Resin Cement–Zirconia Bond Strengthening by Exposure to Low-Temperature Atmospheric Pressure Multi-Gas Plasma
Abstract
:1. Introduction
2. Materials and Methods
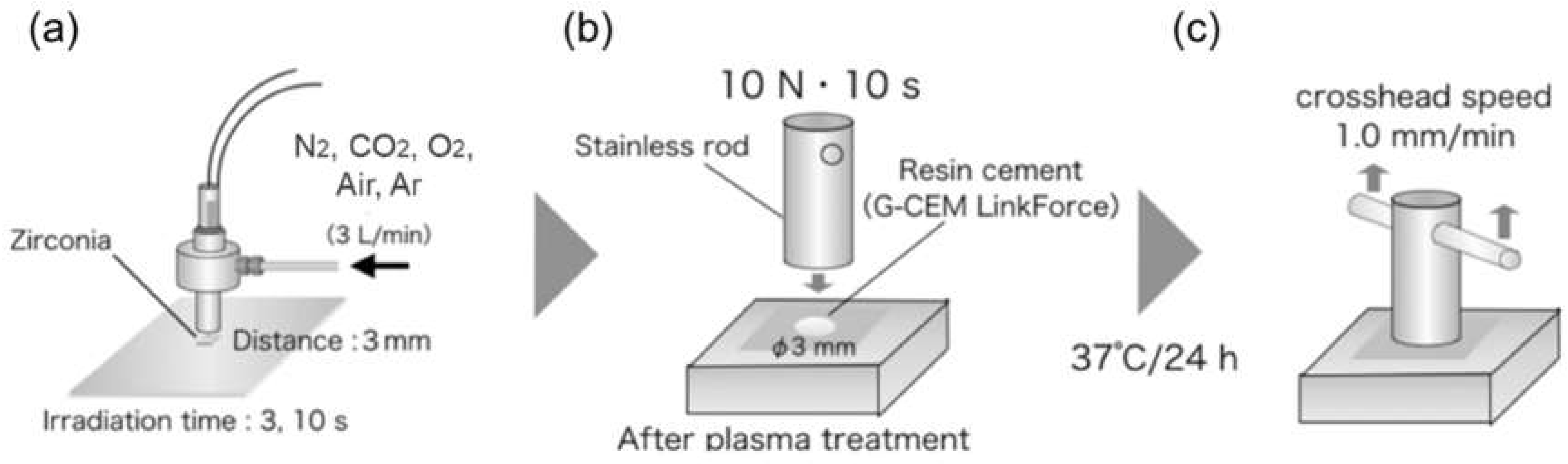
2.1. Preparation of Specimens
2.2. Evaluation of Tensile Bond Strength between Zirconia and Cement
2.3. Evaluation of Wettability of Zirconia Surface
2.4. Analysis of Zirconia Surface Topography
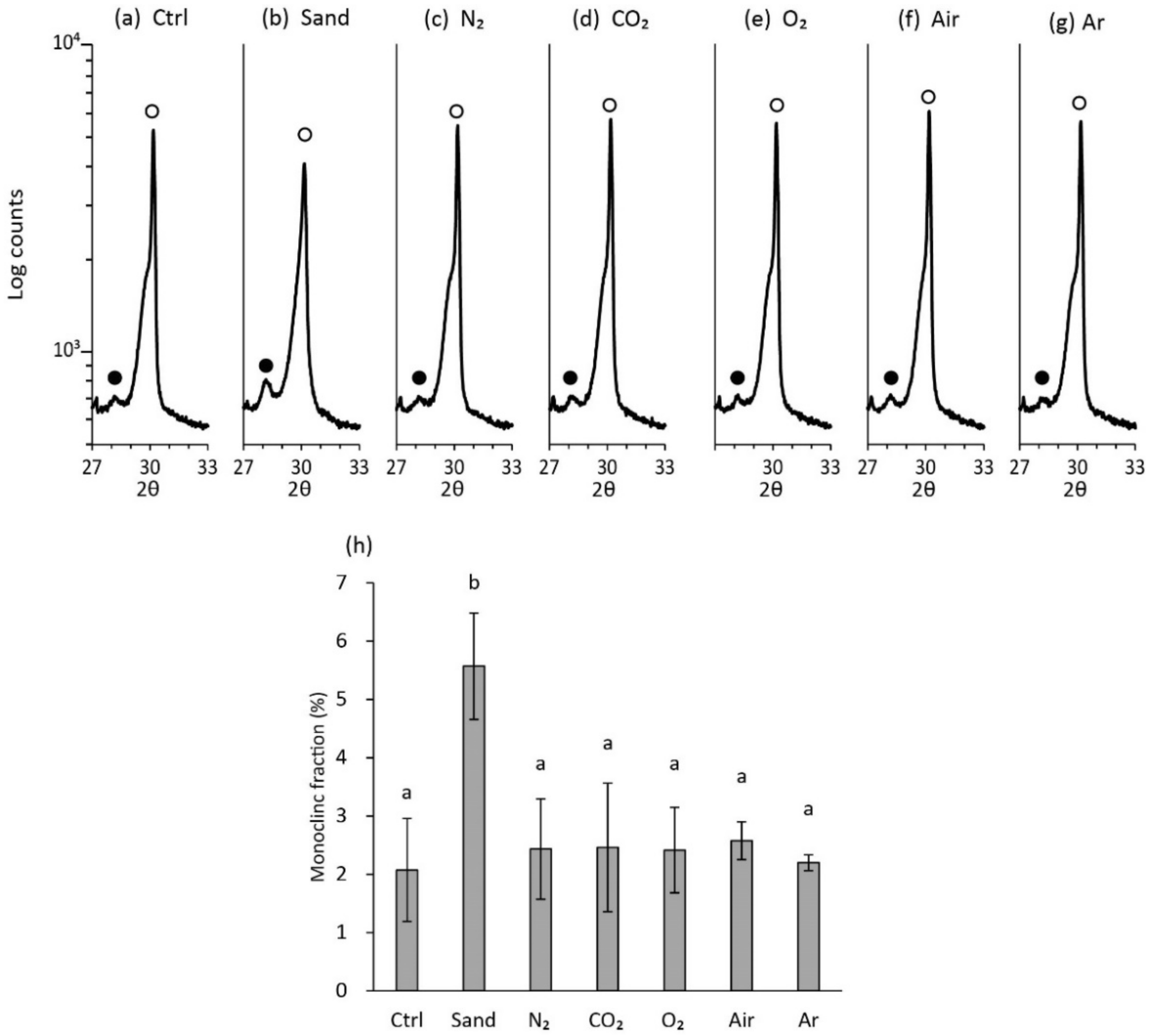
2.5. Analysis of Crystalline Structure of Zirconia Surface
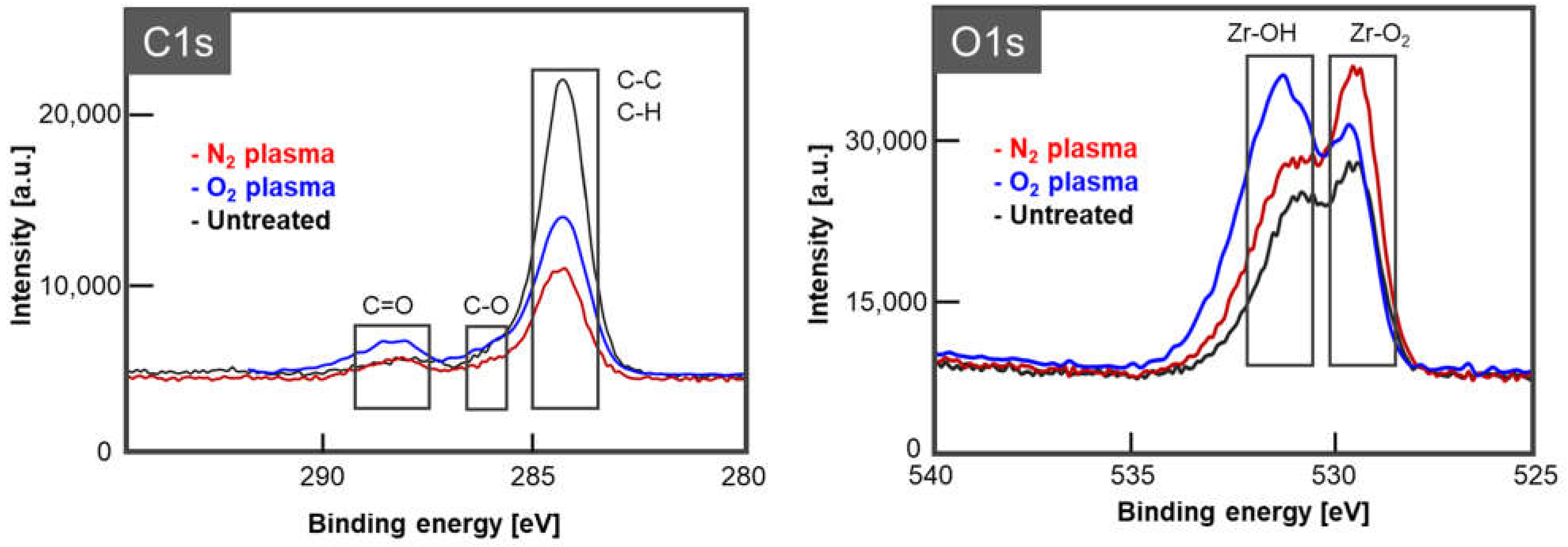
2.6. Analysis of Zirconia Surface Chemical Composition
3. Results
3.1. Tensile Bond Strength between Zirconia and Cement
3.2. Water Contact Angle of Zirconia Surface
3.3. Zirconia Surface Topography
3.4. Crystalline Structure of Zirconia Surface
3.5. Zirconia Surface Chemical Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Denry, I.; Kelly, J.R. State of the Art of Zirconia for Dental Applications. Dent. Mater. 2008, 24, 299–307. [Google Scholar] [CrossRef]
- Miyazaki, T.; Nakamura, T.; Matsumura, H.; Ban, S.; Kobayashi, T. Current Status of Zirconia Restoration. J. Prosthodont. Res. 2013, 57, 236–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okada, M.; Taketa, H.; Torii, Y.; Irie, M.; Matsumoto, T. Optimal Sandblasting Conditions for Conventional-Type Yttria-Stabilized Tetragonal Zirconia Polycrystals. Dent. Mater. 2019, 35, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.S.; Zhang, Y.; Sky Driver, M.; Caruso, A.N.; Yu, Q.; Wang, Y. Surface Modification of Several Dental Substrates by Non-Thermal, Atmospheric Plasma Brush. Dent. Mater. 2013, 29, 871–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takamatsu, T.; Hirai, H.; Sasaki, R.; Miyahara, H.; Okino, A. Surface Hydrophilization of Polyimide Films Using Atmospheric Damage-Free Multigas Plasma Jet Source. IEEE Trans. Plasma Sci. 2013, 41, 119–125. [Google Scholar] [CrossRef]
- Takamatsu, T.; Uehara, K.; Sasaki, Y.; Miyahara, H.; Matsumura, Y.; Iwasawa, A.; Ito, N.; Azuma, T.; Kohno, M.; Okino, A. Investigation of reactive species using various gas plasmas. Rsc Adv. 2014, 4, 39901–39905. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Wang, C.; Ma, S.Q.; Liu, Z.H.; Zang, C.C.; Zhang, W.Y.; Sun, Y.C. High Bonding Strength Between Zirconia and Composite Resin Based on Combined Surface Treatment for Dental Restorations. J. Appl. Biomater. Funct. Mater. 2020, 18, 2280800020928655. [Google Scholar] [CrossRef]
- Ito, Y.; Okawa, T.; Fukumoto, T.; Tsurumi, A.; Tatsuta, M.; Fujii, T.; Tanaka, J.; Tanaka, M. Influence of Atmospheric Pressure Low-Temperature Plasma Treatment on the Shear Bond Strength Between Zirconia and Resin Cement. J. Prosthodont. Res. 2016, 60, 289–293. [Google Scholar] [CrossRef]
- Ahn, J.J.; Kim, D.S.; Bae, E.B.; Kim, G.C.; Jeong, C.M.; Huh, J.B.; Lee, S.H. Effect of Non-Thermal Atmospheric Pressure Plasma (NTP) and Zirconia Primer Treatment on Shear Bond Strength between Y-TZP and Resin Cement. Materials 2020, 13, 3934. [Google Scholar] [CrossRef]
- Kim, D.S.; Ahn, J.J.; Bae, E.B.; Kim, G.C.; Jeong, C.M.; Huh, J.B.; Lee, S.H. Influence of Non-Thermal Atmospheric Pressure Plasma Treatment on Shear Bond Strength between Y-TZP and Self-Adhesive Resin Cement. Materials 2019, 12, 3321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabari, K.; Hosseinpour, S.; Mohammad-Rahimi, H. The Impact of Plasma Treatment of Cercon® Zirconia Ceramics on Adhesion to Resin Composite Cements and Surface Properties. J. Lasers. Med. Sci. 2017, 8 (Suppl. 1), S56–S61. [Google Scholar] [CrossRef] [Green Version]
- Takamatsu, T.; Uehara, K.; Sasaki, Y.; Hidekazu, M.; Matsumura, Y.; Iwasawa, A.; Ito, N.; Kohno, M.; Azuma, T.; Okino, A. Microbial Inactivation in the Liquid Phase Induced by Multigas Plasma Jet. PLoS ONE 2015, 10, e0132381. [Google Scholar]
- Takamatsu, T.; Miyahara, H.; Azuma, T.; Okino, A. Decomposition of Tetrodotoxin Using Multi-Gas Plasma Jet. J. Toxicol. Sci. 2014, 39, 281–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurosawa, M.; Takamatsu, T.; Kawano, H.; Hayashi, Y.; Miyahara, H.; Ota, S.; Okino, A.; Yoshida, M. Endoscopic Hemostasis in Porcine Gastrointestinal Tract Using CO2 Low-Temperature Plasma Jet. J. Surg. Res. 2019, 234, 334–342. [Google Scholar] [CrossRef] [Green Version]
- Yanagawa, Y.; Kawano, H.; Kobayashi, T.; Miyahara, H.; Okino, A.; Mitsuhara, I. Direct Protein Introduction into Plant Cells Using a Multi-Gas Plasma Jet. PLoS ONE 2017, 12, e0171942. [Google Scholar] [CrossRef] [Green Version]
- Abonti, T.R.; Kaku, M.; Kojima, S.; Sumi, H.; Kojima, S.; Yamamoto, T.; Yashima, Y.; Miyahara, H.; Okino, A.; Kawata, T.; et al. Irradiation Effects of Low Temperature Multi Gas Plasma Jet on Oral Bacteria. Dent. Mater. J. 2016, 35, 822–828. [Google Scholar] [CrossRef] [Green Version]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. Suggested Guidelines for the Topographic Evaluation of Implant Surfaces. Int. J. Oral Maxillofac. Implants 2000, 15, 331–344. [Google Scholar] [PubMed]
- Garvie, R.; Nicholson, P. Phase Analysis in Zirconia Systems. J. Am. Ceram. Soc. 1972, 55, 303–305. [Google Scholar] [CrossRef]
- Toraya, H.; Yoshimura, M.; Somiya, S. Calibration Curve for Quantitative-Analysis of the Monoclinic-Tetragonal ZrO2 System by X-ray-Diffraction. J. Am. Ceram. Soc. 1984, 67, 119–121. [Google Scholar]
- Okazaki, Y.; Tanaka, H.; Matsumoto, K.I.; Hori, M.; Toyokuni, S. Non-Thermal Plasma-Induced DMPO-OH Yields Hydrogen Peroxide. Arch. Biochem. Biophys. 2021, 705, 108901. [Google Scholar] [CrossRef]
- Huang, C.P.; Dong, C.; Tang, Z. Advanced Chemical Oxidation: Its Present Role and Potential Future in Hazardous Waste Treatment. Waste Manag. 1993, 13, 361–377. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y. Hydroxyl Radicals Based Advanced Oxidation Processes (AOPs) for Remediation of Soils Contaminated with Organic Compounds: A Review. Chem. Eng. J. 2016, 284, 582–598. [Google Scholar] [CrossRef]
- Carey, J.H. An Introduction to Advanced Oxidation Processes (AOP) for Destruction of Organics in Wastewater. Water Qual. Res. J. 1992, 27, 1–22. [Google Scholar] [CrossRef]
- Pizzorni, M.; Lertora, E.; Mandolfino, C.; Vicini, S.; Salerno, M.; Salerno, M.; Prato, M. Comparative characterization of the surface state of Ti-6Al-4V substrates in different pre-bonding conditions. J. Adv. Join. Processes 2021, 3, 100058. [Google Scholar] [CrossRef]
- Pizzorni, M.; Lertora, E.; Gambaro, C.; Mandolfino, C.; Salerno, M.; Prato, M. Low-pressure plasma treatment of CFRP substrates for epoxy-adhesive bonding: An investigation of the effect of various process gases. Int. J. Adv. Manuf. Technol. 2019, 102, 3021–3035. [Google Scholar] [CrossRef]
- Nagaoka, N.; Yoshihara, K.; Feitosa, V.P.; Tamada, Y.; Irie, M.; Yoshida, Y.; Van Meerbeek, B.; Hayakawa, S. Chemical Interaction Mechanism of 10-MDP With Zirconia. Sci. Rep. 2017, 7, 45563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.C.; Hsieh, J.P.; Chen, Y.C.; Kang, L.L.; Hwang, C.S.; Chuang, S.F. Promoting Porcelain-Zirconia Bonding Using Different Atmospheric Pressure Gas Plasmas. Dent. Mater. 2018, 34, 1188–1198. [Google Scholar] [CrossRef]
- Gauthier, M.A.; Stangel, I.; Ellis, T.H.; Zhu, X.X. Oxygen Inhibition in Dental Resins. J. Dent. Res. 2005, 84, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, A.; Shishido, S.; Nakamura, K.; Harada, A.; Katsuda, Y.; Kanno, T.; Egusa, H. Impact of Adhesive Primer and Light-Curing on Polymerization Kinetics of Self-Adhesive Resin Cement in Association with Free Radical Reaction. Eur. J. Oral Sci. 2021, 129. in press. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.M.; Min, B.K.; Kim, Y.K.; Kwon, T.Y. Influence of Sandblasting Particle Size and Pressure on Resin Bonding Durability to Zirconia: A Residual Stress Study. Materials 2020, 13, 5629. [Google Scholar] [CrossRef] [PubMed]
- Oshita, T.; Kawano, H.; Takamatsu, T.; Miyahara, H.; Okino, A. Temperature Controllable Atmospheric Plasma Source. IEEE Trans. Plasma Sci. 2015, 43, 1987–1992. [Google Scholar] [CrossRef]
- Kawano, H.; Takamatsu, T.; Matsumura, Y.; Miyahara, H.; Iwasawa, A.; Okino, A. Influence of Gas Temperature in Atmospheric Non-Equilibrium Plasma on Bactericidal Effect. Biocontrol. Sci. 2018, 23, 167–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chevalier, J.; Cales, B.; Drouin, J.M. Low-Temperature Aging of Y-TZP Ceramics. J. Am. Ceram. Soc. 1999, 82, 2150–2154. [Google Scholar] [CrossRef]



| Sa (µm) | Str | Sdr (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ave | SD | Stats. | Ave | SD | Stats. | Ave | SD | Stats. | |
| Ctrl | 0.1123 | 0.0075 | a | 0.883 | 0.067 | a | 4.55 | 0.22 | a |
| Sand | 0.4713 | 0.0319 | b | 0.925 | 0.015 | a | 34.97 | 0.67 | b |
| N2 | 0.1127 | 0.0051 | a | 0.916 | 0.039 | a | 4.81 | 0.31 | a |
| CO2 | 0.1110 | 0.0010 | a | 0.903 | 0.008 | a | 4.92 | 0.51 | a |
| O2 | 0.1140 | 0.0040 | a | 0.904 | 0.006 | a | 4.93 | 0.95 | a |
| Air | 0.1050 | 0.0036 | a | 0.856 | 0.054 | a | 4.73 | 0.25 | a |
| Ar | 0.1140 | 0.0020 | a | 0.839 | 0.154 | a | 4.75 | 0.66 | a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoda, N.; Abe, Y.; Suenaga, Y.; Matsudate, Y.; Hoshino, T.; Sugano, T.; Nakamura, K.; Okino, A.; Sasaki, K. Resin Cement–Zirconia Bond Strengthening by Exposure to Low-Temperature Atmospheric Pressure Multi-Gas Plasma. Materials 2022, 15, 631. https://doi.org/10.3390/ma15020631
Yoda N, Abe Y, Suenaga Y, Matsudate Y, Hoshino T, Sugano T, Nakamura K, Okino A, Sasaki K. Resin Cement–Zirconia Bond Strengthening by Exposure to Low-Temperature Atmospheric Pressure Multi-Gas Plasma. Materials. 2022; 15(2):631. https://doi.org/10.3390/ma15020631
Chicago/Turabian StyleYoda, Nobuhiro, Yuri Abe, Yuma Suenaga, Yoshiki Matsudate, Tomohiro Hoshino, Takehiko Sugano, Keisuke Nakamura, Akitoshi Okino, and Keiichi Sasaki. 2022. "Resin Cement–Zirconia Bond Strengthening by Exposure to Low-Temperature Atmospheric Pressure Multi-Gas Plasma" Materials 15, no. 2: 631. https://doi.org/10.3390/ma15020631
APA StyleYoda, N., Abe, Y., Suenaga, Y., Matsudate, Y., Hoshino, T., Sugano, T., Nakamura, K., Okino, A., & Sasaki, K. (2022). Resin Cement–Zirconia Bond Strengthening by Exposure to Low-Temperature Atmospheric Pressure Multi-Gas Plasma. Materials, 15(2), 631. https://doi.org/10.3390/ma15020631







