Effect of Hydrogen on Corrosion Behavior of 321 Stainless Steel in NH4Cl Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrochemical Hydrogen Charging
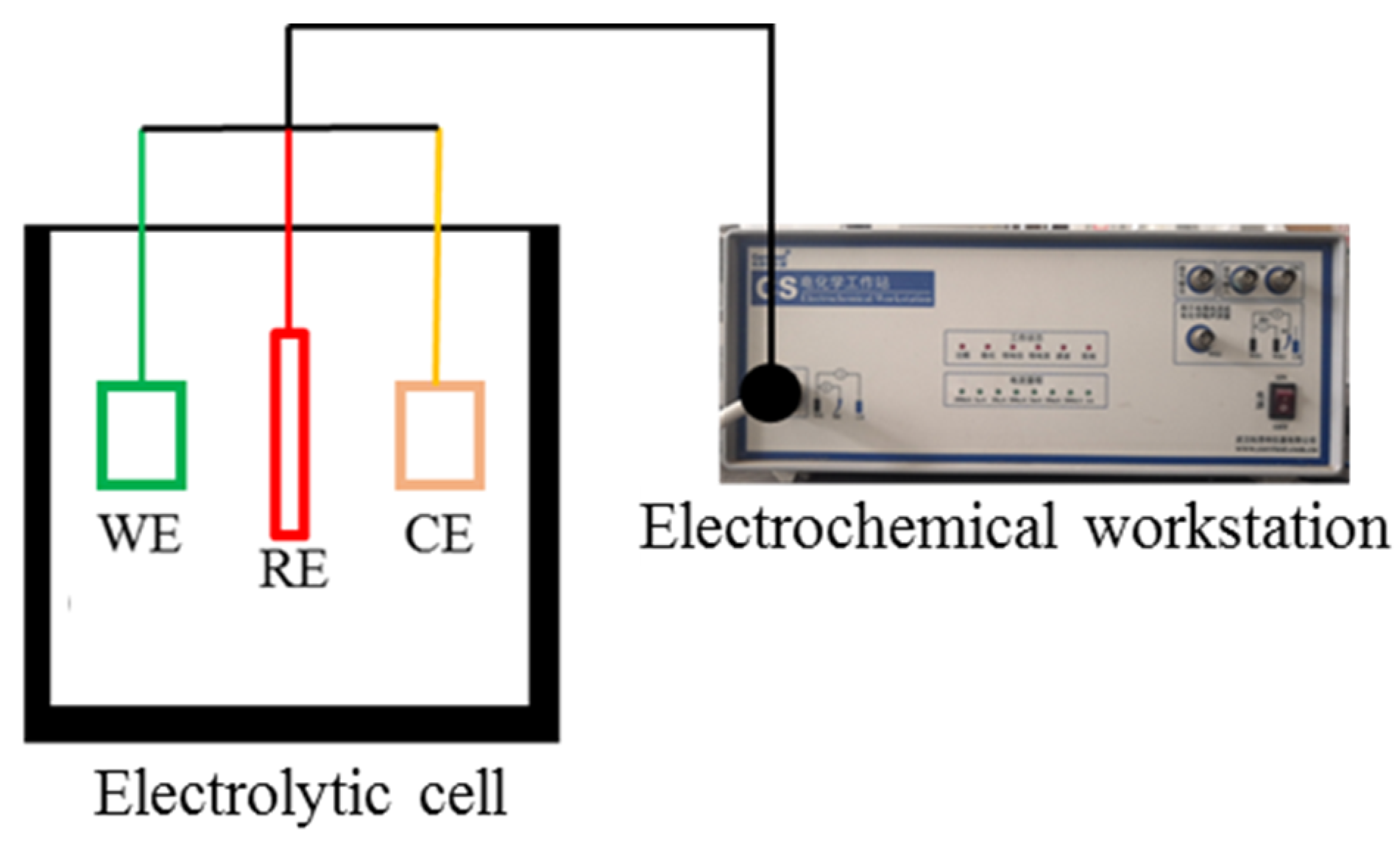
2.3. Electrochemical Test
2.4. Characterization
3. Results and Discussion
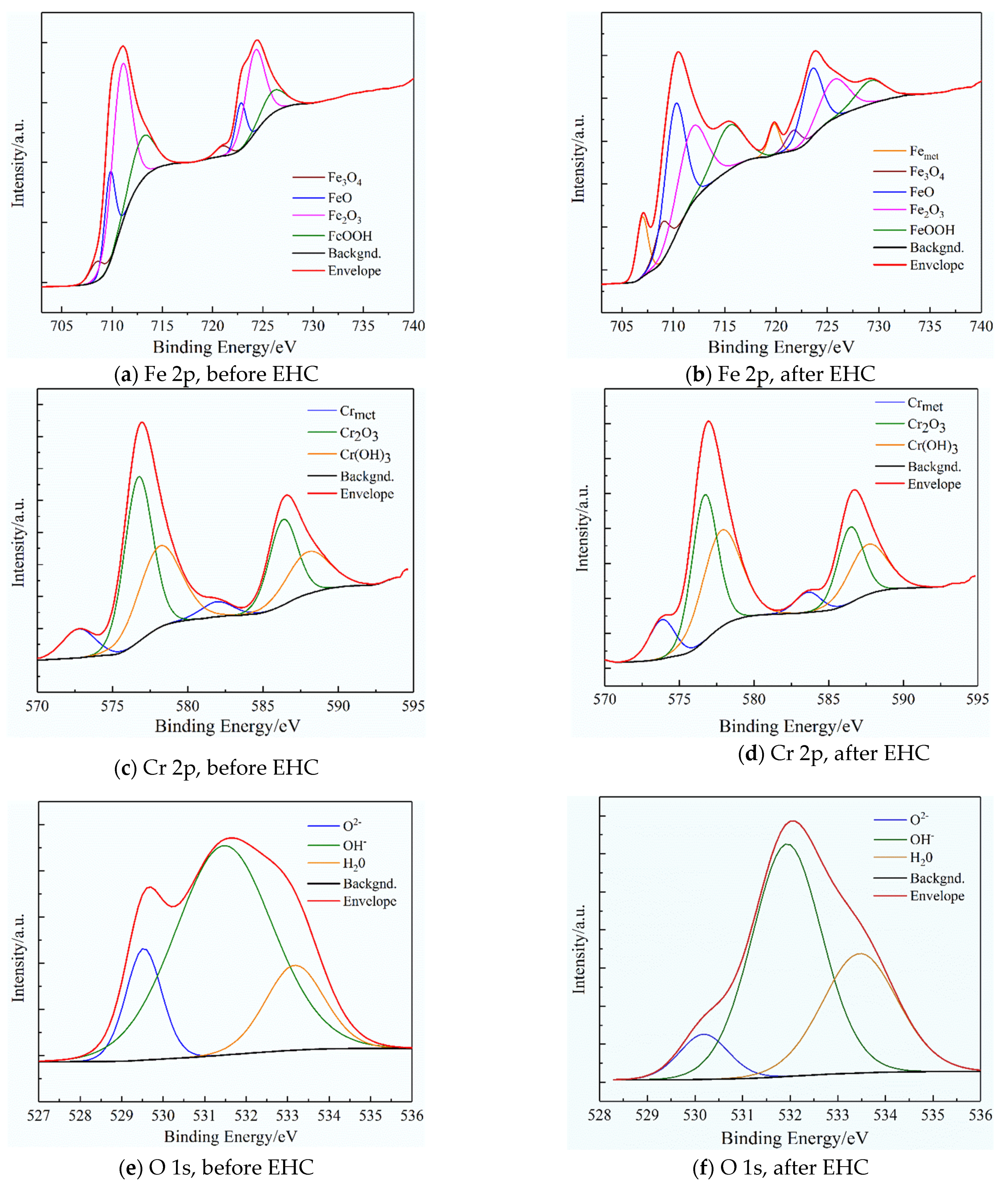
3.1. Effect of Hydrogen on Passive Film Structure of 321 Stainless Steel
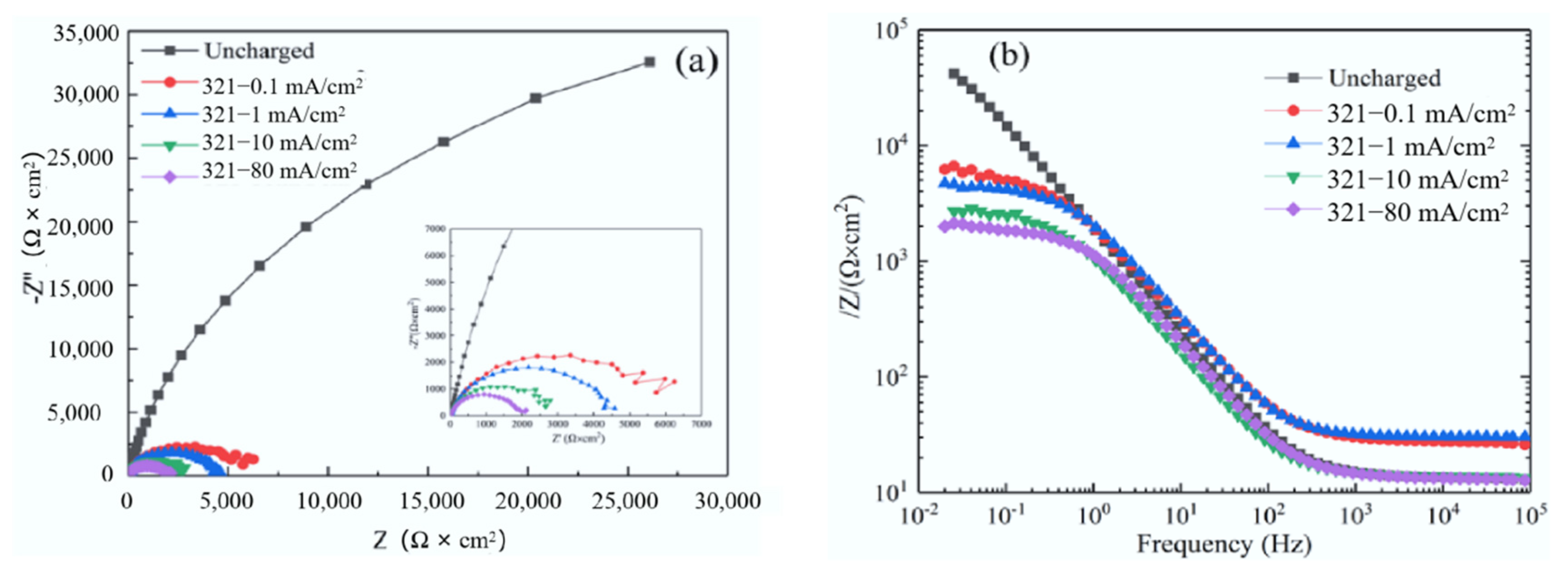
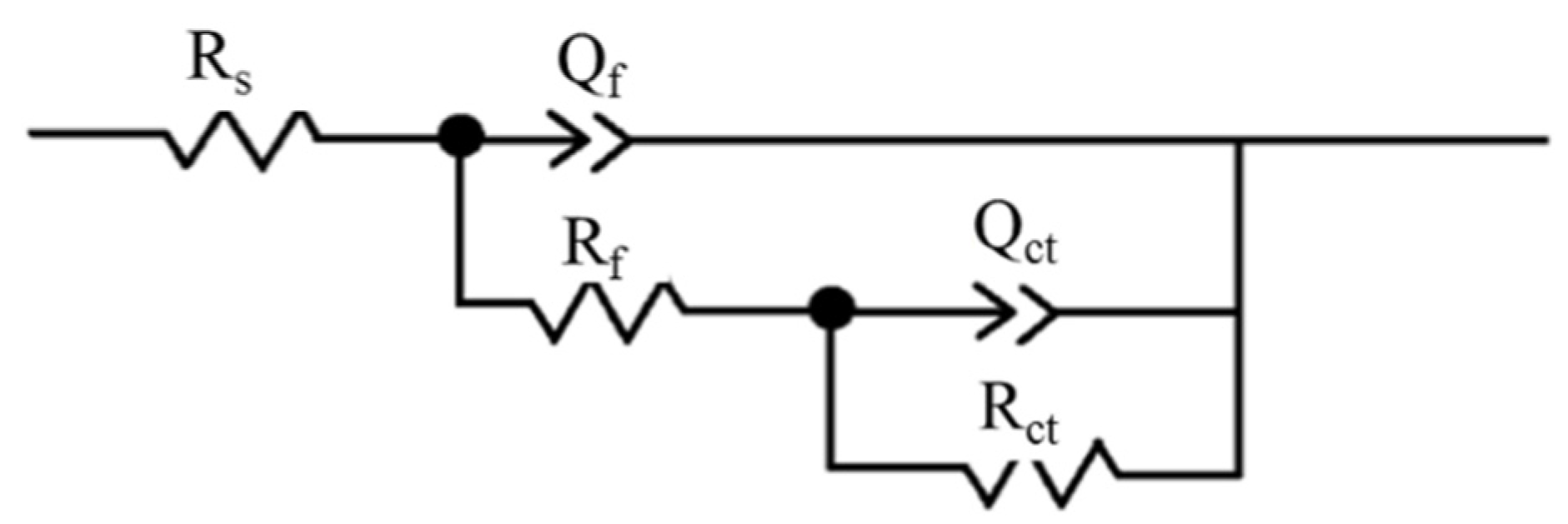
3.2. Effect of Hydrogen on Electrochemical Properties of 321 Stainless Steel
3.3. Corrosion Morphology Analysis
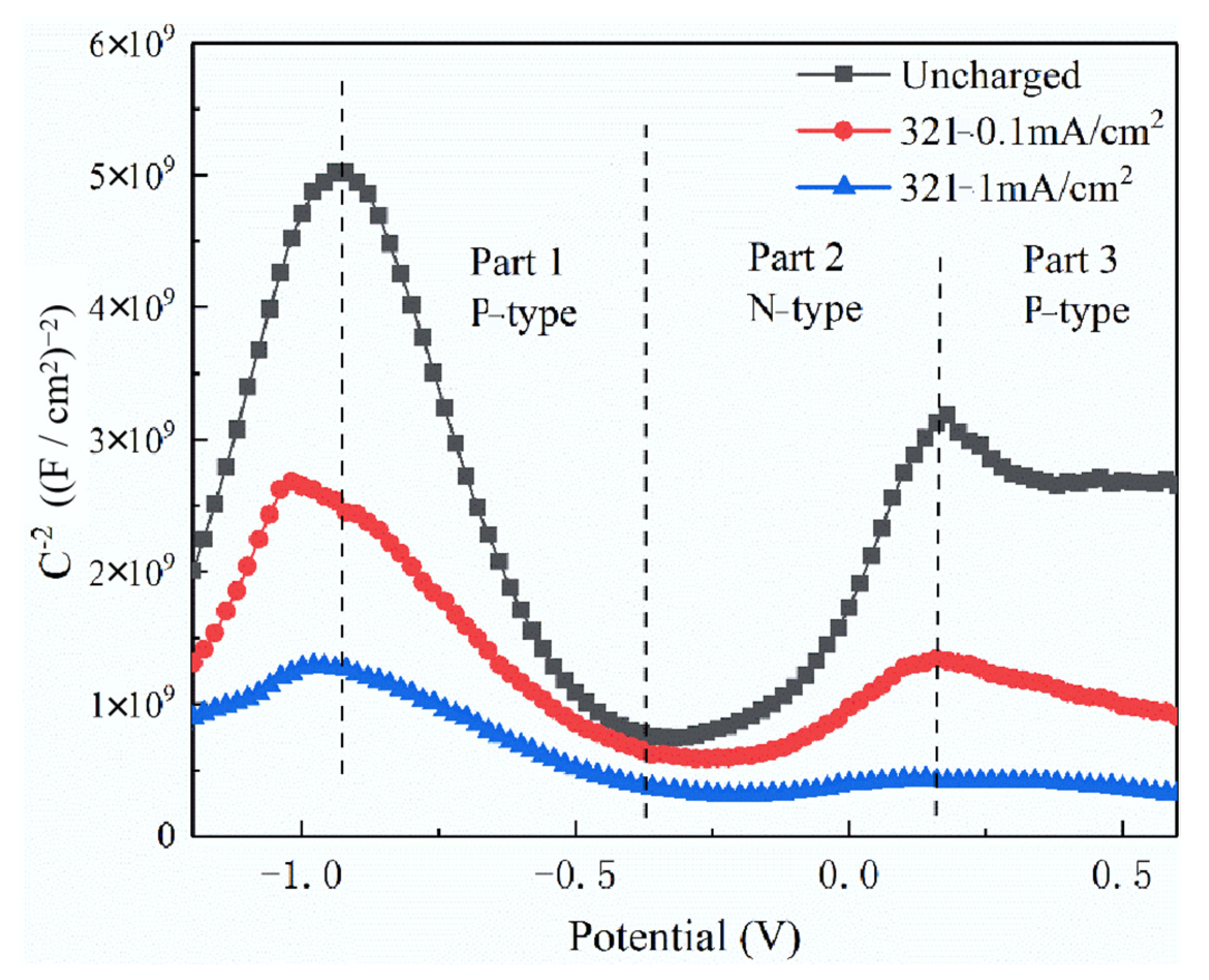
3.4. Effect of Hydrogen on Semiconductor Characteristics of Passive Film
4. Conclusions
- (1)
- Hydrogen can change the structure and chemical composition of a passivation film on 321 stainless steel and promote the reduction of Fe3+ to Fe2+ and the conversion of O−- to OH− and H2O. At the same time, it reduces the stability of the passivation film.
- (2)
- The polarization resistance decreases with the increase in hydrogen charging current density, which improves the corrosion rate of the metal. Hydrogen can increase the thermodynamic and kinetic tendency of corrosion reaction and cooperate with Cl− to promote the occurrence of pitting corrosion.
- (3)
- Under high hydrogen concentrations (10−80 mA/cm2), hydrogen can promote the transformation of the pitting structure of 321 stainless steel from “small and deep” to “large and shallow”.f Hydrogen reduces the protective performance of passive film and inhibits the formation of the passive film.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuguang, L.; Daoyou, L.; Shun, L.I. Corrosion Analysis and Protection Measures of Heat Exchanger in Hydrofining Unit. Liaoning Chem. Ind. 2020, 49, 699–701. [Google Scholar]
- Liang, W.; Fang, Y. Leakage analysis for a high pressure heat exchanger in hydrorefining unit. Pet. Refin. Eng. 2019, 49, 31–35. [Google Scholar]
- Ma, H. Analysis on corrosion cracking of tube bundles in 0Cr18Ni10Ti stainless steel heat exchangers. Petro-Chem. Equip. Technol. 2019, 40, 10–13. [Google Scholar]
- Panahi, H.; Eslami, A.; Golozar, M.; Laleh, A.A. An investigation on corrosion failure of a shell-and-tube heat exchanger in a natural gas treating plant. Eng. Fail. Anal. 2020, 118, 104918. [Google Scholar] [CrossRef]
- Wu, Y.M. Calculations estimate process stream depositions. Oil Gas J. 1994, 118, 38–41. [Google Scholar]
- Shargay, C.A.; Jacobs, G.E.; Price, M.D. Ammonium Salt Corrosion in Hydrotreating Unit Stripper Column Overhead Systems; NACE International: Houston, TX, USA, 1999. [Google Scholar]
- Ou, G.; Jin, H.; Xie, H.; Cao, J.; Qiu, J. Prediction of ammonium salt deposition in hydroprocessing air cooler tubes. Eng. Fail. Anal. 2011, 18, 1458–1464. [Google Scholar] [CrossRef]
- Wang, H.B.; Li, Y.; Cheng, G.X.; Wu, W.; Zhang, Y.H. A study on the corrosion behavior of carbon steel exposed to a H2S-containing NH4Cl medium. J. Mater. Eng. Perform. 2018, 27, 2492–2504. [Google Scholar] [CrossRef]
- Toba, K.; Ueyama, M.; Kawano, K.; Sakai, J. Corrosion of Carbon Steel and Alloys in Concentrated Ammonium Chloride Solutions. Corrosion 2012, 68, 1049–1056. [Google Scholar] [CrossRef]
- Saleem, B.; Ahmed, F.; Rafiq, M.A.; Ajmal, M.; Ali, L. Stress corrosion failure of an X52 grade gas pipeline. Eng. Fail. Anal. 2014, 46, 157–165. [Google Scholar] [CrossRef]
- Sui, R.; Wang, W.; Liu, Y.; Li, D.; Li, W. Root cause analysis of stress corrosion at tube-to-tube sheet joints of a waste heat boiler. Eng. Fail. Anal. 2014, 45, 398–405. [Google Scholar] [CrossRef]
- Alvisi, P.P.; Lins, V.D.F.C. Acid salt corrosion in a hydrotreatment plant of a petroleum refinery. Eng. Fail. Anal. 2008, 15, 1035–1041. [Google Scholar] [CrossRef]
- Xu, X.Q.; Niu, J.; Li, C.Z.; Huang, H.J.; Yin, C.X. Comparative Study on Hydrogen Embrittlement Susceptibility in Heat-Affected Zone of TP321 Stainless Steel. Mater. Sci. Forum 2020, 993, 568–574. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, H.; Yu, C.; Jin, H.; Wang, C.; Ou, G. Analysis on the corrosion failure of U-tube heat exchanger in hydrogenation unit. Eng. Fail. Anal. 2021, 125, 105448. [Google Scholar] [CrossRef]
- Xu, S.; Wang, C.; Wang, W. Failure Analysis of stress corrosion cracking in heat exchanger tubes during start-up operation. Eng. Fail. Anal. 2015, 51, 1–8. [Google Scholar] [CrossRef]
- Li, J.; Zhong, X.; Wang, T.; Shang, T.; Hu, J.; Zhang, Z.; Zeng, D.; Hou, D.; Shi, T. Synergistic effect of erosion and hydrogen on properties of passive film on 2205 duplex stainless steel. J. Mater. Sci. Technol. 2020, 67, 1–10. [Google Scholar] [CrossRef]
- Ningshen, S.; Mudali, U.K. Hydrogen effects on pitting corrosion and semiconducting properties of nitrogen-containing type 316L stainless steel. Electrochim. Acta 2009, 54, 6374–6382. [Google Scholar] [CrossRef]
- Xiuqing, X.; Junwei, A.; Chen, W.; Jing, N. Study on the hydrogen embrittlement susceptibility of AISI 321 stainless steel. Eng. Fail. Anal. 2021, 122, 105212. [Google Scholar] [CrossRef]
- Yin, L.; Liu, Y.; Dai, N.; Qian, S.; Wan, Y.; Wu, J.; Li, J.; Jiang, Y. Effect of Hydrogen Charging Conditions on Hydrogen Blisters and Pitting Susceptibility of 445J1M Ferritic Stainless Steel. J. Electrochem. Soc. 2018, 165, C1007–C1016. [Google Scholar] [CrossRef]
- Frankel, G.S.; Stockert, L.; Hunkeler, F.; Boehni, H. Metastable Pitting of Stainless Steel. Corrosion 1987, 43, 429–436. [Google Scholar] [CrossRef]
- Khalaj, G.; Khalaj, M.-J. Investigating the corrosion of the Heat-Affected Zones (HAZs) of API-X70 pipeline steels in aerated carbonate solution by electrochemical methods. Int. J. Press. Vessel. Pip. 2016, 145, 1–12. [Google Scholar] [CrossRef]
- Peng, X. Research on Microstructure Evolution and Corrosion Property of 316LN Stainless Steel for Nuclear Primary Pipe. Ph.D. Thesis, Nanjing University of Aeronautics and Astronautics, Nanjing, China, 2018. [Google Scholar]
- Yao, J. The Microelectrochemical Behavior and the Stability of Passive Film on 2205 Duplex Stainless Steel. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2019. [Google Scholar]


| C | Si | Mn | S | Ni | Cr | Ti | Fe |
|---|---|---|---|---|---|---|---|
| 0.02 | 0.63 | 2.23 | 0.70 | 10.03 | 18.05 | 0.28 | Bal. |
| Spectrum | Component | Binding Energy/eV | Peak Area /% | |
|---|---|---|---|---|
| before EHC | after EHC | |||
| Fe 2p3/2 | Fe | 706.7 | 0 | 9.11 |
| Fe3O4 | 708.8 | 5.19 | 9.17 | |
| FeO | 709.6 | 17 | 33.28 | |
| Fe2O3 | 710.8 | 59.66 | 28.96 | |
| FeOOH | 711.4 | 18.15 | 19.48 | |
| Cr 2p3/2 | Cr | 574.1 | 7.43 | 11.79 |
| Cr2O3 | 576.5 | 53.25 | 44.20 | |
| Cr(OH)3 | 577.4 | 39.32 | 44.01 | |
| O 1s | O2− | 530.2 | 13.61 | 8.03 |
| OH− | 531.3 | 69.33 | 59.32 | |
| H2O | 533.2 | 17.06 | 32.65 | |
| Condition | Rs/Ω·cm2 | Qf/F·cm−2 | Rf/Ω·cm2 | Qct/F·cm−2 | Rct/Ω·cm2 |
|---|---|---|---|---|---|
| Without EHC | 13.0 | 2.03 × 10−5 | 1.39 × 104 | 9.45 × 10−5 | 8.20 × 104 |
| 0.1 mA/cm2 | 14.4 | 3.46 × 10−5 | 2.98 × 103 | 7.89 × 10−5 | 6.75 × 103 |
| 1 mA/cm2 | 10.0 | 1.51 × 10−5 | 3.05 × 103 | 8.02 × 10−5 | 4.43 × 103 |
| 10 mA/cm2 | 10.2 | 5.02 × 10−5 | 1.38 × 103 | 1.64 × 10−4 | 2.76 × 103 |
| 80 mA/cm2 | 10.0 | 3.77 × 10−5 | 1.42 × 103 | 1.11 × 10−4 | 2.04 × 103 |
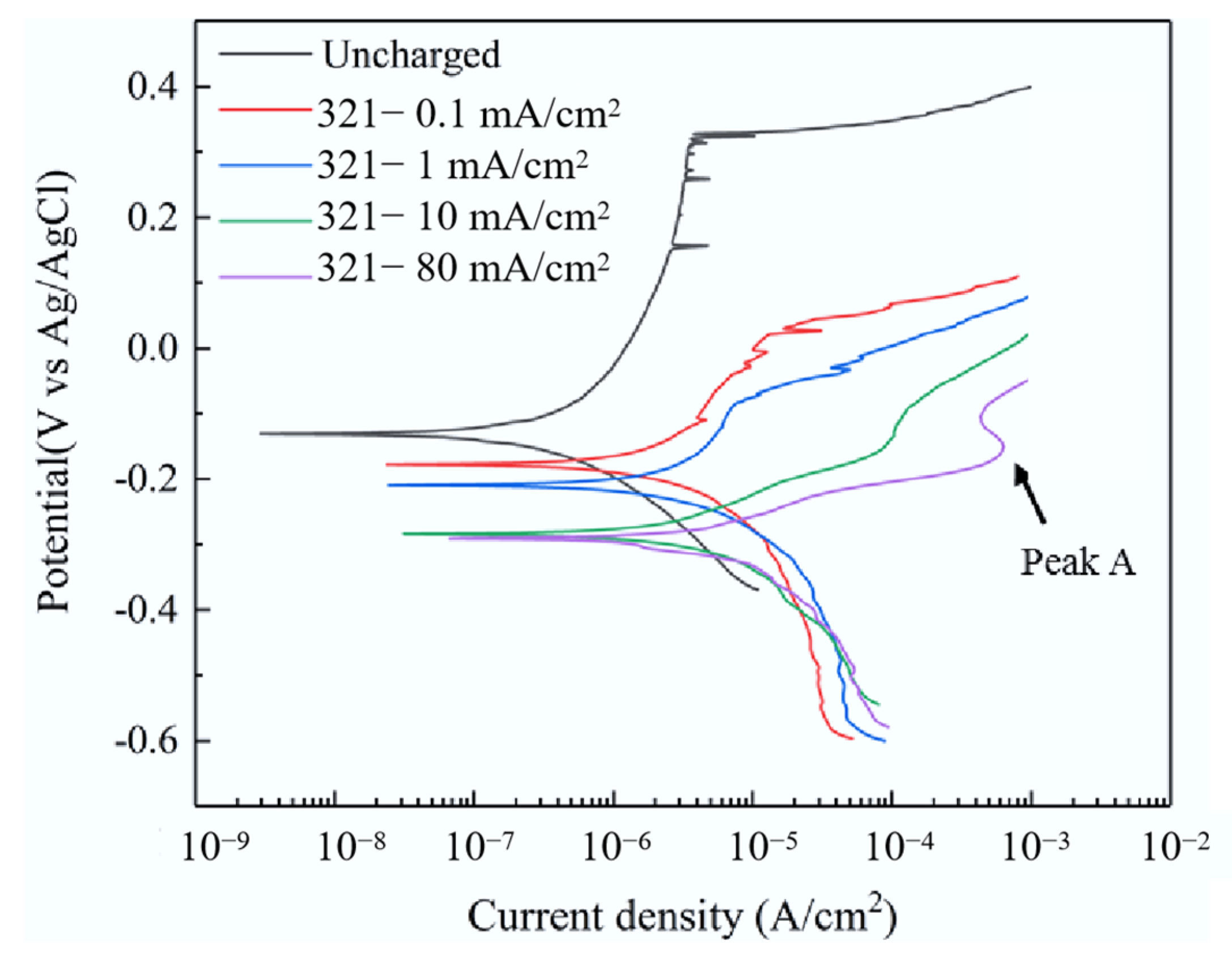
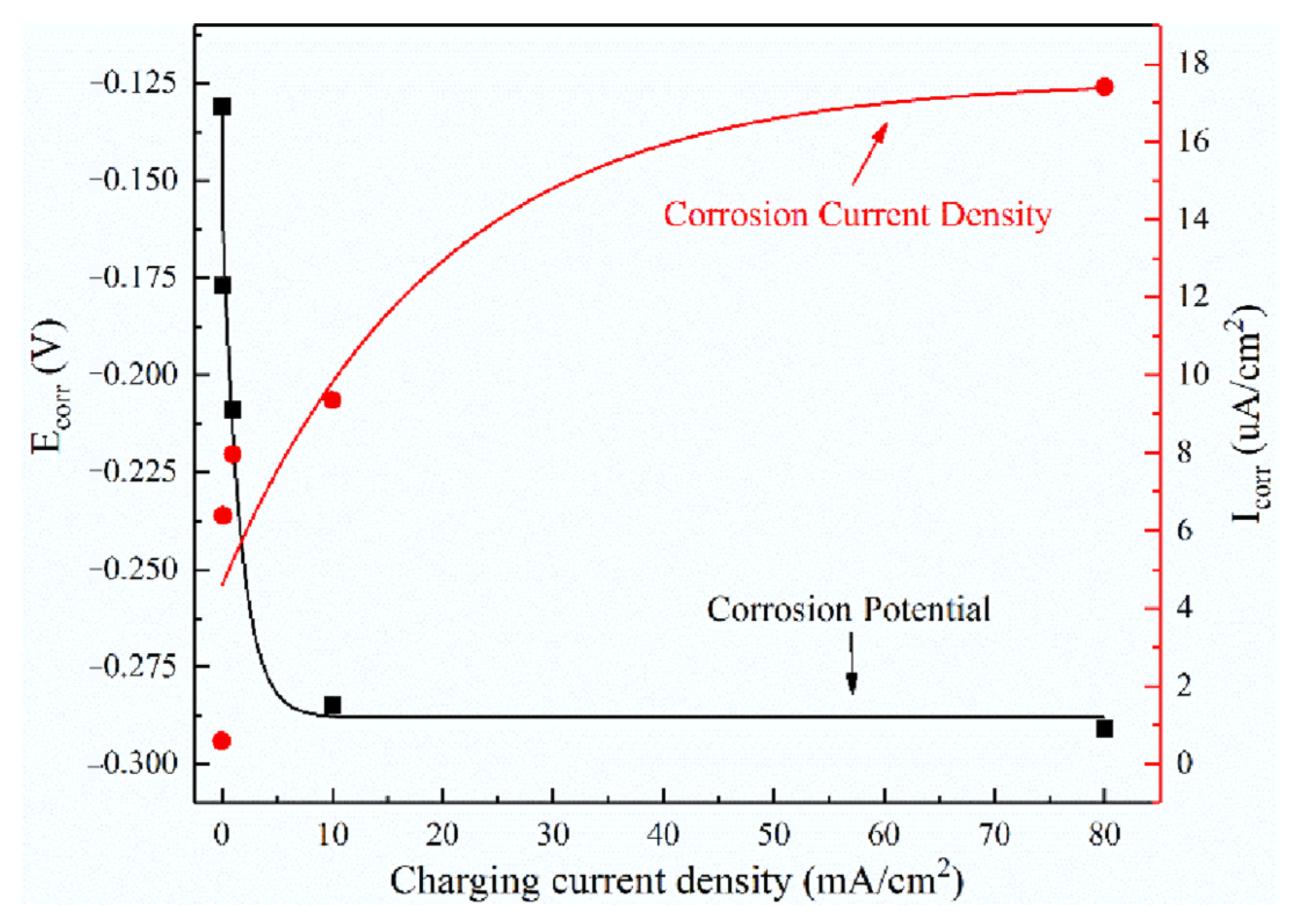
| Condition | Ecorr/V | Icorr/μA·cm−2 | Epit/V | Ipass/μA·cm−2 |
|---|---|---|---|---|
| Without EHC | −0.131 | 0.593 | 0.315 | 3.612 |
| 0.1 mA/cm2 | −0.177 | 6.384 | 0.021 | 12.884 |
| 1 mA/cm2 | −0.209 | 7.965 | −0.084 | 7.517 |
| 10 mA/cm2 | −0.285 | 9.349 | / | / |
| 80 mA/cm2 | −0.291 | 17.418 | / | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Wang, W.; Suo, T.; Jiang, L.; Yin, X.; Cheng, G.; Fu, A.; Yang, F. Effect of Hydrogen on Corrosion Behavior of 321 Stainless Steel in NH4Cl Solution. Materials 2022, 15, 7010. https://doi.org/10.3390/ma15197010
Xu X, Wang W, Suo T, Jiang L, Yin X, Cheng G, Fu A, Yang F. Effect of Hydrogen on Corrosion Behavior of 321 Stainless Steel in NH4Cl Solution. Materials. 2022; 15(19):7010. https://doi.org/10.3390/ma15197010
Chicago/Turabian StyleXu, Xiuqing, Wei Wang, Tao Suo, Lei Jiang, Xiangkun Yin, Guangxu Cheng, Anqing Fu, and Fang Yang. 2022. "Effect of Hydrogen on Corrosion Behavior of 321 Stainless Steel in NH4Cl Solution" Materials 15, no. 19: 7010. https://doi.org/10.3390/ma15197010





