State-of-the-Art Review on Engineering Uses of Calcium Phosphate Compounds: An Eco-Friendly Approach for Soil Improvement
Abstract
1. Introduction
2. Existing Methods of Soil Improvement
2.1. Conventional Methods
2.2. Ecofriendly Methods
2.2.1. MICP
Advantages of MICP
- -
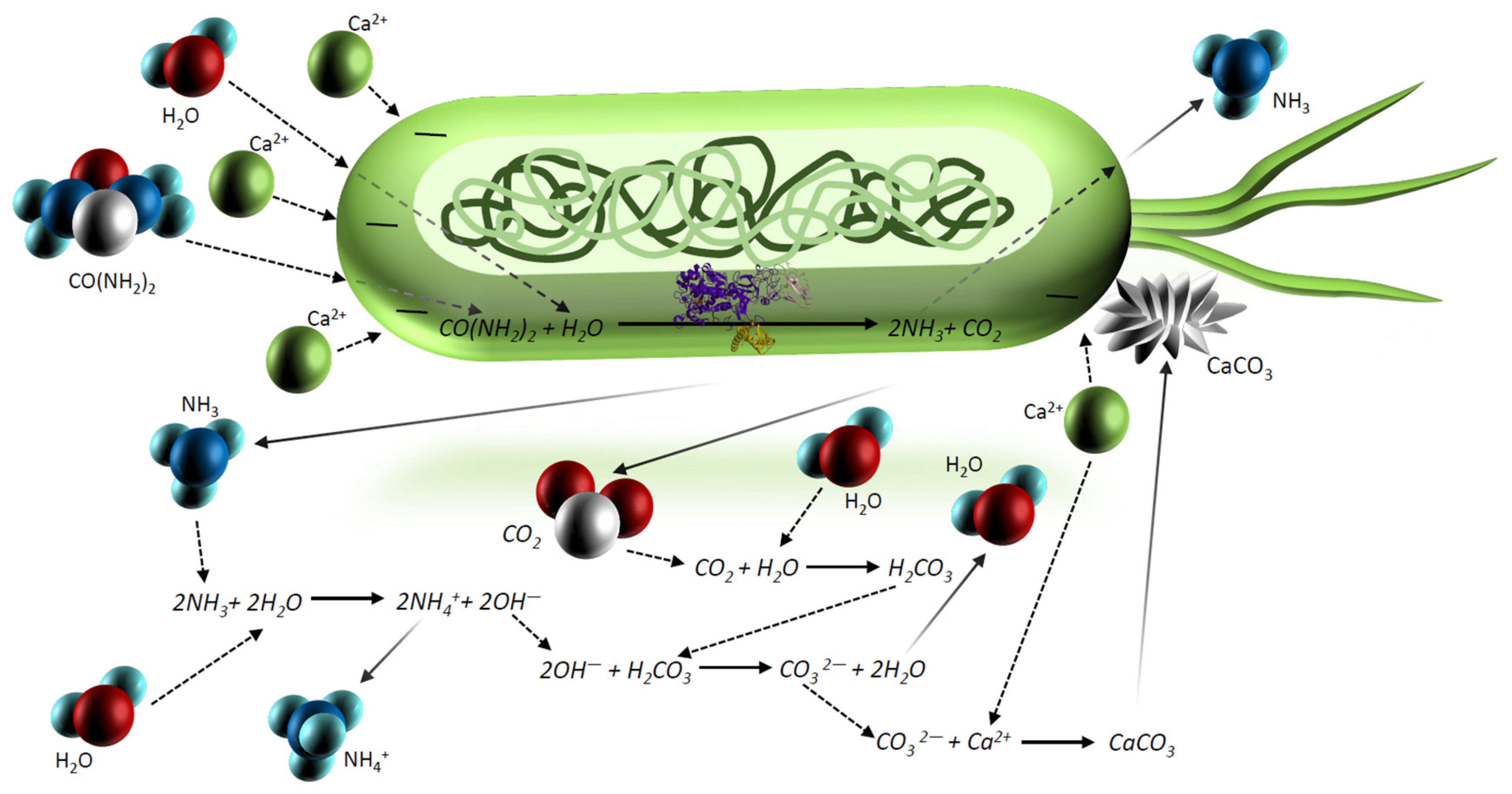
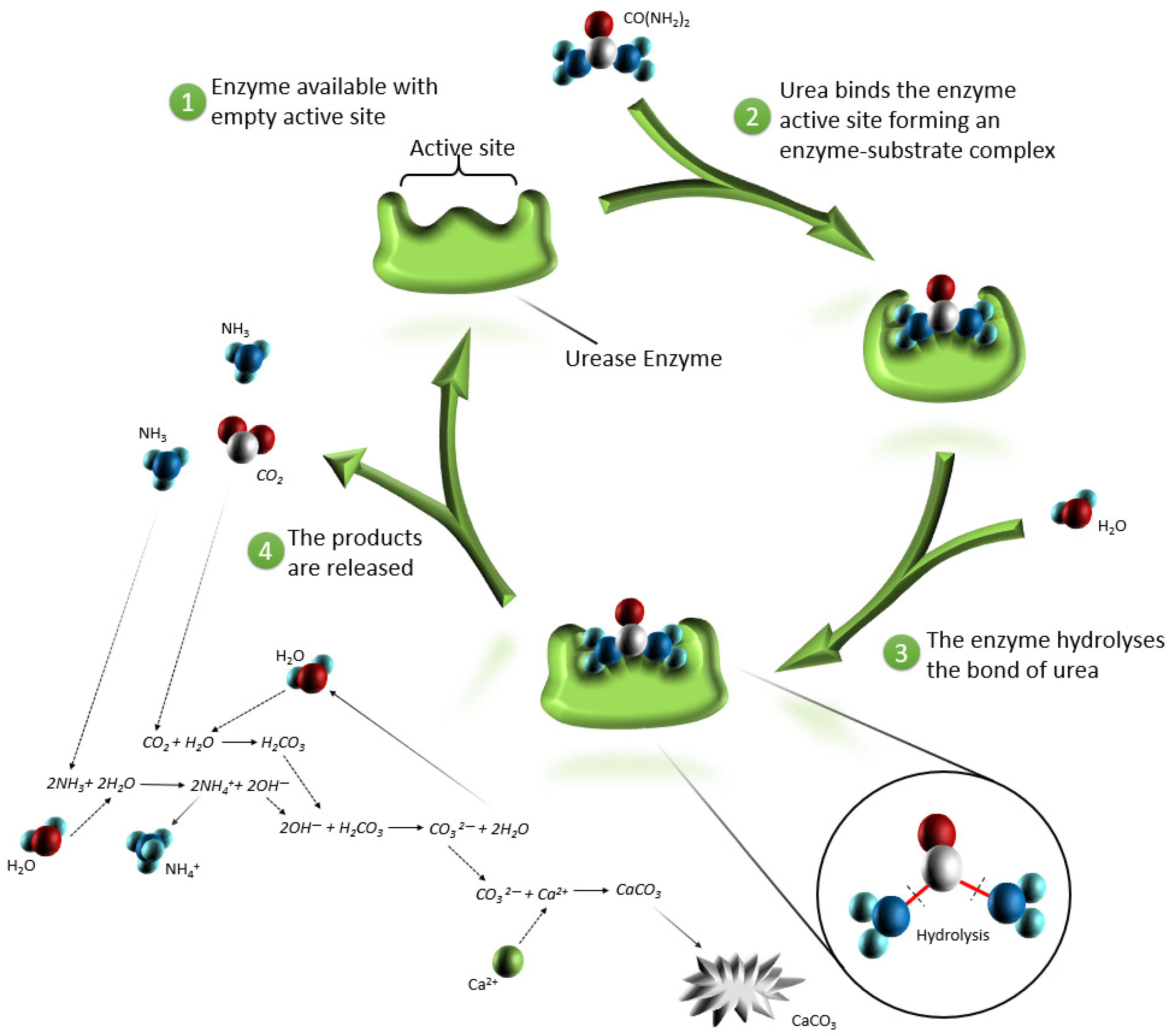
- MICP can solve a range of important geotechnical and environmental challenges such as soil reinforcement, reducing the risk of ground degradation to landslides, preventing liquefaction during earthquakes, stabilizing oil, the production of bio-concrete materials, heavy metal and radionuclide retention, sewage treatment, concrete repair, the modification of mortar, tunnel wall stabilization, enhance oil recoverability and reservoir profile control as well as water plugging, CO2 capture, and storage [4,19,22,23,27].
- -
- This method is superior to conventional methods since traditional methods are more complex in terms of construction and are time-consuming, energy-intensive, and low productive [28].
- -
- -
- By applying some species of bacteria to the soil, atmospheric CO2 levels can be reduced. These bacteria do not produce nitrogen-based by-products, making them more environmentally friendly [19]. The following bacteria have the potential to be used: sulfate-reducing bacteria increases the transformation rate of CO2 into solid minerals [30], cyanobacteria during MICP absorb CO2 from the air and utilize it to precipitate CaCO3 [31], and bacillus mucilaginous produces carbonic anhydrase, which can remove CO2 from the air to precipitate CaCO3 [32].
- -
- Unlike conventional methods, MICP leaves the soil structure unaffected throughout the entire period of treatment [23].
Limitations of MICP
- -
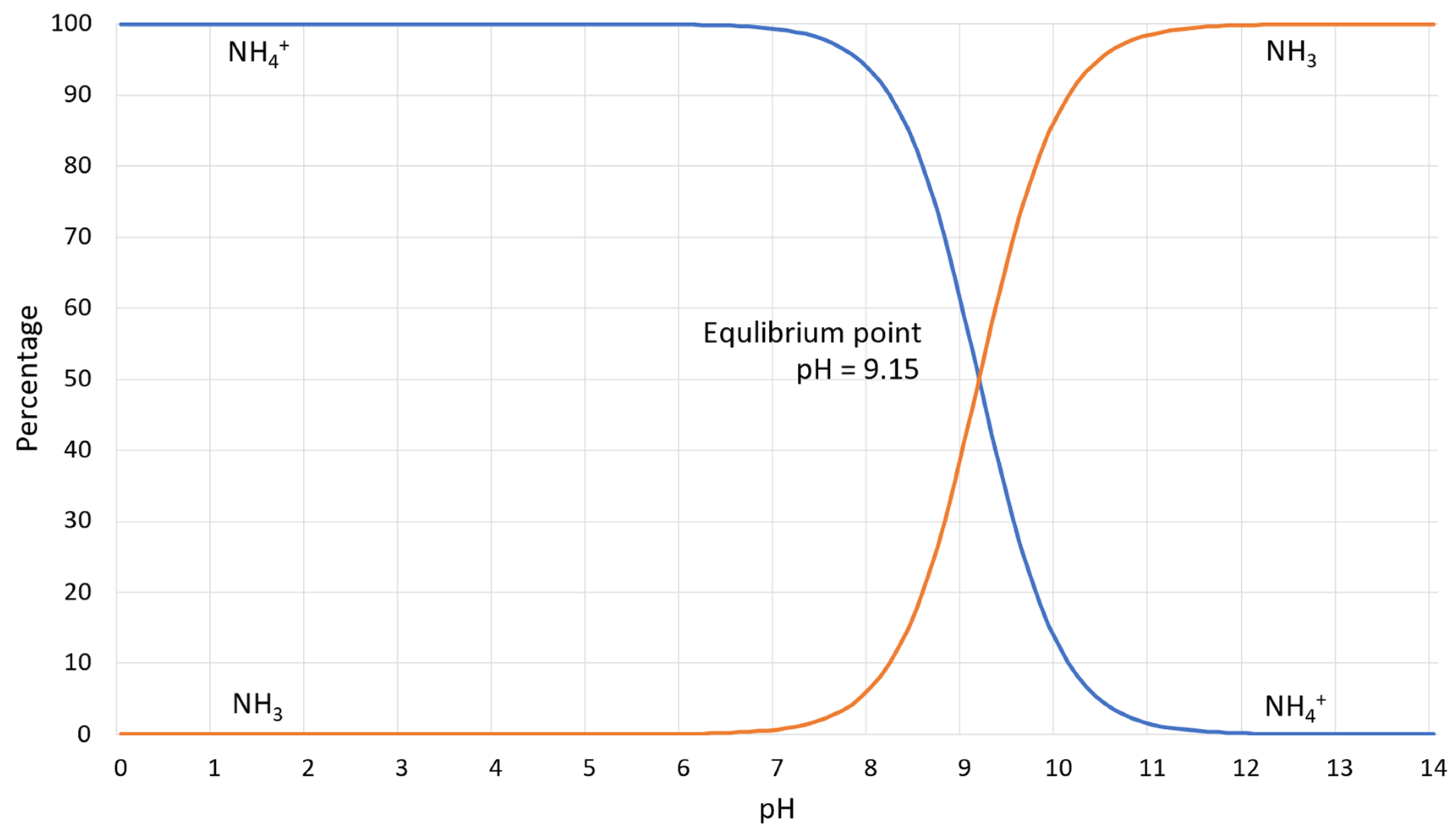
- Ammonium and ammonia produced by the enzymatic reaction are dangerous and harmful substances, and in large concentrations, causes toxic effects on human health and has impacts on the flora and deposition of nitrogen in the atmosphere [12,21,24]. Figure 3 illustrates the percentage dependence of free ammonia and ionized ammonia in solution when the pH is changed from 0 to 14 at 25 °C. According to Figure 3, under pH 6, almost 99% of all ammonia remains in ionized form, whereas after pH 7, it rapidly converts to free ammonia and contaminates the environment. At pH 7.15, the percentage of ammonia passes the 1% level, and increases rapidly after pH 8, reaching the equilibrium point of 50% at pH 9.15. At pH 11.15, the percentage of ammonium passes the 99% level and the increase slows down considerably.
- -
- The reaction pathway is slower and more complicated than in the case of chemicals [21].
- -
- -
- -
2.2.2. EICP
Advantages of EICP
- -
- -
- The UCS of soils treated with EICP can reach 6.5 MPa [16].
- -
- EICP requires less monitoring than MICP and is less energy consuming [41].
- -
- Urease extracted from plants can be a cost-effective alternative to chemical purified urease [34].
- -
- In comparison to the MICP method, the EICP method is biologically safer since it does not involve bacteria [48].
Limitation of EICP
- -
- The cost of EICP is prohibitive because 57–98% of the cost of the treatment solution comes from the enzyme urease [49]. The price of urease from Canavalia ensiformis (Jack bean) is 12.8 US $/g with a urease activity of 1 U/mg [50]. According to our approximate calculations, the use of urease in the open field will cost about 100,000 to 200,000 $US/m3.
- -
- -
- Using the enzyme instead of bacteria results in the removal of binding points, potentially reducing the efficacy of the method and the strength [16].
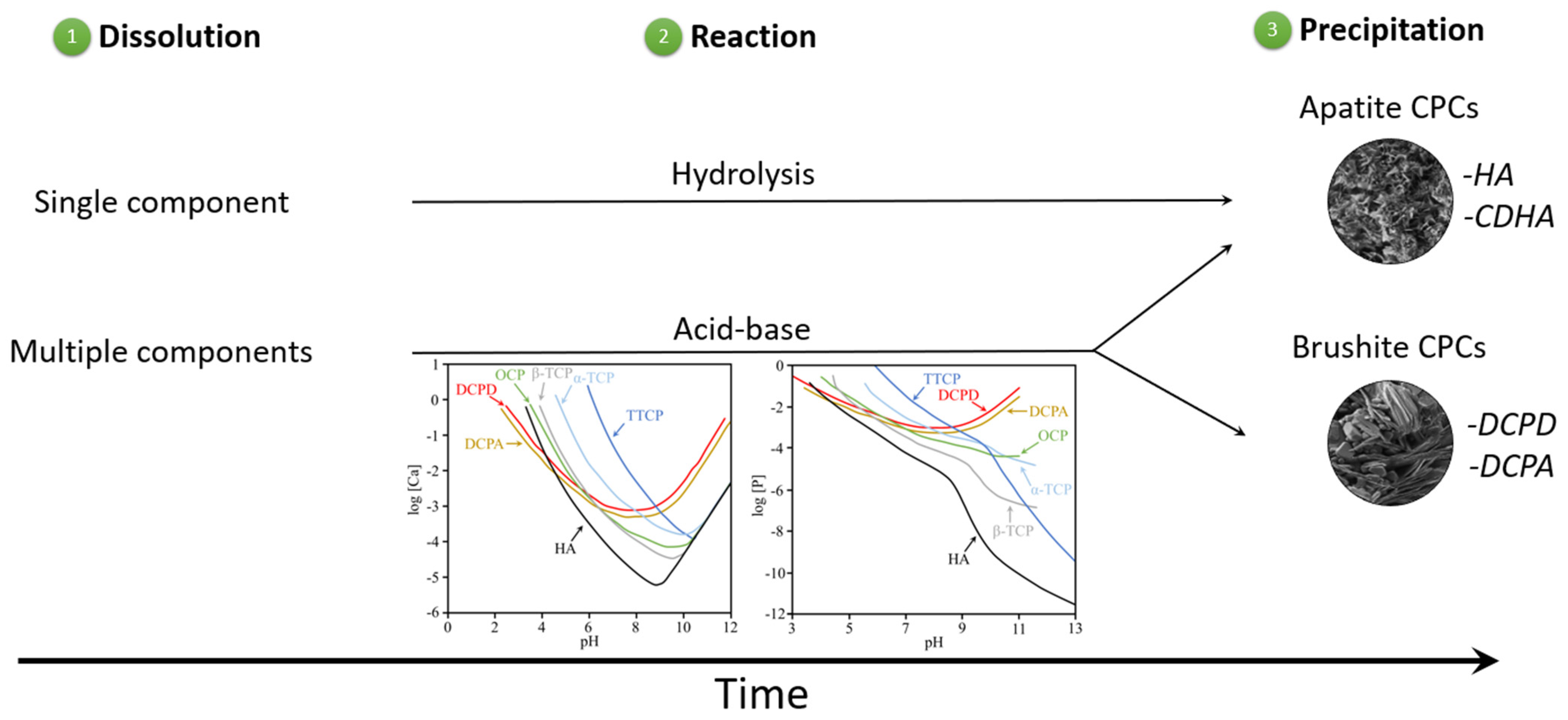
3. Calcium Phosphate Compounds
3.1. Mechanisms of Soil Improvements Using CPCs
- i.
- Reaction between calcium phosphate compounds alone
- ii.
- Reaction between calcium and carboxylic acid
| Precipitation Source | Ca and P Source | Soil Type | Addition | Chemical Concentration | Treatment Duration (Days) | Precipitation Type | Crystal Morphology | UCS (kPa) | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|
| Microbially mediated reaction between calcium phosphate compounds | ||||||||||
| Acidotolerant urease-producing bacteria (Staphylococcus saprophyticus) | Feed bone meal | Cracked stone | - | 1:1 (Ca/Urea) | 2 | Hydroxyapatite | Rod-like and plate-like microparticles | ND | [68] | |
| Dimorphic phytase-active (Arxula adeninivorans) | Calcium phytate | Glass beads | - | - | 3 | Monetite, whitlockite and hydroxyapatite | Needle-like crystals | ND | [69] | |
| Soil-derived bacteria | Ca2+ and PO43− | Alluvial topsoil | - | 1:1 (Ca2+/PO43−) | 5 | Hydroxyapatite and calcite | Bacteria-like hydroxyapatite and rhombohedral calcite | ND | [70] | |
| Enzymatically mediated reaction between calcium phosphate compounds | ||||||||||
| Acid urease (Nagapshin) | Bone meal powder (Cows) | Toyoura sand | - | 0.25:1 (Ca/Urea) | 16 | Brushite | Amorphous-like | 1620 | [71] | |
| Phytase enzyme | Sodium glycerophosphate (SGP) | Lead-zinc tailings pond sample | Mg2+ | 1.5 M SGP | 3 | Newberyite and lead phosphate | ND | 2700 | [73] | |
| Enzymatically mediated reaction between calcium phosphate compounds | ||||||||||
| Urease (watermelon seeds (Citrullus vulgaris) extract) | Chemicals (DPP and CA) | Toyoura sand | - | 1.5:0.75 M (DAP:CA) + urease (solid—liquid ratio of 0.005) | 28 | ND | Specific crystal structure could not be identified | 125.6 | [72] | |
| Chemical reaction between calcium phosphate compounds | ||||||||||
| Chemicals (diammonium phosphate (DAP) and calcium acetate (CA)) | Toyoura sand | 10% of tricalcium phosphate (TCP) powder | 1.5:0.75 (DAP:CA) | 28 | ND | Whisker-like crystal | 261.4 | [74,75] | ||
| Chemicals (dipotassium phosphate (DPP) and CA) | Toyoura sand | 10% of scallop shell (SS) powder | 1.2 M: 0.6 M (DPP:CA) | 56 | ND | Not clearly identify a crystal formation among sand particles | 156.9 | [76,77] | ||
| Chemicals (DAP and CA) | Toyoura sand | Phosphate powders | 10% of tricalcium phosphate (TCP) powder | 1.5:0.75 M (DAP:CA) | 28 | ND | Whisker-like crystal | 250 | [78] | |
| 1% magnesium phosphate (MgP) powder | 14 | Numerous 10-μm-long crystals | 75 | |||||||
| Carbonate powders | 5% calcium carbonate (CC) powder | 56 | Unified structures of sand particles and CPC precipitation | 250 | ||||||
| 1% magnesium carbonate (MgC) powder | 28 | Numerous 10-μm-long crystals without unification with sand particles | 110 | |||||||
| Chemical reaction between calcium phosphate compounds | ||||||||||
| Chemicals (DAP and CA) | Toyoura sand | - | 1.5:0.75 M (DAP:CA) | 14 | Hydroxyapatite | Whisker-like crystal | 63.5 | [79] | ||
| Chemicals (DAP and calcium nitrate (CN)) | 1.0 M:0.5 M (DAP:CN) | 14 | Plate-like crystals | 20 | ||||||
| Chemicals (DAP and CA) | Toyoura sand | - | 1.5:0.75 M (DAP:CA) | 28 | Hydroxyapatite | Whisker-like crystal | 87.6 | [80] | ||
| Reaction between calcium and carboxylic acids | ||||||||||
| Chemicals (DAP and CA) | Toyoura sand | Extract from agricultural alkaline and acidic soil (source of microorganisms) and amino acid source (asparagine (Asn), glutamine (Gln) and glycine (Gly)) | 1.5:0.75 M (DA:CA) + 0.1 M amino acid | 28 | ND | Whisker-like crystal | 50–100 | [83] | ||
| Chemicals (DAP and CN) | 1.0 M:0.5 M (DAP: CN) + 0.1 M amino acid | Plate-like crystals | ||||||||
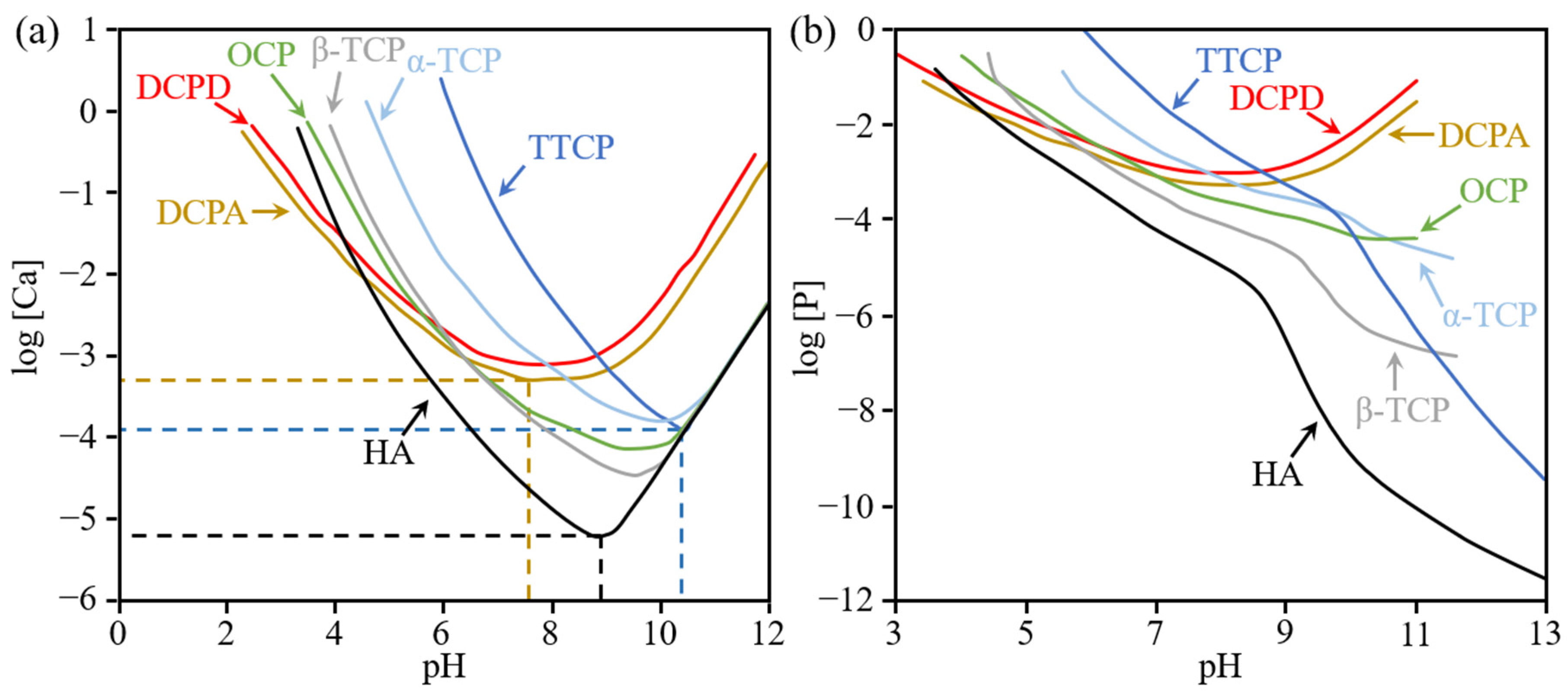
| Ca/P Ratio | Compound | Abbreviation | Formula | Solubility at 25 °C, g/L | pH Stability Range in Aquatic Solutions at 25 °C |
|---|---|---|---|---|---|
| 0.5 | Monocalcium phosphate monohydrate | MCPM | ~18 | 0.0–2.0 | |
| 0.5 | Monocalcium phosphate anhydrate | MCPA | ~17 | a | |
| 1.0 | Dicalcium phosphate dihydrate | DCPD | ~0.088 | 2.0–6.0 | |
| 1.0 | Dicalcium phosphate anhydrate | DCPA | ~0.048 | a | |
| 1.33 | Octacalcium phosphate | OCP | ~0.0081 | 5.5–7.0 | |
| 1.5 | α-tricalcium phosphate | α-TCP | ~0.0025 | b | |
| 1.5 | β-tricalcium phosphate | β-TCP | ~0.0005 | b | |
| 1.2–2.2 | Amorphous calcium phosphate | ACP | c | 5–12 | |
| 1.5–1.67 | Calcium-deficient hydroxyapatite | CDHA | ~0.0094 | 6.5–9.5 | |
| 1.67 | Hydroxyapatite | HA | ~0.0003 | 9.5–12 | |
| 2.0 | Tetracalcium phosphate | TTCP | ~0.0007 | b |
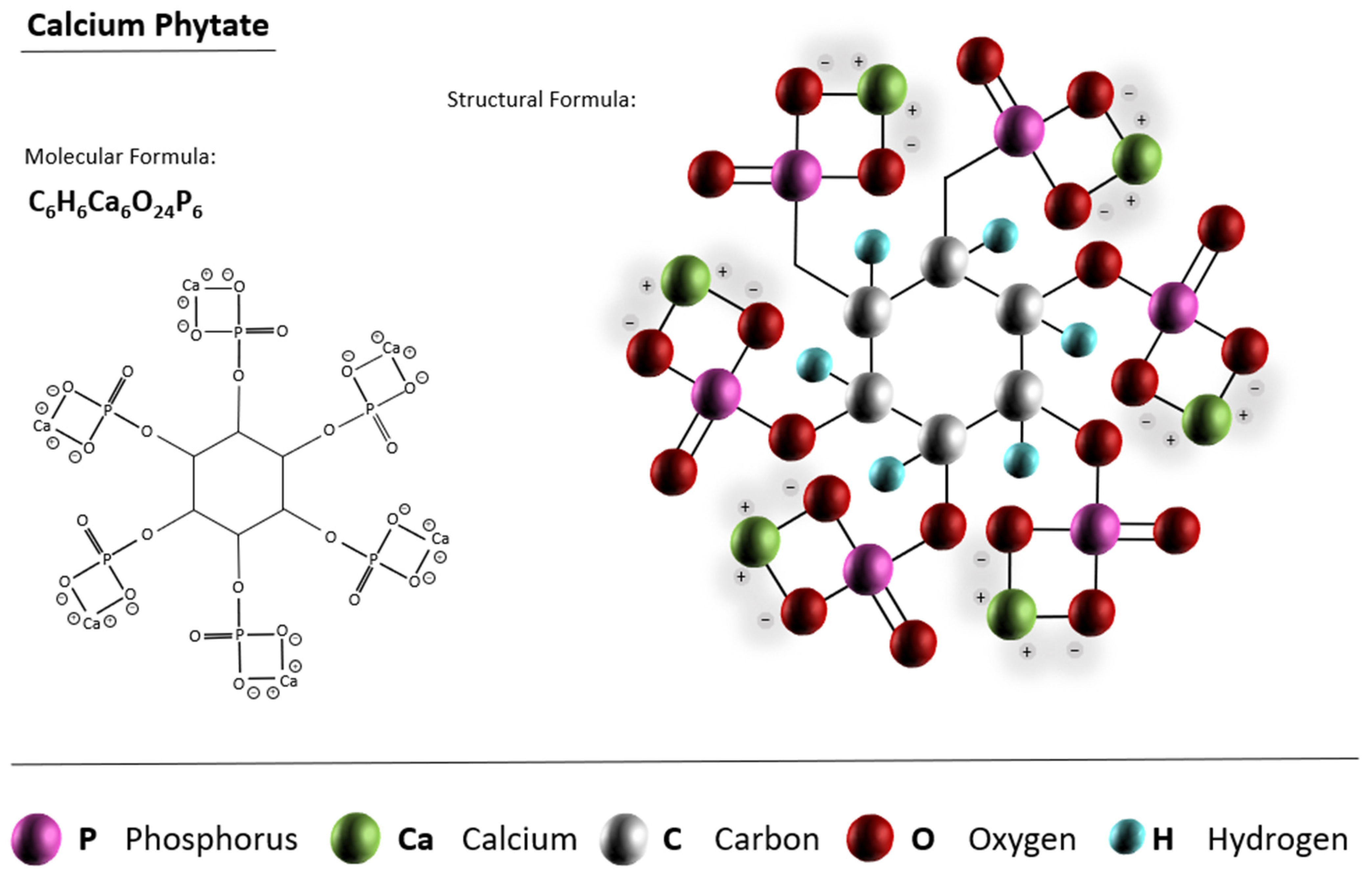
3.1.1. CPCs from Calcium Phytate
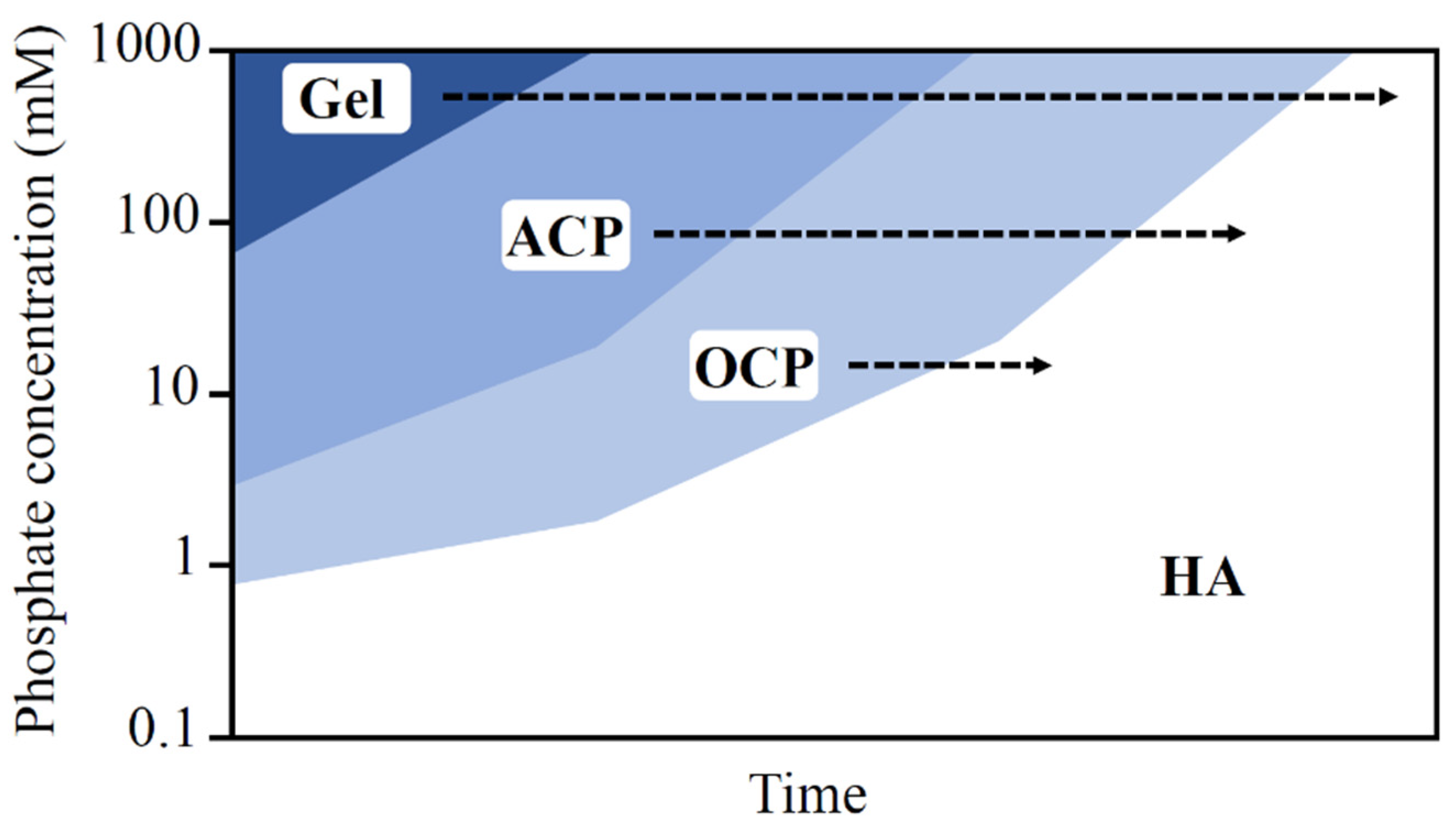
3.1.2. Microbially Induced CPCs Precipitation
- i.
- Monetatite (dicalcium phosphate anhydrate) precipitation by acidotolerant bacteria [27]
- ii.
- iii.
- Hydroxyapatite precipitation by acidic urease [68]
3.2. Prospects and Merits
4. Conclusions
5. Future Perspectives
- i.
- Alternative resources for calcium and phosphate need to be found. Most of the chemicals that are available on the market are aimed at the medical field. These are costly and are not suitable for soil improvement on a large scale. Animal bones are an excellent option, however, since the natural hydroxyapatite needs to be separated from the fatty inclusions, which requires acids, it is not an ideal candidate for use outside the laboratory.
- ii.
- For the deposition of CPCs, the atomic concentration of phosphorus and calcium in the system and the pH of the medium must be taken into account. In field applications, it is challenging to control the pH of the soil in such a small range to obtain the desired compound. Introducing bacteria may simplify this, although the problem of ammonium contamination in the environment will emerge. To overcome such an issue, it is necessary to investigate new methods of incorporating CPCs into the soil and control the parameters accurately.
- iii.
- In a long-term perspective, the low durability problem should be solved. The investigation of the effect of the application of CPCs to the soil could have an impact on the strength of the soil. Since this is a novel approach to ground reinforcement, the combination of the MICP and EICP research results over many decades and the combination of them for applying with CPCs can reveal its potential and make this material an ideal analogue for cement in the future.
- iv.
- In order to improve the existing soil stabilization methods using CPCs, the problem of ammonium must be addressed. Using bacteria or urease in this method, 6% ammonium in a gaseous form contaminates the atmosphere. Therefore, in future studies, this should be taken into account and correlated by adding different additives or by changing the parameters of the precipitation reactions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Devi, K.S.; Lakshimi, V.V.; Alakanandana, A. Impacts of cement industry on environment-an overview. Asia Pac. J. Res. 2017, 1, 156–161. [Google Scholar]
- Imbabi, M.S.; Carrigan, C.; McKenna, S. Trends and developments in green cement and concrete technology. Int. J. Sustain. Built Environ. 2012, 1, 194–216. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries 2022: U.S. Geological Survey; U.S. Geological Survey: Reston, VA, USA, 2022; pp. 44–45. [CrossRef]
- Song, M.; Ju, T.; Meng, Y.; Han, S.; Lin, L.; Jiang, J. A review on the applications of microbially induced calcium carbonate precipitation in solid waste treatment and soil remediation. Chemosphere 2021, 290, 133229. [Google Scholar] [CrossRef]
- Chen, C.; Xu, R.; Tong, D.; Qin, X.; Cheng, J.; Liu, J.; Zhang, Q. A striking growth of CO2 emissions from the global cement industry driven by new facilities in emerging countries. Environ. Res. Lett. 2022, 17, 044007. [Google Scholar] [CrossRef]
- Nie, S.; Zhou, J.; Yang, F.; Lan, M.; Li, J.; Zhang, Z.; Sanjayan, J.G. Analysis of theoretical carbon dioxide emissions from cement production: Methodology and application. J. Clean. Prod. 2022, 334, 130270. [Google Scholar] [CrossRef]
- Mishra, U.C.; Sarsaiya, S.; Gupta, A. A systematic review on the impact of cement industries on the natural environment. Environ. Sci. Pollut. Res. 2022, 29, 18440–18451. [Google Scholar] [CrossRef]
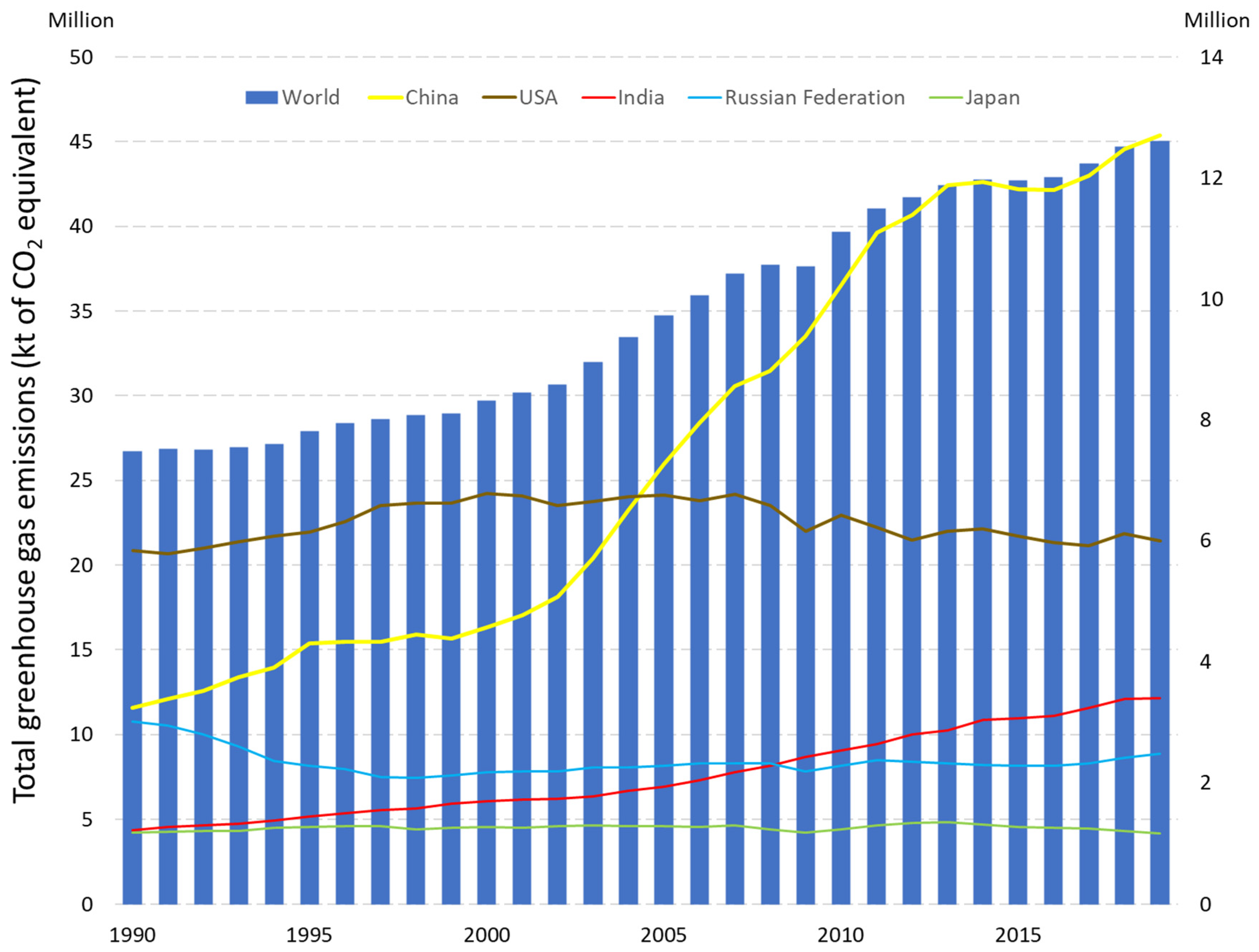
- Total Greenhouse Gas Emissions (kt of CO2 Equivalent). Available online: https://data.worldbank.org/indicator/EN.ATM.GHGT.KT.CE?locations=JP-US-1W (accessed on 17 August 2022).
- Ivanov, V.; Stabnikov, V.; Stabnikova, O.; Kawasaki, S. Environmental safety and biosafety in construction biotechnology. World J. Microbiol. Biotechnol. 2019, 35, 26. [Google Scholar] [CrossRef]
- Nicholson, P.G. Soil Improvement and Ground Modification Methods; Butterworth-Heinemann: Oxford, UK, 2014; pp. 3–11. ISBN 9780124080768. [Google Scholar]
- Assadi-Langroudi, A.; O’Kelly, B.C.; Barreto, D.; Cotecchia, F.; Dicks, H.; Ekinci, A.; van Paassen, L. Recent advances in nature-inspired solutions for ground engineering (NiSE). Int. J. Geosynth. Ground Eng. 2022, 8, 1–36. [Google Scholar] [CrossRef]
- Haouzi, F.Z.; Courcelles, B. Major applications of MICP sand treatment at multi-scale levels: A review. In Proceedings of the GeoEdmonton, Edmonton, AB, Canada, 23–26 September 2018. [Google Scholar]
- Gowthaman, S.; Nakashima, K.; Kawasaki, S. A state-of-the-art review on soil reinforcement technology using natural plant fiber materials: Past findings, present trends and future directions. Materials 2018, 11, 553. [Google Scholar] [CrossRef]
- Verma, H.; Ray, A.; Rai, R.; Gupta, T.; Mehta, N. Ground improvement using chemical methods: A review. Heliyon 2021, 7, e07678. [Google Scholar] [CrossRef]
- Han, J. Principles and Practice of Ground Improvement; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 245–246. ISBN 9781118259917. [Google Scholar]
- Arab, M.G.; Alsodi, R.; Almajed, A.; Yasuhara, H.; Zeiada, W.; Shahin, M.A. State-of-the-art review of enzyme-induced calcite precipitation (EICP) for ground improvement: Applications and prospects. Geosciences 2021, 11, 492. [Google Scholar] [CrossRef]
- National Research Council. Geological and Geotechnical Engineering in the New Millennium: Opportunities for Research and Technological Innovation; National Academies Press: Washington, DC, USA, 2006; pp. 20–90. ISBN 0309100097. [Google Scholar]
- Boquet, E.; Boronat, A.; Ramos-Cormenzana, A. Production of calcite (calcium carbonate) crystals by soil bacteria is a general phenomenon. Nature 1973, 246, 527–529. [Google Scholar] [CrossRef]
- Chen, J.; Liu, B.; Zhong, M.; Jing, C.; Guo, B. Research status and development of microbial induced calcium carbonate mineralization technology. PLoS ONE 2022, 17, e0271761. [Google Scholar] [CrossRef]
- Whiffin, V.S. Microbial CaCO3 Precipitation for the Production of Biocement. Ph.D. Thesis, Murdoch University, Perth, WA, Australia, September 2004. [Google Scholar]
- Han, L.; Li, J.; Xue, Q.; Chen, Z.; Zhou, Y.; Poon, C.S. Bacterial-induced mineralization (BIM) for soil solidification and heavy metal stabilization: A critical review. Sci. Total Environ. 2020, 746, 140967. [Google Scholar] [CrossRef]
- Omoregie, A.I.; Palombo, E.A.; Nissom, P.M. Bioprecipitation of calcium carbonate mediated by ureolysis: A review. Environ. Eng. Res. 2021, 26, 200379. [Google Scholar] [CrossRef]
- Terzis, D.; Laloui, L. A decade of progress and turning points in the understanding of bio-improved soils: A review. Geomech. Energy Environ. 2019, 19, 100116. [Google Scholar] [CrossRef]
- Dharmakeerthi, R.S.; Thenabadu, M.W. Urease activity in soils: A review. J. Natl. Sci. Found. Sri Lanka 1996, 24, 159–195. [Google Scholar] [CrossRef]
- Chen, L.; Song, Y.; Huang, J.; Lai, C.; Jiao, H.; Fang, H.; Song, X. Critical review of solidification of sandy soil by microbially induced carbonate precipitation (MICP). Crystals 2021, 11, 1439. [Google Scholar] [CrossRef]
- Amarakoon, G.G.N.N.; Kawasaki, S. Factors affecting sand solidification using MICP with Pararhodobacter sp. Mater. Trans. 2018, 59, 72–81. [Google Scholar] [CrossRef]
- Ivanov, V.; Chu, J.; Stabnikov, V. Basics of construction microbial biotechnology. In Biotechnologies and Biomimetics for Civil Engineering; Springer: Cham, Switzerland, 2015; pp. 21–56. [Google Scholar] [CrossRef]
- Liu, J.; Li, G.; Li, X.A. Geotechnical engineering properties of soils solidified by microbially induced CaCO3 precipitation (MICP). Adv. Civ. Eng. 2021, 2021, 6683930. [Google Scholar] [CrossRef]
- Choi, S.G.; Chang, I.; Lee, M.; Lee, J.H.; Han, J.T.; Kwon, T.H. Review on geotechnical engineering properties of sands treated by microbially induced calcium carbonate precipitation (MICP) and biopolymers. Constr. Build. Mater. 2020, 246, 118415. [Google Scholar] [CrossRef]
- Paul, V.G.; Wronkiewicz, D.J.; Mormile, M.R. Impact of elevated CO2 concentrations on carbonate mineral precipitation ability of sulfate-reducing bacteria and implications for CO2 sequestration. Appl. Geochem. 2017, 78, 250–271. [Google Scholar] [CrossRef]
- Obst, M.; Dynes, J.J.; Lawrence, J.R.; Swerhone, G.D.; Benzerara, K.; Karunakaran, C.; Hitchcock, A.P. Precipitation of amorphous CaCO3 (aragonite-like) by cyanobacteria: A STXM study of the influence of EPS on the nucleation process. Geochim. Cosmochim. Acta 2009, 73, 4180–4198. [Google Scholar] [CrossRef]
- Zhan, Q.; Yu, X.; Pan, Z.; Qian, C. Microbial-induced synthesis of calcite based on carbon dioxide capture and its cementing mechanism. J. Clean. Prod. 2021, 278, 123398. [Google Scholar] [CrossRef]
- Prajapati, S. Cation Exchange for Ammonia Removal from Wastewater. Master’s Thesis, Tampere University of Technology, Tampere, Finland, May 2014. [Google Scholar]
- Saif, A.; Cuccurullo, A.; Gallipoli, D.; Perlot, C.; Bruno, A.W. Advances in enzyme induced carbonate precipitation and application to soil improvement: A review. Materials 2022, 15, 950. [Google Scholar] [CrossRef] [PubMed]
- Gaol, C.L.; Ganzer, L.; Mukherjee, S.; Alkan, H. Investigation of clogging in porous media induced by microorganisms using a microfluidic application. Environ. Sci. Water Res. Technol. 2021, 7, 441–454. [Google Scholar] [CrossRef]
- Kavazanjian, E.; Hamdan, N. Enzyme induced carbonate precipitation (EICP) columns for ground improvement. In Proceedings of the IFCEE, San Antonio, TX, USA, 17–21 March 2015. [Google Scholar]
- Sumner, J.B. The isolation and crystallization of the enzyme urease. Preliminary paper. In A Source Book in Chemistry, 1900–1950; Harvard University Press: Cambridge, UK, 2013; pp. 322–327. [Google Scholar] [CrossRef]
- Nemati, M.; Voordouw, G. Modification of porous media permeability, using calcium carbonate produced enzymatically in situ. Enzym. Microb. Technol. 2003, 33, 635–642. [Google Scholar] [CrossRef]
- Yasuhara, H.; Hayashi, K.; Okamura, M. Evolution in mechanical and hydraulic properties of calcite-cemented sand mediated by biocatalyst. In Proceedings of the Geo-Frontiers 2011, Dallas, TX, USA, 13–16 March 2011. [Google Scholar]
- Šudomová, M.; Hassan, S.T. Gossypol from Gossypium spp. inhibits Helicobacter pylori clinical strains and urease enzyme activity: Bioactivity and safety assessments. Sci. Pharm. 2022, 90, 29. [Google Scholar] [CrossRef]
- Almajed, A.; Lateef, M.A.; Moghal, A.A.B.; Lemboye, K. State-of-the-art review of the applicability and challenges of microbial-induced calcite precipitation (MICP) and enzyme-induced calcite precipitation (EICP) techniques for geotechnical and geoenvironmental applications. Crystals 2021, 11, 370. [Google Scholar] [CrossRef]
- Ahenkorah, I.; Rahman, M.M.; Karim, M.R.; Beecham, S.; Saint, C. A review of enzyme induced carbonate precipitation (EICP): The role of enzyme kinetics. Sustain. Chem. 2021, 2, 92–114. [Google Scholar] [CrossRef]
- Mazzei, L.; Musiani, F.; Ciurli, S. The structure-based reaction mechanism of urease, a nickel dependent enzyme: Tale of a long debate. JBIC J. Biol. Inorg. Chem. 2020, 25, 829–845. [Google Scholar] [CrossRef] [PubMed]
- Dessy, A.; Abyor, N.; Hadi, H. An overview of biocement production from microalgae. Int. J. Sci. Eng. 2011, 2, 31–33. [Google Scholar]
- Ahenkorah, I.; Rahman, M.M.; Karim, M.R.; Beecham, S. Enzyme induced calcium carbonate precipitation and its engineering application: A systematic review and meta-analysis. Constr. Build. Mater. 2021, 308, 125000. [Google Scholar] [CrossRef]
- Putra, H.; Yasuhara, H.; Fauzan, M. Review of enzyme-induced calcite precipitation as a ground-improvement technique. Infrastructures 2020, 5, 66. [Google Scholar] [CrossRef]
- Xu, K.; Huang, M.; Zhen, J.; Xu, C.; Cui, M. Field implementation of enzyme-induced carbonate precipitation technology for reinforcing a bedding layer beneath an underground cable duct. J. Rock Mech. Geotech. Eng. 2022, in press. [Google Scholar] [CrossRef]
- Cui, M.J.; Lai, H.J.; Hoang, T.; Chu, J. One-phase-low-pH enzyme induced carbonate precipitation (EICP) method for soil improvement. Acta Geotech. 2021, 16, 481–489. [Google Scholar] [CrossRef]
- Almajed, A.A. Enzyme Induced Carbonate Precipitation (EICP) for Soil Improvement. Ph.D. Thesis, Arizona State University, Tempe, AZ, USA, August 2017. [Google Scholar]
- Urease Powder, 1units/MG 9002-13-5. Available online: https://www.sigmaaldrich.com/US/en/product/sigma/94280 (accessed on 17 August 2022).
- National Effluent Standards. Ministry of the Environment, Government of Japan. Available online: https://www.env.go.jp/en/water/wq/nes.html (accessed on 17 August 2022).
- Ivanov, V.; Stabnikov, V. Environmental safety of biotechnological materials and processes. In Bio-Based Materials and Biotechnologies for Eco-Efficient Construction; Woodhead Publishing: Cambridge, UK, 2020; pp. 359–375. [Google Scholar] [CrossRef]
- Yu, X.; Jiang, J. Mineralization and cementing properties of bio-carbonate cement, bio-phosphate cement, and bio-carbonate/phosphate cement: A review. Environ. Sci. Pollut. Res. 2018, 25, 21483–21497. [Google Scholar] [CrossRef]
- Yu, X.; Chu, J.; Yang, Y.; Qian, C. Reduction of ammonia production in the biocementation process for sand using a new biocement. J. Clean. Prod. 2021, 286, 124928. [Google Scholar] [CrossRef]
- Gowthaman, S.; Mohsenzadeh, A.; Nakashima, K.; Kawasaki, S. Removal of ammonium by-products from the effluent of bio-cementation system through struvite precipitation. Mater. Today Proc. 2022, 61, 243–249. [Google Scholar] [CrossRef]
- Putra, H.; Yasuhara, H.; Kinoshita, N. Applicability of natural zeolite for NH-forms removal in enzyme-mediated calcite precipitation technique. Geosciences 2017, 7, 61. [Google Scholar] [CrossRef]
- Su, F.; Yang, Y.; Qi, Y.; Zhang, H. Combining microbially induced calcite precipitation (MICP) with zeolite: A new technique to reduce ammonia emission and enhance soil treatment ability of MICP technology. J. Environ. Chem. Eng. 2022, 10, 107770. [Google Scholar] [CrossRef]
- Kim, S.I.; Heo, W.; Lee, S.J.; Kim, Y.J. Isolation and characterization of effective bacteria that reduce ammonia emission from livestock manure. Microorganisms 2021, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Qiu, J.; Wang, Y.; Gu, X. Calcium acetate as calcium source used to biocement for improving performance and reducing ammonia emission. J. Clean. Prod. 2022, 348, 131286. [Google Scholar] [CrossRef]
- Keykha, H.A.; Asadi, A. Solar powered electro-bio-stabilization of soil with ammonium pollution prevention system. Adv. Civ. Eng. Mater 2017, 6, 360–371. [Google Scholar] [CrossRef]
- Lee, M.; Gomez, M.G.; San Pablo, A.; Kolbus, C.M.; Graddy, C.M.; DeJong, J.T.; Nelson, D.C. Investigating ammonium by-product removal for ureolytic bio-cementation using meter-scale experiments. Sci. Rep. 2019, 9, 18313. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.C.; Eanes, E.D. Calcium phosphate cements. Monogr. Oral Sci. 2001, 18, 148–163. [Google Scholar] [PubMed]
- Chow, L.C. Next generation calcium phosphate-based biomaterials. Dent. Mater. J. 2009, 28, 1–10. [Google Scholar] [CrossRef]
- Schmitz, J.P.; Hollinger, J.O.; Milam, S.B. Reconstruction of bone using calcium phosphate bone cements: A critical review. J. Oral Maxillofac. Surg. 1999, 57, 1122–1126. [Google Scholar] [CrossRef]
- De Aza, P.N.; Guitian, F.; Santos, C.; De Aza, S.; Cusco, R.; Artus, L. Vibrational properties of calcium phosphate compounds. 2. Comparison between hydroxyapatite and β-tricalcium phosphate. Chem. Mater. 1997, 9, 916–922. [Google Scholar] [CrossRef]
- Al-Sanabani, J.S.; Madfa, A.A.; Al-Sanabani, F.A. Application of calcium phosphate materials in dentistry. Int. J. Biomater. 2013, 2013, 876132. [Google Scholar] [CrossRef]
- Lodoso-Torrecilla, I.; van den Beucken, J.J.; Jansen, J.A. Calcium phosphate cements: Optimization toward biodegradability. Acta Biomater. 2021, 119, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, V.; Stabnikov, V.; Kawasaki, S. Ecofriendly calcium phosphate and calcium bicarbonate biogrouts. J. Clean. Prod. 2019, 218, 328–334. [Google Scholar] [CrossRef]
- Roeselers, G.; Van Loosdrecht, M.C.M. Microbial phytase-induced calcium-phosphate precipitation—A potential soil stabilization method. Folia Microbiol. 2010, 55, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Schmittner, K.E.; Giresse, P. Micro-environmental controls on biomineralization: Superficial processes of apatite and calcite precipitation in Quaternary soils, Roussillon, France. Sedimentology 1999, 46, 463–476. [Google Scholar] [CrossRef]
- Gowthaman, S.; Yamamoto, M.; Nakashima, K.; Ivanov, V.; Kawasaki, S. Calcium phosphate biocement using bone meal and acid urease: An eco-friendly approach for soil improvement. J. Clean. Prod. 2021, 319, 128782. [Google Scholar] [CrossRef]
- Dilrukshi, R.A.N.; Watanabe, J.; Kawasaki, S. Strengthening of sand cemented with calcium phosphate com-pounds using plant-derived urease. GEOMATE J. 2016, 11, 2461–2467. [Google Scholar]
- Han, L.J.; Li, J.S.; Xue, Q.; Guo, M.Z.; Wang, P.; Poon, C.S. Enzymatically induced phosphate precipitation (EIPP) for stabilization/solidification (S/S) treatment of heavy metal tailings. Constr. Build. Mater. 2022, 314, 125577. [Google Scholar] [CrossRef]
- Kawasaki, S.; Akiyama, M. Effect of addition of phosphate powder on unconfined compressive strength of sand cemented with calcium phosphate compound. Mater. Trans. 2013, 54, 2079–2084. [Google Scholar] [CrossRef]
- Kawasaki, S.; Akiyama, M. Soil reinforcement using calcium phosphate compounds. ASEAN Eng. J. 2016, 5, 5–13. [Google Scholar] [CrossRef]
- Amarakoon, G.G.N.N.; Koreeda, T.; Kawasaki, S. Effect on unconfined compressive strength of sand test pieces cemented with calcium phosphate compound. GEOMATE J. 2014, 7, 1070–1075. [Google Scholar] [CrossRef]
- Amarakoon, G.G.N.N.; Koreeda, T.; Kawasaki, S. Improvement in the unconfined compressive strength of sand test pieces cemented with calcium phosphate compound by addition of calcium carbonate powders. Mater. Trans. 2014, 55, 1391–1399. [Google Scholar] [CrossRef]
- Kawasaki, S.; Akiyama, M. Enhancement of unconfined compressive strength of sand test pieces cemented with calcium phosphate compound by addition of various powders. Soils Found. 2013, 53, 966–976. [Google Scholar] [CrossRef]
- Akiyama, M.; Kawasaki, S. Novel grout material comprised of calcium phosphate compounds: In vitro evaluation of crystal precipitation and strength reinforcement. Eng. Geol. 2012, 125, 119–128. [Google Scholar] [CrossRef]
- Kawasaki, S.; Akiyama, M. Unique grout material composed of calcium phosphate compounds. GEOMATE J. 2013, 4, 429–435. [Google Scholar] [CrossRef]
- Barralet, J.E.; Tremayne, M.; Lilley, K.J.; Gbureck, U. Modification of calcium phosphate cement with α-hydroxy acids and their salts. Chem. Mater. 2005, 17, 1313–1319. [Google Scholar] [CrossRef]
- Tamimi, F.; Sheikh, Z.; Barralet, J. Dicalcium phosphate cements: Brushite and monetite. Acta Biomater. 2012, 8, 474–487. [Google Scholar] [CrossRef]
- Akiyama, M.; Kawasaki, S. Microbially mediated sand solidification using calcium phosphate compounds. Eng. Geol. 2012, 137, 29–39. [Google Scholar] [CrossRef]
- Zhang, F.; Jin, Y.; Ye, B. A try to give a unified description of Toyoura sand. Soils Found. 2010, 50, 679–693. [Google Scholar] [CrossRef]
- Gil, F.J.; Ginebra, M.P.; Driessens, F.C.M.; Planell, J.A.; Best, S.M. Calcium phosphate bone cements for clinical applications. Part I: Solution chemistry. J. Mater. Sci. Mater. Med. 1999, 10, 169–176. [Google Scholar] [CrossRef]
- Gil, F.J.; Ginebra, M.P.; Driessens, F.C.M.; Planell, J.A.; Best, S.M. Calcium phosphate bone cements for clinical applications. Part II: Precipitate formation during setting reactions. J. Mater. Sci. Mater. Med. 1999, 10, 177–183. [Google Scholar] [CrossRef]
- Amjad, Z. Calcium Phosphates in Biological and Industrial Systems; Kluwer Academic Publishers: Boston, MA, USA, 1998; pp. 1–15. ISBN 9781461375210. [Google Scholar]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef] [PubMed]
- Ambard, A.J.; Mueninghoff, L. Calcium phosphate cement: Review of mechanical and biological properties. J. Prosthodont. 2006, 15, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Moutin, T.; Gal, J.Y.; El Halouani, H.; Picot, B.; Bontoux, J.J.W.R. Decrease of phosphate concentration in a high rate pond by precipitation of calcium phosphate: Theoretical and experimental results. Water Res. 1992, 26, 1445–1450. [Google Scholar] [CrossRef]
- Dilrukshi, R.A.N.; Kawasaki, S. Plant-derived urease induced sand cementation used in geotechnical engineering applications. In Proceedings of the International Conference on Geomechanics, Geo-Energy and Geo-Resources, Melbourne, VI, Australia, 28–29 September 2016. [Google Scholar]
- Posner, A.S.; Betts, F. Synthetic amorphous calcium phosphate and its relation to bone mineral structure. Acc. Chem. Res. 1975, 8, 273–281. [Google Scholar] [CrossRef]
- Takagi, S.; Chow, L.C.; Ishikawa, K. Formation of hydroxyapatite in new calcium phosphate cements. Biomaterials 1998, 19, 1593–1599. [Google Scholar] [CrossRef]
- Tung, M.S. Calcium phosphates: Structure, composition, solubility, and stability. In Calcium Phosphates in Bio-Logical and Industrial Systems; Springer: Boston, MA, USA, 1998; pp. 1–19. [Google Scholar]
- Sharma, N.; Angural, S.; Rana, M.; Puri, N.; Kondepudi, K.K.; Gupta, N. Phytase producing lactic acid bacteria: Cell factories for enhancing micronutrient bioavailability of phytate rich foods. Trends Food Sci. Technol. 2020, 96, 1–12. [Google Scholar] [CrossRef]
- Hussain, S.M.; Hanif, S.; Sharif, A.; Bashir, F.; Iqbal, H. Unrevealing the sources and catalytic functions of phytase with multipurpose characteristics. Catal. Lett. 2022, 152, 1358–1371. [Google Scholar] [CrossRef]
- Maga, J.A. Phytate: Its chemistry, occurrence, food interactions, nutritional significance, and methods of analysis. J. Agric. Food Chem. 1982, 30, 1–9. [Google Scholar] [CrossRef]
- Kumar, V.; Sinha, A.K. General aspects of phytases. In Enzymes in Human and Animal Nutrition; Academic Press: London, UK, 2018; pp. 53–72. [Google Scholar] [CrossRef]
- Mullaney, E.J.; Daly, C.B.; Ullah, A.H. Advances in phytase research. Adv. Appl. Microbiol. 2000, 47, 157–199. [Google Scholar] [CrossRef]
- Liu, X.; Han, R.; Cao, Y.; Turner, B.L.; Ma, L.Q. Enhancing phytate availability in soils and phytate-p acquisition by plants: A review. Environ. Sci. Technol. 2022, 56, 9196–9219. [Google Scholar] [CrossRef]
- Stabnikov, V.; Ivanov, V.; Chu, J. Construction Biotechnology: A new area of biotechnological research and applications. World J. Microbiol. Biotechnol. 2015, 31, 1303–1314. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, C.; Liu, Q.; Xu, C.; Xiong, Y.L. Ca2+-selective electrode: A simple method to measure the phytase-aided release of bound calcium in soymilk. J. Food Compos. Anal. 2015, 39, 43–47. [Google Scholar] [CrossRef]
- Sano, K.; Fukuhara, H.; Nakamura, Y. Phytase of the yeast Arxula adeninivorans. Biotechnol. Lett. 1999, 21, 33–38. [Google Scholar] [CrossRef]
- Romano, N.; Kumar, V. Phytase in animal feed. In Enzymes in Human and Animal Nutrition; Academic Press: London, UK, 2018; pp. 73–88. [Google Scholar] [CrossRef]
- Sattari, S.Z.; Bouwman, A.F.; Giller, K.E.; van Ittersum, M.K. Residual soil phosphorus as the missing piece in the global phosphorus crisis puzzle. Proc. Natl. Acad. Sci. USA 2012, 109, 6348–6353. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ruiz-Agudo, E.; Putnis, C.V.; Menneken, M.; Putnis, A. Kinetics of calcium phosphate nucleation and growth on calcite: Implications for predicting the fate of dissolved phosphate species in alkaline soils. Environ. Sci. Technol. 2012, 46, 834–842. [Google Scholar] [CrossRef]
- Wahid, F.; Fahad, S.; Danish, S.; Adnan, M.; Yue, Z.; Saud, S.; Datta, R. Sustainable management with mycorrhizae and phosphate solubilizing bacteria for enhanced phosphorus uptake in calcareous soils. Agriculture 2020, 10, 334. [Google Scholar] [CrossRef]
- Dilrukshi, R.A.N.; Kawasaki, S. Effective Use of Plant-Derived Urease in the Field of Geoenvironmental/Geotechnical Engineering. J. Civ. Environ. Eng. 2016, 6, 1. [Google Scholar]
- Lei, Y.; Song, B.; van der Weijden, R.D.; Saakes, M.; Buisman, C.J. Electrochemical induced calcium phosphate precipitation: Importance of local pH. Environ. Sci. Technol. 2017, 51, 11156–11164. [Google Scholar] [CrossRef]


| Compound | Hydrolysis |
|---|---|
| Monocalcium phosphate | |
| Dicalcium phosphate | |
| Octacalcium phosphate | |
| Tricalcium phosphate | |
| Hydroxyapatite | |
| Tetracalcium phosphate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avramenko, M.; Nakashima, K.; Kawasaki, S. State-of-the-Art Review on Engineering Uses of Calcium Phosphate Compounds: An Eco-Friendly Approach for Soil Improvement. Materials 2022, 15, 6878. https://doi.org/10.3390/ma15196878
Avramenko M, Nakashima K, Kawasaki S. State-of-the-Art Review on Engineering Uses of Calcium Phosphate Compounds: An Eco-Friendly Approach for Soil Improvement. Materials. 2022; 15(19):6878. https://doi.org/10.3390/ma15196878
Chicago/Turabian StyleAvramenko, Maksym, Kazunori Nakashima, and Satoru Kawasaki. 2022. "State-of-the-Art Review on Engineering Uses of Calcium Phosphate Compounds: An Eco-Friendly Approach for Soil Improvement" Materials 15, no. 19: 6878. https://doi.org/10.3390/ma15196878
APA StyleAvramenko, M., Nakashima, K., & Kawasaki, S. (2022). State-of-the-Art Review on Engineering Uses of Calcium Phosphate Compounds: An Eco-Friendly Approach for Soil Improvement. Materials, 15(19), 6878. https://doi.org/10.3390/ma15196878







