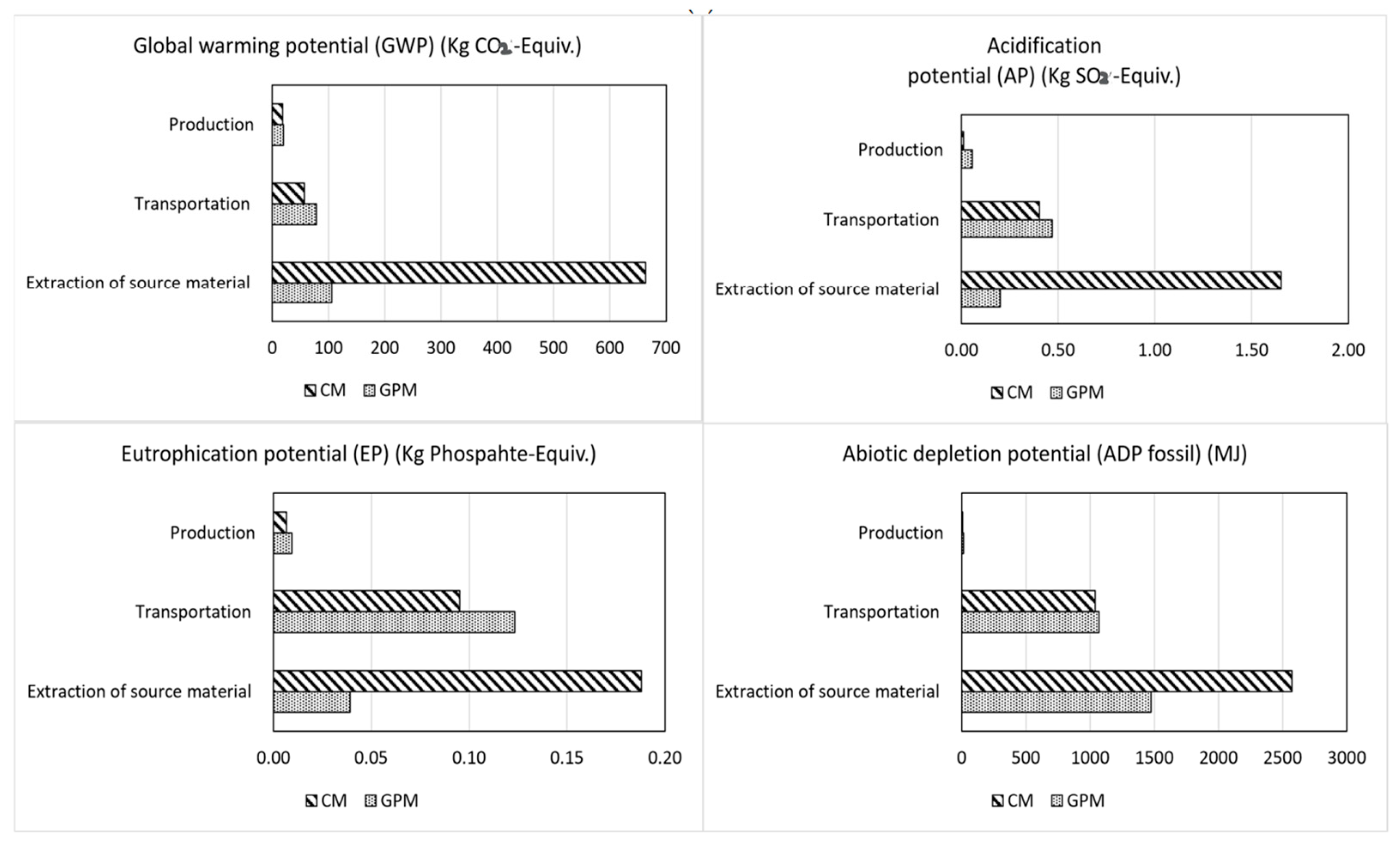
12. Cost–Benefit
13. Applications
14. Limitations of the Review
15. Future Research Areas
- i.
- Presently, there is limited research on the physico-chemo-mechanical performance and behavior of a binary system of geopolymerized fly ash-phosphogypsum binder mortar at ambient curing conditions. There is a need to fully understand the hydration mechanism, crack mechanism, volumetric phase assemblages, failure mode, and resistance to physical and chemical attacks.
- ii.
- Further studies are required to fully understand the performance of reclaimed fly ash for geopolymer production. The development of techniques to extract silica from coal ash tailings is a potential source of creating value from waste.
- iii.
- The development of alternative activators to hydroxides and silicates with less environmental impact and minimal cost. The production of alkaline activators produces CO2 via the electrolysis of salts; therefore, there is a need for new bio-materials to replace the existing alkaline activators.
- iv.
- The utilization of a one-part activator is a research route that seems promising and needs long-term performance studies. There is a need to understand the influence on microstructure development, dosage optimization, and mechanical strength. Just like ordinary Portland cement, the development of “just add” water geopolymer is still a novel area that requires further study.
- v.
- Most research is limited to high-temperature heat curing conditions, which are expensive and energy intensive. Therefore, research on low-temperature ambient curing conditions should be performed to reduce energy requirements. The level of acceptance of geopolymers can be expanded if they can be sustainably and economically produced at low energy and cost.
- vi.
- The use of in situ curing requires materials that should not only reduce the setting time but also improve the early age strength. The correlation between setting time and early age strength gain of geopolymer at ambient temperature needs further study.
- vii.
- Long-term repetitive tests under different laboratory and in situ testing conditions are required to comprehensively define the durability and microstructural properties of geopolymers to facilitate the development of test methods and validation techniques. Additional long-term curing studies for geopolymer are needed since the 28-day curing regime was designed for ordinary Portland cement concrete.
- viii.
- There is very limited research on the prediction and optimization of geopolymer concrete strength concerning mix design parameters such as precursor properties, Si/Al ratio, Ca/(Si+Al), water/solid ratio, Al/Na2O ratio, H2O/Na2O ratio, and curing conditions. There is a need to develop a harmonized fit-for-purpose mix design standard that considers all geopolymer production variables.
- ix.
- There is a need to understand the shear strength and stress-strain behavior of geopolymer structural members to increase industrial application.
16. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Kheimi, M.; Aziz, I.H.; Abdullah, M.M.A.B.; Almadani, M.; Razak, R.A. Waste Material via Geopolymerization for Heavy-Duty Application: A Review. Materials 2022, 15, 3205. [Google Scholar] [CrossRef] [PubMed]
- Davidovits, J. Geopolymer Chemistry and Applications, 5th ed.; Institut Géopolymère: Saint-Quentin, France, 2020. [Google Scholar]
- McCaffrey, R. Climate change and the cement industry. Glob Cem Lime Mag (Environ. Spec. Issue) 2002, 15, 19. [Google Scholar]
- Burduhos Nergis, D.D.; Abdullah, M.M.A.B.; Vizureanu, P.; Faheem, M.T.M. Geopolymers and Their Uses: Review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 374, 012019. [Google Scholar] [CrossRef]
- Habert, G.; d’Espinose de Lacaillerie, J.B.; Roussel, N. An environmental evaluation of geopolymer based concrete production: Reviewing current research trends. J. Clean. Prod. 2011, 19, 1229–1238. [Google Scholar] [CrossRef]
- Nodehi, M.; Taghvaee, V.M. Alkali-Activated Materials and Geopolymer: A Review of Common Precursors and Activators Addressing Circular Economy. Circ. Econ. Sustain. 2021, 2, 165–196. [Google Scholar] [CrossRef]
- Biernacki, J.J.; Bullard, J.W.; Sant, G.; Brown, K.; Glasser, F.P.; Jones, S.; Ley, T.; Livingston, R.; Nicoleau, L.; Olek, J.; et al. Cements in the 21st century: Challenges, perspectives, and opportunities. J. Am. Ceram. Soc. 2017, 100, 2746–2773. [Google Scholar] [CrossRef]
- A global forecast for the construction industry to 2030. In Global Construction 2030; Oxford Economics: Oxford, UK, 2022.
- World’s First Public Building with Structural Geopolymer Concrete. Available online: https://www.geopolymer.org/news/worlds-first-public-building-with-structural-geopolymer-concrete/ (accessed on 9 April 2022).
- Ultimate Green Geopolymer Concrete-Geocement. Available online: https://www.kiranglobal.com/geocement/ (accessed on 9 April 2022).
- Glasby, T.; Day, J.; Genrich, R.; Kemp, M. Commercial scale geopolymer concrete construction. In Proceedings of the Saudi International Building and Constructions Technology Conference, Riyadh, Saudi Arabia, 11–12 May 2015. [Google Scholar]
- Cao, R.; Fang, Z.; Jin, M.; Shang, Y. Application of Machine Learning Approaches to Predict the Strength Property of Geopolymer Concrete. Materials 2022, 15, 2400. [Google Scholar] [CrossRef]
- Ferdous, W.; Manalo, A.; Khennane, A.; Kayali, O. Geopolymer concrete-filled pultruded composite beams—Concrete mix design and application. Cem. Concr. Compos. 2015, 58, 1–13. [Google Scholar] [CrossRef]
- Li, X.; Ma, X.; Zhang, S.; Zheng, E. Mechanical Properties and Microstructure of Class C Fly Ash-Based Geopolymer Paste and Mortar. Materials 2013, 6, 1485–1495. [Google Scholar] [CrossRef]
- Morsy, M.S.; Alsayed, S.H.; Al-Salloum, Y.; Almusallam, T.H. Effect of Sodium Silicate to Sodium Hydroxide Ratios on Strength and Microstructure of Fly Ash Geopolymer Binder. Arab. J. Sci. Eng. 2014, 39, 4333–4339. [Google Scholar] [CrossRef]
- Prachasaree, W.; Limkatanyu, S.; Hawa, A.; Sukontasukkul, P.; Chindaprasirt, P. Manuscript title: Development of strength prediction models for fly ash based geopolymer concrete. J. Build. Eng. 2020, 32, 101704. [Google Scholar] [CrossRef]
- GlobalABC Roadmap for Building and Construction 2020–2050: Towards a Zero-Emission, Efficient and Resilient Buildings and Construction Sector; International Energy Agency Technology Report: Paris, France, 2020.
- IEA. Cement Technology Roadmap Plots Path to Cutting CO2 Emissions 24% by 2050; IEA: Paris France, 2018. [Google Scholar]
- Barcelo, L.; Kline, J.; Walenta, G.; Gartner, E. Cement and carbon emissions. Mater. Struct. 2013, 47, 1055–1065. [Google Scholar] [CrossRef]
- Huntzinger, D.N.; Eatmon, T.D. A life-cycle assessment of Portland cement manufacturing: Comparing the traditional process with alternative technologies. J. Clean. Prod. 2009, 17, 668–675. [Google Scholar] [CrossRef]
- Gopalakrishnan, R.; Kaveri, R. Using graphene oxide to improve the mechanical and electrical properties of fiber-reinforced high-volume sugarcane bagasse ash cement mortar. Eur. Phys. J. Plus 2021, 136, 202. [Google Scholar] [CrossRef]
- Yang, H.; Liu, L.; Yang, W.; Liu, H.; Ahmad, W.; Ahmad, A.; Aslam, F.; Joyklad, P. A comprehensive overview of geopolymer composites: A bibliometric analysis and literature review. Case Stud. Constr. Mater. 2022, 16, e00830. [Google Scholar] [CrossRef]
- Shi, C.; Jimenez, A.F.; Palomo, A. New cements for the 21st century: The pursuit of an alternative to Portland cement. Cem. Concr. Res. 2011, 41, 750–763. [Google Scholar] [CrossRef]
- McLellan, B.C.; Williams, R.P.; Lay, J.; van Riessen, A.; Corder, G.D. Costs and carbon emissions for geopolymer pastes in comparison to ordinary portland cement. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef]
- Cao, Y.; Cui, Y.; Yu, X.; Li, T.; Chang, I.-S.; Wu, J. Bibliometric analysis of phosphogypsum research from 1990 to 2020 based on literatures and patents. Environ. Sci. Pollut. Res. 2021, 28, 66845–66857. [Google Scholar] [CrossRef]
- Dwivedi, A.; Jain, M.K. Fly ash—Waste management and overview: A Review. Recent Res. Sci. Technol. 2014, 6, 30–35. [Google Scholar]
- Sett, R. Flyash: Characteristics, Problems, and Possible Utilization. Adv. Appl. Sci. Res. 2017, 8, 32–50. [Google Scholar]
- Alabi, A.S.; Mahachi, J. Chloride ion penetration performance of recycled concrete with different geopolymers. Mater. Today: Proc. 2020, 38, 762–766. [Google Scholar] [CrossRef]
- Almutairi, A.L.; Tayeh, B.A.; Adesina, A.; Isleem, H.F.; Zeyad, A.M. Potential applications of geopolymer concrete in construction: A review. Case Stud. Constr. Mater. 2021, 15, e00733. [Google Scholar] [CrossRef]
- Rashad, A.M. Metakaolin as cementitious material: History, scours, production and composition—A comprehensive overview. Constr. Build. Mater. 2013, 41, 303–318. [Google Scholar] [CrossRef]
- Rashad, A.M. A comprehensive overview about the influence of different admixtures and additives on the properties of alkali-activated fly ash. Mater. Des. 2014, 53, 1005–1025. [Google Scholar] [CrossRef]
- Hassan, A.; Arif, M.; Shariq, M. Use of geopolymer concrete for a cleaner and sustainable environment—A review of mechanical properties and microstructure. J. Clean. Prod. 2019, 223, 704–728. [Google Scholar] [CrossRef]
- Van Deventer, J.S.; Provis, J.; Duxson, P. Technical and commercial progress in the adoption of geopolymer cement. Miner. Eng. 2012, 29, 89–104. [Google Scholar] [CrossRef]
- Shobeiri, V.; Bennett, B.; Xie, T.; Visintin, P. A comprehensive assessment of the global warming potential of geopolymer concrete. J. Clean. Prod. 2021, 297, 126669. [Google Scholar] [CrossRef]
- Cong, P.; Cheng, Y. Advances in geopolymer materials: A comprehensive review. J. Traffic Transp. Eng. 2021, 8, 283–314. [Google Scholar] [CrossRef]
- Jin, M.; Zheng, Z.; Sun, Y.; Chen, L.; Jin, Z. Resistance of metakaolin-MSWI fly ash based geopolymer to acid and alkaline environments. J. Non-Crystalline Solids 2016, 450, 116–122. [Google Scholar] [CrossRef]
- Naghizadeh, A.; Ekolu, S.O. Investigation of mixture factors influencing alkali-silica reaction in fly ash-based geopolymer mortars. In Proceedings of the 71st RILEM Annual Week ICACMS 2017, Chennal, India, 3–8 September 2017; pp. 395–400. [Google Scholar]
- Rashad, A.M. Phosphogypsum as a construction material. J. Clean. Prod. 2017, 166, 732–743. [Google Scholar] [CrossRef]
- Raza, A.; El Ouni, M.H.; Azab, M.; Ali, K.; Haider, H.; Rashedi, A. A scientometric review on mechanical and durability performance of geopolymer Paste: Effect of various raw materials. Constr. Build. Mater. 2022, 345, 128297. [Google Scholar] [CrossRef]
- Amran, M.; Debbarma, S.; Ozbakkaloglu, T. Fly ash-based eco-friendly geopolymer concrete: A critical review of the long-term durability properties. Constr. Build. Mater. 2020, 270, 121857. [Google Scholar] [CrossRef]
- Aldawsari, S.; Kampmann, R.; Harnisch, J.; Rohde, C. Setting Time, Microstructure, and Durability Properties of Low Calcium Fly Ash/Slag Geopolymer: A Review. Materials 2022, 15, 876. [Google Scholar] [CrossRef]
- Giacobello, F.; Ielo, I.; Belhamdi, H.; Plutino, M.R. Geopolymers and Functionalization Strategies for the Development of Sustainable Materials in Construction Industry and Cultural Heritage Applications: A Review. Materials 2022, 15, 1725. [Google Scholar] [CrossRef] [PubMed]
- Luhar, I.; Luhar, S.; Abdullah, M.M.A.B.; Razak, R.A.; Vizureanu, P.; Sandu, A.V.; Matasaru, P.-D. A State-of-the-Art Review on Innovative Geopolymer Composites Designed for Water and Wastewater Treatment. Materials 2021, 14, 7456. [Google Scholar] [CrossRef]
- Davidovits, J. Why Alkali-Activated Materials (AAM) Are Not Geopolymers? Available online: https://www.geopolymer.org/faq/alkali-activated-materials-geopolymers/ (accessed on 12 April 2022).
- Provis, J.L. Geopolymers and other alkali activated materials: Why, how, and what? Mater. Struct. 2014, 47, 11–25. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Chadegani, A.A.; Salehi, H.; Yunus, M.; Farhadi, H.; Fooladi, M.; Farhadi, M.; Ebrahim, N.A. A Comparison between Two Main Academic Literature Collections: Web of Science and Scopus Databases. Asian Soc. Sci. 2013, 9, 18–26. [Google Scholar] [CrossRef]
- Bergman, E.M.L. Finding Citations to Social Work Literature: The Relative Benefits of Using Web of Science, Scopus, or Google Scholar. J. Acad. Libr. 2012, 38, 370–379. [Google Scholar] [CrossRef]
- Meho, L.I. Using Scopus’s CiteScore for assessing the quality of computer science conferences. J. Inf. 2019, 13, 419–433. [Google Scholar] [CrossRef]
- Ahmad, W.; Ahmad, A.; Ostrowski, K.A.; Aslam, F.; Joyklad, P. A scientometric review of waste material utilization in concrete for sustainable construction. Case Stud. Constr. Mater. 2021, 15, e00683. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, H.; Feng, P.; Zhang, P. A review on shrinkage-reducing methods and mechanisms of alkali-activated/geopolymer systems: Effects of chemical additives. J. Build. Eng. 2022, 49, 104056. [Google Scholar] [CrossRef]
- Wohlin, C. Guidelines for Snowballing in Systematic Literature Studies and a Replication in Software Engineering. In Proceedings of the 18th International Conference on Evaluation and Assessment in Software Engineering, London, UK, 13–14 May 2014; pp. 1–10. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Whiting, P.; Savović, J.; Higgins, J.P.T.; Caldwell, D.M.; Reeves, B.C.; Shea, B.; Davies, P.; Kleijnen, J.; Churchill, R.; ROBIS group. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. J. Clin. Epidemiol. 2016, 69, 225–234. [Google Scholar] [CrossRef]
- Dhasindrakrishna, K.; Pasupathy, K.; Ramakrishnan, S.; Sanjayan, J. Progress, current thinking and challenges in geopolymer foam concrete technology. Cem. Concr. Compos. 2020, 116, 103886. [Google Scholar] [CrossRef]
- Van Deventer, J. Progress in the Adoption of Geopolymer Cement. In Handbook of Low Carbon Concrete; Nazari, A., Sanjayan, J.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 217–262. [Google Scholar] [CrossRef]
- Provis, J.L.; Bernal, S.A. Milestones in the analysis of alkali-activated binders. J. Sustain. Cem. Mater. 2014, 4, 74–84. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers: Inorganic polymeric new materials. J. Therm. Anal. 1991, 37, 1633–16556. [Google Scholar] [CrossRef]
- Sotelo-Piña, C.; Aguilera-González, E.N.; Martínez-Luévanos, A. Geopolymers: Past, Present, and Future of Low Carbon Footprint Eco-Materials. In Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–21. [Google Scholar] [CrossRef]
- Pacheco-Torgal, J.A. Labrincha, C. Leonelli, A. Palomo, and P. Chindaprasirt. In Handbook of Alkali-Activated Cements, Mortars, and Concretes; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Colangelo, F.; Farina, I.; Travaglioni, M.; Salzano, C.; Cioffi, R.; Petrillo, A. Eco-efficient industrial waste recycling for the manufacturing of fibre reinforced innovative geopolymer mortars: Integrated waste management and green product development through LCA. J. Clean. Prod. 2021, 312, 127777. [Google Scholar] [CrossRef]
- de Azevedo, A.R.G.; Marvila, M.T.; de Oliveira, L.B.; Ferreira, W.M.; Colorado, H.; Teixeira, S.R.; Vieira, C.M.F. Circular economy and durability in geopolymers ceramics pieces obtained from glass polishing waste. Int. J. Appl. Ceram. Technol. 2021, 18, 1891–1900. [Google Scholar] [CrossRef]
- Heah, C.Y.; Kamarudin, H.; Al Bakri, A.M.M.; Bnhussain, M.; Luqman, M.; Nizar, I.K.; Ruzaidi, C.M.; Liew, Y.M. Kaolin-based geopolymers with various NaOH concentrations. Int. J. Miner. Met. Mater. 2013, 20, 313–322. [Google Scholar] [CrossRef]
- Jamil, N.H.; Abdullah, M.M.A.B.; Pa, F.C.; Mohamad, H.; Ibrahim, W.M.A.W.; Chaiprapa, J. Influences of SiO2, Al2O3, CaO and MgO in phase transformation of sintered kaolin-ground granulated blast furnace slag geopolymer. J. Mater. Res. Technol. 2020, 9, 14922–14932. [Google Scholar] [CrossRef]
- Kaya, M.; Koksal, F.; Gencel, O.; Munir, M.J.; Kazmi, S.M.S. Influence of micro Fe2O3 and MgO on the physical and mechanical properties of the zeolite and kaolin based geopolymer mortar. J. Build. Eng. 2022, 52, 104443. [Google Scholar] [CrossRef]
- Longhi, M.; Rodríguez, E.D.; Walkley, B.; Zhang, Z.; Kirchheim, A.P. Metakaolin-based geopolymers: Relation between formulation, physicochemical properties and efflorescence formation. Compos. Part B Eng. 2020, 182, 107671. [Google Scholar] [CrossRef]
- Sun, K.; Peng, X.; Chu, S.; Wang, S.; Zeng, L.; Ji, G. Utilization of BOF steel slag aggregate in metakaolin-based geopolymer. Constr. Build. Mater. 2021, 300, 124024. [Google Scholar] [CrossRef]
- Tchakouté, H.K.; Rüscher, C.H.; Kong, S.; Kamseu, E.; Leonelli, C. Geopolymer binders from metakaolin using sodium waterglass from waste glass and rice husk ash as alternative activators: A comparative study. Constr. Build. Mater. 2016, 114, 276–289. [Google Scholar] [CrossRef]
- Nikolov, A.; Nugteren, H.; Rostovsky, I. Optimization of geopolymers based on natural zeolite clinoptilolite by calcination and use of aluminate activators. Constr. Build. Mater. 2020, 243, 118257. [Google Scholar] [CrossRef]
- Rożek, P.; Król, M.; Mozgawa, W. Geopolymer-zeolite composites: A review. J. Clean. Prod. 2019, 230, 557–579. [Google Scholar] [CrossRef]
- Ulloa, N.; Baykara, H.; Cornejo, M.; Rigail-Cedeño, A.; Paredes, C.; Villalba, J.L. Application-oriented mix design optimization and characterization of zeolite-based geopolymer mortars. Constr. Build. Mater. 2018, 174, 138–149. [Google Scholar] [CrossRef]
- Kaze, R.C.; Naghizadeh, A.; Tchadjie, L.; Adesina, A.; Djobo, J.N.Y.; Nemaleu, J.G.D.; Kamseu, E.; Melo, U.C.; Tayeh, B.A. Lateritic soils based geopolymer materials: A review. Constr. Build. Mater. 2022, 344, 128157. [Google Scholar] [CrossRef]
- Gualtieri, M.L.; Romagnoli, M.; Pollastri, S.; Gualtieri, A. Inorganic polymers from laterite using activation with phosphoric acid and alkaline sodium silicate solution: Mechanical and microstructural properties. Cem. Concr. Res. 2015, 67, 259–270. [Google Scholar] [CrossRef]
- Kaze, C.R.; Lemougna, P.N.; Alomayri, T.; Assaedi, H.; Adesina, A.; Das, S.K.; Lecomte-Nana, G.-L.; Kamseu, E.; Melo, U.C.; Leonelli, C. Characterization and performance evaluation of laterite based geopolymer binder cured at different temperatures. Constr. Build. Mater. 2020, 270, 121443. [Google Scholar] [CrossRef]
- Djobo, J.N.Y.; Elimbi, A.; Tchakouté, H.K.; Kumar, S. Volcanic ash-based geopolymer cements/concretes: The current state of the art and perspectives. Environ. Sci. Pollut. Res. 2016, 24, 4433–4446. [Google Scholar] [CrossRef]
- Tchadjié, L.N.; Ekolu, S.O.; Quainoo, H.; Tematio, P. Incorporation of activated bauxite to enhance engineering properties and microstructure of volcanic ash geopolymer mortar composites. J. Build. Eng. 2021, 41, 102384. [Google Scholar] [CrossRef]
- Tchakoute, H.; Elimbi, A.; Yanne, E.; Djangang, C. Utilization of volcanic ashes for the production of geopolymers cured at ambient temperature. Cem. Concr. Compos. 2013, 38, 75–81. [Google Scholar] [CrossRef]
- Chen, K.; Wu, D.; Zhang, Z.; Pan, C.; Shen, X.; Xia, L.; Zang, J. Modeling and optimization of fly ash–slag-based geopolymer using response surface method and its application in soft soil stabilization. Constr. Build. Mater. 2021, 315, 125723. [Google Scholar] [CrossRef]
- Harris, D. Ash as an internationally traded commodity. In Coal Combustion Products (CCP’s); Robl, T., Oberlink, A., Jones, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 509–529. [Google Scholar] [CrossRef]
- Ash at Work: Applications, Science, and Sustainability of Coal Ash; ACAA: Farmington Hills, MI, USA, 2021.
- Mashifana, T.; Sithole, N. Utilization of fly ash: Basic oxygen furnace slag as a raw material in geopolymerization. IOP Conf. Series: Mater. Sci. Eng. 2019, 652, 012060. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Hamdan, S.; van Deventer, J.S.J. Modification of phase evolution in alkali-activated blast furnace slag by the incorporation of fly ash. Cem. Concr. Compos. 2014, 45, 125–135. [Google Scholar] [CrossRef]
- Naghizadeh, A.; Ekolu, S. Method for comprehensive mix design of fly ash geopolymer mortars. Constr. Build. Mater. 2019, 202, 704–717. [Google Scholar] [CrossRef]
- Rajmohan, B.; Nayaka, R.R.; Kumar, K.R.; Kaleemuddin, K. Mechanical and durability performance evaluation of heat cured low calcium fly ash based sustainable geopolymer concrete. Mater. Today: Proc. 2022, 58, 1337–1343. [Google Scholar] [CrossRef]
- Zhuang, X.Y.; Chen, L.; Komarneni, S.; Zhou, C.H.; Tong, D.S.; Yang, H.M.; Yu, W.H.; Wang, H. Fly ash-based geopolymer: Clean production, properties and applications. J. Clean. Prod. 2016, 125, 253–267. [Google Scholar] [CrossRef]
- Somna, R.; Saowapun, T.; Somna, K.; Chindaprasirt, P. Rice husk ash and fly ash geopolymer hollow block based on NaOH activated. Case Stud. Constr. Mater. 2022, 16, e01092. [Google Scholar] [CrossRef]
- Naghizadeh, A.; Ekolu, S.O. Behaviour of fly ash geopolymer binders under exposure to alkaline media. Asian J. Civ. Eng. 2019, 20, 785–798. [Google Scholar] [CrossRef]
- Graytee, A.; Sanjayan, J.G.; Nazari, A. Development of a high strength fly ash-based geopolymer in short time by using microwave curing. Ceram. Int. 2018, 44, 8216–8222. [Google Scholar] [CrossRef]
- Aiken, T.A.; Kwasny, J.; Sha, W. Resistance of fly ash geopolymer binders to organic acids. Mater. Struct. 2020, 53, 115. [Google Scholar] [CrossRef]
- Alanazi, H.; Hu, J.; Kim, Y.-R. Effect of slag, silica fume, and metakaolin on properties and performance of alkali-activated fly ash cured at ambient temperature. Constr. Build. Mater. 2018, 197, 747–756. [Google Scholar] [CrossRef]
- Hua, S.; Wang, K.; Yao, X.; Xu, W.; He, Y. Effects of fibers on mechanical properties and freeze-thaw resistance of phosphogypsum-slag based cementitious materials. Constr. Build. Mater. 2016, 121, 290–299. [Google Scholar] [CrossRef]
- Raut, S.P.; Patil, U.S.; Madurwar, M.V. Utilization of phosphogypsum and rice husk to develop sustainable bricks. Mater. Today: Proc. 2022, 60, 595–601. [Google Scholar] [CrossRef]
- Available online: http://www-pub.iaea.org/MTCD/Publications/PDF/Pub1582_web.pdf (accessed on 11 July 2022).
- Mashifana, T.P.; Okonta, F.N.; Ntuli, F. Geotechnical Properties and Microstructure of Lime-Fly Ash-Phosphogypsum-Stabilized Soil. Adv. Civ. Eng. 2018, 2018, 3640868. [Google Scholar] [CrossRef]
- Wei, Z.; Deng, Z. Research hotspots and trends of comprehensive utilization of phosphogypsum: Bibliometric analysis. J. Environ. Radioact. 2021, 242, 106778. [Google Scholar] [CrossRef] [PubMed]
- Vaičiukynienė, D.; Nizevičienė, D.; Kantautas, A.; Bocullo, V.; Kielė, A. Alkali Activated Paste and Concrete Based on of Biomass Bottom Ash with Phosphogypsum. Appl. Sci. 2020, 10, 5190. [Google Scholar] [CrossRef]
- Vijay, J.J.; Rao, H.S.; Ghorpade, V.G. XRD & SEM studies of Fly-ash and Phosphogypsum based Geopolymer Bricks. Int. J. Eng. Trends Technol. 2021, 69, 225–232. [Google Scholar] [CrossRef]
- Rashad, A.M. Potential use of phosphogypsum in alkali-activated fly ash under the effects of elevated temperatures and thermal shock cycles. J. Clean. Prod. 2015, 87, 717–725. [Google Scholar] [CrossRef]
- Hamdi, N.; Ben Messaoud, I.; Srasra, E. Production of geopolymer binders using clay minerals and industrial wastes. Comptes Rendus. Chim. 2018, 22, 220–226. [Google Scholar] [CrossRef]
- Fidanchevski, E.; Angjusheva, B.; Jovanov, V.; Murtanovski, P.; Vladiceska, L.; Aluloska, N.S.; Nikolic, J.K.; Ipavec, A.; Šter, K.; Mrak, M.; et al. Technical and radiological characterisation of fly ash and bottom ash from thermal power plant. J. Radioanal. Nucl. Chem. Artic. 2021, 330, 685–694. [Google Scholar] [CrossRef]
- Xie, T.; Ozbakkaloglu, T. Behavior of low-calcium fly and bottom ash-based geopolymer concrete cured at ambient temperature. Ceram. Int. 2015, 41, 5945–5958. [Google Scholar] [CrossRef]
- Suksiripattanapong, C.; Krosoongnern, K.; Thumrongvut, J.; Sukontasukkul, P.; Horpibulsuk, S.; Chindaprasirt, P. Properties of cellular lightweight high calcium bottom ash-portland cement geopolymer mortar. Case Stud. Constr. Mater. 2020, 12, e00337. [Google Scholar] [CrossRef]
- Phoo-Ngernkham, T.; Maegawa, A.; Mishima, N.; Hatanaka, S.; Chindaprasirt, P. Effects of sodium hydroxide and sodium silicate solutions on compressive and shear bond strengths of FA–GBFS geopolymer. Constr. Build. Mater. 2015, 91, 1–8. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Effect of GGBFS on setting, workability and early strength properties of fly ash geopolymer concrete cured in ambient condition. Constr. Build. Mater. 2014, 66, 163–171. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P. Fracture properties of GGBFS-blended fly ash geopolymer concrete cured in ambient temperature. Mater. Struct. 2016, 50, 32. [Google Scholar] [CrossRef]
- Aziz, I.H.; Abdullah, M.M.A.B.; Salleh, M.M.; Azimi, E.A.; Chaiprapa, J.; Sandu, A.V. Strength development of solely ground granulated blast furnace slag geopolymers. Constr. Build. Mater. 2020, 250, 118720. [Google Scholar] [CrossRef]
- Sithole, N.T.; Mashifana, T. Geosynthesis of building and construction materials through alkaline activation of granulated blast furnace slag. Constr. Build. Mater. 2020, 264, 120712. [Google Scholar] [CrossRef]
- Nagajothi, S.; Elavenil, S. Effect of GGBS Addition on Reactivity and Microstructure Properties of Ambient Cured Fly Ash Based Geopolymer Concrete. Silicon 2020, 13, 507–516. [Google Scholar] [CrossRef]
- Mashifana, T.; Sebothoma, J.; Sithole, T. Alkaline Activation of Basic Oxygen Furnace Slag Modified Gold Mine Tailings for Building Material. Adv. Civ. Eng. 2021, 2021, 9984494. [Google Scholar] [CrossRef]
- Sithole, N.; Okonta, F.; Ntuli, F. Mechanical Properties and Structure of Fly Ash Modified Basic Oxygen Furnace Slag Based Geopolymer Masonry Blocks. J. Solid Waste Technol. Manag. 2020, 46, 372–383. [Google Scholar] [CrossRef]
- Lee, W.-H.; Cheng, T.-W.; Lin, K.-Y.; Lin, K.-L.; Wu, C.-C.; Tsai, C.-T. Geopolymer Technologies for Stabilization of Basic Oxygen Furnace Slags and Sustainable Application as Construction Materials. Sustainability 2020, 12, 5002. [Google Scholar] [CrossRef]
- Li, L.; Wei, Y.-J.; Li, Z.; Farooqi, M.U. Rheological and viscoelastic characterizations of fly ash/slag/silica fume-based geopolymer. J. Clean. Prod. 2022, 354, 131629. [Google Scholar] [CrossRef]
- Duan, P.; Yan, C.; Zhou, W. Compressive strength and microstructure of fly ash based geopolymer blended with silica fume under thermal cycle. Cem. Concr. Compos. 2017, 78, 108–119. [Google Scholar] [CrossRef]
- Sukontasukkul, P.; Chindaprasirt, P.; Pongsopha, P.; Phoo-Ngernkham, T.; Tangchirapat, W.; Banthia, N. Effect of fly ash/silica fume ratio and curing condition on mechanical properties of fiber-reinforced geopolymer. J. Sustain. Cem. Mater. 2020, 9, 218–232. [Google Scholar] [CrossRef]
- Liu, S.; Liu, W.; Jiao, F.; Qin, W.; Yang, C. Production and resource utilization of flue gas desulfurized gypsum in China—A review. Environ. Pollut. 2021, 288, 117799. [Google Scholar] [CrossRef]
- Guo, X.; Shi, H.; Dick, W.A. Utilization of thermally treated flue gas desulfurization (FGD) gypsum and class-C Fly Ash (CFA) to prepare CFA-based geopolymer. J. Wuhan Univ. Technol. Sci. Ed. 2013, 28, 132–138. [Google Scholar] [CrossRef]
- Sun, Z.; Tang, Q.; Xakalashe, B.S.; Fan, X.; Gan, M.; Chen, X.; Ji, Z.; Huang, X.; Friedrich, B. Mechanical and environmental characteristics of red mud geopolymers. Constr. Build. Mater. 2022, 321, 125564. [Google Scholar] [CrossRef]
- He, J.; Zhang, J.; Yu, Y.; Zhang, G. The strength and microstructure of two geopolymers derived from metakaolin and red mud-fly ash admixture: A comparative study. Constr. Build. Mater. 2012, 30, 80–91. [Google Scholar] [CrossRef]
- Singh, S.; Aswath, M.; Ranganath, R. Effect of mechanical activation of red mud on the strength of geopolymer binder. Constr. Build. Mater. 2018, 177, 91–101. [Google Scholar] [CrossRef]
- Falayi, T. A comparison between fly ash- and basic oxygen furnace slag-modified gold mine tailings geopolymers. Int. J. Energy Environ. Eng. 2019, 11, 207–217. [Google Scholar] [CrossRef]
- Hossain, S.S.; Roy, P.; Bae, C.-J. Utilization of waste rice husk ash for sustainable geopolymer: A review. Constr. Build. Mater. 2021, 310, 125218. [Google Scholar] [CrossRef]
- Basri, M.S.M.; Mustapha, F.; Mazlan, N.; Ishak, M.R. Rice Husk Ash-Based Geopolymer Binder: Compressive Strength, Optimize Composition, FTIR Spectroscopy, Microstructural, and Potential as Fire-Retardant Material. Polymers 2021, 13, 4373. [Google Scholar] [CrossRef]
- Ranjbar, N.; Mehrali, M.; Behnia, A.; Alengaram, U.J.; Jumaat, M.Z. Compressive strength and microstructural analysis of fly ash/palm oil fuel ash based geopolymer mortar. Mater. Des. 2014, 59, 532–539. [Google Scholar] [CrossRef]
- Huseien, G.F.; Asaad, M.A.; Abadel, A.A.; Ghoshal, S.K.; Hamzah, H.K.; Benjeddou, O.; Mirza, J. Drying Shrinkage, Sulphuric Acid and Sulphate Resistance of High-Volume Palm Oil Fuel Ash-Included Alkali-Activated Mortars. Sustainability 2022, 14, 498. [Google Scholar] [CrossRef]
- Xiao, R.; Ma, Y.; Jiang, X.; Zhang, M.; Zhang, Y.; Wang, Y.; Huang, B.; He, Q. Strength, microstructure, efflorescence behavior and environmental impacts of waste glass geopolymers cured at ambient temperature. J. Clean. Prod. 2019, 252, 119610. [Google Scholar] [CrossRef]
- Tho-In, T.; Sata, V.; Boonserm, K.; Chindaprasirt, P. Compressive strength and microstructure analysis of geopolymer paste using waste glass powder and fly ash. J. Clean. Prod. 2018, 172, 2892–2898. [Google Scholar] [CrossRef]
- Jiang, X.; Xiao, R.; Ma, Y.; Zhang, M.; Bai, Y.; Huang, B. Influence of waste glass powder on the physico-mechanical properties and microstructures of fly ash-based geopolymer paste after exposure to high temperatures. Constr. Build. Mater. 2020, 262, 120579. [Google Scholar] [CrossRef]
- Labrincha, J.; Puertas, F.; Schroeyers, W.; Kovler, K.; Pontikes, Y.; Nuccetelli, C.; Krivenko, P.; Kovalchuk, O.; Petropavlovsky, O.; Komljenovic, M.; et al. From NORM by-products to building materials. In Naturally Occurring Radioactive Materials in Construction; Schroeyers, W., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 183–252. [Google Scholar] [CrossRef]
- Naghizadeh, A.; Ekolu, S.O. Pozzolanic Materials and Waste Products for Formulation of Geopolymer Cements in Developing Countries: A Review. Concr. Beton 2017, 151, 22–33. [Google Scholar]
- Chindaprasirt, P.; Chalee, W. Effect of sodium hydroxide concentration on chloride penetration and steel corrosion of fly ash-based geopolymer concrete under marine site. Constr. Build. Mater. 2014, 63, 303–310. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Srinivasula, R.M.; Dinakar, P.; Rao, B.K.; Satpathy, B.N.; Mohanty, A. Effect of the Na2SiO3/NaOH Ratio and NaOH Molarity on the Synthesis of Fly Ash-Based Geopolymer Mortar. In Proceedings of the Geo-Chicago 2016, Chicago, IL, USA, 14–18 August 2016; pp. 336–344. [Google Scholar] [CrossRef]
- Keawthun, M.; Krachodnok, S.; Chaisena, A. Conversion of Waste Glasses into Sodium Silicate Solutions. Int. J. Chem. Sci. 2014, 14, 83–91. [Google Scholar]
- Provis, J.L.; Palomo, A.; Shi, C. Advances in understanding alkali-activated materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Rangan, B.V. Engineering properties of geopolymer concrete. In Geopolymers; Provis, J.L., van Deventer, J.S.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 211–226. [Google Scholar] [CrossRef]
- Ridtirud, C.; Chindaprasirt, P.; Pimraksa, K. Factors affecting the shrinkage of fly ash geopolymers. Int. J. Miner. Met. Mater. 2011, 18, 100–104. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-part alkali-activated materials: A review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Askarian, M.; Tao, Z.; Samali, B.; Adam, G.; Shuaibu, R. Mix composition and characterisation of one-part geopolymers with different activators. Constr. Build. Mater. 2019, 225, 526–537. [Google Scholar] [CrossRef]
- Masi, G.; Filipponi, A.; Bignozzi, M.C. Fly ash-based one-part alkali activated mortars cured at room temperature: Effect of precursor pre-treatments. Open Ceram. 2021, 8, 100178. [Google Scholar] [CrossRef]
- Yahya, Z.; Abdullah, M.M.A.B.; Talib, S.Z.A.; Razak, R.A. Comparative study on early strength of sodium hydroxide (NaOH) activated fly ash based geopolymer. AIP Conf. Proc. 2017, 1887, 20059. [Google Scholar] [CrossRef]
- Naghizadeh, A.; Ekolu, S.; Musonda, I. High temperature heat—Treatment (HTHT) for partial mitigation of alkali attack in hardened fly ash geopolymer binders. Case Stud. Constr. Mater. 2020, 12, e00341. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Use of OPC to improve setting and early strength properties of low calcium fly ash geopolymer concrete cured at room temperature. Cem. Concr. Compos. 2015, 55, 205–214. [Google Scholar] [CrossRef]
- Lim, N.H.A.S.; Samadi, M.; Ariffin, N.F.; Hussin, M.W.; Bhutta, M.A.R.; Sarbini, N.N.; Khalid, N.H.A.; Aminuddin, E. Effect of Curing Conditions on Compressive Strength of FA-POFA-based Geopolymer Mortar. IOP Conf. Series: Mater. Sci. Eng. 2018, 431, 092007. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Rattanasak, U.; Taebuanhuad, S. Role of microwave radiation in curing the fly ash geopolymer. Adv. Powder Technol. 2013, 24, 703–707. [Google Scholar] [CrossRef]
- Tan, J.; Cai, J.; Huang, L.; Yang, Q.; Mao, M.; Li, J. Feasibility of using microwave curing to enhance the compressive strength of mixed recycled aggregate powder based geopolymer. Constr. Build. Mater. 2020, 262, 120897. [Google Scholar] [CrossRef]
- Rabie, M.; Irshidat, M.R.; Al-Nuaimi, N. Ambient and Heat-Cured Geopolymer Composites: Mix Design Optimization and Life Cycle Assessment. Sustainability 2022, 14, 4942. [Google Scholar] [CrossRef]
- Jindal, B.B. Investigations on the properties of geopolymer mortar and concrete with mineral admixtures: A review. Constr. Build. Mater. 2019, 227, 116644. [Google Scholar] [CrossRef]
- Rattanasak, U.; Pankhet, K.; Chindaprasirt, P. Effect of chemical admixtures on properties of high-calcium fly ash geopolymer. Int. J. Miner. Met. Mater. 2011, 18, 364–369. [Google Scholar] [CrossRef]
- Karthik, A.; Sudalaimani, K.; Vijayakumar, C.; Saravanakumar, S. Effect of bio-additives on physico-chemical properties of fly ash-ground granulated blast furnace slag based self cured geopolymer mortars. J. Hazard. Mater. 2018, 361, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Hadi, M.N.S.; Farhan, N.A.; Sheikh, M.N. Design of geopolymer concrete with GGBFS at ambient curing condition using Taguchi method. Constr. Build. Mater. 2017, 140, 424–431. [Google Scholar] [CrossRef]
- Karthik, S.; Mohan, K.S.R. A Taguchi Approach for Optimizing Design Mixture of Geopolymer Concrete Incorporating Fly Ash, Ground Granulated Blast Furnace Slag and Silica Fume. Crystals 2021, 11, 1279. [Google Scholar] [CrossRef]
- Memiş, S.; Bılal, M.A.M. Taguchi optimization of geopolymer concrete produced with rice husk ash and ceramic dust. Environ. Sci. Pollut. Res. 2021, 29, 15876–15895. [Google Scholar] [CrossRef]
- Olivia, M.; Nikraz, H. Properties of fly ash geopolymer concrete designed by Taguchi method. Mater. Des. 2012, 36, 191–198. [Google Scholar] [CrossRef]
- Bondar, D.; Nanukuttan, S.; Provis, J.L.; Soutsos, M. Efficient mix design of alkali activated slag concretes based on packing fraction of ingredients and paste thickness. J. Clean. Prod. 2019, 218, 438–449. [Google Scholar] [CrossRef]
- Borges, P.H.R.; Fonseca, L.F.; Nunes, V.A.; Panzera, T.H.; Martuscelli, C.C. Andreasen Particle Packing Method on the Development of Geopolymer Concrete for Civil Engineering. J. Mater. Civ. Eng. 2014, 26, 692–697. [Google Scholar] [CrossRef]
- Li, N.; Shi, C.; Zhang, Z.; Zhu, D.; Hwang, H.-J.; Zhu, Y.; Sun, T. A mixture proportioning method for the development of performance-based alkali-activated slag-based concrete. Cem. Concr. Compos. 2018, 93, 163–174. [Google Scholar] [CrossRef]
- Mermerdaş, K.; Algın, Z.; Oleiwi, S.M.; Nassani, D.E. Optimization of lightweight GGBFS and FA geopolymer mortars by response surface method. Constr. Build. Mater. 2017, 139, 159–171. [Google Scholar] [CrossRef]
- Mohammed, B.S.; Haruna, S.; Wahab, M.M.B.A.; Liew, M. Optimization and characterization of cast in-situ alkali-activated pastes by response surface methodology. Constr. Build. Mater. 2019, 225, 776–787. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, C.; Wang, X.; Zhang, T.; Wang, Q. Response surface methodology for multi-objective optimization of fly ash-GGBS based geopolymer mortar. Constr. Build. Mater. 2021, 315, 125644. [Google Scholar] [CrossRef]
- Li, N.; Shi, C.; Zhang, Z.; Wang, H.; Liu, Y. A review on mixture design methods for geopolymer concrete. Compos. Part B Eng. 2019, 178, 107490. [Google Scholar] [CrossRef]
- Lahoti, M.; Narang, P.; Tan, K.H.; Yang, E.-H. Mix design factors and strength prediction of metakaolin-based geopolymer. Ceram. Int. 2017, 43, 11433–11441. [Google Scholar] [CrossRef]
- Beulah, M.; Sudhir, M.R.; Chen, S.; Rai, S.; Jain, D. An Empirical Model for Geopolymer Reactions Involving Fly Ash and GGBS. Adv. Mater. Sci. Eng. 2022, 2022, 8801294. [Google Scholar] [CrossRef]
- Gijbels, K.; Iacobescu, R.I.; Pontikes, Y.; Schreurs, S.; Schroeyers, W. Alkali-activated binders based on ground granulated blast furnace slag and phosphogypsum. Constr. Build. Mater. 2019, 215, 371–380. [Google Scholar] [CrossRef]
- Mahdi, S.N.; Babu R, D.V.; Hossiney, N.; Abdullah, M.M.A.B. Strength and durability properties of geopolymer paver blocks made with fly ash and brick kiln rice husk ash. Case Stud. Constr. Mater. 2022, 16, e00800. [Google Scholar] [CrossRef]
- Topark-Ngarm, P.; Chindaprasirt, P.; Sata, V. Setting Time, Strength, and Bond of High-Calcium Fly Ash Geopolymer Concrete. J. Mater. Civ. Eng. 2015, 27, 04014198. [Google Scholar] [CrossRef]
- Song, W.; Zhu, Z.; Peng, Y.; Wan, Y.; Xu, X.; Pu, S.; Song, S.; Wei, Y. Effect of steel slag on fresh, hardened and microstructural properties of high-calcium fly ash based geopolymers at standard curing condition. Constr. Build. Mater. 2019, 229, 116933. [Google Scholar] [CrossRef]
- Part, W.K.; Ramli, M.; Cheah, C.B. An overview on the influence of various factors on the properties of geopolymer concrete derived from industrial by-products. Constr. Build. Mater. 2015, 77, 370–395. [Google Scholar] [CrossRef]
- Deb, P.S.; Nath, P.; Sarker, P.K. The effects of ground granulated blast-furnace slag blending with fly ash and activator content on the workability and strength properties of geopolymer concrete cured at ambient temperature. Mater. Des. 2014, 62, 32–39. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Chareerat, T.; Hatanaka, S.; Cao, T. High-Strength Geopolymer Using Fine High-Calcium Fly Ash. J. Mater. Civ. Eng. 2011, 23, 264–270. [Google Scholar] [CrossRef]
- Yacob, N.S.; ElGawady, M.A.; Sneed, L.H.; Said, A. Shear strength of fly ash-based geopolymer reinforced concrete beams. Eng. Struct. 2019, 196, 109298. [Google Scholar] [CrossRef]
- Mo, K.H.; Alengaram, U.J.; Jumaat, M.Z. Structural performance of reinforced geopolymer concrete members: A review. Constr. Build. Mater. 2016, 120, 251–264. [Google Scholar] [CrossRef]
- Kumaravel, S.; Thirugnanasambandam, S. Flexural behaviour of reinforced low calcium fly ash based geopolymer concrete beam. Glob. J. Res. Eng. 2013, 13, 9–13. [Google Scholar]
- Kumaravel, S.; Thirugnanasambandam, S.; Jeyasehar, C.A. Flexural behavior of geopolymer concrete beams with GGBS. IUP J. Struct. Eng. 2014, 7, 45–54. [Google Scholar]
- Nguyen, K.T.; Le, T.A.; Lee, K. Experimental study on flexural strength of reinforced geopolymer concrete beams. Int. J. Civ. Environ. Eng. 2016, 10, 516–520. [Google Scholar]
- Ahmad, A.; Ahmad, W.; Chaiyasarn, K.; Ostrowski, K.A.; Aslam, F.; Zajdel, P.; Joyklad, P. Prediction of Geopolymer Concrete Compressive Strength Using Novel Machine Learning Algorithms. Polymers 2021, 13, 3389. [Google Scholar] [CrossRef]
- Ahmed, H.U.; Abdalla, A.A.; Mohammed, A.S.; Mohammed, A.A.; Mosavi, A. Statistical Methods for Modeling the Compressive Strength of Geopolymer Mortar. Materials 2022, 15, 1868. [Google Scholar] [CrossRef]
- Huynh, A.T.; Nguyen, Q.D.; Xuan, Q.L.; Magee, B.; Chung, T.; Tran, K.T.; Nguyen, K.T. A Machine Learning-Assisted Numerical Predictor for Compressive Strength of Geopolymer Concrete Based on Experimental Data and Sensitivity Analysis. Appl. Sci. 2020, 10, 7726. [Google Scholar] [CrossRef]
- Wang, Q.; Ahmad, W.; Ahmad, A.; Aslam, F.; Mohamed, A.; Vatin, N.I. Application of Soft Computing Techniques to Predict the Strength of Geopolymer Composites. Polymers 2022, 14, 1074. [Google Scholar] [CrossRef]
- Song, H.; Ahmad, A.; Farooq, F.; Ostrowski, K.A.; Maślak, M.; Czarnecki, S.; Aslam, F. Predicting the compressive strength of concrete with fly ash admixture using machine learning algorithms. Constr. Build. Mater. 2021, 308, 125021. [Google Scholar] [CrossRef]
- Albitar, M.; Ali, M.S.M.; Visintin, P.; Drechsler, M. Durability evaluation of geopolymer and conventional concretes. Constr. Build. Mater. 2017, 136, 374–385. [Google Scholar] [CrossRef]
- Ranjbar, N.; Mehrali, M.; Mehrali, M.; Alengaram, U.J.; Jumaat, M.Z. Graphene nanoplatelet-fly ash based geopolymer composites. Cem. Concr. Res. 2015, 76, 222–231. [Google Scholar] [CrossRef]
- Amran, M.; Al-Fakih, A.; Chu, S.H.; Fediuk, R.; Haruna, S.; Azevedo, A.; Vatin, N. Long-term durability properties of geopolymer concrete: An in-depth review. Case Stud. Constr. Mater. 2021, 15, e00661. [Google Scholar] [CrossRef]
- Provis, J.L.; Arbi, K.; Bernal, S.A.; Bondar, D.; Buchwald, A.; Castel, A.; Chithiraputhiran, S.; Cyr, M.; Dehghan, A.; Dombrowski-Daube, K.; et al. RILEM TC 247-DTA round robin test: Mix design and reproducibility of compressive strength of alkali-activated concretes. Mater. Struct. 2019, 52, 99. [Google Scholar] [CrossRef]
- Abdollahnejad, Z.; Pacheco-Torgal, F.; Félix, T.; Tahri, W.; Aguiar, J. Mix design, properties and cost analysis of fly ash-based geopolymer foam. Constr. Build. Mater. 2015, 80, 18–30. [Google Scholar] [CrossRef]
- Bajpai, R.; Choudhary, K.; Srivastava, A.; Sangwan, K.S.; Singh, M. Environmental impact assessment of fly ash and silica fume based geopolymer concrete. J. Clean. Prod. 2020, 254, 120147. [Google Scholar] [CrossRef]
- Assi, L.N.; Carter, K.; Deaver, E.; Ziehl, P. Review of availability of source materials for geopolymer/sustainable concrete. J. Clean. Prod. 2020, 263, 121477. [Google Scholar] [CrossRef]
- Sandanayake, M.; Gunasekara, C.; Law, D.; Zhang, G.; Setunge, S. Greenhouse gas emissions of different fly ash based geopolymer concretes in building construction. J. Clean. Prod. 2018, 204, 399–408. [Google Scholar] [CrossRef]
- You, S.; Ho, S.W.; Li, T.; Maneerung, T.; Wang, C.-H. Techno-economic analysis of geopolymer production from the coal fly ash with high iron oxide and calcium oxide contents. J. Hazard. Mater. 2018, 361, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Shikuku, V.O.; Sylvain, T. Application of Geopolymer Composites in Wastewater Treatment. In Polymer Nanocomposites for Advanced Engineering and Military Applications; Ramdani, N., Ed.; IGI Global: Hershey, PA, USA, 2019; pp. 131–149. [Google Scholar] [CrossRef]
- Whyte, C.; de Bres, D. Geopolymer Concrete: Concrete from Waste Materials. 2016. Available online: https://sustainabletech.co.za/wp-content/uploads/2021/05/Geo-Polymer-Concrete-Systems.pdf (accessed on 15 August 2022).








| Option | Inclusion Criteria Applied |
|---|---|
| Language | English |
| Publication date | 2011–2022 (April) |
| Subject area | Engineering; Material Science; Environmental Science |
| Source type | Journal |
| Document Type | Article, Review |
| Availability | Full text |
| Country | Oxides (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | MnO | K2O | Na2O | TiO2 | P2O5 | SO3 | LOI | |
| South Africa [89] | 56.45 | 30.27 | 3.58 | 4.59 | 1.06 | - | 0.77 | 0.14 | 1.57 | 0.38 | - | 0.42 |
| India [86] | 61.16 | 30.48 | 4.62 | 1.75 | 0.18 | - | 0.18 | 0.76 | 1.56 | 0.27 | 0.19 | 0.60 |
| China [80] | 54.6 | 27.2 | 11.6 | 2.2 | 1.0 | - | 0.7 | 1.0 | 0.5 | - | - | 1.0 |
| Australia [90] | 51.11 | 25.56 | 12.48 | 4.3 | 1.45 | 0.15 | 0.7 | 0.77 | 1.32 | 0.885 | 0.24 | 0.57 |
| United Kingdom [91] | 46.78 | 22.52 | 9.15 | 2.24 | 1.33 | 0.05 | 4.09 | 0.89 | 1.05 | - | 0.90 | 3.57 |
| United States of America [92] | 56.52 | 22.75 | 4.56 | 8.53 | 2.64 | - | 1.16 | 0.69 | - | - | 0.4 | 0.35 |
| Country | Oxides (%) | % | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | MnO | K2O | Na2O | TiO2 | P2O5 | SO3 | F | LOI | |
| South Africa [96] | 1.37 | 0.23 | 0.121 | 44 | - | - | - | - | - | 1.28 | 51 | 1.06 | - |
| India [94] | 1.75 | 0.13 | 0.16 | 38.87 | 0.02 | - | - | 0.11 | 0.05 | 1.04 | 52.94 | 0.32 | 3.97 |
| China [93] | 4.86 | 4.38 | - | 31.05 | 0.26 | - | 0.41 | - | 0.2 | 3.57 | 30.95 | - | 22.91 |
| United Kingdom [95] | 2.4 | 0.40 | 0.23 | 40 | 0.04 | - | 0.03 | 0.13 | 0.03 | 0.95 | 52 | 0.14 | 6.40 |
| United States of America [95] | 3 | 0.3 | 0.2 | 31 | - | - | - | - | 0.04 | 2.25 | 55 | 0.2 | 17.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsimbe, J.; Dinka, M.; Olukanni, D.; Musonda, I. Geopolymer: A Systematic Review of Methodologies. Materials 2022, 15, 6852. https://doi.org/10.3390/ma15196852
Matsimbe J, Dinka M, Olukanni D, Musonda I. Geopolymer: A Systematic Review of Methodologies. Materials. 2022; 15(19):6852. https://doi.org/10.3390/ma15196852
Chicago/Turabian StyleMatsimbe, Jabulani, Megersa Dinka, David Olukanni, and Innocent Musonda. 2022. "Geopolymer: A Systematic Review of Methodologies" Materials 15, no. 19: 6852. https://doi.org/10.3390/ma15196852
APA StyleMatsimbe, J., Dinka, M., Olukanni, D., & Musonda, I. (2022). Geopolymer: A Systematic Review of Methodologies. Materials, 15(19), 6852. https://doi.org/10.3390/ma15196852







