Flower-like Superhydrophobic Surfaces Fabricated on Stainless Steel as a Barrier against Corrosion in Simulated Acid Rain
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemical
2.2. Devices
- -
- Electrochemical measurements:
- -
- Gamry 600™potentiostat/galvanostat, Warminster, PA, USA controlled by an electrochemical program.
- -
- Processing and analysis of experimental data using the following software programs: CorrView, CorrWare, Zplot and ZView programs from Scribner Associates, Southern Pines, NC, USA (all version 2.80).
- -
- ATR-FTIR: SHIMADZU-IRAffinity-1, Shimadzu Europa GmbH, Duisburg, F.R. Germany.
- -
- Scanning Electron Microscope (SEM): FEI Sirion 400 NC, Eindhoven, the Netherlands.
- -
- Goniometer: Data Physics OCA 35, Filderstadt, Germany.
2.3. Preparation of the As-Prepared Hydrophobic Layers
2.4. Electrochemical Characterisation
2.5. Surface Characterisation
2.5.1. Contact Angle
2.5.2. ATR-FTIR Analysis
2.5.3. Surface Investigation by SEM/EDX
3. Results and Discussion
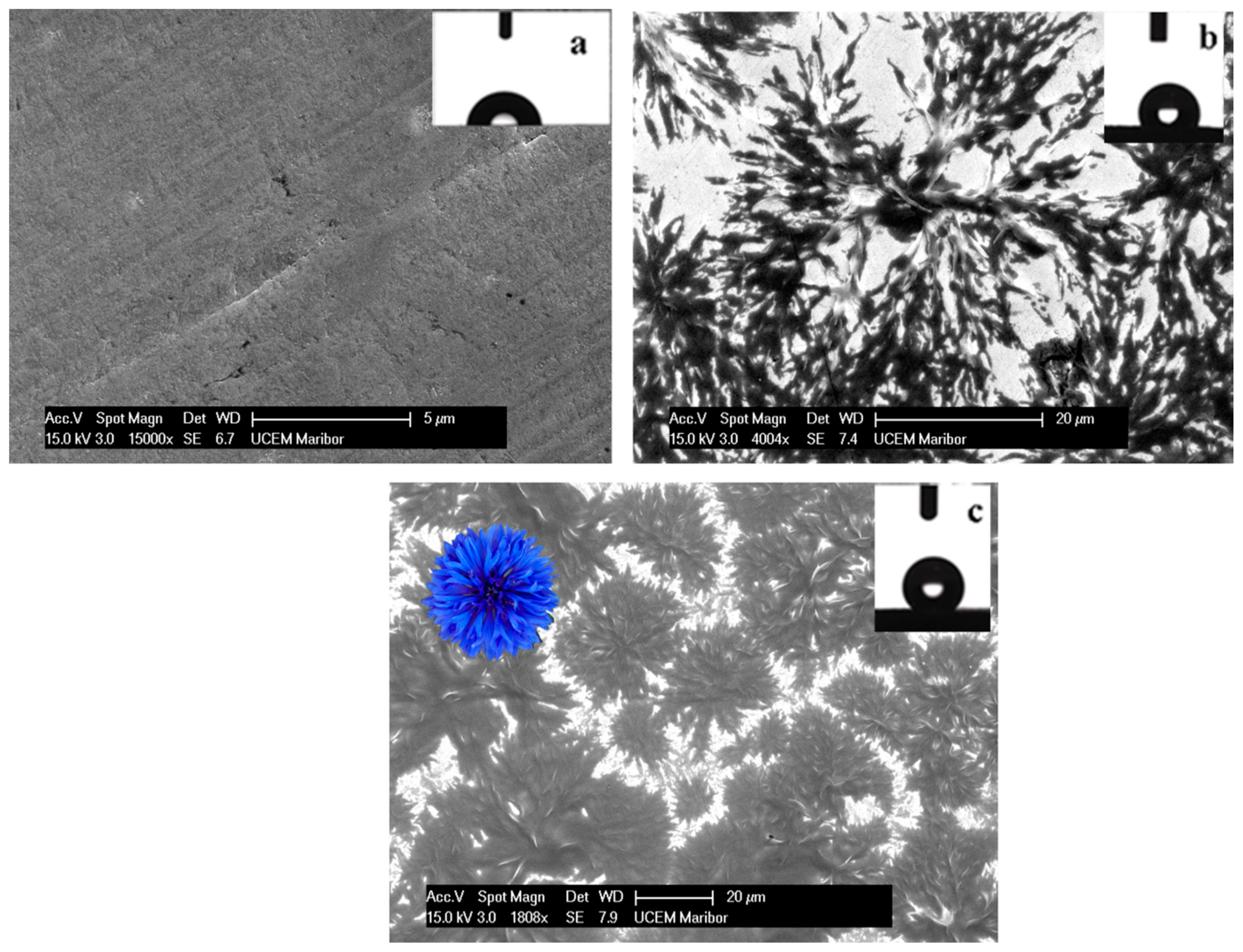
3.1. Morphology of the As-Prepared Hydrophobic Layers
Contact Angle Measurements
3.2. Electrochemical and Corrosion-Resistance Measurements
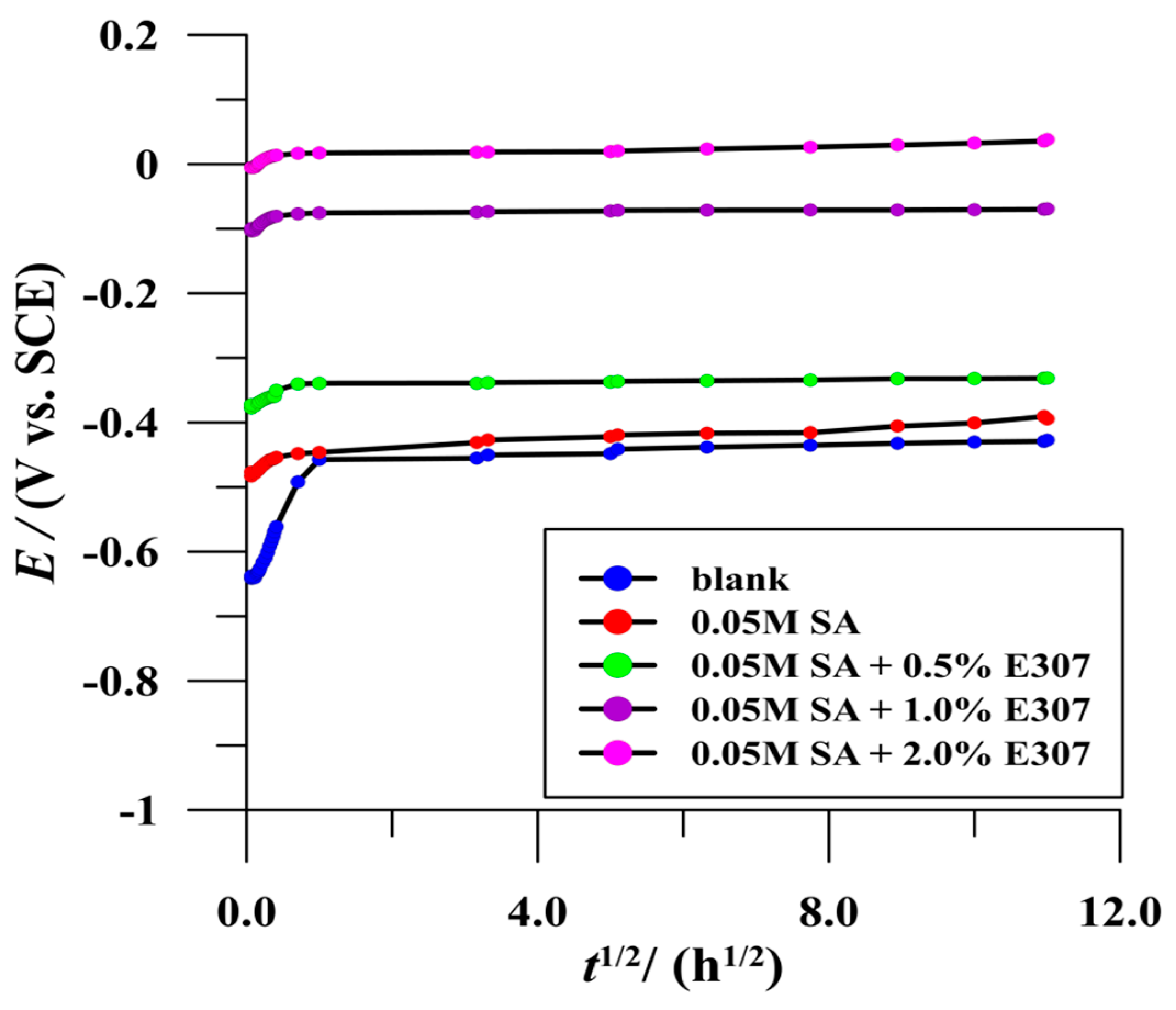
3.2.1. Open Circuit Potential Measurements
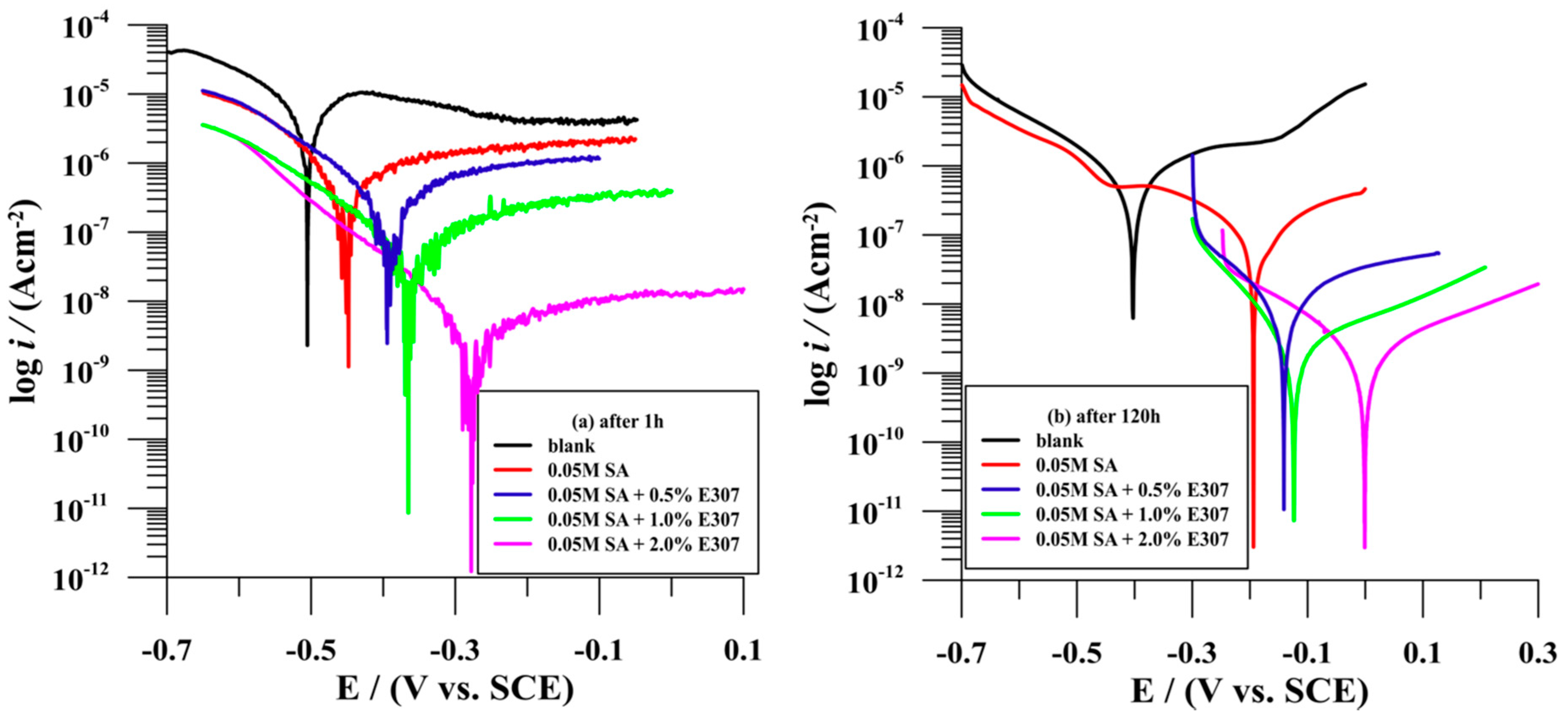
3.2.2. Potentiodynamic Polarisation Test
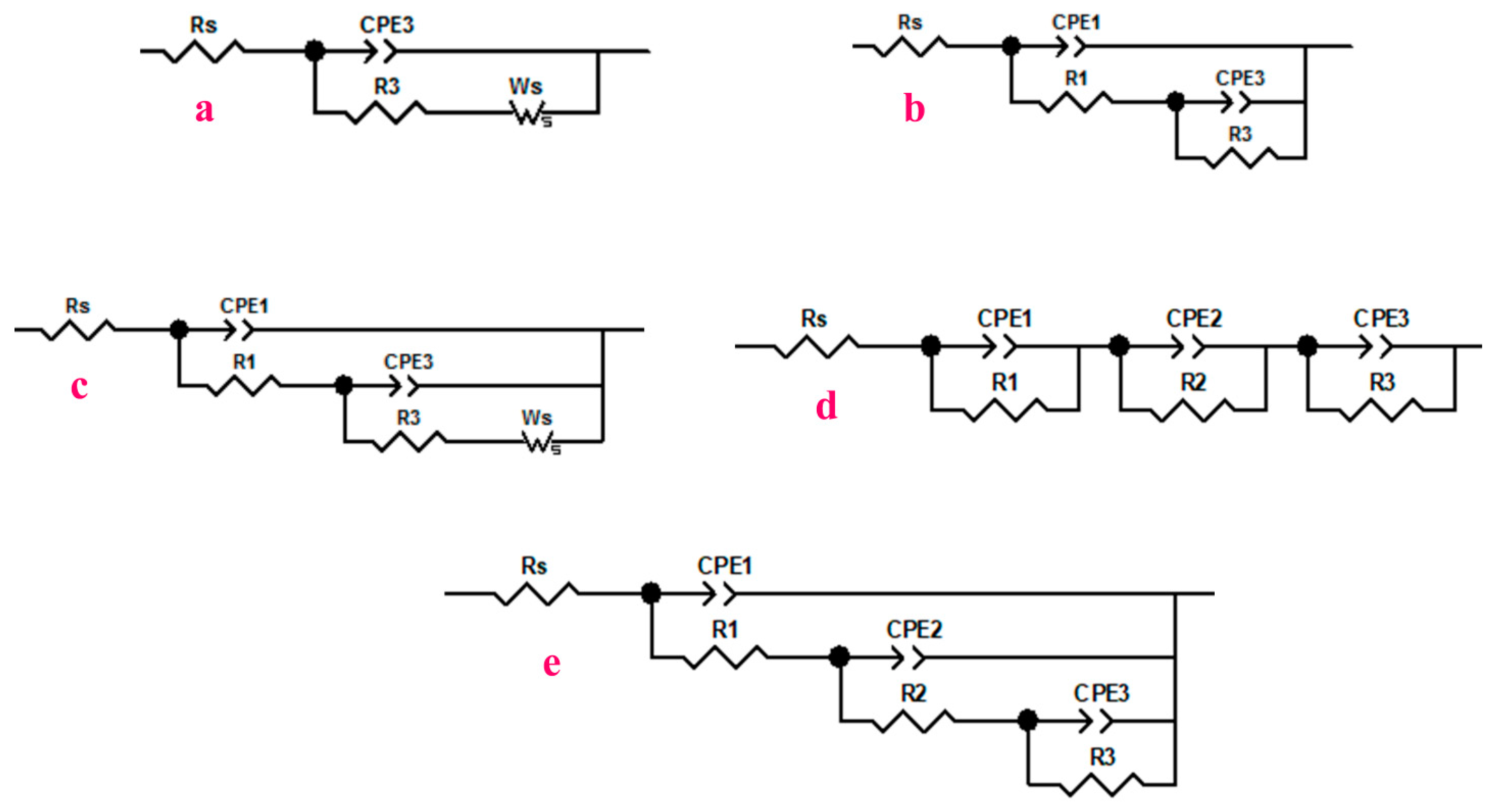
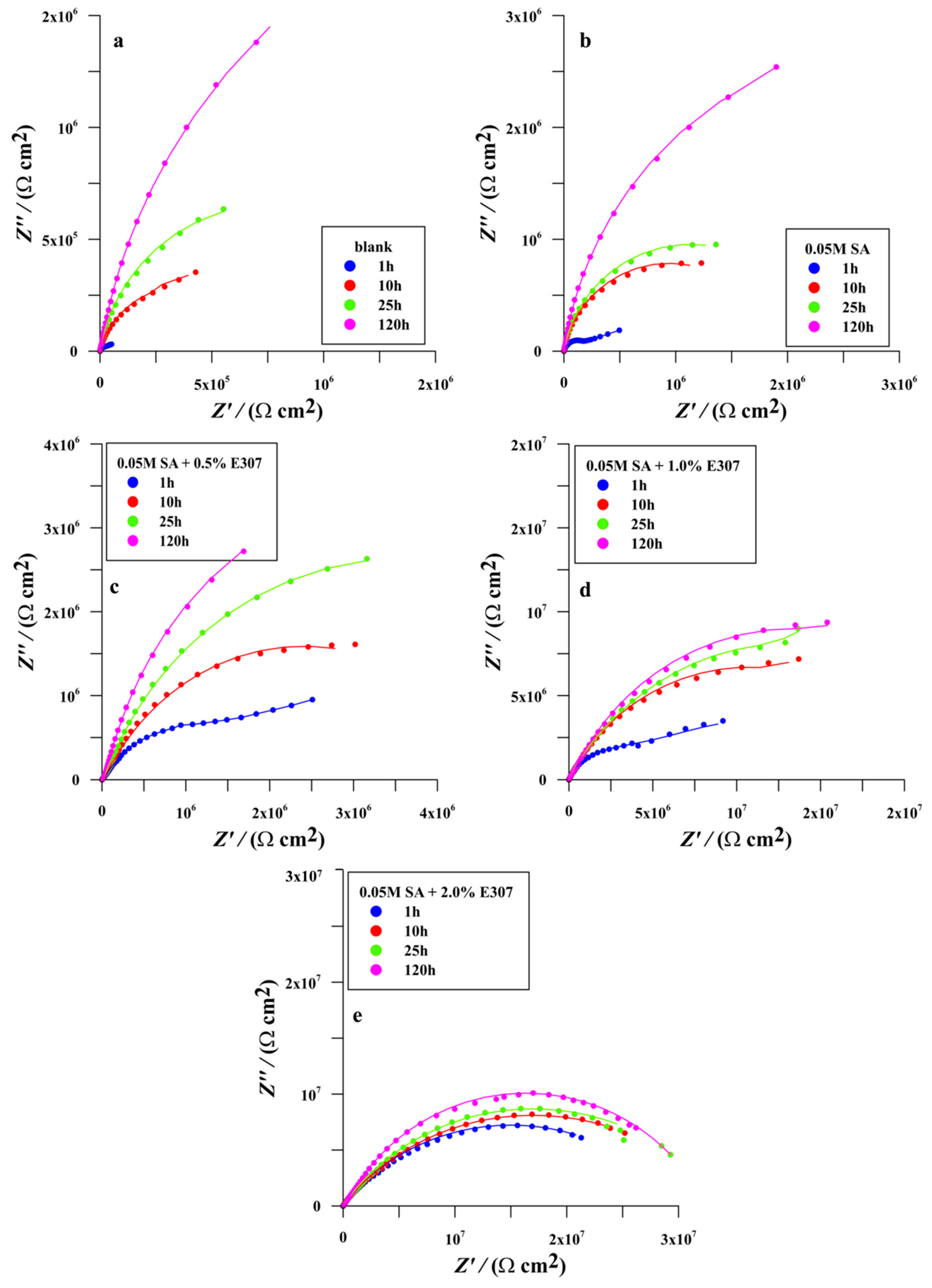
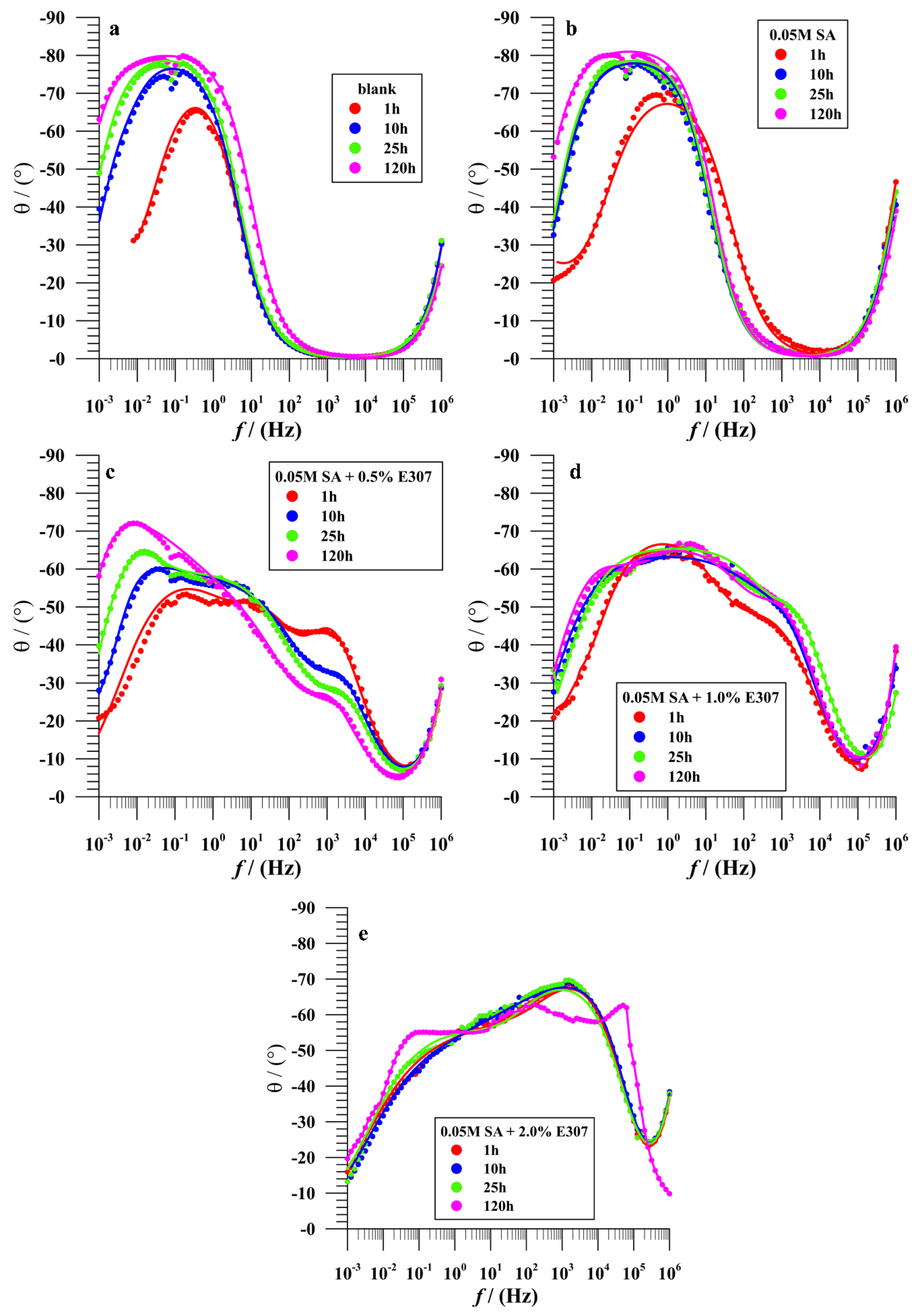
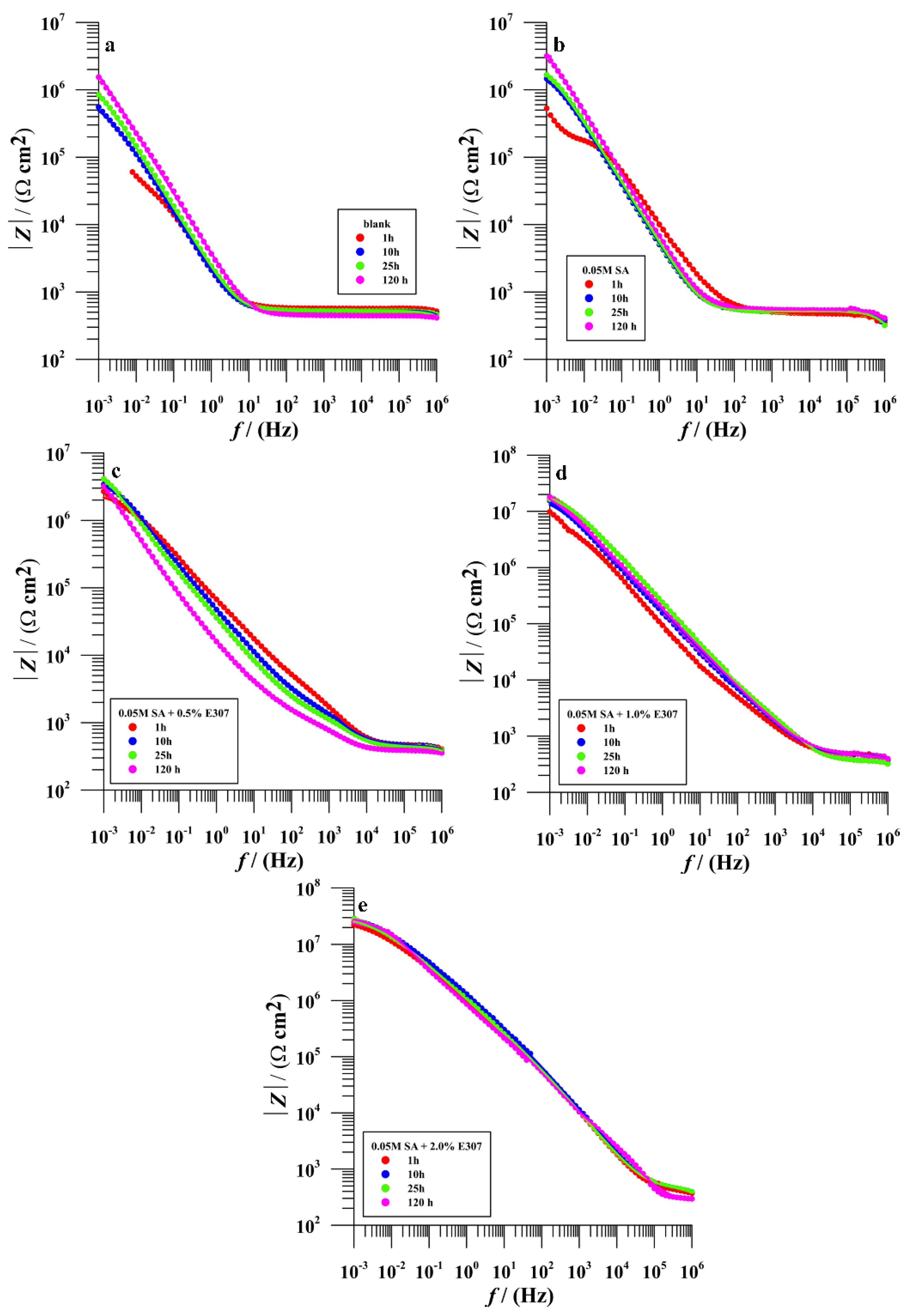
3.2.3. Electrochemical Impedance Spectroscopy (EIS)
Bare Surface
Surface Modified in Stearic Acid (SA)
Surface Modified in Surface Modified in the Mixtures of (SA + x%E307, x = 0.5%, 1.0% and 2.0%)
3.3. Surface Characterisation/Characterisation of Coating Material
3.3.1. ATR–FTIR Analysis
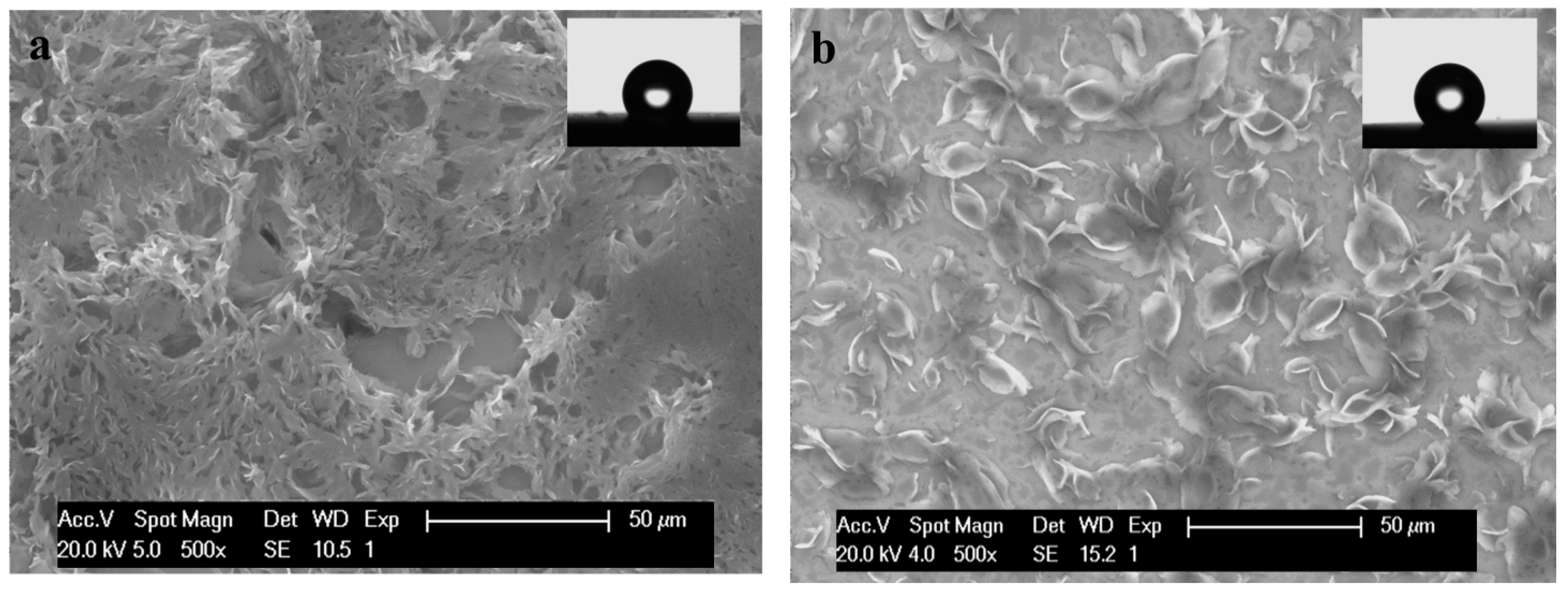
3.3.2. SEM- EDAX
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lv, Y.; Niu, D.; Liu, X.; Li, Y.C. Corrosion Damage and Life Prediction of Concrete Structure in a 41-Year-Old Steelworks. Materials 2022, 15, 5893. [Google Scholar] [CrossRef] [PubMed]
- Chaouiki, A.; Chafiq, M.; Rbaa, M.; Lgaz, H.; Salghi, R.; Lakhrissi, B.; Ali, I.H.; Masroor, S.; Cho, Y. New 8-Hydroxyquinoline-Bearing Quinoxaline Derivatives as Effective Corrosion Inhibitors for Mild Steel in HCl: Electrochemical and Computational Investigations. Coatings 2020, 10, 811. [Google Scholar] [CrossRef]
- Chaouiki, A.; Chafiq, M.; Lgaz, H.; Al-Hadeethi, M.R.; Ali, I.H.; Masroor, S.; Chung, I.M. Green Corrosion Inhibition of Mild Steel by Hydrazone Derivatives in 1.0 M HCl. Coatings 2020, 10, 640. [Google Scholar] [CrossRef]
- D’Elia, M.F.; Magni, M.; Romanò, T.; Trasatti, S.P.M.; Niederberger, M.; Caseri, W.R. Smart Anticorrosion Coatings Based on Poly(Phenylene Methylene): An Assessment of the Intrinsic Self-Healing Behavior of the Copolymer. Polymers 2022, 14, 3457. [Google Scholar] [CrossRef]
- Zhao, X.; Jiang, D.; Ma, L.; Zeng, X.; Li, Z.; Huang, G. Special Issue: Corrosion Effects and Smart Coatings of Corrosion Protection. Coatings 2022, 12, 1378. [Google Scholar] [CrossRef]
- Kordas, G. Corrosion Barrier Coatings: Progress and Perspectives of the Chemical Route. Corros. Mater. Degrad. 2022, 3, 376–413. [Google Scholar] [CrossRef]
- Chen, X.; Tang, J.; Wei, H.; Zhang, H.; Tang, Y.; Zhao, X.; Zuo, Y. Effect of Cerium Tartrate on the Corrosion Resistance of Epoxy Coating on Aluminum Alloy and Its Mechanism. Coatings 2022, 12, 785. [Google Scholar] [CrossRef]
- Liu, R.; Liu, X.; Yang, H.; Jie, H.; Li, T.; Lyu, K.; Shah, S.P. Effect of CeO2–GO Nanocomposite on the Anticorrosion Properties of Epoxy Coating in Simulated Acid Rain Solution. Polymers 2022, 14, 3573. [Google Scholar] [CrossRef] [PubMed]
- Kaskah, S.E.; Ehrenhaft, G.; Gollnick, J.; Fischer, C.B. N-b-Hydroxyethyl Oleyl Imidazole as Synergist to Enhance the Corrosion Protection Effect of Natural Cocoyl Sarcosine on Steel. Corros. Mater. Degrad. 2022, 3, 536–552. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, W.; Liu, H.; Liu, L.; Wang, F.; Qiao, Z. Single Dense Layer of PEO Coating on Aluminum Fabricated by “Chain-like” Discharges. Materials 2022, 15, 4635. [Google Scholar] [CrossRef]
- Wang, Z.; Zhitomirsky, I. Surfactants for Electrophoretic Deposition of Polyvinylidene Fluoride–Silica Composites. Surfaces 2022, 5, 308–317. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, Y.; Wang, J.; Li, R.; Liu, X.; Ai, S. Facile and Eco-Friendly Preparation of Mild Steel Based Superhydrophobic Surfaces without Chemical Modifications. Coatings 2022, 12, 737. [Google Scholar] [CrossRef]
- Baskar, P.; Rathinapriya, P.; Prabakaran, M. Use of Trochodendron Aralioides Extract as Green Corrosion Inhibitor for Mild Steel in 1M HCl Solutions. Processes 2022, 10, 1480. [Google Scholar] [CrossRef]
- Qian, H.; Liu, W.; Chang, W.; Hao, X.; Zhang, D. D-Cysteine Functionalized Superhydrophobic Nanocomposite Coating with Multiple-Action Antibacterial Property and Enhanced Mechanical Durability. Coatings 2022, 12, 1158. [Google Scholar] [CrossRef]
- Fu, S.; Zhang, S.; Xiang, Q.; Tan, W.; Li, W.; Chen, S.; Guo, L. Experimental and Theoretical Investigation of Corrosion Inhibition Effect of Multi-Active Compounds on Mild Steel in 1 M HCl. Int. J. Electrochem. Sci. 2019, 14, 6855–6873. [Google Scholar] [CrossRef]
- Abdallah, M.; Soliman, K.A.; Al-Gorair, A.S.; Al Bahir, A.; Al-Fahemi, J.H.; Motawea, M.S.; Al-Juaid, S.S. Enhancing the Inhibition and Adsorption Performance of SABIC Iron Corrosion in Sulfuric Acid by Expired Vitamins. Experimental and Computational Approach. RSC Adv. 2021, 11, 17092–17107. [Google Scholar] [CrossRef]
- Anejjar, A.; Id, O.; Mouden, E.; Batah, A.; Bouskri, A.; Rjoub, A. Corrosion Inhibition Potential of Ascorbic Acid on Carbon Steel in Acid Media. Appl. J. Environ. Eng. Sci. 2017, 3, 36–46. [Google Scholar]
- Fuchs-Godec, R.; Pavlović, M.G.; Tomić, M.V. The Inhibitive Effect of Vitamin-C on the Corrosive Performance of Steel in HCL Solutions. Int. J. Electrochem. Sci. 2013, 8, 1511–1519. [Google Scholar]
- Fuchs-Godec, R.; Pavlovic, M.G.; Tomic, M.V. The Inhibitive Effect of Vitamin-C on the Corrosive Performance of Steel in HCl Solutions—Part II. Int. J. Electrochem. Sci. 2015, 10, 10502–10512. [Google Scholar]
- Hippolyte, C.N.; Serge, B.Y.; Sagne, A.; Creus, J.; Albert, T. Nicotinamide Inhibition Properties for Copper Corrosion in 3.5% NaCl Solution: Experimental and Theorical Investigations. J. Mater. Sci. Chem. Eng. 2018, 06, 100–121. [Google Scholar] [CrossRef]
- Ju, H.; Li, Q.; Ju, Y. lin Corrosion Inhibition of Vitamins B for Hot Dipped Zn Coating Steel in Hydrochloric Acid. Adv. Mater. Res. 2012, 562–564, 184–187. [Google Scholar] [CrossRef]
- Sheoran, A.S.; Sheoran, V.; Choudhary, R.P. G Eochemistry of a Cid M Ine D Rainage. Environ. Res. 2010, 4, 653–665. [Google Scholar]
- Malhotra, S.; Singh, G. Vitamins: Potential Inhibitors for Nickel in Acidic Media. Surf. Eng. 2005, 21, 187–192. [Google Scholar] [CrossRef]
- Ramanathan, R.; Aloysius, A.; Christy, A.; Anthony, N. Calcium D-Pantothenate as Green Corrosion Inhibitor on Mild Steel in 240 Ppm NaCl Solution. Int. J. Innov. Technol. Explor. Eng. 2019, 9, 435–442. [Google Scholar] [CrossRef]
- Ahmed, R.A. Investigation of Corrosion Inhibition of Vitamins B1 and C on Mild Steel in 0.5 M HCl Solution: Experimental and Computational Approach. Orient. J. Chem. 2016, 32, 295–304. [Google Scholar] [CrossRef]
- Fuchs-Godec, R.; Zerjav, G. Corrosion Resistance of High-Level-Hydrophobic Layers in Combination with Vitamin E—(α-Tocopherol) as Green Inhibitor. Corros. Sci. 2015, 97, 7–16. [Google Scholar] [CrossRef]
- Fuchs-Godec, R. A Synergistic Effect between Stearic Acid and (+)-α-Tocopherol as a Green Inhibitor on Ferritic Stainless Steel Corrosion Inhibition in 3.0% NaCl Solution. Coatings 2021, 11, 971. [Google Scholar] [CrossRef]
- Fuchs–Godec, R. Effect of α-Tocopherol as a Green Inhibitor on Chloride-Induced Corrosion of Steel. Int. J. Electrochem. Sci. 2019, 14, 10396–10409. [Google Scholar] [CrossRef]
- Yang, H.; Gao, Y.; Qin, W.; Sun, J.; Huang, Z.; Li, Y.; Li, B.; Sun, J. A Robust Superhydrophobic Surface on AA3003 Aluminum Alloy with Intermetallic Phases In-Situ Pinning Effect for Corrosion Protection. J. Alloys Compd. 2022, 898, 163038. [Google Scholar] [CrossRef]
- Peethan, A.; Pais, M.; Rao, P.; Singh, K.; George, S.D. Wettability Tailored Superhydrophobic and Oil-Infused Slippery Aluminium Surface for Improved Anti-Corrosion Performance. Mater. Chem. Phys. 2022, 290, 126517. [Google Scholar] [CrossRef]
- Abbasi, S.; Nouri, M.; Sabour Rouhaghdam, A. A Novel Combined Method for Fabrication of Stable Corrosion Resistance Superhydrophobic Surface on Al Alloy. Corros. Sci. 2019, 159, 108144. [Google Scholar] [CrossRef]
- Xia, Y.; Fan, G.; Chen, K.; Chen, Y.; He, Z.; Ou, J. Preparation and Anti-Corrosion Performances of Grass-like Microstructured Superhydrophobic Surface on Copper via Solution-Immersion. Mater. Lett. 2022, 323, 3–6. [Google Scholar] [CrossRef]
- Chen, X.; Wang, P.; Zhang, D.; Ou, J. Effect of Surface Nanostructure on Enhanced Atmospheric Corrosion Resistance of a Superhydrophobic Surface. Colloids Surf. Physicochem. Eng. Asp. 2022, 647, 129058. [Google Scholar] [CrossRef]
- Ouyang, Y.; Chen, Z.; Guo, E.; Qiu, R.; Wang, X.; Kang, H.; Wang, T. Bioinspired Superhydrophobic Surface via One-Step Electrodeposition and Its Corrosion Inhibition for Mg-Li Alloy. Colloids Surf. Physicochem. Eng. Asp. 2022, 648, 129145. [Google Scholar] [CrossRef]
- Tang, Y.; Cai, Y.; Wang, L.; Luo, X.; Wang, B.; Song, Q.; Liu, Z. Formation Mechanism of Superhydrophobicity of Stainless Steel by Laser-Assisted Decomposition of Stearic Acid and Its Corrosion Resistance. Opt. Laser Technol. 2022, 153, 108190. [Google Scholar] [CrossRef]
- Heshmati, M.R.; Amiri, S.; Hosseini-Zori, M. Synthesis and Characterization of Superhydrophobic-Superoleophobic and Anti-Corrosion Coatings via Sol-Gel Process. Open J. Org. Polym. Mater. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Ge, Y.; Cheng, J.; Wang, X.; Xue, L.; Zhu, S.; Zhang, B.; Hong, S.; Wu, Y.; Zhang, X.; Liang, X.B. Formation and Properties of Superhydrophobic Al Coatings on Steel. ACS Omega 2021, 6, 18383–18394. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Y.; Tan, X.; Zhang, Z.; Jiang, Z.; Lian, J.; Wen, C.; Ren, L. Superhydrophobic Brass Surfaces with Tunable Water Adhesion Fabricated by Laser Texturing Followed by Heat Treatment and Their Anti-Corrosion Ability. Appl. Surf. Sci. 2022, 575, 151596. [Google Scholar] [CrossRef]
- Domantovsky, A.G.; Emelyanenko, K.A.; Emelyanenko, A.M.; Boinovich, L.B. The Influence of Prolonged High-Concentration Ozone Exposure on Superhydrophobic Coatings in Static and High-Speed Flow Atmospheres. Materials 2022, 15, 5725. [Google Scholar] [CrossRef]
- Wan, Y.; Chen, M.; Liu, W.; Shen, X.X.; Min, Y.; Xu, Q. The Research on Preparation of Superhydrophobic Surfaces of Pure Copper by Hydrothermal Method and Its Corrosion Resistance. Electrochim. Acta 2018, 270, 310–318. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, B.; Song, W. Improving the Anti-Corrosion and Anti-Wear Performance of Anodic Coating on the Surface of AA 5052 via Hydro-Thermal Treatment. Materials 2022, 15, 1447. [Google Scholar] [CrossRef] [PubMed]
- Subeshan, B.; Asmatulu, R. Corrosion Mitigation of Metals and Alloys via Superhydrophobic Coatings with Plasma Surface and Heat Treatment Processes. Eng. Fail. Anal. 2022, 139, 106437. [Google Scholar] [CrossRef]
- Parvate, S.; Dixit, P.; Chattopadhyay, S. Superhydrophobic Surfaces: Insights from Theory and Experiment. J. Phys. Chem. 2020, 124, 1323–1360. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, R.; Long, F.; Zhang, X.; Liu, J.; Hu, W.; Liu, L. New Perspectives on Structural Parameters and Hydrophobic Model Inspired by a Superhydrophobic Cu Cone-Flower Coating. Mater. Des. 2021, 206, 109827. [Google Scholar] [CrossRef]
- Liu, C.; Su, F.; Liang, J.; Huang, P. Facile Fabrication of Superhydrophobic Cerium Coating with Micro-Nano Flower-like Structure and Excellent Corrosion Resistance. Surf. Coat. Technol. 2014, 258, 580–586. [Google Scholar] [CrossRef]
- Shi, Y.L.; Yang, W.; Bai, J.; Feng, X.J.; Wang, Y.S. Fabrication of Flower-like Copper Film with Reversible Superhydrophobicity-Superhydrophilicity and Anticorrosion Properties. Surf. Coat. Technol. 2014, 253, 148–153. [Google Scholar] [CrossRef]
- Al Zoubi, W.; Ko, Y.G. Flowerlike Organic-Inorganic Coating Responsible for Extraordinary Corrosion Resistance via Self-Assembly of an Organic Compound. ACS Sustain. Chem. Eng. 2018, 6, 3546–3555. [Google Scholar] [CrossRef]
- Li, H.; Peng, Y.; Yu, S.; Yin, X. Both Slender Pillars and Hierarchical Structures Achieving Superhydrophobicity and the Comparison of Their Properties. Appl. Surf. Sci. 2020, 505, 144524. [Google Scholar] [CrossRef]
- Zhang, L.; Gong, Z.; Jiang, B.; Sun, Y.; Chen, Z.; Gao, X.; Yang, N. Ni-Al Layered Double Hydroxides (LDHs) Coated Superhydrophobic Mesh with Flower-like Hierarchical Structure for Oil/Water Separation. Appl. Surf. Sci. 2019, 490, 145–156. [Google Scholar] [CrossRef]
- Zhang, B.; Feng, H.; Lin, F.; Wang, Y.; Wang, L.; Dong, Y.; Li, W. Wettability of Stearic Acid Modified Chrome Oxide Layer on Copper Substrate. Micro Nano Lett. 2017, 12, 157–160. [Google Scholar] [CrossRef]
- Liu, T.; Yin, Y.; Chen, S.; Chang, X.; Cheng, S. Super-Hydrophobic Surfaces Improve Corrosion Resistance of Copper in Seawater. Electrochim. Acta 2007, 52, 3709–3713. [Google Scholar] [CrossRef]
- De Leeuw, N.H. Computer Simulations of Structures and Properties of the Biomaterial Hydroxyapatite. J. Mater. Chem. 2010, 20, 5376–5389. [Google Scholar] [CrossRef]
- Szabelski, P.; De Feyter, S.; Drach, M.; Lei, S. Computer Simulation of Chiral Nanoporous Networks on Solid Surfaces. Langmuir 2010, 26, 9506–9515. [Google Scholar] [CrossRef] [PubMed]
- Jo Tauviqirrahman, M.; Fadhli Afif, M.; Paryanto, P.; Jamari, J.; Caesarendra, W. Investigation of the Tribological Performance of Heterogeneous Slip/No-Slip Journal Bearing Considering Thermo-Hydrodynamic Effects. Fluids 2021, 6, 48. [Google Scholar] [CrossRef]
- Teisala, H.; Butt, H. Hierarchical Structures for Superhydrophobic and Superoleophobic Surfaces Hannu Teisala* and Hans-Jürgen Butt Max Planck Institute for Polymer Research Department of Physics at Interfaces Many Surfaces Possessing Robust Super Liquid Repellency, Are Hier. Langmuir 2018, 35, 10689–10703. [Google Scholar] [CrossRef]
- Ellingson, L.A.; Shedlosky, T.J.; Bierwagen, G.P.; de la Rie, E.R.; Brostoff, L.B. The Use of Electrochemical Impedance Spectroscopy in the Evaluation of Coatings for Outdoor Bronze. Stud. Conserv. 2004, 49, 53–62. [Google Scholar] [CrossRef]
- Hernández, H.H.; Reynoso, A.M.R.; González, J.C.T.; Morán, C.O.G. Electrochemical Impedance Spectroscopy (EIS): A Review Study of Basic Aspects of the Corrosion Mechanism Applied to Steels. In Electrochemical Impedance Spectroscopy; IntechOpen: Vienna, Austria, 2020; pp. 1–35. [Google Scholar]
- Kashkovskiy, R.; Strelnikova, K.; Fedotova, A.; Strelnikova, K.; Fedotova, A. Application of Electrochemical Impedance Spectroscopy to Study Hydrogen Sulphide Corrosion of Steel and Its Inhibition: A Review Sulphide Corrosion of Steel and Its Inhibition: A Review. Corros. Eng. Sci. Technol. 2019, 54, 2782. [Google Scholar] [CrossRef]
- Luka, Z. Evaluation of Model and Dispersion Parameters and Their Effects on the Formation of Constant-Phase Elements in Equivalent Circuits. J. Electroanal. Chem. 1999, 464, 68–75. [Google Scholar]
- Ribeiro, D.V. Use of Electrochemical Impedance Spectroscopy (EIS) to Monitoring the Corrosion of Reinforced Concrete Para Monitoramento Da Corrosão Em Concreto Armado. Rev. IBRACON Estrut. Mater. 2015, 8, 529–537. [Google Scholar] [CrossRef]
- Walter, G.W. A Review of Impedance Plot Methods used for Corrosion Performance Analysis of Painted Metals. Corros. Sci. 1986, 26, 681–703. [Google Scholar] [CrossRef]
- Canales, C. Electrochemical Impedance Spectroscopy and Its Applications. In 21st Century Nanostructured Materials; Spring: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Margarit-Mattos, I.C.P. EIS and Organic Coatings Performance: Revisiting Some Key Points. Electrochim. Acta 2020, 354, 136725. [Google Scholar] [CrossRef]
- Ammar, S.; Ramesh, K.; Vengadaesvaran, B.; Ramesh, S.; Arof, A.K. Amelioration of Anticorrosion and Hydrophobic Properties of Epoxy/PDMS Composite Coatings Containing Nano ZnO Particles. Prog. Org. Coat. 2016, 92, 54–65. [Google Scholar] [CrossRef]
- Atapour, M.; Wei, Z.; Chaudhary, H.; Lendel, C.; Odnevall, I.; Hedberg, Y. Metal Release from Stainless Steel 316L in Whey Protein—And Simulated Milk Solutions under Static and Stirring Conditions. Food Control 2019, 101, 163–172. [Google Scholar] [CrossRef]
- Atapour, M.; Odnevall, I.; Hedberg, Y. Electrochimica Acta Stainless Steel in Simulated Milk and Whey Protein Solutions e In Fl Uence of Grade on Corrosion and Metal Release. Electrochim. Acta 2020, 331, 135428. [Google Scholar] [CrossRef]
- Rončević, I.Š.; Vladislavić, N.; Buzuk, M. Surface Modifications of the Biodegradable Magnesium Based Implants with Self-Assembled Monolayers Formed by T-BAG Method. Acta Chim. Slov. 2018, 65, 698–708. [Google Scholar] [CrossRef]
- Valderrama, A.C.S.; Rojas De, G.C. Traceability of Active Compounds of Essential Oils in Antimicrobial Food Packaging Using a Chemometric Method by ATR-FTIR. Am. J. Anal. Chem. 2017, 8, 726–741. [Google Scholar] [CrossRef]
- Che Man, Y.B.; Ammawath, W.; Mirghani, M.E.S. Determining α-Tocopherol in Refined Bleached and Deodorized Palm Olein by Fourier Transform Infrared Spectroscopy. Food Chem. 2005, 90, 323–327. [Google Scholar] [CrossRef]
- Jeevahan, J.; Chandrasekaran, M.; Britto Joseph, G.; Durairaj, R.B.; Mageshwaran, G. Superhydrophobic Surfaces: A Review on Fundamentals, Applications, and Challenges. J. Coat. Technol. Res. 2018, 15, 231–250. [Google Scholar] [CrossRef]
- Silva, S.D.; Feliciano, R.P.; Boas, L.V.; Bronze, M.R. Application of FTIR-ATR to Moscatel Dessert Wines for Prediction of Total Phenolic and Flavonoid Contents and Antioxidant Capacity. Food Chem. 2014, 150, 489–493. [Google Scholar] [CrossRef]
- Krilov, D.; Kosović, M.; Serec, K. Spectroscopic Studies of Alpha Tocopherol Interaction with a Model Liposome and Its Influence on Oxidation Dynamics. Spectrochim. Acta -Part A Mol. Biomol. Spectrosc. 2014, 129, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Barthlott, W.; Mail, M.; Neinhuis, C. Superhydrophobic Hierarchically Structured Surfaces in Biology: Evolution, Structural Principles and Biomimetic Applications. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20160191. [Google Scholar] [CrossRef] [PubMed]
- Latthe, S.S.; Terashima, C.; Nakata, K.; Fujishima, A. Superhydrophobic Surfaces Developed by Mimicking Hierarchical Surface Morphology of Lotus Leaf. Molecules 2014, 19, 4256–4283. [Google Scholar] [CrossRef]
- Fay, F.; Linossier, I.; Langlois, V.; Haras, D.; Vallee-Rehel, K. SEM and EDX Analysis: Two Powerful Techniques for the Study of Antifouling Paints. Prog. Org. Coat. 2005, 54, 216–223. [Google Scholar] [CrossRef]
- Aydin, I.; Cetinel, H.; Pasinli, A.; Yuksel, M. Preparation of Hydroxyapatite Coating by Using Citric Acid Sodium Citrate Buffer System in the Biomimetic Procedure. Mater. Test. 2013, 55, 782–788. [Google Scholar] [CrossRef]


| Corrosive Media Acid Rain, pH = 3 | icorr (μAcm−2) | Ecorr (V/SCE) | Rp (MΩ cm2) | βc (mV/dec) | βa (mV/dec) | % ηicorr | % ηRp | |
|---|---|---|---|---|---|---|---|---|
| after 1 h bare surface AISI 410S | ||||||||
| 5.731 | −0.504 | 0.020 | −120.83 | 141.02 | ||||
| modified surface | wt% E307 | |||||||
| 0* | 0.630 | −0.448 | 0.120 | −127.3 | 321.28 | 85.8 | 83.4 | |
| 0.5 | 0.244 | −0.395 | 0.271 | −143.9 | 313.31 | 95.5 | 92.7 | |
| 1.0 | 0.092 | −0.387 | 0.528 | −149.6 | 332.21 | 98.3 | 96.2 | |
| 2.0 | 0.0025 | −0.275 | 9.010 | −146.0 | 339.37 | 99.9 | 99.8 | |
| after 120 h, bare surface AISI 410S | ||||||||
| 0.997 | −0.402 | 0.054 | −121.3 | 272.5 | ||||
| modified surface | wt% E307 | |||||||
| 0* | 0.141 | −0.193 | 0.317 | −142.2 | 232.7 | 85.8 | 83.1 | |
| 0.5 | 0.008 | −0.141 | 3.249 | −141.2 | 292.1 | 99.2 | 98.3 | |
| 1.0 | 0.0025 | −0.123 | 11.730 | −137.7 | 307.2 | 99.7 | 99.5 | |
| 2.0 | 0.0020 | −0.001 | 18.690 | −157.3 | 315.4 | 99.8 | 99.7 |
| Corrosive Media Acid Rain, pH = 3 | Rs (Ωcm2) | R1 (kΩcm2) | n1 | C1 (µFcm−2) | R2 (MΩcm2) | n2 | C2 (µFcm−2) | R3 (MΩ cm2) | n3 | C3 (µFcm−2) | Rp-EIS (MΩ cm2) | % ηRp-EIS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| bare surface | 1 h | 165.5 | / | / | / | / | / | / | 0.048 | 0.862 | 124.30 | 0.120 | |
| 10 h | 163.2 | / | / | / | / | / | / | 0.646 | 0.850 | 259.21 | 0.271 | ||
| 25 h | 159.7 | 0.272 | 0.892 | 62.67 | / | / | / | 0.801 | 0.392 | 17.29 | 0.528 | ||
| 120 h | 155.5 | 0.387 | 0.901 | 60.15 | / | / | / | 1.264 | 0.406 | 1.22 | 9.010 | ||
| modified surface SA | 1 h | 241.5 | 0.540 | 0.956 | 3.14 | / | / | / | 0.219 | 0.781 | 26.91 | 0.220 | 77.91 |
| 10 h | 236.1 | 0.791 | 0.903 | 15.08 | / | / | / | 1.898 | 0.829 | 41.06 | 1.899 | 97.44 | |
| 25 h | 232.8 | 0.657 | 0.908 | 13.70 | / | / | / | 2.257 | 0.895 | 22.23 | 2.258 | 97.85 | |
| 120 h | 240.0 | 0.617 | 0.925 | 11.14 | / | / | / | 6.047 | 0.911 | 16.44 | 6.048 | 99.20 | |
| modified surface (SA + 0.5%E307) | 1 h | 339.8 | 1.954 | 0.782 | 0.220 | 0.021 | 0.663 | 3.044 | 2.922 | 0.761 | 8.20 | 2.945 | 98.35 |
| 10 h | 335.1 | 0.826 | 0.766 | 0.209 | 0.064 | 0.592 | 12.24 | 4.738 | 0.757 | 21.45 | 4.803 | 98.99 | |
| 25 h | 349.5 | 0.603 | 0.760 | 0.208 | 0.132 | 0.602 | 20.30 | 6.311 | 0.853 | 31.06 | 6.444 | 99.25 | |
| 120 h | 339.0 | 0.350 | 0.838 | 0.601 | 0.158 | 0.523 | 19.12 | 8.280 | 0.906 | 47.01 | 8.437 | 99.42 | |
| modified surface (SA + 1.0%E307) | 1 h | 346.2 | 9.893 | 0.731 | 0.266 | 0.005 | 0.731 | 0.266 | 5.830 | 0.803 | 1.865 | 5.845 | 99.17 |
| 10 h | 340.4 | 15.102 | 0.726 | 0.219 | 3.850 | 0.768 | 0.625 | 16.212 | 0.768 | 0.625 | 20.081 | 99.76 | |
| 25 h | 341.6 | 8.492 | 0.755 | 0.109 | 7.900 | 0.744 | 0.660 | 16.432 | 0.615 | 0.622 | 24.322 | 99.80 | |
| 120 h | 350.2 | 10.724 | 0.761 | 0.150 | 6.901 | 0.722 | 1.249 | 20.105 | 0.890 | 0.746 | 27.010 | 99.82 | |
| modified surface (SA + 2.0%E307) | 1 h | 475.3 | 101.1 | 0.808 | 0.025 | 0.326 | 0.651 | 0.052 | 25.801 | 0.538 | 1.240 | 26.231 | 99.81 |
| 10 h | 484.8 | 220.6 | 0.789 | 0.034 | 1.094 | 0.658 | 0.080 | 26.180 | 0.694 | 0.401 | 27.492 | 99.82 | |
| 25 h | 473.9 | 225.4 | 0.777 | 0.044 | 3.474 | 0.594 | 0.080 | 28.121 | 0.699 | 0.349 | 31.820 | 99.85 | |
| 120 h | 479.5 | 563.9 | 0.990 | 0.007 | 4.074 | 0.668 | 0.120 | 28.592 | 0.713 | 0.214 | 33.231 | 99.86 |
| 3473 cm−1 | −OH stretching band |
| 2927 cm−1 2868 cm−1 | symmetric and asymmetric stretching vibrations of CH2 and CH3 |
| 1461 cm−1 | asymmetric bending of the phenyl skeleton and methyl group |
| 1378 cm−1 | symmetric bending of the methyl group |
| 1262 cm−1 | −CH2 |
| 1086 cm−1 | plane bending of phenyl group |
| 919 cm−1 | for trans = CH2 stretching |
| 1207 cm−1 1110–1107 cm−1 1068–1062 cm−1 | stretching vibration of C−O |
| After Fabrication | After 120 h | of Immersion | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Bare Surface | Modified Surface (SA) | Modified Surface (SA + 2%E307) | Modified Surface (SA) | Modified Surface (SA + 2%E307) | |||||
| Element | wt% | Element | wt% | Element | wt% | Element | wt% | Element | wt% |
| C K | 0.06 | C K | 43.04 | C K | 51.02 | C K | 41.74 | C K | 47.88 |
| Si K | 0.55 | Si K | 0.37 | Si K | 0.17 | Si K | 0.41 | Si K | 0.25 |
| Cr K | 15.74 | Cr K | 7.52 | Cr K | 6.71 | Cr K | 8.02 | Cr K | 7.37 |
| Fe K | 83.11 | Fe K | 49.08 | Fe K | 42.11 | Fe K | 49.83 | Fe K | 44.51 |
| Total | 100.00 | Total | 100.00 | Total | 100.00 | Total | 100.00 | Total | 100.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuchs-Godec, R. Flower-like Superhydrophobic Surfaces Fabricated on Stainless Steel as a Barrier against Corrosion in Simulated Acid Rain. Materials 2022, 15, 7104. https://doi.org/10.3390/ma15207104
Fuchs-Godec R. Flower-like Superhydrophobic Surfaces Fabricated on Stainless Steel as a Barrier against Corrosion in Simulated Acid Rain. Materials. 2022; 15(20):7104. https://doi.org/10.3390/ma15207104
Chicago/Turabian StyleFuchs-Godec, Regina. 2022. "Flower-like Superhydrophobic Surfaces Fabricated on Stainless Steel as a Barrier against Corrosion in Simulated Acid Rain" Materials 15, no. 20: 7104. https://doi.org/10.3390/ma15207104
APA StyleFuchs-Godec, R. (2022). Flower-like Superhydrophobic Surfaces Fabricated on Stainless Steel as a Barrier against Corrosion in Simulated Acid Rain. Materials, 15(20), 7104. https://doi.org/10.3390/ma15207104






