Ultra-Fast Thermal Shock Evaluation of Ti2AlC Ceramic
Abstract
1. Introduction
2. Experimental Procedures
2.1. Sample Preparation
2.2. Rapid Thermal Shock Testing
2.3. Characterization
3. Results and Discussion
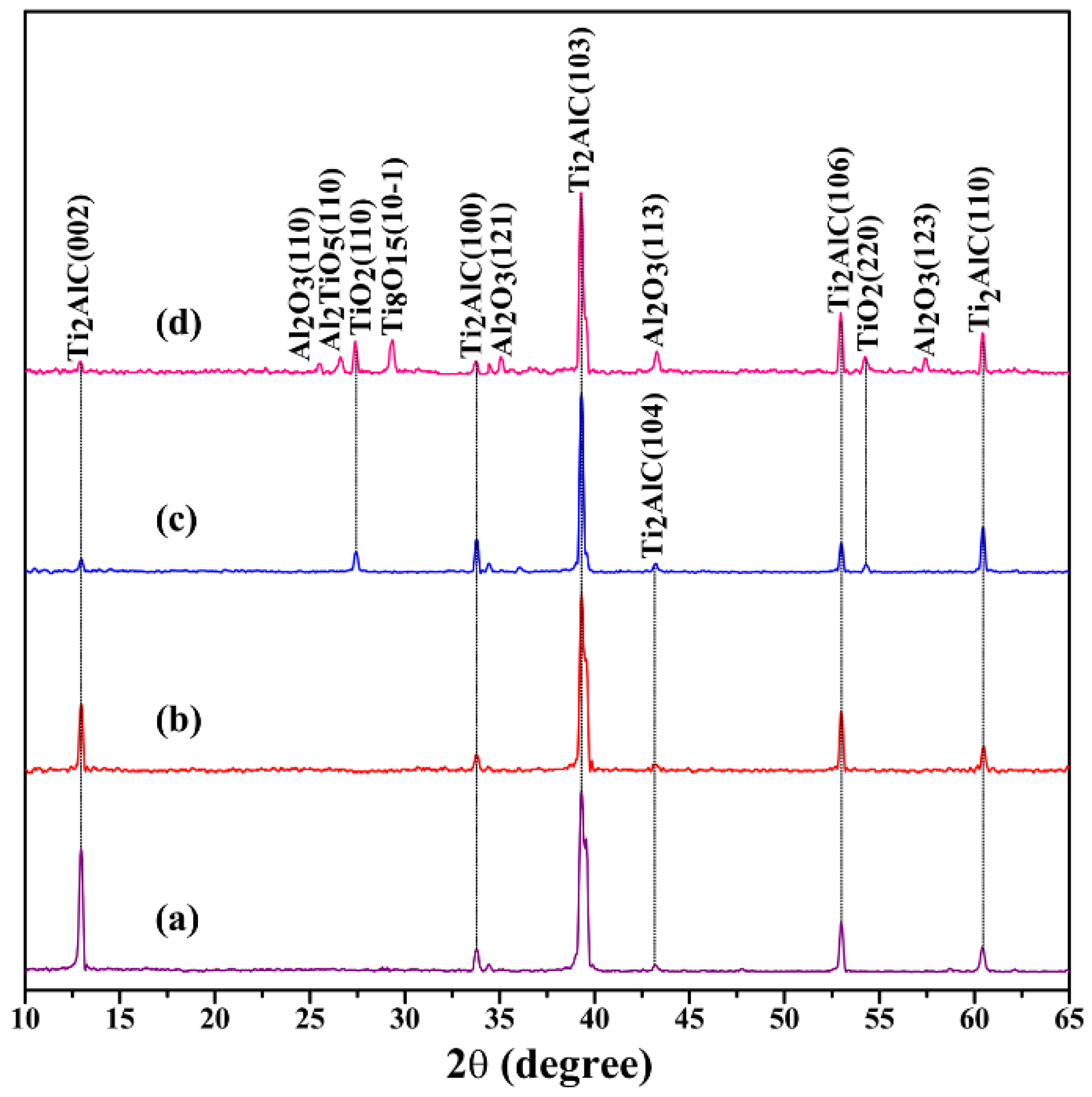
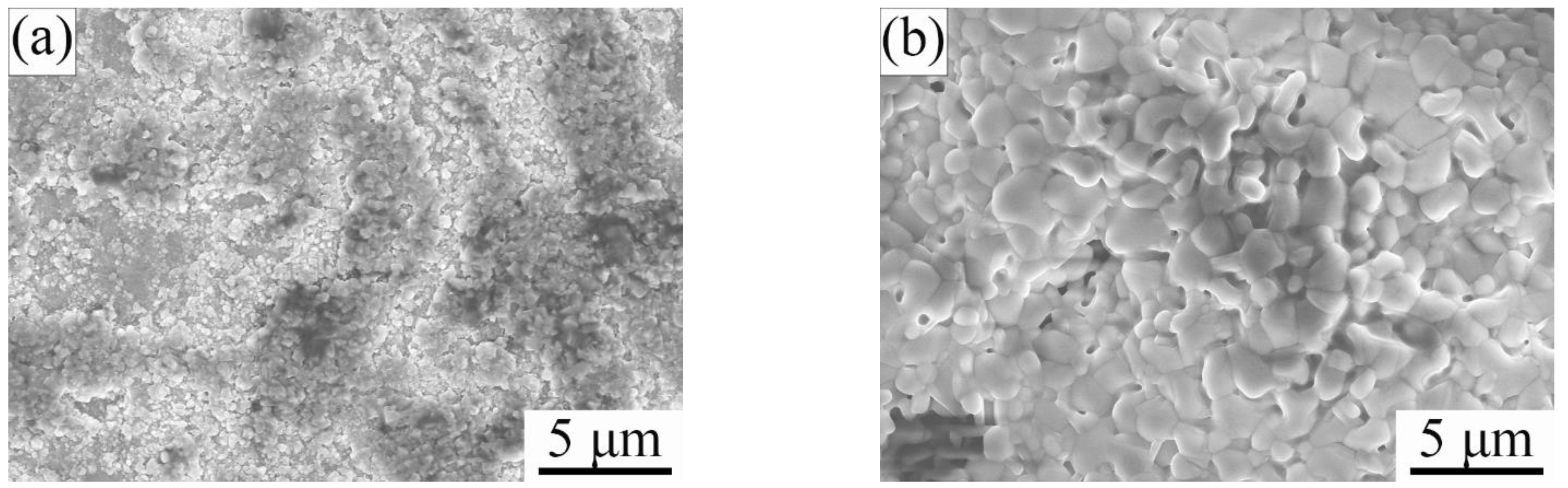
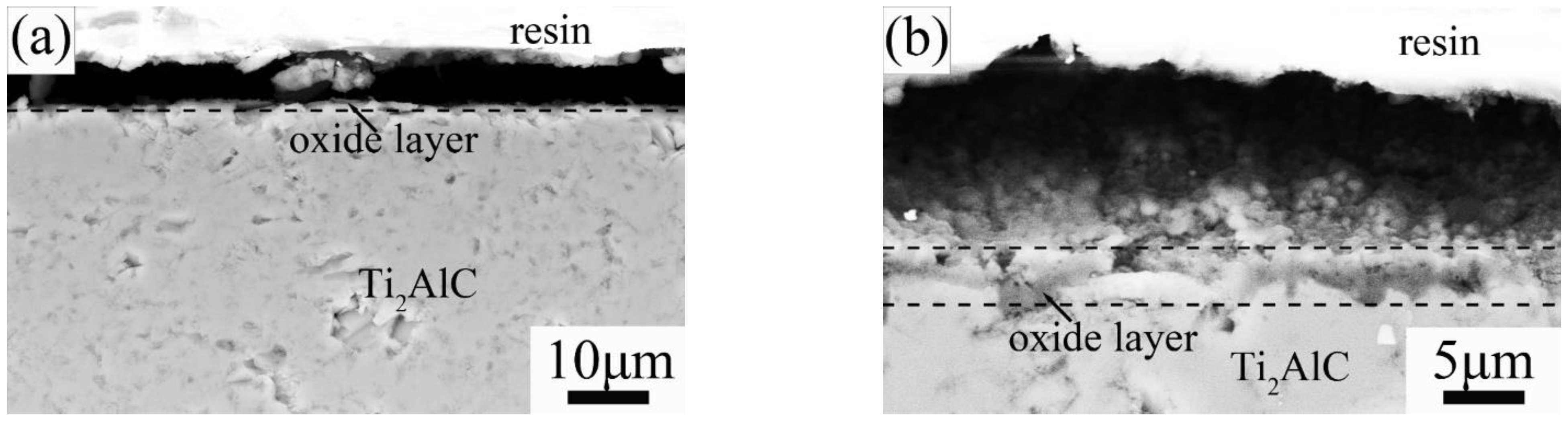
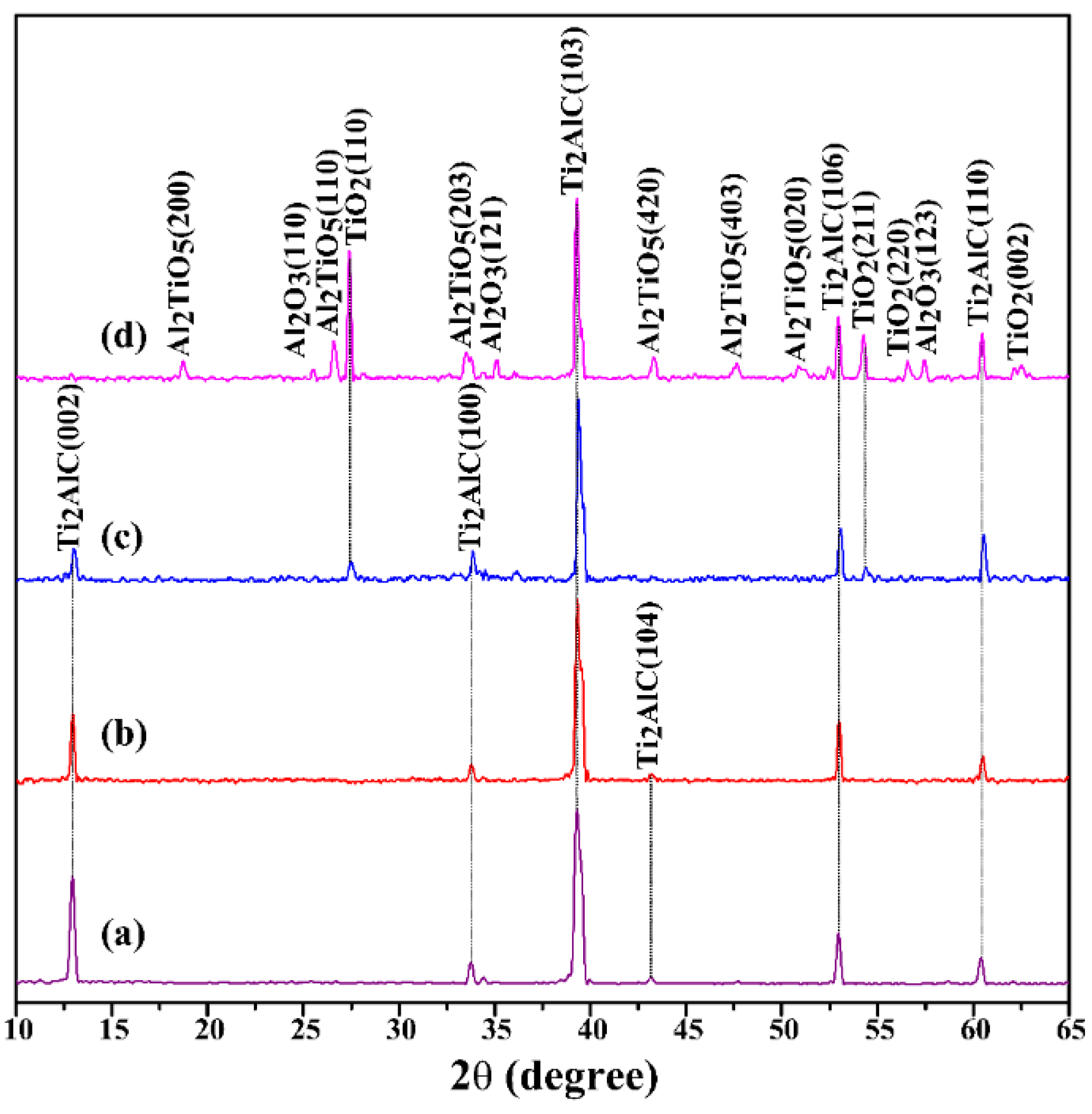
3.1. Phase Composition and Microstructure of Air-Quenched Ti2AlC Ceramic Heated in Air
3.2. Phase Composition and Microstructure of Water-Quenched Ti2AlC Ceramic Heated in Air
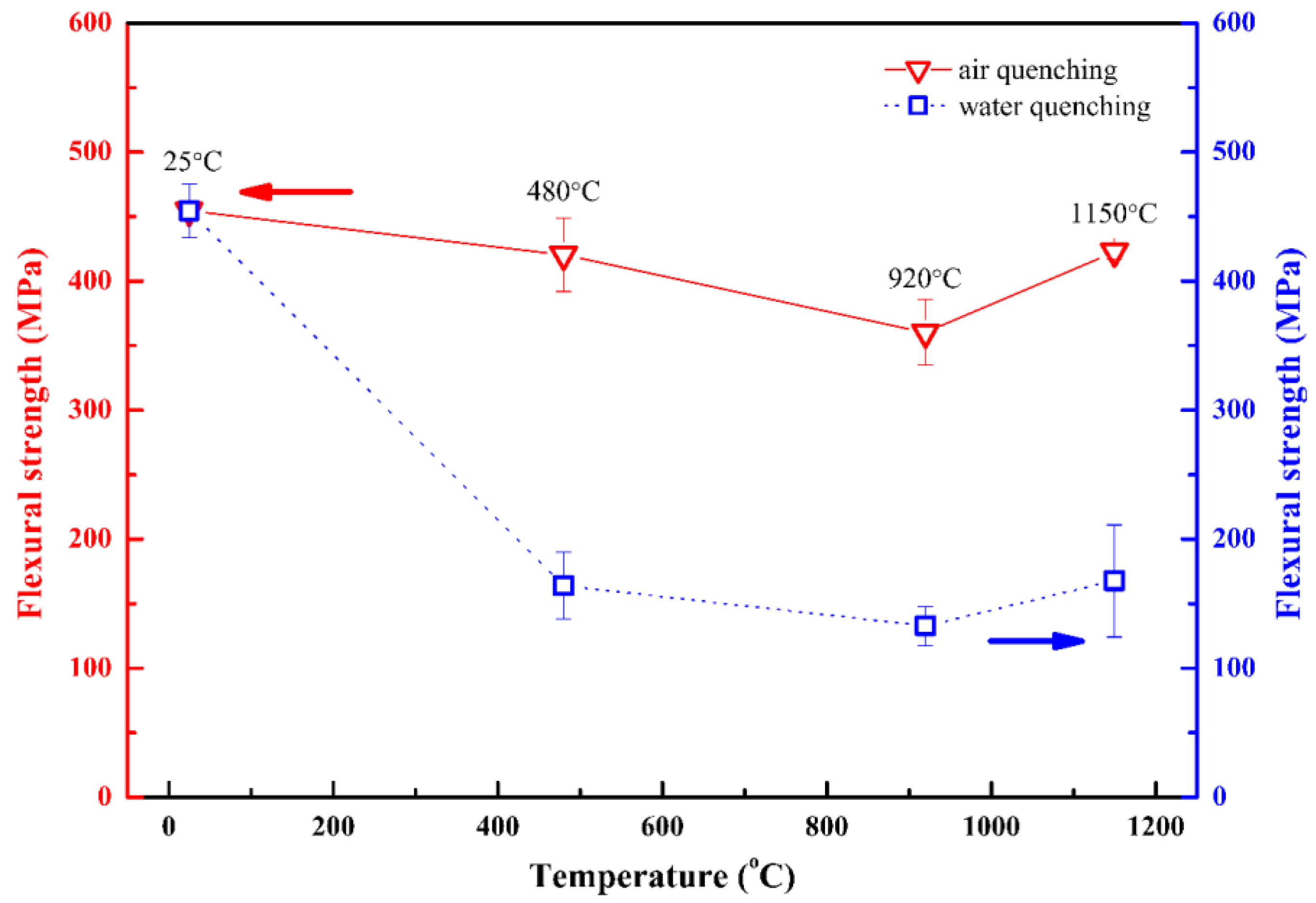
3.3. Residual Flexural Strength of Ti2AlC Ceramic
3.4. Thermal Shock Mechanism of Ti2AlC Ceramic
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Niu, Y.; Fu, S.; Zhang, K.; Dai, B.; Zhang, H.; Grasso, S.; Hu, C. Synthesis, microstructure, and properties of high purity Mo2TiAlC2 ceramics fabricated by spark plasma sintering. J. Adv. Ceram. 2020, 9, 759–768. [Google Scholar] [CrossRef]
- Barsoum, M.; El-Raghy, T. The MAX phases: Unique new carbide and nitride materials. Am. Sci. 2001, 89, 334. [Google Scholar] [CrossRef]
- Sun, Z.M. Progress in research and development on MAX phases: A family of layered ternary compounds. Int. Mater. Rev. 2011, 56, 143–166. [Google Scholar] [CrossRef]
- Barsoum, M.W. The MN+1AXN phases: A new class of solids. Prog. Solid State Chem. 2000, 28, 201–281. [Google Scholar] [CrossRef]
- Gong, Y.; Tian, W.; Zhang, P.; Chen, J.; Zhang, Y.; Sun, Z. Slip casting and pressureless sintering of Ti3AlC2. J. Adv. Ceram. 2019, 8, 367–376. [Google Scholar] [CrossRef]
- Radovic, M.; Barsoum, M.W. MAX phases: Bridging the gap between metals and ceramics. Am. Ceram. Soc. Bull. 2013, 92, 20–27. [Google Scholar]
- Sharma, P.; Singh, K.; Pandey, O.P. Pandey. Investigation on oxidation stability of V2AlC Max phase. Thermochim. Acta 2021, 704, 179010. [Google Scholar] [CrossRef]
- Zhang, Q.; Fu, S.; Wan, D.; Bao, Y.; Feng, Q.; Grasso, S.; Hu, C. Synthesis and property characterization of ternary laminar Zr2SB ceramic. J. Adv. Ceram. 2022, 11, 825–833. [Google Scholar] [CrossRef]
- Zhang, Q.; Fu, S.; Wan, D.; Bao, Y.; Feng, Q.; Grasso, S.; Hu, C. Rapidly synthesizing Hf2SB ceramics by thermal explosion. J. Eur. Ceram. Soc. 2022, 42, 3780–3786. [Google Scholar] [CrossRef]
- Barsoum, M.W.; Radovic, M. Elastic and mechanical properties of the MAX phases. Annu. Rev. Mater. Res. 2011, 41, 195–227. [Google Scholar] [CrossRef]
- Hu, S.; Li, S.; Li, H.; Zhou, Y. Oxyacetylene torch testing and microstructural characterization of a Cr2AlC ceramic. J. Alloys Compd. 2018, 740, 77–81. [Google Scholar] [CrossRef]
- Hoffman, E.N.; Vinson, D.W.; Sindelar, R.L.; Tallman, D.J.; Kohse, G.; Barsoum, M.W. MAX phase carbides and nitrides: Properties for future nuclear power plant in-core applications and neutron transmutation analysis. Nucl. Eng. Des. 2012, 244, 17–24. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y. Recent progress in theoretical prediction, preparation, and characterization of layered ternary transition-metal carbides. Annu. Rev. Mater. Res. 2009, 39, 415–443. [Google Scholar] [CrossRef]
- Hadi, M.A.; Christopoulos, S.R.G.; Chroneos, A.; Naqib, S.H.; Islam, A.K.M.A. Elastic behaviour and radiation tolerance in Nb-based 211 MAX phases, Mater. Today Commun. 2020, 25, 101499. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, D.; Grasso, S.; Suzuki, T.S.; Kasahara, A.; Tosa, M.; Kim, B.; Sakka, Y.; Zhu, M.; Hu, C. Effect of texture microstructure on tribological properties of tailored Ti3AlC2 ceramic. J. Adv. Ceram. 2017, 6, 120–128. [Google Scholar] [CrossRef]
- Hu, C.; Lin, Z.; He, L.; Bao, Y.; Wang, J.; Li, M.; Zhou, Y. Physical and mechanical properties of bulk Ta4AlC3 ceramic prepared by an in situ reaction synthesis/hot-pressing method. J. Am. Ceram. Soc. 2007, 90, 2542–2548. [Google Scholar] [CrossRef]
- Sundberg, M.; Malmqvist, G.; Magnusson, A.; El-Raghy, T. Alumina forming high temperature silicides and carbides. Ceram. Int. 2004, 30, 1899–1904. [Google Scholar] [CrossRef]
- Tang, C.; Steinbrück, M.; Große, M.; Bergfeldt, T.; Seifert, H.J. Oxidation behavior of Ti2AlC in the temperature range of 1400–1600 °C in steam. J. Nucl. Mater. 2017, 490, 130–142. [Google Scholar] [CrossRef]
- Lin, Z.J.; Zhuo, M.J.; Zhou, Y.C.; Li, M.S.; Wang, J.Y. Microstructural characterization of layered ternary Ti2AlC. Acta Mater. 2006, 54, 1009–1015. [Google Scholar] [CrossRef]
- Cui, B.; Jayaseelan, D.D.; Lee, W.E. TEM study of the early stages of Ti2AlC oxidation at 900 °C. Scr. Mater. 2012, 67, 830–833. [Google Scholar] [CrossRef]
- Yang, H.J.; Pei, Y.T.; Rao, J.C.; de Hosson, J.T.M. Self-healing performance of Ti2AlC ceramic. J. Mater. Chem. 2012, 22, 8304–8313. [Google Scholar] [CrossRef]
- Cui, B.; Jayaseelan, D.D.; Lee, W.E. Microstructural evolution during high-temperature oxidation of Ti2AlC ceramics. Acta Mater. 2011, 59, 4116–4125. [Google Scholar] [CrossRef]
- Yan, M.; Zeng, C.; Li, Z.; Li, X.; Chen, Y. Physical, antioxidant and thermal shock properties of Cu/Ti2AlC conductive composites. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2013, 28, 504–507. [Google Scholar] [CrossRef]
- Lin, Z.J.; Li, M.S.; Wang, J.Y.; Zhou, Y.C. Influence of water vapor on the oxidation behavior of Ti3AlC2 and Ti2AlC. Scr. Mater. 2008, 58, 29–32. [Google Scholar] [CrossRef]
- Tallman, D.J.; Anasori, B.; Barsoum, M.W. A critical review of the oxidation of Ti2AlC, Ti3AlC2 and Cr2AlC in air. Mater. Res. Lett. 2013, 1, 115–125. [Google Scholar] [CrossRef]
- Bai, Y.; Kong, F.; He, X.; Li, N.; Qi, X.; Zheng, Y.; Zhu, C.; Wang, R.; Duff, A.I. Thermal shock behavior of Ti2AlC from 200 °C to 1400 °C. J. Am. Ceram. Soc. 2017, 100, 4190–4198. [Google Scholar] [CrossRef]
- Bao, Y.W.; Wang, X.H.; Zhang, H.B.; Zhou, Y.C. Thermal shock behavior of Ti3AlC2 from between 200 °C and 1300 °C. J. Eur. Ceram. Soc. 2005, 25, 3367–3374. [Google Scholar] [CrossRef]
- Su, X.; Bao, Y.; Wan, D.; Zhang, H.; Xu, L.; Grasso, S.; Hu, C. Thermal shock resistance of Ti3SiC2 ceramic under extremely rapid thermal cycling. J. Alloys Compd. 2021, 866, 158985. [Google Scholar] [CrossRef]
- Hu, B.; Bao, Y.; Su, X.; Wan, D.; Feng, Q.; Grasso, S.; Hu, C. Comparative investigation of ultrafast thermal shock of Ti3AlC2 ceramic in water and air. Int. J. Appl. Ceram. Technol. 2021, 18, 1863–1871. [Google Scholar] [CrossRef]
- Lin, Z.; Zhou, Y.; Li, M.; Wang, J. Hot corrosion and protection of Ti2AlC against Na2SO4 salt in air. J. Eur. Ceram. Soc. 2006, 26, 3871–3879. [Google Scholar] [CrossRef]
- Xu, J.; Gao, Z.; Qian, Y.; Li, M. Ultra-High-Temperature oxidation and thermal stability of Ti2AlC in air at 1600–1800 °C. Oxid. Met. 2016, 86, 327–338. [Google Scholar] [CrossRef]
- Li, S.; Song, G.; Kwakernaak, K.; van der Zwaag, S.; Sloof, W.G. Multiple crack healing of a Ti2AlC ceramic. J. Eur. Ceram. Soc. 2012, 32, 1813–1820. [Google Scholar] [CrossRef]
- Yang, H.J.; Pei, Y.T.; Rao, J.C.; de Hosson, J.T.M.; Li, S.B.; Song, G.M. High temperature healing of Ti2AlC: On the origin of inhomogeneous oxide scale. Scr. Mater. 2011, 65, 135–138. [Google Scholar] [CrossRef]
- Fey, T.; Stumpf, M.; Chmielarz, A.; Colombo, P.; Greil, P.; Potoczek, M. Microstructure, thermal conductivity and simulation of elastic modulus of MAX-phase (Ti2AlC) gel-cast foams. J. Eur. Ceram. Soc. 2018, 38, 3424–3432. [Google Scholar] [CrossRef]
- Cao, J.; Yin, Z.; Li, H.; Gao, G.; Zhang, X. Tribological and mechanical properties of Ti2AlC coating at room temperature and 800 °C. Ceram. Int. 2018, 44, 1046–1051. [Google Scholar] [CrossRef]
- Wang, X.H.; Zhou, Y.C. High-temperature oxidation behavior of Ti2AlC in air. Oxid. Met. 2003, 59, 303–320. [Google Scholar] [CrossRef]

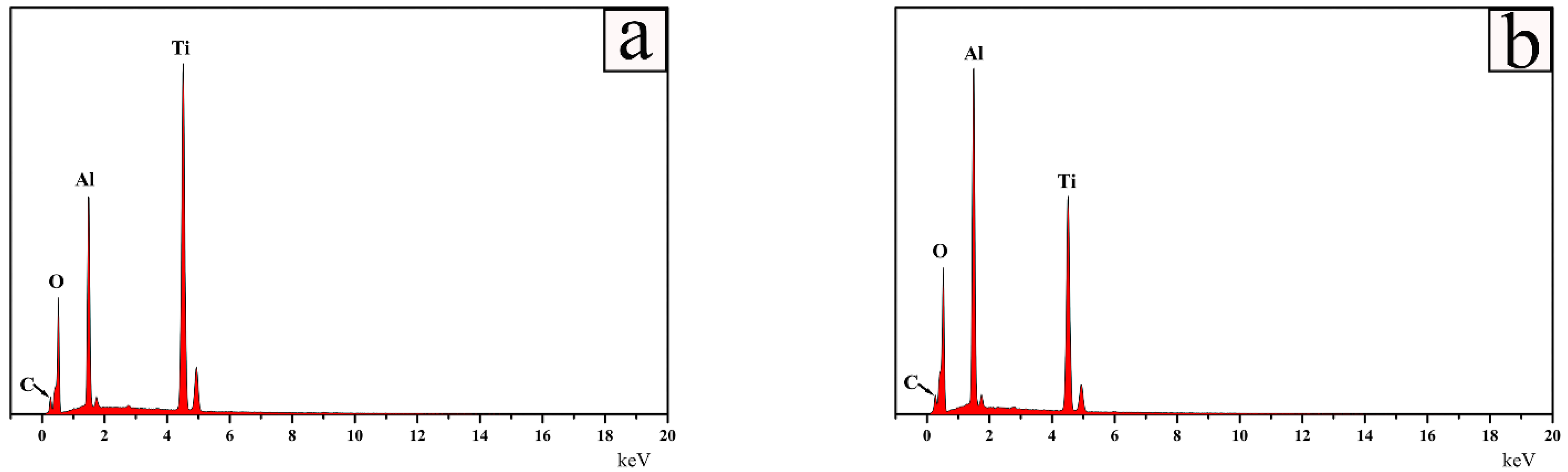


| Divisions | Heating | Quenching |
|---|---|---|
| Group 1 | In air | In air |
| Group 2 | In air | In water |
| Temperature | Phase Compositions |
|---|---|
| 25 °C | Ti2AlC |
| 480 °C | Ti2AlC |
| 920 °C | Ti2AlC, TiO2 |
| 1150 °C | Ti2AlC, TiO2, Al2O3, Al2TiO5, Ti8O15 |
| Temperature | Phase Compositions |
|---|---|
| 25 °C | Ti2AlC |
| 480 °C | Ti2AlC |
| 920 °C | Ti2AlC, TiO2 |
| 1150 °C | Ti2AlC, TiO2, Al2O3, Al2TiO5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, W.; Hu, B.; Fu, S.; Wan, D.; Bao, Y.; Feng, Q.; Grasso, S.; Hu, C. Ultra-Fast Thermal Shock Evaluation of Ti2AlC Ceramic. Materials 2022, 15, 6877. https://doi.org/10.3390/ma15196877
Ding W, Hu B, Fu S, Wan D, Bao Y, Feng Q, Grasso S, Hu C. Ultra-Fast Thermal Shock Evaluation of Ti2AlC Ceramic. Materials. 2022; 15(19):6877. https://doi.org/10.3390/ma15196877
Chicago/Turabian StyleDing, Wei, Baotong Hu, Shuai Fu, Detian Wan, Yiwang Bao, Qingguo Feng, Salvatore Grasso, and Chunfeng Hu. 2022. "Ultra-Fast Thermal Shock Evaluation of Ti2AlC Ceramic" Materials 15, no. 19: 6877. https://doi.org/10.3390/ma15196877
APA StyleDing, W., Hu, B., Fu, S., Wan, D., Bao, Y., Feng, Q., Grasso, S., & Hu, C. (2022). Ultra-Fast Thermal Shock Evaluation of Ti2AlC Ceramic. Materials, 15(19), 6877. https://doi.org/10.3390/ma15196877







