Synthesis of Ti-Al-xNb Ternary Alloys via Laser-Engineered Net Shaping for Biomedical Application: Densification, Electrochemical and Mechanical Properties Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Laser Metal Deposition
2.2.2. Sample Preparation and Analyses
2.2.3. Microhardness
2.2.4. Density
2.2.5. Corrosion Test
3. Results and Discussion
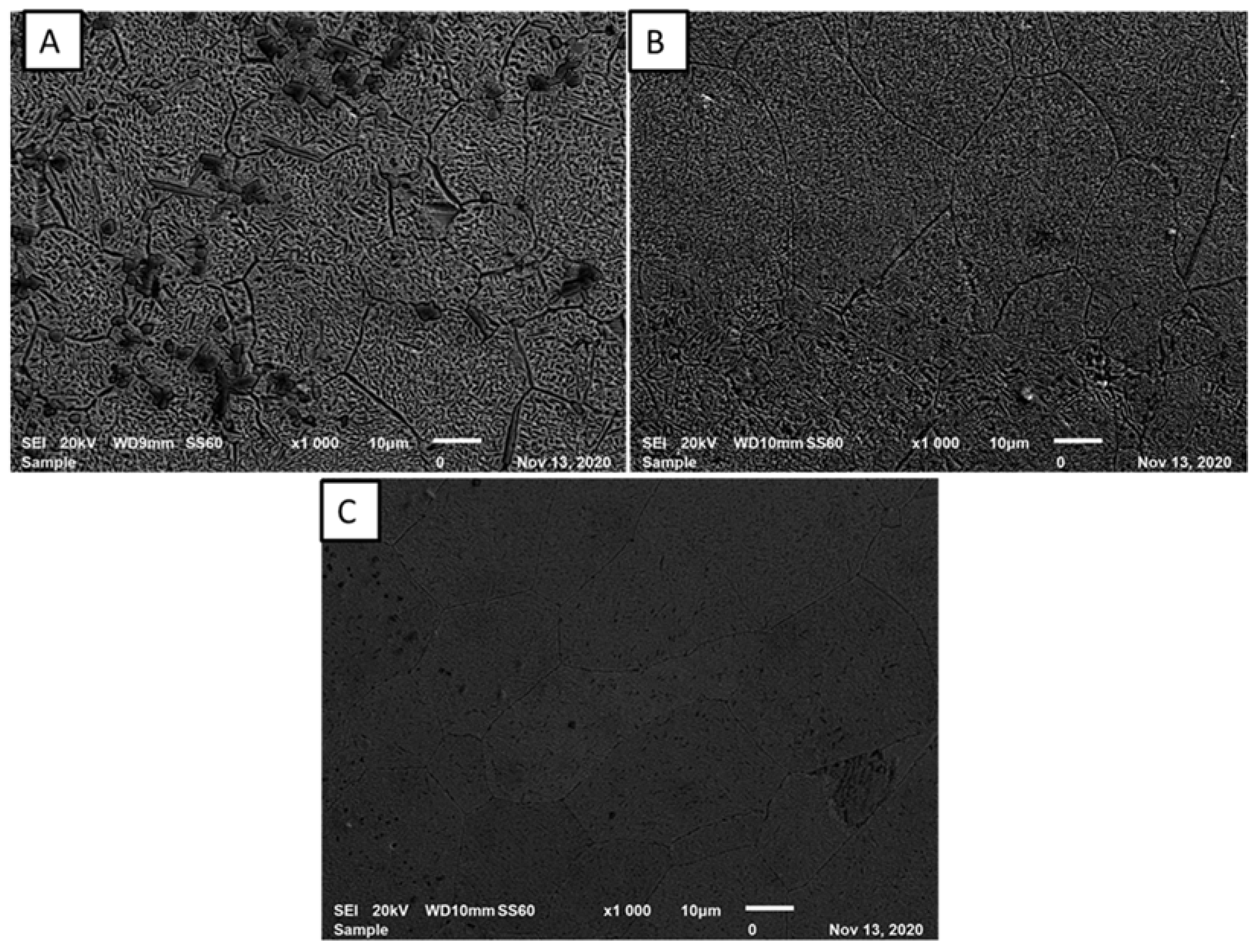
3.1. Microstructural Results
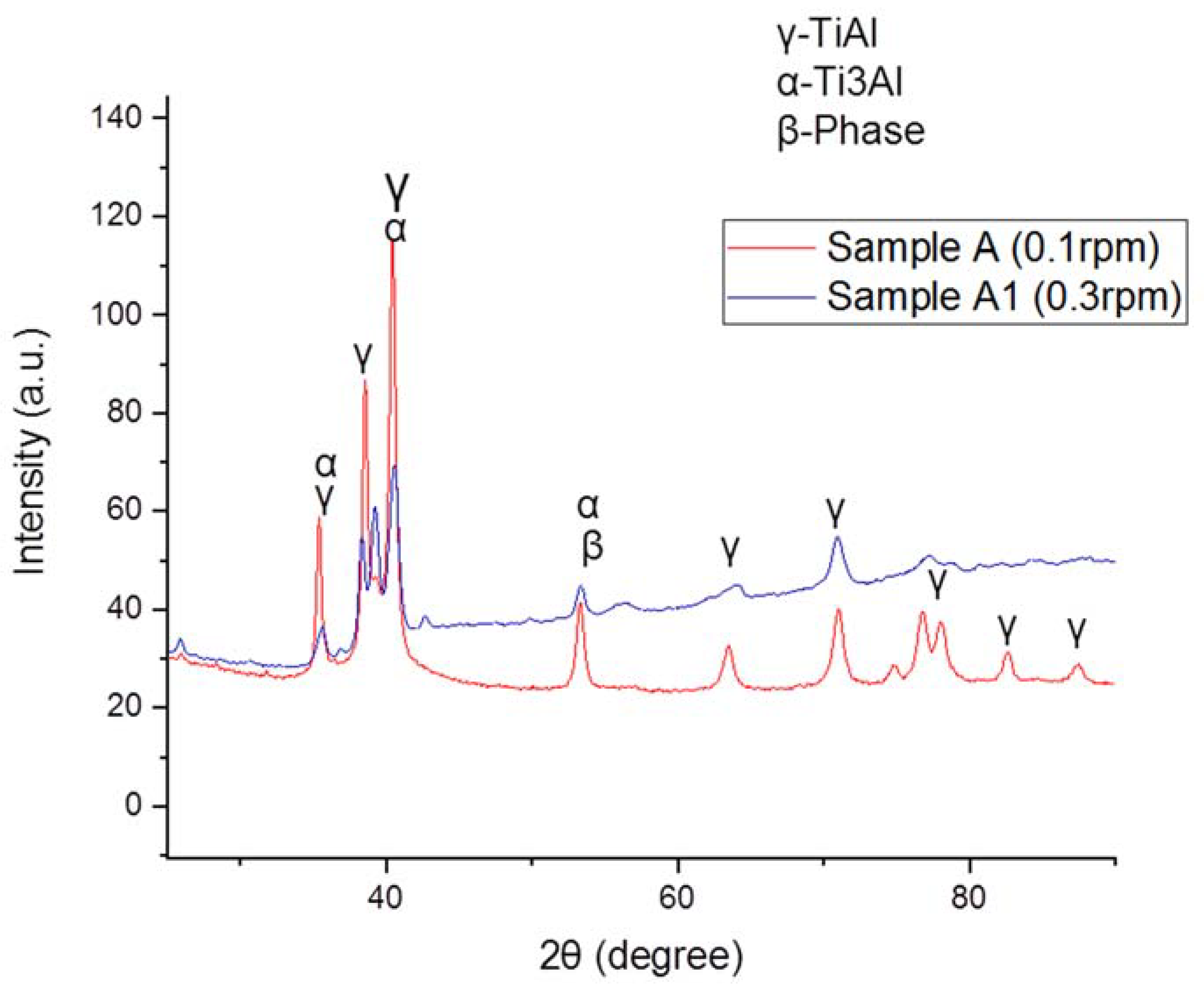
3.2. Phase Analysis Results
3.3. Densification Results
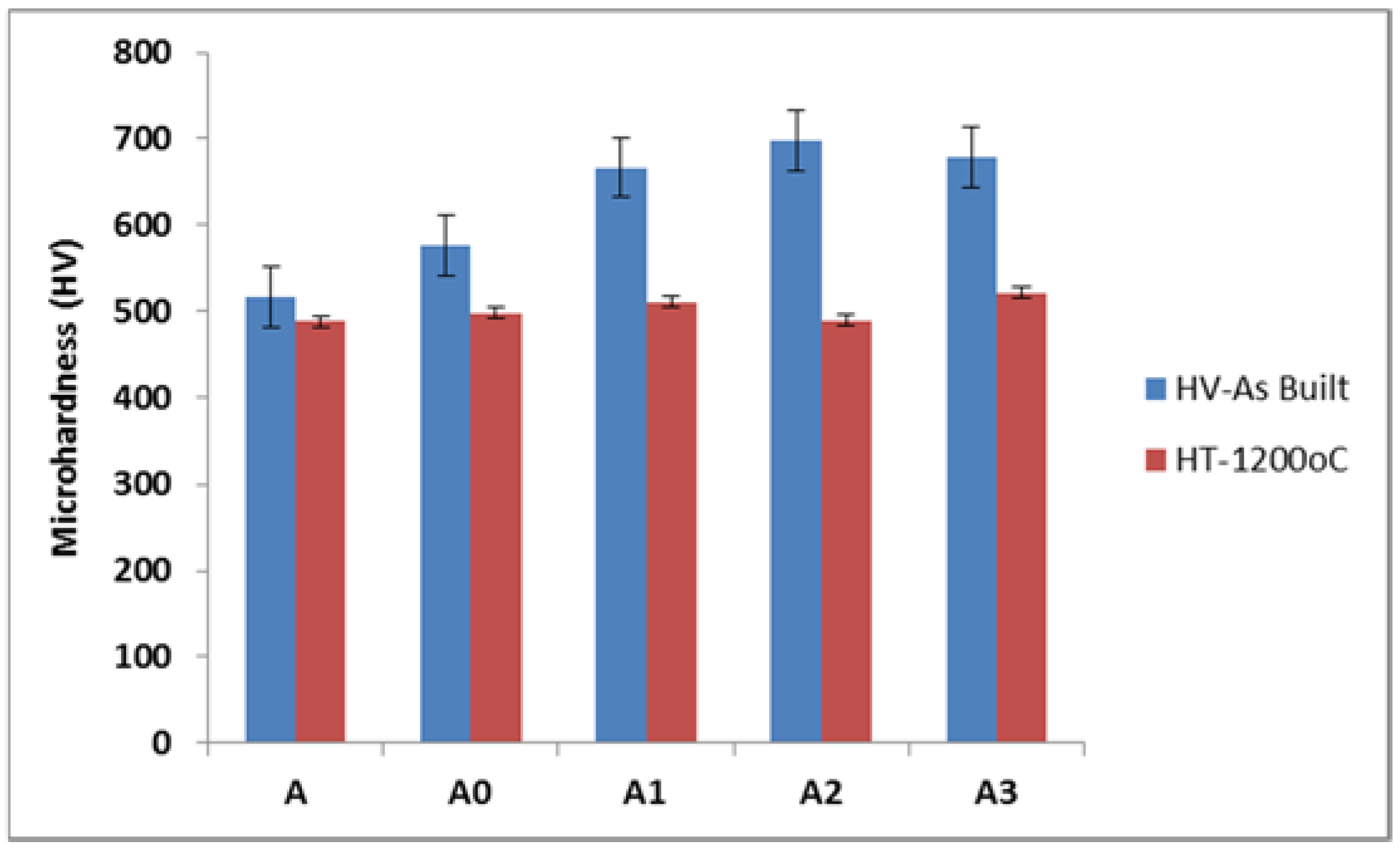
3.4. Microhardness Results
3.5. Corrosion Results
SEM Images of Corroded Samples in 3.65% NaCl Solution
4. Conclusions
- ✓
- Ti-Al-xNb was successfully synthesized as a biomaterial by means of LENS-additive manufacturing and the following conclusions were drawn:
- ✓
- The EDS of the samples suggested that all incorporated elemental powders were available in the fabricated Ti-Al-xNb alloy.
- ✓
- The effect of the Nb feed rate was sufficient to create different microstructural evolutions, as presented by SEM images
- ✓
- Heat-treatment of the developed samples presented a significant decrease in microhardness with a maximum hardness value of 521.4 HV, which suggest that stress relief was achieved.
- ✓
- A high potential of −0.62267 V was evident for sample A2, with a current density of 8.28 × 10−5 A/cm2.
- ✓
- SEM/EDS of corroded samples presented no evidence of rust, however, oxide-protective layers are evident on the surface of the alloys
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rack, H.; Qazi, J. Titanium alloys for biomedical applications. Mater. Sci. Eng. C 2006, 26, 1269–1277. [Google Scholar] [CrossRef]
- Majumdar, P.; Singh, S.; Chakraborty, M. Elastic modulus of biomedical titanium alloys by nano-indentation and ultrasonic techniques—A comparative study. Mater. Sci. Eng. A 2008, 489, 419–425. [Google Scholar] [CrossRef]
- Iijima, D.; Yoneyama, T.; Doi, H.; Hamanaka, H.; Kurosaki, N. Wear properties of Ti and Ti–6Al–7Nb castings for dental prostheses. Biomaterials 2003, 24, 1519–1524. [Google Scholar] [CrossRef]
- Dalmau, A.; Pina, V.G.; Devesa, F.; Amigó, V.; Muñoz, A.I. Electrochemical behavior of near-beta titanium biomedical alloys in phosphate buffer saline solution. Mater. Sci. Eng. C 2015, 48, 55–62. [Google Scholar] [CrossRef]
- Buciumeanu, M.; Bagheri, A.; Shamsaei, N.; Thompson, S.; Silva, F.; Henriques, B. Tribocorrosion behavior of additive manufactured Ti-6Al-4V biomedical alloy. Tribol. Int. 2018, 119, 381–388. [Google Scholar] [CrossRef]
- Leśniak-Ziółkowska, K.; Płonka, J.; Śmiga-Matuszowicz, M.; Brzychczy-Włoch, M.; Kazek-Kęsik, A. Functionalization of PEO layer formed on Ti-15Mo for biomedical application. J. Biomed. Mater. Res. B 2020, 108, 1568–1579. [Google Scholar] [CrossRef]
- Vorobjova, A.; Tishkevich, D.; Shimanovich, D.; Zdorovets, M.; Kozlovskiy, A.; Zubar, T.; Vinnik, D.; Dong, M.; Trukhanov, S.; Trukhanov, A. Electrochemical behaviour of Ti/Al2O3/Ni nanocomposite material in artificial physiological solution: Prospects for biomedical application. Nanomaterials 2020, 10, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarka, M.; Dikici, B.; Niinomi, M.; Ezirmik, K.; Nakai, M.; Yilmazer, H. A systematic study of β-type Ti-based PVD coatings on magnesium for biomedical application. Vacuum 2020, 183, 109850. [Google Scholar] [CrossRef]
- Aissa, C.B.; Khlifi, K. CAE-PVD synthesis and characterization of titanium-based biocompatible coatings deposited on titanium alloy for biomedical application. Mater. Today Proc. 2021, 42, A10–A17. [Google Scholar] [CrossRef]
- Mu, J.; Wang, H.; Qin, B.; Zhang, Y.; Chen, L.; Zeng, C. Improved wear and corrosion resistance of biological compatible TiZrNb films on biomedical Ti6Al4V substrates by optimizing sputtering power. Surf. Coat. Tech. 2021, 428, 127866. [Google Scholar] [CrossRef]
- Zhao, X.; Shi, X.; Zhang, W.; Wang, X.; Zhang, L. TiAl–nHA composite coatings for sintering protection of carbon fiber and improvement of carbon fiber–hydroxyapatite composites interface. J. Alloys Compd. 2020, 854, 157027. [Google Scholar] [CrossRef]
- Cruz, H.V.; Souza, J.C.M.; Henriques, M.; Rocha, L.A. Tribocorrosion and bio-tribocorrosion in the oral environment: The case of dental implants. In Biomedical Tribology; Davim, J.P., Ed.; Nova Science Publishers: New York, NY, USA, 2011; pp. 1–33. [Google Scholar]
- Zakaria, S.M.; Zein, S.H.S.; Othman, M.R.; Yang, F.; Jansen, J.A. Nanophase Hydroxyapatite as a Biomaterial in Advanced Hard Tissue Engineering: A Review. Tissue Eng. Part B Rev. 2013, 19, 431–441. [Google Scholar] [CrossRef]
- Lu, X.W.; Lin, X.; Cao, Y.Q.; Hu, J.; Gao, B.; Huang, W.D. Effects of Processing Parameters and Heat Treatment on Phase Structure of the Hydroxyapatite Coating on Pure Ti Surface by Laser Cladding in-situ Synthesis. Rare Met. Mater. Eng. 2011, 40, 714–717. [Google Scholar]
- Tuukkanen, J.; Nakamura, M. Hydroxyapatite as a nanomaterial for advanced tissue engineering and drug therapy. Curr. Pharm. Des. 2017, 23, 3786–3793. [Google Scholar] [CrossRef] [Green Version]
- Ratha, I.; Datta, P.; Balla, V.K.; Nandi, S.K.; Kundu, B. Effect of doping in hydroxyapatite as coating material on biomedical implants by plasma spraying method: A review. Ceram. Int. 2020, 47, 4426–4445. [Google Scholar] [CrossRef]
- Fras, Z.; Sahebkar, A.; Banach, M. The Use of Aspirin in Contemporary Primary Prevention of Atherosclerotic Cardiovascular Diseases Revisited: The Increasing Need and Call for a Personalized Therapeutic Approach. Am. J. Cardiovasc. Drugs 2020, 21, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Krishna, B.V.; Bose, S.; Bandyopadhyay, A. Low stiffness porous Ti structures for load-bearing implants. Acta Biomater. 2007, 3, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Krishna, B.V.; Bandyopadhyay, A.; Bose, S. Processing and biocompatibility evaluation of laser processed porous titanium. Acta Biomater. 2007, 3, 1007–1018. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Shivaram, A.; Tarafder, S.; Sahasrabudhe, H.; Banerjee, D.; Bose, S. In Vivo Response of Laser Processed Porous Titanium Implants for Load-Bearing Implants. Ann. Biomed. Eng. 2016, 45, 249–260. [Google Scholar] [CrossRef] [Green Version]
- Bandyopadhyay, A.; Mitra, I.; Shivaram, A.; Dasgupta, N.; Bose, S. Direct comparison of additively manufactured porous titanium and tantalum implants towards in vivo osseointegration. Addit. Manuf. 2019, 28, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Maharubin, S.; Hu, Y.; Sooriyaarachchi, D.; Cong, W.; Tan, G.Z. Laser engineered net shaping of antimicrobial and biocompatible titanium-silver alloys. Mater. Sci. Eng. C 2019, 105, 110059. [Google Scholar] [CrossRef]
- Wen, H.; Zeng, C.; Ettefagh, A.H.; Gao, J.; Guo, S. Laser surface treatment of Ti-10Mo alloy under Ar and N2 environment for biomedical application. J. Laser Appl. 2019, 31, 022012. [Google Scholar] [CrossRef]
- Das, M.; Balla, V.; Kumar, T.S.S.; Manna, I. Fabrication of Biomedical Implants using Laser Engineered Net Shaping (LENS™). Trans. Indian Ceram. Soc. 2013, 72, 169–174. [Google Scholar] [CrossRef]
- Vail, N.; Swain, L.; Fox, W.; Aufdlemorte, T.; Lee, G.; Barlow, J. Materials for biomedical applications. Mater. Des. 1999, 20, 123–132. [Google Scholar] [CrossRef]
- Steen, W.M.; Mazumder, J. Laser Material Processing; Springer Science & Business Media: London, UK, 2010. [Google Scholar]
- Arthur, N.; Malabi, K.; Baloyi, P.; Moller, H.; Pityana, S. Influence of process parameters on layer build-up and microstructure of Ti6Al4V (ELI) alloy on the optomec LENS. In Proceedings of the 17th Annual Conference of the Rapid Product Development Association of South Africa (RAPDASA), VUT Southern Gauteng Science and Technology Park, Vaal, South Africa, 2–4 November 2016. [Google Scholar]
- Izadi, M.; Farzaneh, A.; Mohammed, M.; Gibson, I.; Rolfe, B. A review of laser engineered net shaping (LENS) build and process parameters of metallic parts. Rapid Prototyp. J. 2020, 26, 1059–1078. [Google Scholar] [CrossRef]
- Branzoi, I.V.; Iordoc, M.; Branzoi, F. Surface Characterization and Corrosion Behaviour of Ti Based Alloys in Fetal Bovine Serum. UPB Sci. Bull. Ser. B 2009, 71, 31–40. [Google Scholar]
- Malatji, N.; Popoola, A.P.I.; Lengopeng, T.; Pityana, S. Effect of Nb addition on the microstructural, mechanical and electrochemical characteristics of AlCrFeNiCu high-entropy alloy. Int. J. Miner. Metall. Mater. 2020, 27, 1332–1340. [Google Scholar] [CrossRef]
- Kanyane, L.; Adesina, O.; Popoola, P.; Farotade, G.; Malatji, N. Microstructural evolution and corrosion properties of laser clad Ti-Ni on titanium alloy (Ti6Al4V). Procedia Manuf. 2019, 35, 1267–1272. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X.; Wang, X.; Zhang, C.; Chai, L.; Chen, Y. Microstructural investigation of a rapidly solidified Ti–Zr–Fe–Si–Sn–Nb alloy. J. Alloys Compd. 2010, 504, S480–S482. [Google Scholar] [CrossRef]
- Rabadia, C.; Liu, Y.; Cao, G.; Li, Y.; Zhang, C.; Sercombe, T.; Sun, H.; Zhang, L. High-strength β stabilized Ti-Nb-Fe-Cr alloys with large plasticity. Mater. Sci. Eng. A 2018, 732, 368–377. [Google Scholar] [CrossRef]
- Fischer, F.D.; Waitz, T.; Scheu, C.; Cha, L.; Dehm, G.; Antretter, T.; Clemens, H. Study of nanometer-scaled lamellar microstructure in a Ti–45Al–7.5 Nb alloy–Experiments and modeling. Intermetallics 2010, 18, 509–517. [Google Scholar] [CrossRef]
- Sibisi, P.N.; Popoola, A.P.; Arthur, N.K.; Kubjane, S.M.; Ngoveni, A.S.; Kanyane, L.R. Evaluation of hatch distance and powder feed rate effects in TI-6AL-4V alloy developed by LMD technique. In Proceedings of the 19th Annual International RAPDASA Conference, Johannesburg, South Africa, 6–9 November 2018. [Google Scholar]
- Makena, M.I.; Shongwe, M.B.; Ramakokovhu, M.M.; Olubambi, P.A. Effect of sintering parameters on densification, corrosion and wear behaviour of Ni-50Fe alloy prepared by spark plasma sintering. J. Alloys Compd. 2017, 699, 1166–1179. [Google Scholar] [CrossRef]
- Liu, H.W.; Bishop, D.P.; Plucknett, K.P. Densification behaviour and microstructural evolution of Ti-48Al consolidated by spark plasma sintering. J. Mater. Sci. 2016, 52, 613–627. [Google Scholar] [CrossRef]
- Masina, B.N.; Skhosane, S.; Hoosain, S.; Tlotleng, M. Effect of in-situ heat treatment and process parameters on the laser-deposited IN718 microstructure and mechanical properties. MRS Adv. 2020, 5, 1245–1257. [Google Scholar] [CrossRef]
- Distl, B.; Dehm, G.; Stein, F. Effect of Oxygen on High-temperature Phase Equilibria in Ternary Ti-Al-Nb Alloys. Z. Anorg. Allg. Chem. 2020, 646, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Lazurenko, D.V.; Laptev, I.S.; Golkovsky, M.G.; Stark, A.; Paul, J.; Bataev, I.; Ruktuev, A.A.; Song, L.; Gollwitzer, C.; Pyczak, F. Influence of the Ti/Al/Nb ratio on the structure and properties on intermetallic layers obtained on titanium by non-vacuum electron beam cladding. Mater. Charact. 2020, 163, 110246. [Google Scholar] [CrossRef]
- Tlotleng, M.; Lengopeng, T.; Seerane, M.N.; Pityana, S.L. Effects of Heat-Treatment on the Microstructure of TiAl-Nb Produced with Laser Metal Deposition Technique; David L. Lawrence Convention Center: Pittsburgh, PA, USA, 2017. [Google Scholar]
- Hoosain, S.E.; Pityana, S.; Freemantle, C.S.; Tlotleng, M. Heat Treatment of In Situ Laser-Fabricated Titanium Aluminide. Metals 2018, 8, 655. [Google Scholar] [CrossRef] [Green Version]
- Seidel, A.; Saha, S.; Maiwald, T.; Moritz, J.; Polenz, S.; Marquardt, A.; Kaspar, J.; Finaske, T.; Lopez, E.; Brueckner, F.; et al. Intrinsic Heat Treatment Within Additive Manufacturing of Gamma Titanium Aluminide Space Hardware. JOM 2019, 71, 1513–1519. [Google Scholar] [CrossRef]
- Manivasagam, G.; Dhinasekaran, D.; Rajamanickam, A. Biomedical implants: Corrosion and its prevention-a review. Recent Pat. Corros. Sci. 2010, 2, 40–54. [Google Scholar] [CrossRef] [Green Version]
- Krzywicka, M.; Antoszewski, B.; Palka, K.; Tofil, S. Ti6Al7Nb Alloy Laser Micromachining-Surface Properties. In Proceedings of the 2018 Conference on Electrotechnology: Processes, Models, Control and Computer Science (EPMCCS), Kielce, Poland, 12–14 November 2018. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Niinomi, M.; Akahori, T.; Fukui, H.; Toda, H. Corrosion resistance and biocompatibility of Ti–Ta alloys for biomedical applications. Mater. Sci. Eng. A 2005, 398, 28–36. [Google Scholar] [CrossRef]
- Shi, Y.Z.; Collins, L.; Feng, R.; Zhang, C.; Balke, N.; Liaw, P.K.; Yang, B. Homogenization of AlxCoCrFeNi high-entropy alloys with improved corrosion resistance. Corros. Sci. 2018, 133, 12–131. [Google Scholar] [CrossRef]
- Kumar, N.; Fusco, M.; Komarasamy, M.; Mishra, R.; Bourham, M.; Murty, K. Understanding effect of 3.5 wt.% NaCl on the corrosion of Al0.1CoCrFeNi high-entropy alloy. J. Nucl. Mater. 2017, 495, 154–163. [Google Scholar] [CrossRef]
- Rodriguez, A.A.; Tylezak, J.H.; Gao, M.C.; Jablonski, P.D.; Detrois, M.; Ziomek-Moroz, M.; Hawk, J.A. Effect of Molybdenum on the Corrosion Behaviour of High-Entropy Alloys CoCrFeNi2 and CoCrFeNi2Mo0.25 under Sodium Chloride Aqueous Conditions. Adv. Mater. Sci. Eng. 2018, 2018, 11. [Google Scholar] [CrossRef] [Green Version]
- Nene, S.; Frank, M.; Liu, K.; Sinha, S.; Mishra, R.; McWilliams, B.; Cho, K. Corrosion-resistant high entropy alloy with high strength and ductility. Scr. Mater. 2019, 166, 168–172. [Google Scholar] [CrossRef]





| Parameter | Symbol | Set-Value | Unit |
|---|---|---|---|
| Laser power | P | 450 | W |
| Laser spot size | D | 1.4 | mm |
| Deposition speed | S | 26 | in/min |
| Al powder | M-Al | 2.4 | L/min |
| Ti powder | M-Ti | 4.2 | L/min |
| Nb powder | M-Nb | 1.0 | L/min |
| Nb Feed Rate (g/min) | |
|---|---|
| Sample Code | |
| A | 0.041 |
| A0 | 0.043 |
| A1 | 0.052 |
| A2 | 0.055 |
| A3 | 0.061 |
| Elements in Atomic % | Theoretical Density (g/cm3) | Actual Density (g/cm3) | |||
|---|---|---|---|---|---|
| Sample | Ti | Al | Nb | ||
| A | 56.22 (56) | 38.75 (39) | 5.04 (5) | 4.06 | 4.0245 |
| A1 | 47.34 (47) | 42.44 (42) | 10.22 (10) | 4.22 | 4.2035 |
| A3 | 51.34 (51) | 34.89 (35) | 13.77 (14) | 4.50 | 4.4585 |
| Sample Code | Ecorr, Obs (V) | Jcorr (A/cm²) | Corrosion Rate (mm/Year) | Polarization Resistance (Ω) |
|---|---|---|---|---|
| A | −0.80245 | 6.99 × 10−5 | 0.021175 | 1258.5 |
| A0 | −1.1062 | 3.63 × 10−5 | 0.082186 | 1140.83 |
| A1 | −1.0843 | 0.000267 | 0.081057 | 1128.75 |
| A2 | −0.62267 | 8.28 × 10−5 | 0.01023 | 2536.41 |
| A3 | −0.94834 | 0.000181 | 0.06053 | 1464.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanyane, L.R.; Popoola, A.P.I.; Pityana, S.; Tlotleng, M. Synthesis of Ti-Al-xNb Ternary Alloys via Laser-Engineered Net Shaping for Biomedical Application: Densification, Electrochemical and Mechanical Properties Studies. Materials 2022, 15, 544. https://doi.org/10.3390/ma15020544
Kanyane LR, Popoola API, Pityana S, Tlotleng M. Synthesis of Ti-Al-xNb Ternary Alloys via Laser-Engineered Net Shaping for Biomedical Application: Densification, Electrochemical and Mechanical Properties Studies. Materials. 2022; 15(2):544. https://doi.org/10.3390/ma15020544
Chicago/Turabian StyleKanyane, Lehlogonolo Rudolf, Abimbola Patricia Idowu Popoola, Sisa Pityana, and Monnamme Tlotleng. 2022. "Synthesis of Ti-Al-xNb Ternary Alloys via Laser-Engineered Net Shaping for Biomedical Application: Densification, Electrochemical and Mechanical Properties Studies" Materials 15, no. 2: 544. https://doi.org/10.3390/ma15020544
APA StyleKanyane, L. R., Popoola, A. P. I., Pityana, S., & Tlotleng, M. (2022). Synthesis of Ti-Al-xNb Ternary Alloys via Laser-Engineered Net Shaping for Biomedical Application: Densification, Electrochemical and Mechanical Properties Studies. Materials, 15(2), 544. https://doi.org/10.3390/ma15020544







