Flexible Perfluoropolyethers-Functionalized CNTs-Based UHMWPE Composites: A Study on Hydrogen Evolution, Conductivity and Thermal Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemical Treatment of Carbonaceous Materials
2.3. Preparation of UHMWPE-Based Composites
2.4. Characterization
3. Results and Discussion
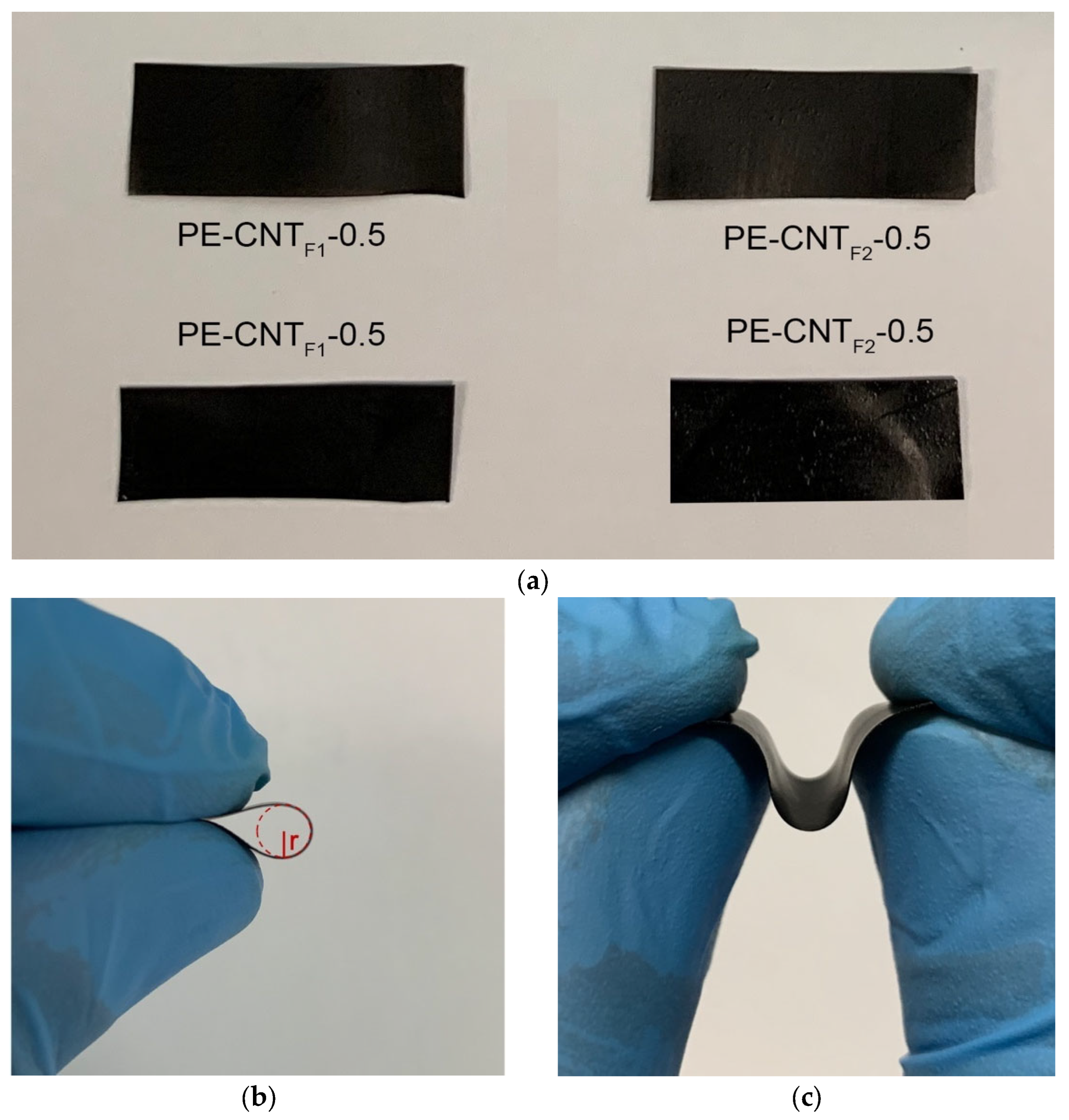
3.1. Preparation of Flexible PFPE-Functionalized CNTs-Based UHMWPE Composites
3.2. Electrical Resistivity
3.3. Morphology SEM and TEM
3.4. Hydrogen Evolution Reaction (HER): Voltammetry and Electrochemical AFM (EC-AFM)
3.5. Thermogravimetric Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiu, H.; Yang, J. Chapter 2—Structure and Properties of Carbon Nanotubes. In Industrial Applications of Carbon Nanotubes Chen (Micro and Nano Technologies); Peng, H., Li, Q., Tao, B.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 47–69. [Google Scholar]
- Tarfaoui, M.; Lafdi, K.; EL Moumen, A. Mechanical properties of carbon nanotubes based polymer composites. Compos. Part B Eng. 2016, 103, 113–121. [Google Scholar] [CrossRef]
- Shin, D.; Ko, Y.; Cho, J. Layer-by-layer assembled (high-energy carbon nanotube/conductive carbon nanotube)n nanocomposites for high volumetric capacitance supercapacitor electrodes. RSC Adv. 2016, 6, 21844–21853. [Google Scholar] [CrossRef]
- Liu, L.; Niu, Z.; Chen, J. Flexible supercapacitors based on carbon nanotubes. Chin. Chem. Lett. 2018, 29, 571–581. [Google Scholar] [CrossRef]
- Licht, S.; Douglas, A.; Ren, J.; Carter, R.; Lefler, M.; Pint, C.L. Carbon Nanotubes Produced from Ambient Carbon Dioxide for Environmentally Sustainable Lithium-Ion and Sodium-Ion Battery Anodes. ACS Central Sci. 2016, 2, 162–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arai, S.; Fukuoka, R. A carbon nanotube-reinforced noble tin anode structure for lithium-ion batteries. J. Appl. Electrochem. 2016, 46, 331–338. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Lei, H.; Cao, R.; Zhang, M. Cobalt Corrole on Carbon Nanotube as a Synergistic Catalyst for Oxygen Reduction Reaction in Acid Media. Electrochim. Acta 2015, 171, 81–88. [Google Scholar] [CrossRef]
- Padmavathi, R.; Devi, A.S.; Saranya, N.; Gnanasundaram, P.; Sangeetha, D. Synthesis and characterization of Pt supported on multiwalled carbon nanotubes for improved catalytic performance in fuel cell applications. J. Porous Mater. 2015, 22, 647–658. [Google Scholar] [CrossRef]
- Subianto, S.; Pica, M.; Casciola, M.; Cojocaru, P.; Merlo, L.; Hards, G.; Jones, D. Physical and chemical modification routes leading to improved mechanical properties of perfluorosulfonic acid membranes for PEM fuel cells. J. Power Sources 2013, 233, 216–230. [Google Scholar] [CrossRef]
- Xiao, Y.; Lin, J.-Y.; Wu, J.; Tai, S.-Y.; Yue, G.; Lin, T.-W. Dye-sensitized solar cells with high-performance polyaniline/multi-wall carbon nanotube counter electrodes electropolymerized by a pulse potentiostatic technique. J. Power Sources 2013, 233, 320–325. [Google Scholar] [CrossRef]
- Jeon, I.; Matsuo, Y.; Maruyama, S. Single-Walled Carbon Nanotubes in Solar Cells BT—Single-Walled Carbon Nanotubes: Preparation, Properties and Applications; Li, Y., Maruyama, S., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 271–298. [Google Scholar]
- Rother, M.; Brohmann, M.; Yang, S.; Grimm, S.B.; Schießl, S.P.; Graf, A.; Zaumseil, J. Aerosol-Jet Printing of Polymer-Sorted (6,5) Carbon Nanotubes for Field-Effect Transistors with High Reproducibility. Adv. Electron. Mater. 2017, 3, 1700080. [Google Scholar] [CrossRef]
- Mohan, M.; Sharma, V.K.; Kumar, E.A.; Gayathri, V. Hydrogen Storage in Carbon Materials—A Review. Energy Storage 2019, 1, e35. [Google Scholar] [CrossRef]
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen Storage for Mobility: A Review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A. Investigation on platinum loaded multi-walled carbon nanotubes for hydrogen storage applications. Int. J. Hydrog. Energy 2020, 45, 2967–2974. [Google Scholar] [CrossRef]
- Chaudhari, R.; Khanna, S.; Vora, J.; Patel, V.K.; Paneliya, S.; Pimenov, D.Y.; Giasin, K.; Wojciechowski, S. Experimental investigations and optimization of MWCNTs-mixed WEDM process parameters of nitinol shape memory alloy. J. Mater. Res. Technol. 2021, 15, 2152–2169. [Google Scholar] [CrossRef]
- Khanna, S.; Patel, R.; Marathey, P.; Chaudari, R.; Vora, J.; Banerjee, R.; Ray, A.; Mukhopadhyay, I. Growth of titanium dioxide nanorod over shape memory material using chemical vapor deposition for energy conversion application. Mater. Today Proc. 2019, 28, 475–479. [Google Scholar] [CrossRef]
- Wang, S.; Huang, Y.; Chang, E.; Zhao, C.; Ameli, A.; Naguib, H.E.; Park, C.B. Evaluation and modeling of electrical conductivity in conductive polymer nanocomposite foams with multiwalled carbon nanotube networks. Chem. Eng. J. 2021, 411, 128382. [Google Scholar] [CrossRef]
- Candelario, V.M.; Moreno, R.; Guiberteau, F.; Ortiz, A.L. Enhancing the sliding-wear resistance of SiC nanostructured ceramics by adding carbon nanotubes. J. Eur. Ceram. Soc. 2016, 36, 3083–3089. [Google Scholar] [CrossRef]
- Yan, X.; Gu, J.; Zheng, G.; Guo, J.; Galaska, A.M.; Yu, J.; Alam Khan, M.; Sun, L.; Young, D.P.; Zhang, Q.; et al. Lowly loaded carbon nanotubes induced high electrical conductivity and giant magnetoresistance in ethylene/1-octene copolymers. Polymer 2016, 103, 315–327. [Google Scholar] [CrossRef]
- Kil, T.; Jin, D.; Yang, B.; Lee, H. A comprehensive micromechanical and experimental study of the electrical conductivity of polymeric composites incorporating carbon nanotube and carbon fiber. Compos. Struct. 2021, 268, 114002. [Google Scholar] [CrossRef]
- Xu, S.; Liu, J.; Li, Q. Mechanical properties and microstructure of multi-walled carbon nanotube-reinforced cement paste. Constr. Build. Mater. 2015, 76, 16–23. [Google Scholar] [CrossRef]
- Mondal, S.; Ghosh, S.; Ganguly, S.; Das, P.; Ravindren, R.; Sit, S.; Chakraborty, G.; Das, N.C. Highly conductive and flexible nano-structured carbon-based polymer nanocomposites with improved electromagnetic-interference-shielding performance. Mater. Res. Express 2017, 4, 105039. [Google Scholar] [CrossRef]
- Erdem, T.; Idris, M.; Demir, H.V.; Tuncel, D. Macromol. Mater. Eng. 11/2017. Macromol. Mater. Eng. 2017, 302. [Google Scholar] [CrossRef] [Green Version]
- Su, Z.; Wang, H.; Ye, X.; Tian, K.; Huang, W.; He, J.; Guo, Y.; Tian, X. Anisotropic thermally conductive flexible polymer composites filled with hexagonal born nitride (h-BN) platelets and ammine carbon nanotubes (CNT-NH2): Effects of the filler distribution and orientation. Compos. Part A Appl. Sci. Manuf. 2018, 109, 402–412. [Google Scholar] [CrossRef]
- Gardea, F.; Lagoudas, D.C. Characterization of electrical and thermal properties of carbon nanotube/epoxy composites. Compos. Part B Eng. 2014, 56, 611–620. [Google Scholar] [CrossRef]
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Park, S.-J.; Lee, W.R. A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites. J. Ind. Eng. Chem. 2015, 21, 11–25. [Google Scholar] [CrossRef]
- Graf, A.; Tropf, L.C.; Zakharko, Y.; Zaumseil, J.; Gather, M. Near-infrared exciton-polaritons in strongly coupled single-walled carbon nanotube microcavities. Nat. Commun. 2016, 7, 13078. [Google Scholar] [CrossRef] [Green Version]
- Pal, G.; Kumar, S. Multiscale modeling of effective electrical conductivity of short carbon fiber-carbon nanotube-polymer matrix hybrid composites. Mater. Des. 2016, 89, 129–136. [Google Scholar] [CrossRef]
- Dintcheva, N.; Arrigo, R.; Gambarotti, C.; Carroccio, S.; Filippone, G.; Cicogna, F.; Guenzi, M. α-Tocopherol-induced radical scavenging activity in carbon nanotubes for thermo-oxidation resistant ultra-high molecular weight polyethylene-based nanocomposites. Carbon 2014, 74, 14–21. [Google Scholar] [CrossRef]
- Zare, Y.; Rhee, K.Y. Development and modification of conventional Ouali model for tensile modulus of polymer/carbon nanotubes nanocomposites assuming the roles of dispersed and networked nanoparticles and surrounding interphases. J. Colloid Interface Sci. 2017, 506, 283–290. [Google Scholar] [CrossRef]
- Castellino, M.; Rovere, M.; Shahzad, M.I.; Tagliaferro, A. Conductivity in carbon nanotube polymer composites: A comparison between model and experiment. Compos. Part A Appl. Sci. Manuf. 2016, 87, 237–242. [Google Scholar] [CrossRef]
- Talaeemashhadi, S.; Sansotera, M.; Gambarotti, C.; Famulari, A.; Bianchi, C.L.; Guarda, P.A.; Navarrini, W. Functionalization of multi-walled carbon nanotubes with perfluoropolyether peroxide to produce superhydrophobic properties. Carbon 2013, 59, 150–159. [Google Scholar] [CrossRef]
- Gola, M.; Sansotera, M.; Navarrini, W.; Bianchi, C.L.; Stampino, P.G.; Latorrata, S.; Dotelli, G. Perfluoropolyether-functionalized gas diffusion layers for proton exchange membrane fuel cells. J. Power Sources 2014, 258, 351–355. [Google Scholar] [CrossRef]
- Sansotera, M.; Navarrini, W.; Resnati, G.; Metrangolo, P.; Famulari, A.; Bianchi, C.L.; Guarda, P.A. Preparation and characterization of superhydrophobic conductive fluorinated carbon blacks. Carbon 2010, 48, 4382–4390. [Google Scholar] [CrossRef]
- Sansotera, M.; Navarrini, W.; Gola, M.; Dotelli, G.; Stampino, P.G.; Bianchi, C.L. Conductivity and superhydrophobic effect on PFPE-modified porous carbonaceous materials. Int. J. Hydrog. Energy 2012, 37, 6277–6284. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; Morici, E.; Arrigo, R.; Zerillo, G.; Marona, V.; Sansotera, M.; Magagnin, L.; Navarrini, W. High performance composites containing perfluoropolyethers-functionalized carbon-based nanoparticles: Rheological behavior and wettability. Compos. Part B Eng. 2016, 95, 29–39. [Google Scholar] [CrossRef]
- Donnadio, A.; D’Amato, R.; Marmottini, F.; Panzetta, G.; Pica, M.; Battocchio, C.; Capitani, D.; Ziarelli, F.; Casciola, M. On the evolution of proton conductivity of Aquivion membranes loaded with CeO2 based nanofillers: Effect of temperature and relative humidity. J. Membr. Sci. 2019, 574, 17–23. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Zeng, L.; Li, R.; Tian, H.; Fu, X.; Wang, Y.; Zhong, W.-H. A review of the electrical and mechanical properties of carbon nanofiller-reinforced polymer composites. J. Mater. Sci. 2019, 54, 1036–1076. [Google Scholar] [CrossRef]
- Kumar, S.S.; Herrero, J.S.; Irusta, S.; Scott, K. The effect of pretreatment of Vulcan XC-72R carbon on morphology and electrochemical oxygen reduction kinetics of supported Pd nano-particle in acidic electrolyte. J. Electroanal. Chem. 2010, 647, 211–221. [Google Scholar] [CrossRef]
- Guarda, P.; Barchiesi, E.; Fontana, G.; Petricci, S.; Pianca, M.; Marchionni, G. Peroxidic perfluoropolyether from tetrafluoroethylene oxidation: Micro structural analysis by NMR spectroscopy and mechanistic considerations. J. Fluor. Chem. 2005, 126, 141–153. [Google Scholar] [CrossRef]
- Slobodian, P.; Riha, P.; Olejnik, R.; Saha, P. Electromechanical properties of carbon nanotube networks under compression. Meas. Sci. Technol. 2011, 22, 124006. [Google Scholar] [CrossRef]
- Pang, H.; Chen, C.; Bao, Y.; Chen, J.; Ji, X.; Lei, J.; Li, Z.-M. Electrically conductive carbon nanotube/ultrahigh molecular weight polyethylene composites with segregated and double percolated structure. Mater. Lett. 2012, 79, 96–99. [Google Scholar] [CrossRef]
- Luo, C.; Xie, H.; Wang, Q.; Luo, G.; Liu, C. A Review of the Application and Performance of Carbon Nanotubes in Fuel Cells. J. Nanomater. 2015, 2015, 4. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Wang, Y.; An, B.; Huang, R.; Wang, C.; Zhou, Z.-Y.; Lin, W. Networking Pyrolyzed Zeolitic Imidazolate Frameworks by Carbon Nanotubes Improves Conductivity and Enhances Oxygen-Reduction Performance in Polymer-Electrolyte-Membrane Fuel Cells. Adv. Mater. 2017, 29, 1604556. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Yan, D.X.; Bao, Y.; Chen, J.B.; Chen, C.; Li, Z.M. Super-tough conducting carbon nano-tube/ultrahigh-molecular-weight polyethylene composites with segregated and double-percolated structure. J. Mater. Chem. 2012, 22, 23568–23575. [Google Scholar] [CrossRef]
- Haghgoo, M.; Ansari, R.; Hassanzadeh-Aghdam, M. Prediction of electrical conductivity of carbon fiber-carbon nanotube-reinforced polymer hybrid composites. Compos. Part B Eng. 2019, 167, 728–735. [Google Scholar] [CrossRef]
- Hu, H.L.; Zhang, G.; Xiao, L.G.; Wang, H.J.; Zhang, Q.S.; Zhao, Z.D. Preparation and electrical conductivity of graphene/ultrahigh molecular weight polyethylene composites with a segregated structure. Carbon 2012, 50, 4596–4599. [Google Scholar] [CrossRef]
- Sadeghi, S.; Arjmand, M.; Otero Navas, I.; Zehtab Yazdi, A.; Sundararaj, U. Effect of Nanofiller Geometry on Network Formation in Polymeric Nanocomposites: Comparison of Rheological and Electrical Properties of Multiwalled Carbon Nanotube and Graphene Nanoribbon. Macromolecules 2017, 50, 3954–3967. [Google Scholar] [CrossRef]
- Sharma, V.; Prakash, U.; Kumar, B.M. Surface composites by friction stir processing: A review. J. Mater. Process. Technol. 2015, 224, 117–134. [Google Scholar] [CrossRef]
- Lebedev, O.; Ozerin, A.N.; Kechek’Yan, A.S.; Golubev, E.K.; Shevchenko, V.G.; Kurkin, T.S.; Beshenko, M.A.; Sergeev, V.G. Strengthened electrically conductive composite materials based on ultra-high-molecular-weight polyethylene reactor powder and nanosized carbon fillers. Nanotechnologies Russ. 2015, 10, 42–52. [Google Scholar] [CrossRef]
- Sun, H.; Wang, T.; Xu, Y.; Gao, W.; Li, P.; Niu, Q.J. Fabrication of polyimide and functionalized multi-walled carbon nanotubes mixed matrix membranes by in-situ polymerization for CO2 separation. Sep. Purif. Technol. 2017, 177, 327–336. [Google Scholar] [CrossRef]
- Prosini, P.P.; Pozio, A.; Botti, S.; Ciardi, R. Electrochemical studies of hydrogen evolution, storage and oxidation on carbon nanotube electrodes. J. Power Sources 2003, 118, 265–269. [Google Scholar] [CrossRef]
- Lombardi, I.; Bestetti, M.; Mazzocchia, C.; Cavallotti, P.L.; Ducati, U. Electrochemical Characterization of Carbon Nanotubes for Hydrogen Storage. Electrochem. Solid-State Lett. 2004, 7, A115–A118. [Google Scholar] [CrossRef]
- Sansotera, M.; Gola, M.; Dotelli, G.; Navarrini, W. The role of perfluoropolyethers in the development of polymeric proton exchange membrane fuel cells. In Fluorinated Polymers: Volume 2: Applications; Ameduri., B., Sawada, H., Eds.; Royal Society of Chemistry: London, UK, 2016; pp. 356–376. [Google Scholar]
- Kumar, R.M.; Sharma, S.K.; Kumar, B.M.; Lahiri, D. Effects of carbon nanotube aspect ratio on strengthening and tribological behavior of ultra high molecular weight polyethylene composite. Compos. Part A Appl. Sci. Manuf. 2015, 76, 62–72. [Google Scholar] [CrossRef]
- Zamani, P.; Higgins, D.C.; Hassan, F.; Fu, X.; Choi, J.-Y.; Hoque, A.; Jiang, G.; Chen, Z. Highly active and porous graphene encapsulating carbon nanotubes as a non-precious oxygen reduction electrocatalyst for hydrogen-air fuel cells. Nano Energy 2016, 26, 267–275. [Google Scholar] [CrossRef]
- Wang, C.; Waje, M.; Wang, X.; Tang, J.M.; Haddon, R.C.; Yan, Y. Proton Exchange Membrane Fuel Cells with Carbon Nanotube Based Electrodes. Nano Lett. 2004, 4, 345–348. [Google Scholar] [CrossRef]
- Moreno, N.G.; Gervasio, D.; García, A.G.; Robles, J.F.P. Polybenzimidazole-multiwall carbon nanotubes composite membranes for polymer electrolyte membrane fuel cells. J. Power Sources 2015, 300, 229–237. [Google Scholar] [CrossRef]
- Ebenezer, D.; Neelima, K.; Jagannatham, M.; Haridoss, P. Carbon Nanotubes and Nanohorn Hybrid Composite Buckypaper as Microporous Layer for Proton Exchange Membrane Fuel Cell. Fuel Cells 2016, 16, 349–355. [Google Scholar] [CrossRef]
- Berber, M.R.; Hafez, I.H.; Fujigaya, T.; Nakashima, N. A highly durable fuel cell electrocatalyst based on doublepolymer-coated carbon nanotubes. Sci. Rep. 2015, 5, 16711. [Google Scholar] [CrossRef] [Green Version]
- MoghadamEsfahani, R.A.; Vankova, S.K.; Easton, E.B.; Ebralidze, I.I.; Specchia, S. A hybrid Pt/NbO/CNTs catalyst with high activity and durability for oxygen reduction reaction in PEMFC. Renew. Energy 2020, 154, 913–924. [Google Scholar] [CrossRef]
- Jung, D.W.; Park, S.; Ahn, C.Y.; Kim, J.B.; Oh, E.S. Performance Comparison of Pt1–xPdx/Carbon Nanotubes Catalysts in Both Electrodes of Polymer Electrolyte Membrane Fuel Cells. Fuel Cells 2012, 12, 398–405. [Google Scholar] [CrossRef]
- Shahgaldi, S.; Hamelin, J. Improved carbon nanostructures as a novel catalyst support in the cathode side of PEMFC: A critical review. Carbon 2015, 94, 705–728. [Google Scholar] [CrossRef]
- Niaz, S.; Manzoor, T.; Pandith, A.H. Hydrogen storage: Materials, methods and perspectives. Renew. Sustain. Energy Rev. 2015, 50, 457–469. [Google Scholar] [CrossRef]
- Gribov, E.N.; Kuznetsov, A.N.; Golovin, V.A.; Krasnikov, D.V.; Kuznetsov, V.L. Effect of modification of multi-walled carbon nanotubes with nitrogen-containing polymers on the electrochemical performance of Pt/CNT catalysts in PEMFC. Mater. Renew. Sustain. Energy 2019, 8, 7. [Google Scholar] [CrossRef] [Green Version]
- Kojima, Y. Hydrogen storage materials for hydrogen and energy carriers. Int. J. Hydrog. Energy 2019, 44, 18179–18192. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sansotera, M.; Marona, V.; Marziani, P.; Dintcheva, N.T.; Morici, E.; Arrigo, R.; Bussetti, G.; Navarrini, W.; Magagnin, L. Flexible Perfluoropolyethers-Functionalized CNTs-Based UHMWPE Composites: A Study on Hydrogen Evolution, Conductivity and Thermal Stability. Materials 2022, 15, 6883. https://doi.org/10.3390/ma15196883
Sansotera M, Marona V, Marziani P, Dintcheva NT, Morici E, Arrigo R, Bussetti G, Navarrini W, Magagnin L. Flexible Perfluoropolyethers-Functionalized CNTs-Based UHMWPE Composites: A Study on Hydrogen Evolution, Conductivity and Thermal Stability. Materials. 2022; 15(19):6883. https://doi.org/10.3390/ma15196883
Chicago/Turabian StyleSansotera, Maurizio, Valeria Marona, Piergiorgio Marziani, Nadka Tzankova Dintcheva, Elisabetta Morici, Rossella Arrigo, Gianlorenzo Bussetti, Walter Navarrini, and Luca Magagnin. 2022. "Flexible Perfluoropolyethers-Functionalized CNTs-Based UHMWPE Composites: A Study on Hydrogen Evolution, Conductivity and Thermal Stability" Materials 15, no. 19: 6883. https://doi.org/10.3390/ma15196883









