Thermodynamic Simulation of Environmental and Population Protection by Utilization of Technogenic Tailings of Enrichment
Abstract
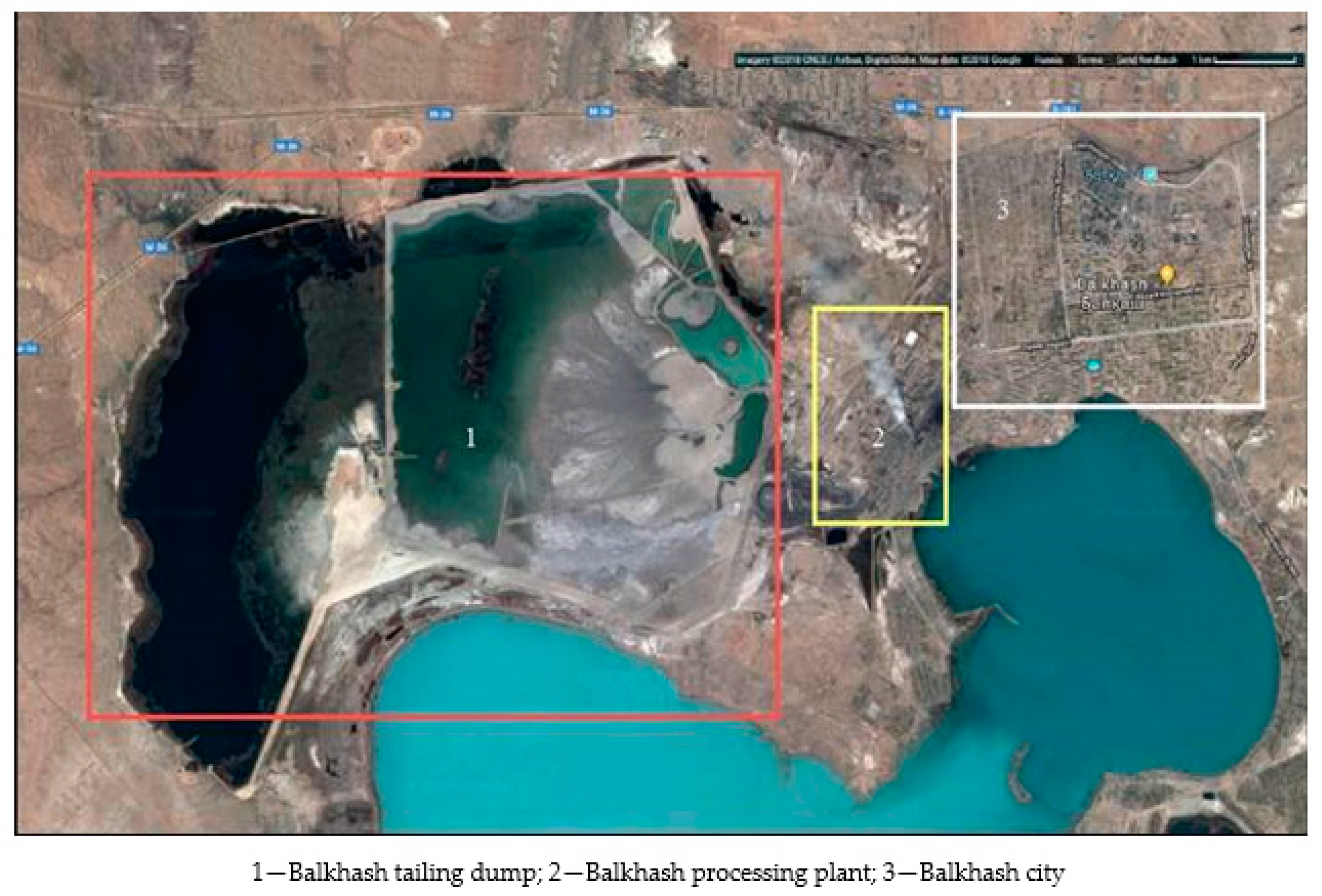
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
- −
- Technogenic mineral formations of the mining and metallurgical industry, containing Si, Al, and Fe oxides in their composition and being in a pulverized fraction are capable of harming human health and the environment, but at the same time act as a silicon-aluminum-iron-containing component for clinker charge in the following calculated optimal ratio of limestone and industrial waste in the raw mixture 80.9–82.5% and 17.5–19.1%, respectively;
- −
- From the thermodynamic calculation of the formation of mineralogical phase formation of the sinter clinker simulation, it follows that in the first row of chemical Equations (4)–(7) at a temperature value of 1400 °C, depending on ΔGTo, the following sequence of clinker mineral formation is represented: Ca3SiO5 (−348.6 kJ); Ca4Al2Fe2O10 (−342.1 kJ); Ca3Al2O6 (−285 kJ); and Ca2SiO4 (−284.1 kJ);
- −
- From the thermodynamic calculation of the formation of mineralogical phase formation of sinter clinker modeling, it follows that in the second series of chemical Equations (8)–(11) in the presence of zinc ferrite at a temperature value of 1400 °C, depending on ΔGTo, the following sequence of clinker mineral formation is represented: Ca3Al2O6 (−1874.1 kJ); Ca3SiO5 (−1319.3 kJ); Ca2SiO4 (−1254.8 kJ); and Ca4Al2Fe2O10 (−331.6 kJ);
- −
- In chemical Equations (8)–(11) in the process of mineralogical phase formation of sinter clinker modeling in the presence of zinc ferrite, contributes, under equal conditions, to a decrease in temperature by 100–200 K and intensification of the flow of equations in comparison with known chemical Equations (4)–(7) with energy savings from 10.5 to 1589.1 kJ;
- −
- In chemical Equations (8)–(11), in the process of mineralogical phase formation of sinter clinker modeling in the presence of zinc ferrite, Zn can be distilled as a gas at T = 1200–1400 K.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shaigazinova, A.E. Waste management of mining and processing of mineral raw materials as an important environmental protection measure. In Proceedings of the Republican Scientific and Theoretical Conference “Seifullin Readings–9: A New Vector of Development of Higher Education and Science” Dedicated to the Day of the First President of the Republic of Kazakhstan, Astana, Kazakhstan, 23–28 November 2013. [Google Scholar]
- Litvinenko, V.S. Digital Economy as a Factor in the Technological Development of the Mineral Sector. Nat. Resour. Res. 2020, 29, 1521–1541. [Google Scholar] [CrossRef]
- The Strategic Plan for Development of the Republic of Kazakhstan until the Year 2025, Has Been Approved by the Decree of the President of the Republic of Kazakhstan No. 636. Available online: https://adilet.zan.kz/rus/docs/U1800000636 (accessed on 15 February 2018).
- Kazakhstan in a New Reality: Time for Action. President of Kazakhstan Kassym-Jomart Tokayev’s State of the Nation Address. Available online: https://www.akorda.kz/en/president-kassym-jomart-tokayev-delivers-his-state-of-the-nation-address-to-the-people-of-kazakhstan-28624 (accessed on 1 September 2020).
- Petrenko, E.S.; Vechkinzova, E.A.; Urazbekov, A.K. Context analysis and prospects of development of the mining and metallurgical industry of Kazakhstan. Ekonom. Otnosh. 2019, 9, 2661–2676. [Google Scholar] [CrossRef]
- Khoroshavin, L.B.; Perepelitsyn, V.A.; Kochkin, D.K. Problems of technogenic resources. Refract. Ind. Ceram. 1998, 39, 366–368. [Google Scholar] [CrossRef]
- Mussapyrova, L.; Nadirov, R.; Baláž, P.; Rajňák, M.; Bureš, R.; Baláž, M. Selective room-temperature leaching of copper from mechanically activated copper smelter slag. J. Mater. Res. Technol. 2021, 12, 2011–2025. [Google Scholar] [CrossRef]
- Kolesnikov, A.; Fediuk, R.; Amran, M.; Klyuev, S.; Klyuev, A.; Volokitina, I.; Naukenova, A.; Shapalov, S.; Utelbayeva, A.; Kolesnikova, O.; et al. Modeling of Non-Ferrous Metallurgy Waste Disposal with the Production of Iron Silicides and Zinc Distillation. Materials 2022, 15, 2542. [Google Scholar] [CrossRef]
- Volokitina, I.; Kolesnikov, A.; Fediuk, R.; Klyuev, S.; Sabitov, L.; Volokitin, A.; Zhuniskaliyev, T.; Kelamanov, B.; Yessengaliev, D.; Yerzhanov, A.; et al. Study of the Properties of Antifriction Rings under Severe Plastic Deformation. Materials 2022, 15, 2584. [Google Scholar] [CrossRef]
- Pavlík, Z.; Keppert, M.; Pavlíková, M.; Žumár, J.; Fořt, J.; Černý, R. Mechanical, hygric, and durability properties of cement mortar with MSWI bottom ash as partial silica sand replacement. Cement. Wapno. Beton. 2014, 19, 67–80. [Google Scholar]
- Sariev, O.; Kim, S.; Zhumagaliev, Y.; Kelamanov, B.; Sultanov, M.; Nurgali, N. Viscosity and crystallization temperature of ferroalloy slags from Kazakhstan ore. Metalurgija 2020, 59, 525–528. [Google Scholar]
- Shalimova, A.V.; Filin, A.E. Development of a mathematical forecasing model for occupational injuries in mining. MIAB Min. Inf. Anal. Bull. 2021, 2–1, 209–219. [Google Scholar] [CrossRef]
- Vasilyeva, N.; Fedorova, E.; Kolesnikov, A. Big Data as a Tool for Building a Predictive Model of Mill Roll Wear. Symmetry 2021, 13, 859. [Google Scholar] [CrossRef]
- Zinovieva, O.M.; Kuznetsov, D.S.; Merkulova, A.M.; Smirnova, N.A. Digitalization of industrial safety management systems in mining. MIAB Min. Inf. Anal. Bull. 2021, 2–1, 113–123. [Google Scholar] [CrossRef]
- Filin, A.E.; Zinovieva, O.M.; Kolesnikova, L.A.; Merkulova, A.M. Prospects of safety control in combination of mining and metallurgy industries. Eurasian Min. 2018, 1, 31–34. [Google Scholar] [CrossRef]
- Nadirov, K.S.; Zhantasov, M.K.; Sakybayev, B.A.; Orynbasarov, A.; Bimbetova, G.; Sadyrbayeva, A.; Kolesnikov, A.; Ashirbayev, H.; Zhantasova, D.; Tuleuov, A. The study of the gossypol resin impact on adhesive properties of the intermediate layer of the pipeline three-layer rust protection coating. Int. J. Adhes. Adhesiv. 2017, 78, 195–199. [Google Scholar] [CrossRef]
- Filin, A.E.; Ovchinnikova, T.I.; Zinovieva, O.M.; Merkulova, A.M. Advance of pulsating ventilation in mining. Gorn. Zhurnal 2020, 3, 67–71. [Google Scholar] [CrossRef]
- Ferreira, W.L.; Reis, É.L.; Lima, R.M. Incorporation of residues from the minero-metallurgical industry in the production of clay–lime brick. J. Clean. Prod. 2015, 87, 505–510. [Google Scholar] [CrossRef] [Green Version]
- Peng, Z.; Gregurek, D.; Wenzl, C.; White, J.F. White Slag Metallurgy and Metallurgical Waste Recycling. J. Metall. 2016, 68, 2313–2315. [Google Scholar] [CrossRef] [Green Version]
- Dairabay, D.; Brener, A.; Golubev, V. Kinetic Equations of Aggregation Processes in Disperse Systems with Allowance for Age-Dependent Clusters Properties. Chem. Eng. Transact. 2016, 47, 193–198. [Google Scholar] [CrossRef]
- Serikbaev, B.E.; Zolkin, A.L.; Kenzhibaeva, G.S. Processing of Non-Ferrous Metallurgy Waste Slag for its Complex Recovery as a Secondary Mineral Raw Material. Refract. Ind. Ceram. 2021, 62, 375–380. [Google Scholar] [CrossRef]
- Pavlik, Z.; Jirickova, M.; Cerny, R.; Sobczuk, H.; Suchorab, Z. Determination of moisture diffusivity using the time domain reflectometry (TDR) method. J. Build. Phys. 2006, 30, 59–70. [Google Scholar] [CrossRef]
- Kolesnikov, A.; Fediuk, R.; Kolesnikova, O.; Zhanikulov, N.; Zhakipbayev, B.; Kuraev, R.; Akhmetova, E.; Shal, A. Processing of Waste from Enrichment with the Production of Cement Clinker and the Extraction of Zinc. Materials 2022, 15, 324. [Google Scholar] [CrossRef] [PubMed]
- Aghazadeh-Ghomi, M.; Pourabbas, Z. Rapid synthesis of zinc oxide nanoparticles from an alkaline zinc solution via direct precipitation. J. Mat. Sci. Mat. Electron. 2021, 32, 24363–24368. [Google Scholar] [CrossRef]
- Zaid, P.; Elite, E.; Kurdowski, V. The importance of zinc in the production of Portland cement. Cem. Applic. 2018, 2, 37–46. [Google Scholar]
- Gineys, N.; Aouad, G.; Sorrentino, F.; Damidot, D. Incorporation of trace elements in Portland cement clinker: Threshold limits for Cu, Ni, Sn or Zn. Cem. Concr. Res. 2011, 41, 1177–1184. [Google Scholar] [CrossRef]
- Perez-Bravo, R.; Alvarez-Pinazo, G.; Compana, J.M.; Santacruz, I.; Losilla, E.R.; Bruque, S.; de la Torre, A.G. Alite sulfoaluminate clinker: Rietveld mineralogical and SEM-EDX analysis. Adv. Cem. Res. 2014, 26, 10–20. [Google Scholar] [CrossRef]
- Mussapyrova, L.A. Copper smelter slag leaching by using H2SO4 in the presence of dichromate. J. Chem. Technol. Metall. 2019, 54, 657–662. [Google Scholar]
- Nadirov, K.S.; Zhantasov, M.K.; Bimbetova, G.Z.; Sadyrbayeva, A.S.; Orynbasarov, A.K.; Kutzhanova, A.N.; Turemuratov, R.S.; Botabaev, N.E.; Zhantasova, D. Examination of optimal parameters of oxy-ethylation of fatty acids with a view to obtaining demulsifiers for deliquefaction in the system of skimming and treatment of oil: A method to obtain demulsifier from fatty acids. Chem. Today 2016, 34, 72–77. [Google Scholar]
- Nadirov, R.K. Recovery of Valuable Metals from Copper Smelter Slag by Sulfation Roasting. Trans. Ind. Inst. Metals 2019, 72, 603–607. [Google Scholar] [CrossRef]
- Efremova, S. Scientific and technical solutions to the problem of utilization of waste from plant- and mineral-based industries. Russ. J. Gen. Chem. 2012, 82, 963–968. [Google Scholar] [CrossRef]
- Fechet, R.; Zlagnean, M.; Moanta, A.; Ciobanu, L. Mining wastes-sampling, processing and using in manufacture portland cement. Rom. J. Min. Dep. 2010, 84, 67–70. [Google Scholar]
- Available online: https://info.geology.gov.kz/ru/informatsiya/spravochnik-mestorozhdenij-kazakhstana/tverdye-poleznye-iskopaemye/item/mynaral (accessed on 28 June 2022).
- Chigarkin, A.V. Regional Geoecology of Kazakhstan; Kazak Universities: Almaty, Kazakhstan, 2000; p. 224. [Google Scholar]
- Klassen, V.K.; Borisov, I.N.; Manuilov, V.E. Technogenic Materials in Cement Production; Monograph-Belgorod: Beijing, China, 2008; p. 126. [Google Scholar]
- Babushkin, V.I.; Matveyev, G.M.; Mchedlov-Petrosyan, O.P. Thermodynamics of Silicates; Stroyizdat: Moscow, Russia, 1986; p. 408. [Google Scholar]
- Roine, A. Outokumpu HSC Chemistry for Windows. Chemical Reaction and Equilibrium Loftware with Extensive Thermochemical Database; Outokumpu Research OY: Pori, Finland, 2002; p. 268. [Google Scholar]
- Sergeeva, I.V.; Botabaev, N.E.; Al’Zhanova, A.Z.; Ashirbaev, K.A. Thermodynamic simulation of chemical and phase transformations in the system of oxidized manganese ore—Carbon. Izv. Ferr. Metall. 2017, 60, 759–765. [Google Scholar] [CrossRef] [Green Version]
- Zhakipbaev, B.Y.; Zhanikulov, N.N.; Kuraev, R.M.; Shal, A.L. Review of technogenic waste and methods of its processing for the purpose of complex utilization of tailings from the enrichment of non-ferrous metal ores as a component of the raw material mixture in the production of cement clinker. Rasayan. J. Chem. 2021, 14, 997–1005. [Google Scholar] [CrossRef]
- Kolesnikov, A.S. Thermodynamic simulation of silicon and iron reduction and zinc and lead distillation in zincoligonite ore-carbon systems. Russ. J. Non-ferrous Met. 2014, 55, 513–518. [Google Scholar] [CrossRef]
- For Utility Model Raw Mix for the Production of Cement Clinker. Patent 6995, 8 April 2022.
- Demidov, A.I. Thermodynamic characteristics of a quasi-binary system CaO—Si1/2O in a solid state. Sci. Technol. Ved. SPbPU. 2018, 24, 134–139. [Google Scholar] [CrossRef]
- Pavlíková, M.; Zemanová, L.; Záleská, M.; Pokorný, J.; Lojka, M.; Jankovský, O.; Pavlík, Z. Ternary Blended Binder for Production of a Novel Type of Lightweight Repair Mortar. Materials 2019, 12, 996. [Google Scholar] [CrossRef] [Green Version]
- Rog, G.; Kozlowska-Rog, A.; Zakula-Sokol, K. Thermodynamic functions of calcium aluminate. J. Chem. Thermodyn. 1993, 25, 807–810. [Google Scholar]
- Zeleznik, F.J.; Gordon, S. Calculation of complex chemical equilibria. Ind. Eng. Chem. 1968, 60, 27–57. [Google Scholar] [CrossRef]
- Amran, M.; Fediuk, R.; Abdelgader, H.S.; Murali, G.; Ozbakkaloglu, T.; Lee, Y.H. Fiber-reinforced alkali-activated concrete: A review. J. Build. Eng. 2022, 45, 103638. [Google Scholar] [CrossRef]
- Pratskova, S.E.; Burmistrov, V.A.; Starikova, A.A. Thermodynamic modeling of oxide melts of CaO—Al2O3—SiO2: Systems. Russ. J. Chem. Chem. Tech. 2020, 63, 45–50. (In Russian) [Google Scholar] [CrossRef]
- Tennis, P.D.; Jennings, H.M. A model for two types of calcium silicate hydrate in the microstructure of Portland cement pastes. Cem. Concr. Res. 2000, 30, 855–863. [Google Scholar] [CrossRef]
- Fukuda, K. Crystal Chemistry of Cement-Clinker Minerals and Melt Differentiation Reaction of Interstitial Melt. Nihon Kessho Gakkaishi 2011, 53, 81–85. [Google Scholar] [CrossRef] [Green Version]
- Hanein, T.; Glasser, F.; Bannerman, M. Thermodynamic data for cement clinkering. Cem. Concr. Res. 2020, 132, 106043. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, Y.; Zhang, L.; Yang, K.; Guan, X.; Zhao, R. Insights on Substitution Preference of Pb Ions in Sulfoaluminate Cement Clinker Phases. Materials 2021, 14, 44. [Google Scholar] [CrossRef]
- Fediuk, R.S.; Lesovik, V.S.; Liseitsev, Y.L.; Timokhin, R.A.; Bituyev, A.V.; Zaiakhanov, M.Y.; Mochalov, A.V. Composite binders for concretes with improved shock resistance. Mag. Civ. Eng. 2019, 85, 145. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, K.; Chen, Y.; Fan, G.; Zhang, L.; Guo, B.; Guan, X.; Zhao, R. Revealing the substitution preference of zinc in ordinary Portland cement clinker phases: A study from experiments and DFT calculations. J. Hazard. Mater. 2021, 409, 124504. [Google Scholar] [CrossRef]
- Jankovsky, O.; Pavlikova, M.; Sedmidubskz, D.; Bousa, D.; Lojka, M.; Pokorny, J.; Zaleska, M.; Pavlik, Z. Study on pozzolana activity of wheat straw ash as potential admixture for blended cements. Ceram.Silik. 2017, 61, 327–339. [Google Scholar] [CrossRef] [Green Version]
- Zaleska, M.; Pavlik, Z.; Pavlikova, M.; Scheinherrova, L.; Pokorny, J.; Trnik, A.; Svora, P.; Fort, J.; Jankovsky, O.; Suchorab, Z. Biomass ash-based mineral admixture prepared from municipal sewage sludge and its application in cement composites. Clean Technol. Environ. Policy 2018, 20, 159–171. [Google Scholar] [CrossRef]
- Amran, M.; Murali, G.; Fediuk, R.; Vatin, N.; Vasilev, Y.; Abdelgader, H. Palm Oil Fuel Ash-Based Eco-Efficient Concrete: A Critical Review of the Short-Term Properties. Materials 2021, 14, 332. [Google Scholar] [CrossRef]
- Fediuk, R.; Smoliakov, A.; Stoyushko, N. Increase in composite binder activity. IOP Conf. Ser. Mater. Sci. Eng. 2016, 156, 012042. [Google Scholar] [CrossRef]
- Tolstoy, A.; Lesovik, V.; Fediuk, R.; Amran, M.; Gunasekaran, M.; Vatin, N.; Vasilev, Y. Production of Greener High-Strength Concrete Using Russian Quartz Sandstone Mine Waste Aggregates. Materials 2020, 13, 5575. [Google Scholar] [CrossRef]
- Fediuk, R. Reducing permeability of fiber concrete using composite binders. Spec. Top. Rev. Porous Media 2018, 9, 79–89. [Google Scholar] [CrossRef]
- Semenov, P.A.; Uzunian, A.V.; Davidenko, A.M.; Derevschikov, A.A.; Goncharenko, Y.M.; Kachanov, V.A.; Khodyrev, V.Y.; Meschanin, A.P.; Minaev, N.G.; Mochalov, V.V.; et al. First study of radiation hardness of lead tungstate crystals at low temperatures. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2007, 582, 575–580. [Google Scholar] [CrossRef] [Green Version]
- Fediuk, R.; Pak, A.; Kuzmin, D. Fine-Grained Concrete of Composite Binder. IOP Conf. Ser. Mater. Sci. Eng. 2017, 262, 012025. [Google Scholar] [CrossRef]

| T, °C | Reaction Gibbs Energy, G, kJ | |||
|---|---|---|---|---|
| 2CaCO3 + SiO2 → Ca2SiO4 + 2CO2 | 3CaCO3 + SiO2 → Ca3SiO5 + 3CO2 | 3CaCO3 + Al2O3 → Ca3Al2O6 + 3CO2 | 4CaCO3 + Al2O3 + Fe2O3 → Ca4Al2Fe2O10 + 4CO2 | |
| 600 | −571.2 | −518.7 | −1012.8 | 579.1 |
| 700 | −650.2 | −612.9 | −1114 | 466.4 |
| 800 | −731.2 | −709 | −1217.2 | 353.3 |
| 900 | −825 | −817.7 | −1322.4 | 239.7 |
| 1000 | −910.7 | −918.1 | −1429.5 | 125.9 |
| 1100 | −998.2 | −1020.1 | −1538.2 | 11.8 |
| 1200 | −1073.6 | −1109.8 | −1648.7 | −102.5 |
| 1300 | −1163.4 | −1213.9 | −1760.7 | −216.9 |
| 1400 | −1254.8 | −1319.3 | −1874.1 | −331.6 |
| 1500 | −1347.8 | −1426.2 | −1989.1 | −446.4 |
| 1600 | −1442.3 | −1534.4 | −2096.2 | −523 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolesnikova, O.; Syrlybekkyzy, S.; Fediuk, R.; Yerzhanov, A.; Nadirov, R.; Utelbayeva, A.; Agabekova, A.; Latypova, M.; Chepelyan, L.; Volokitina, I.; et al. Thermodynamic Simulation of Environmental and Population Protection by Utilization of Technogenic Tailings of Enrichment. Materials 2022, 15, 6980. https://doi.org/10.3390/ma15196980
Kolesnikova O, Syrlybekkyzy S, Fediuk R, Yerzhanov A, Nadirov R, Utelbayeva A, Agabekova A, Latypova M, Chepelyan L, Volokitina I, et al. Thermodynamic Simulation of Environmental and Population Protection by Utilization of Technogenic Tailings of Enrichment. Materials. 2022; 15(19):6980. https://doi.org/10.3390/ma15196980
Chicago/Turabian StyleKolesnikova, Olga, Samal Syrlybekkyzy, Roman Fediuk, Almas Yerzhanov, Rashid Nadirov, Akmaral Utelbayeva, Aktolkyn Agabekova, Marina Latypova, Larissa Chepelyan, Irina Volokitina, and et al. 2022. "Thermodynamic Simulation of Environmental and Population Protection by Utilization of Technogenic Tailings of Enrichment" Materials 15, no. 19: 6980. https://doi.org/10.3390/ma15196980
APA StyleKolesnikova, O., Syrlybekkyzy, S., Fediuk, R., Yerzhanov, A., Nadirov, R., Utelbayeva, A., Agabekova, A., Latypova, M., Chepelyan, L., Volokitina, I., Vatin, N. I., Kolesnikov, A., & Amran, M. (2022). Thermodynamic Simulation of Environmental and Population Protection by Utilization of Technogenic Tailings of Enrichment. Materials, 15(19), 6980. https://doi.org/10.3390/ma15196980










