Failure Analysis of Ultra-High Molecular Weight Polyethylene Tibial Insert in Total Knee Arthroplasty
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Samples: Patients Data and Associated Pathologies
2.2. Characterization Methods
3. Results and Discussion
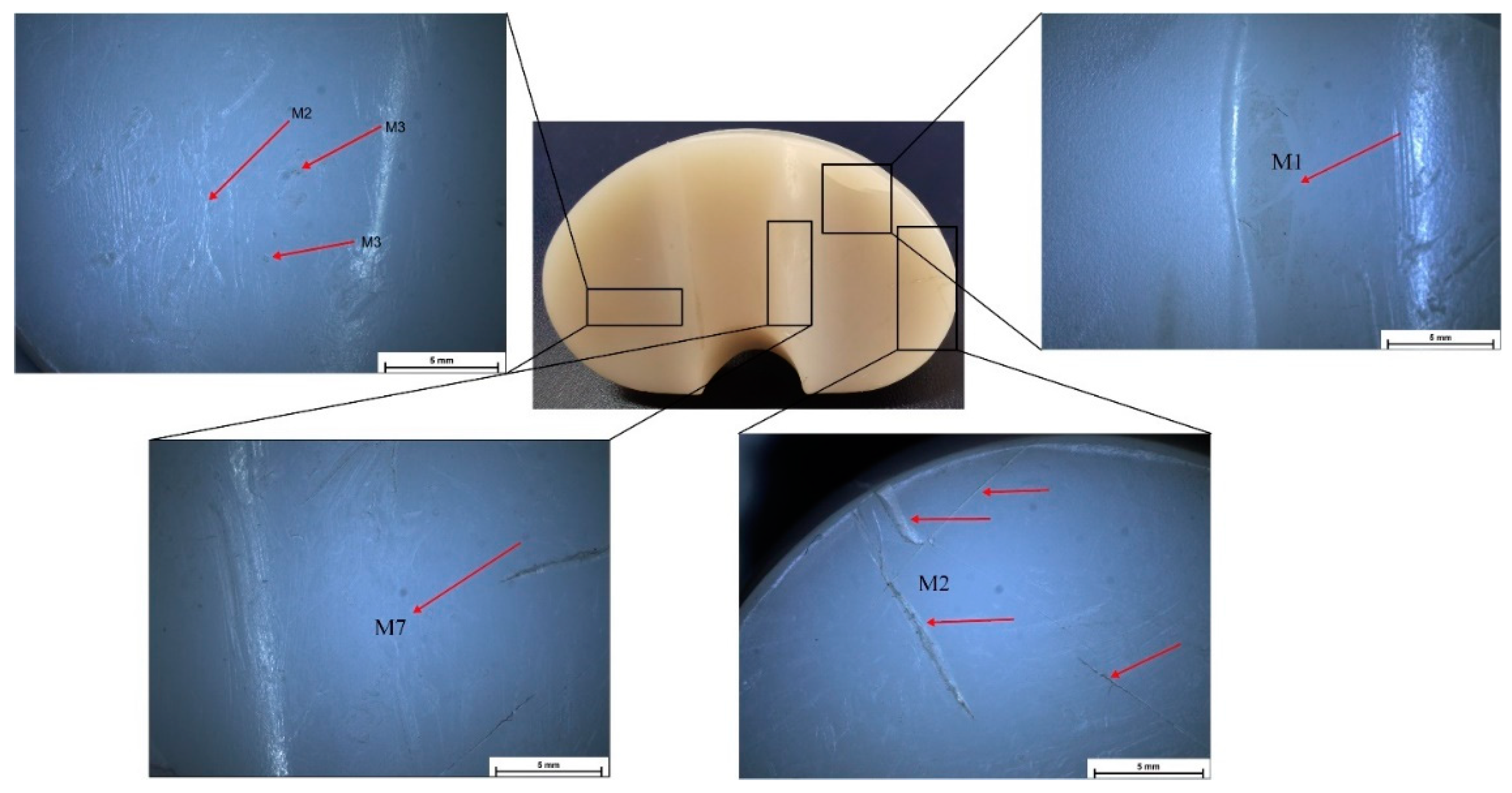
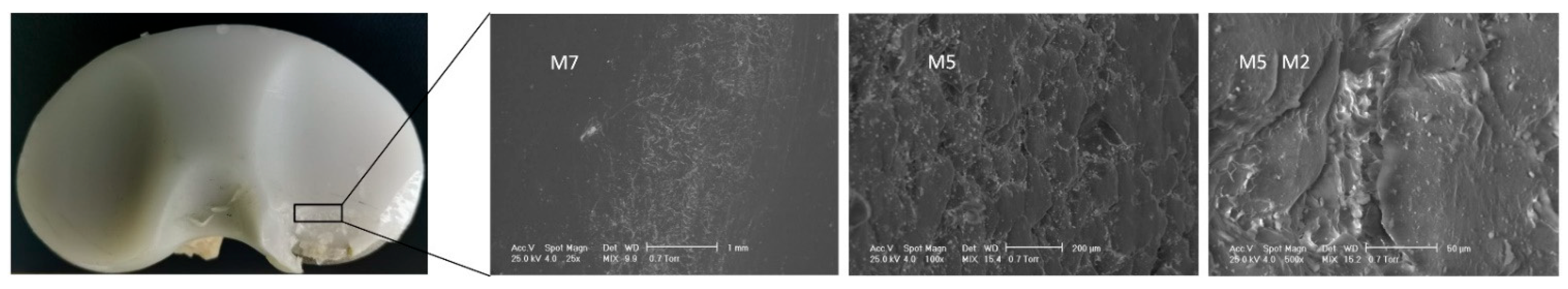
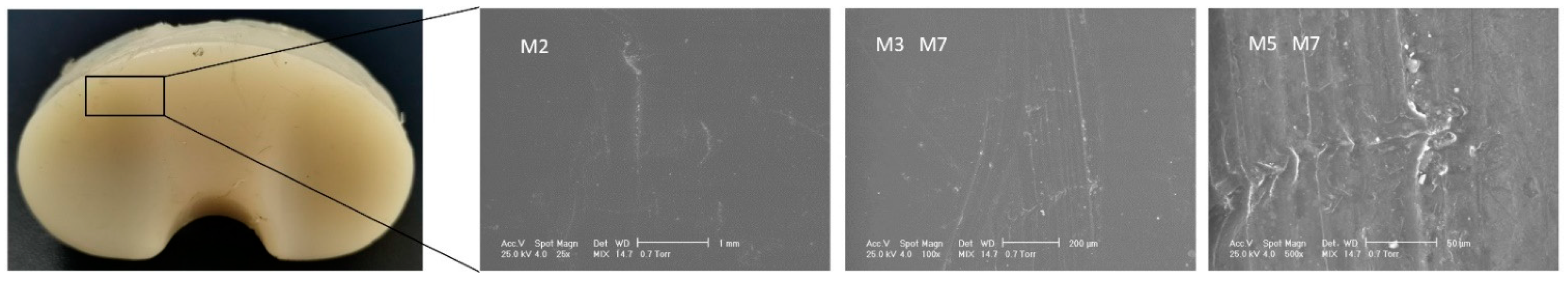
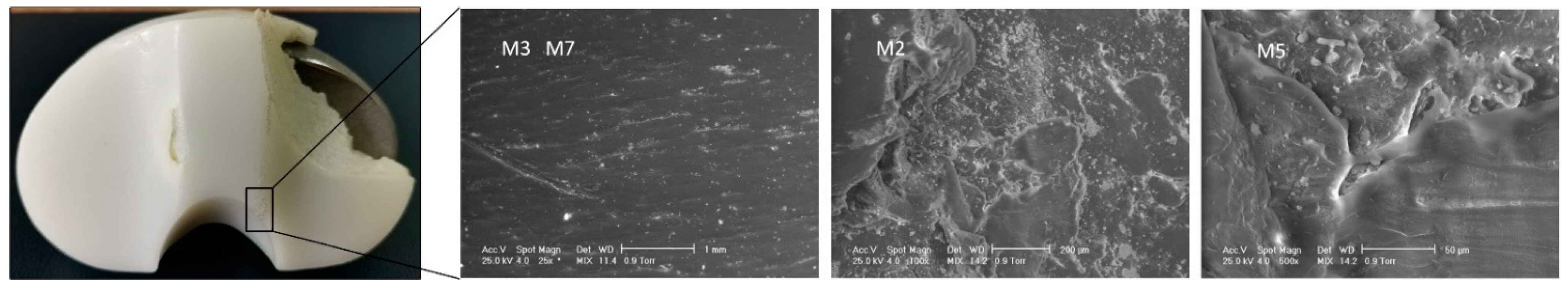
3.1. Surface Deterioration Mechanisms
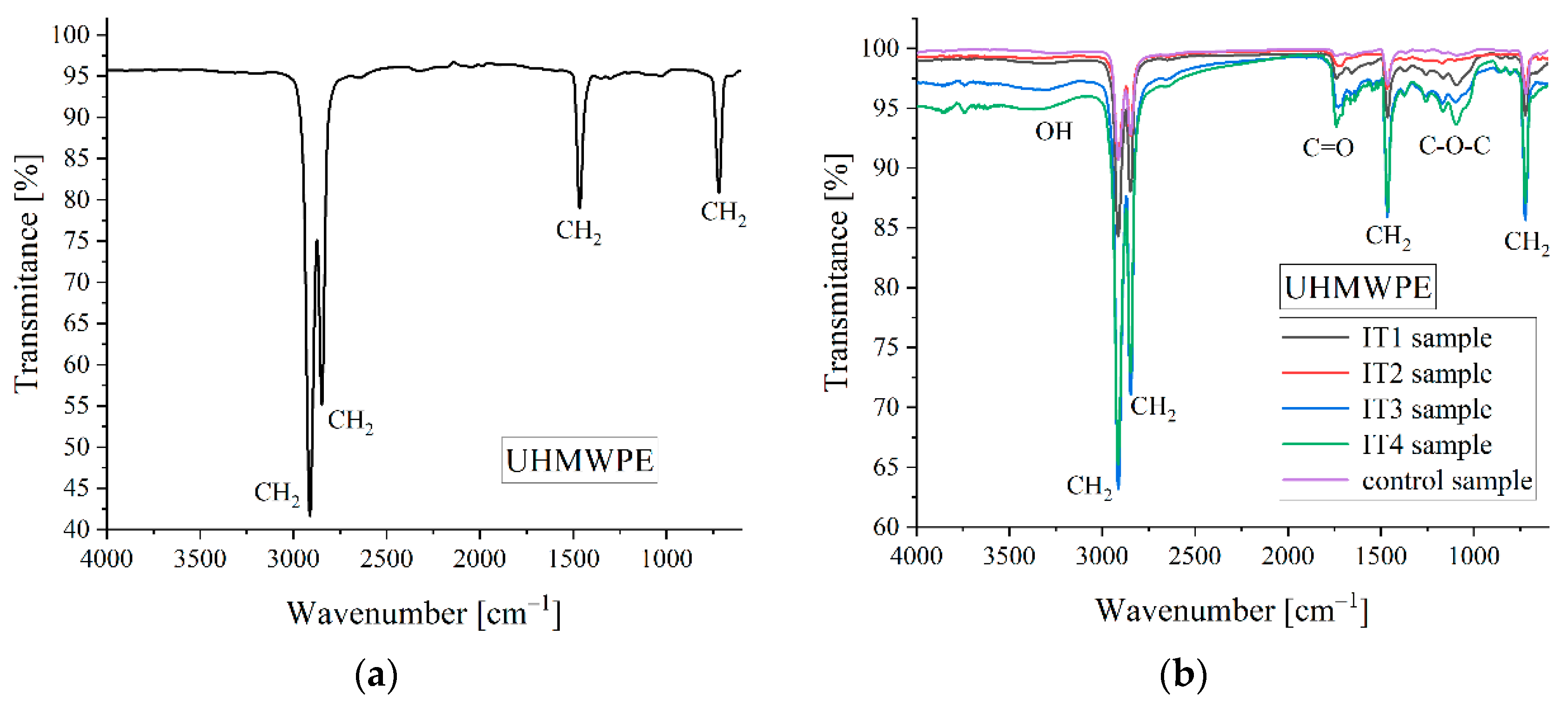
3.2. Oxidative Degradation Analysis
3.3. Mechanical Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maradit, K.H.; Larson, D.R.; Crowson, C.S.; Kremers, W.K.; Washington, R.E.; Steiner, C.A.; Jiranek, W.A.; Berry, D.J. Prevalence of Total Hip and Knee Replacement in the United States. J. Bone Jt. Surg.-Am. Vol. 2015, 97, 1386–1397. [Google Scholar] [CrossRef]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of Primary and Revision Hip and Knee Arthroplasty in the United States from 2005 to 2030. J. Bone Joint Surg. Am. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Gibon, E.; Goodman, M.J.; Goodman, S.B. Patient Satisfaction After Total Knee Arthroplasty. Orthop. Clin. N. Am. 2017, 48, 421–431. [Google Scholar] [CrossRef]
- Beswick, A.D.; Wylde, V.; Gooberman-Hill, R.; Blom, A.; Dieppe, P. What Proportion of Patients Report Long-Term Pain after Total Hip or Knee Replacement for Osteoarthritis? A Systematic Review of Prospective Studies in Unselected Patients. BMJ Open 2012, 2, e000435. [Google Scholar] [CrossRef]
- Wylde, V.; Hewlett, S.; Learmonth, I.D.; Dieppe, P. Persistent Pain after Joint Replacement: Prevalence, Sensory Qualities, and Postoperative Determinants. Pain 2011, 152, 566–572. [Google Scholar] [CrossRef]
- Carbonell-Blasco, P.; Martín-Martínez, J.M.; Antoniac, I.V. Synthesis and Characterization of Polyurethane Sealants Containing Rosin Intended for Sealing Defect in Annulus for Disc Regeneration. Int. J. Adhes. Adhes. 2013, 42, 11–20. [Google Scholar] [CrossRef]
- Bhandari, M.; Smith, J.; Miller, L.E.; Block, J.E. Clinical and Economic Burden of Revision Knee Arthroplasty. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2012, 5, 10859. [Google Scholar] [CrossRef]
- Nica, M.; Cretu, B.; Ene, D.; Antoniac, I.; Gheorghita, D.; Ene, R. Failure Analysis of Retrieved Osteosynthesis Implants. Materials 2020, 13, 1201. [Google Scholar] [CrossRef]
- Iulian, A.; Cosmin, S.; Aurora, A. Adhesion Aspects in Biomaterials and Medical Devices. J. Adhes. Sci. Technol. 2016, 30, 1711–1715. [Google Scholar] [CrossRef]
- Ferche, O.; Moldoveanu, A.; Cinteza, D.; Toader, C.; Moldoveanu, F.; Voinea, A.; Taslitchi, C. From Neuromotor Command to Feedback: A Survey of Techniques for Rehabilitation through Altered Perception. In Proceedings of the 2015 E-Health and Bioengineering Conference (EHB), Iasi, Romania, 19–21 November 2015; pp. 1–4. [Google Scholar]
- Miculescu, M.; Ion, O.A. Regulation and Certification of (Bio)Medical Engineers: A Case Study on Romania. Int. J. Environ. Res. Public Health 2022, 19, 9004. [Google Scholar] [CrossRef]
- Antoniac, I.; Laptoiu, D.; Tecu, C.; Milea, C.; Gradinaru, S. Synthetic Materials for Osteochondral Tissue Engineering. Adv. Exp. Med. Biol. 2018, 1058, 31–52. [Google Scholar] [CrossRef]
- Bane, M.; Miculescu, F.; Blajan, A.I.; Dinu, M.; Antoniac, I. Failure Analysis of Some Retrieved Orthopedic Implants Based on Materials Characterization. Solid State Phenom. 2012, 188, 114–117. [Google Scholar] [CrossRef]
- Antoniac, I.V.; Miculescu, F.; Laptoiu, D.; Antoniac, A.; Niculescu, M.; Grecu, D. Retrieval Analysis of Hip Prostheses. In Handbook of Bioceramics and Biocomposites; Antoniac, I.V., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 901–933. ISBN 978-3-319-12460-5. [Google Scholar]
- Buechel, F.F.; Pappas, M.J. Properties of Materials Used in Orthopaedic Implant Systems. In Principles of Human Joint Replacement: Design and Clinical Application; Buechel, F.F., Pappas, M.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–35. ISBN 978-3-642-23011-0. [Google Scholar]
- ODEP. Available online: https://www.odep.org.uk/ (accessed on 19 April 2022).
- Vertullo, C.J.; Lewis, P.L.; Graves, S.; Kelly, L.; Lorimer, M.; Myers, P. Twelve-Year Outcomes of an Oxinium Total Knee Replacement Compared with the Same Cobalt-Chromium Design: An Analysis of 17,577 Prostheses from the Australian Orthopaedic Association National Joint Replacement Registry. J. Bone Jt. Surg. Am. 2017, 99, 275–283. [Google Scholar] [CrossRef]
- Bonnheim, N.B.; Citters, D.W.V.; Ries, M.D.; Pruitt, L.A. Oxidized Zirconium Components Maintain a Smooth Articular Surface Except Following Hip Dislocation. J. Arthroplast. 2021, 36, 1437–1444. [Google Scholar] [CrossRef]
- Purcell, A.; Buckner, S.; Brindley, G.; Grimes, J. A Unique Case of Extra-Articular Extravasation of Metal Into the Lower Leg Resulting From Oxidized Zirconium Wear Particles From Total Knee Arthroplasty. Arthroplast. Today 2020, 6, 988–992. [Google Scholar] [CrossRef]
- Piconi, C.; Maccauro, G.; Muratori, F.; Brach Del Prever, E. Alumina and Zirconia Ceramics in Joint Replacements. J. Appl. Biomater. Biomech. 2003, 1, 19–32. [Google Scholar]
- Nakamura, S.; Minoda, Y.; Nakagawa, S.; Kadoya, Y.; Takemura, S.; Kobayashi, A.; Mizokawa, S.; Ohta, Y.; Takahashi, S.; Yamamura, K.; et al. Clinical Results of Alumina Medial Pivot Total Knee Arthroplasty at a Minimum Follow-up of 10 years. Knee 2017, 24, 434–438. [Google Scholar] [CrossRef]
- Tsukamoto, R.; Williams, P.A.; Clarke, I.C.; Pezzotti, G.; Shoji, H.; Akagi, M.; Yamamoto, K. Y-TZP Zirconia Run against Highly Crosslinked UHMWPE Tibial Inserts: Knee Simulator Wear and Phase-Transformation Studies. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 86B, 145–153. [Google Scholar] [CrossRef]
- Bergschmidt, P.; Bader, R.; Ganzer, D.; Hauzeur, C.; Lohmann, C.; Rüther, W.; Tigani, D.; Rani, N.; Prats, F.L.; Zorzi, C.; et al. Ceramic Femoral Components in Total Knee Arthroplasty—Two Year Follow-Up Results of an International Prospective Multi-Centre Study. Open Orthop. J. 2012, 6, 172–178. [Google Scholar] [CrossRef]
- Affatato, S.; Erani, P.; Fersini, M.; Contaldi, V.; Terrizzi, A.R.; Licciulli, A. Preliminary In Vitro Wear Assessment of Ceramic Cemented Femoral Components Coupled with Polyethylene Menisci. Materials 2021, 14, 2112. [Google Scholar] [CrossRef]
- Bioceramics and Biocomposites: From Research to Clinical Practice|Wiley. Available online: https://www.wiley.com/en-us/Bioceramics+and+Biocomposites%3A+From+Research+to+Clinical+Practice-p-9781119049340 (accessed on 27 August 2022).
- Panayotov, I.V.; Orti, V.; Cuisinier, F.; Yachouh, J. Polyetheretherketone (PEEK) for Medical Applications. J. Mater. Sci. Mater. Med. 2016, 27, 118. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Devine, J.N. PEEK Biomaterials in Trauma, Orthopedic, and Spinal Implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.W.; Kirch, M.; Birchler, F.; Eckert, K.L.; Mayer, J.; Wintermantel, E.; Sittig, C.; Pfund-Klingenfuss, I.; Textor, M.; Spencer, N.D.; et al. Surface Activation of Polyetheretherketone (PEEK) and Formation of Calcium Phosphate Coatings by Precipitation. J. Mater. Sci. Mater. Med. 1997, 8, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Qu, X.; Zhao, Y.; Yuan, Z.; Zheng, L.; Long, T.; Yao, Q.; Yue, B.; Wang, Y. Preliminary Study on Immediate Postoperative CT Images and Values of the Modular Polyetheretherketone Based Total Knee Arthroplasty: An Observational First-in-Human Trial. Front. Surg. 2022, 9, 809699. [Google Scholar] [CrossRef]
- Ran, J.; Jiang, F.; Sun, X.; Chen, Z.; Tian, C.; Zhao, H. Microstructure and Mechanical Properties of Ti-6Al-4V Fabricated by Electron Beam Melting. Crystals 2020, 10, 972. [Google Scholar] [CrossRef]
- Saboori, A.; Gallo, D.; Biamino, S.; Fino, P.; Lombardi, M. An Overview of Additive Manufacturing of Titanium Components by Directed Energy Deposition: Microstructure and Mechanical Properties. Appl. Sci. 2017, 7, 883. [Google Scholar] [CrossRef]
- Trabecular MetalTM Technology|Zimmer Biomet EU. Available online: https://www.zimmerbiomet.eu/en/products-and-solutions/specialties/hip/trabecular-metal-technology.html (accessed on 16 April 2022).
- Meneghini, R.M.; de Beaubien, B.C. Early Failure of Cementless Porous Tantalum Monoblock Tibial Components. J. Arthroplast. 2013, 28, 1505–1508. [Google Scholar] [CrossRef]
- First In-Patient TKA With PEEK-OPTIMA Femoral Component|Orthopedics This Week. Available online: https://ryortho.com/breaking/first-in-patient-tka-with-peek-optima-femoral-component/ (accessed on 19 April 2022).
- Dalury, D.F.; Pomeroy, D.L.; Gonzales, R.A.; Gruen, T.A.; Adams, M.J.; Empson, J.A. Midterm Results of All-Polyethylene Tibial Components in Primary Total Knee Arthroplasty. J. Arthroplast. 2009, 24, 620–624. [Google Scholar] [CrossRef]
- Meier, E.; Gelse, K.; Trieb, K.; Pachowsky, M.; Hennig, F.F.; Mauerer, A. First Clinical Study of a Novel Complete Metal-Free Ceramic Total Knee Replacement System. J. Orthop. Surg. Res. 2016, 11, 21. [Google Scholar] [CrossRef]
- Bistolfi, A.; Giustra, F.; Bosco, F.; Sabatini, L.; Aprato, A.; Bracco, P.; Bellare, A. Ultra-High Molecular Weight Polyethylene (UHMWPE) for Hip and Knee Arthroplasty: The Present and the Future. J. Orthop. 2021, 25, 98–106. [Google Scholar] [CrossRef]
- Sobieraj, M.C.; Rimnac, C.M. Ultra High Molecular Weight Polyethylene: Mechanics, Morphology, and Clinical Behavior. J. Mech. Behav. Biomed. Mater. 2009, 2, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Bracco, P.; Bellare, A.; Bistolfi, A.; Affatato, S. Ultra-High Molecular Weight Polyethylene: Influence of the Chemical, Physical and Mechanical Properties on the Wear Behavior. A Review. Materials 2017, 10, 791. [Google Scholar] [CrossRef]
- Currier, B.H.; Currier, J.H.; Franklin, K.J.; Mayor, M.B.; Reinitz, S.D.; Van Citters, D.W. Comparison of Wear and Oxidation in Retrieved Conventional and Highly Cross-Linked UHMWPE Tibial Inserts. J. Arthroplast. 2015, 30, 2349–2353. [Google Scholar] [CrossRef]
- Rojanasopondist, P.; Muratoglu, O. Polyethylene in Total Knee Arthroplasty. Available online: https:///musculoskeletalkey.com/polyethylene-in-total-knee-arthroplasty/ (accessed on 18 April 2022).
- Matz, J.; Lanting, B.A.; Howard, J.L. Understanding the Patellofemoral Joint in Total Knee Arthroplasty. Can. J. Surg. 2019, 62, 57–65. [Google Scholar] [CrossRef]
- Gaman, L.; Radoi, M.P.; Delia, C.E.; Luzardo, O.P.; Zumbado, M.; Rodríguez-Hernández, Á.; Stoian, I.; Gilca, M.; Boada, L.D.; Henríquez-Hernández, L.A. Concentration of Heavy Metals and Rare Earth Elements in Patients with Brain Tumours: Analysis in Tumour Tissue, Non-Tumour Tissue, and Blood. Int. J. Environ. Health Res. 2021, 31, 741–754. [Google Scholar] [CrossRef]
- Antoniac, I.; Negrusoiu, M.; Mardare, M.; Socoliuc, C.; Zazgyva, A.; Niculescu, M. Adverse Local Tissue Reaction after 2 Revision Hip Replacements for Ceramic Liner Fracture. Medicine 2017, 96, e6687. [Google Scholar] [CrossRef]
- Papacocea, M.T.; Badarau, I.A.; Radoi, M.; Papacocea, I.R. The Predictive Role of Biochemical Plasma Factors in Patients with Severe Traumatic Brain Injuries. Rev. Chim. 2019, 70, 1754–1757. [Google Scholar] [CrossRef]
- Zhang, L.; Haddouti, E.-M.; Welle, K.; Burger, C.; Kabir, K.; Schildberg, F.A. Local Cellular Responses to Metallic and Ceramic Nanoparticles from Orthopedic Joint Arthroplasty Implants. Int. J. Nanomed. 2020, 15, 6705–6720. [Google Scholar] [CrossRef]
- Marinescu, R.; Antoniac, I.; Laptoiu, D.; Antoniac, A.; Grecu, D. Complications Related to Biocomposite Screw Fixation in ACL Reconstruction Based on Clinical Experience and Retrieval Analysis. Mater. Plast. 2015, 52, 340–344. [Google Scholar]
- ASTM International-ASTM F0561-97-Practice for Retrieval and Analysis of Implanted Medical Devices, and Associated Tissues. Available online: https://www.astm.org/f0561-97.html (accessed on 27 August 2022).
- Antoniac, I.V.; Burcea, M.; Ionescu, R.D.; Balta, F. IOL’s Opacification: A Complex Analysis Based on the Clinical Aspects, Biomaterials Used and Surface Characterization of Explanted IOL’s. Mater. Plast. 2015, 5, 109–112. [Google Scholar]
- Jain, R.; Kapoor, D. The Dynamic Interface: A Review. J. Int. Soc. Prev. Community Dent. 2015, 5, 354–358. [Google Scholar] [CrossRef]
- Branemark, P.; Albrektsson, T. Tissue Integrated Prosthesis Osseointegration. In Clinical Dentistry; Quintessence Publishing Co. Inc.: Chicago, IL, USA, 1985. [Google Scholar]
- Albrektsson: The Interface Zone of Inorganic ImplantsIn. Available online: https://scholar.google.com/scholar_lookup?journal=Annals+of+Biomedical+Engineering&title=The+interface+of+inorganic+implants+in+vivo;+titanium+implants+in+bone&author=T+Albrektsson&author=PI+Br%C3%A5nemark&author=HA+Hansson&author=B+Kasemo&author=K+Larsson&volume=11&publication_year=1983&pages=1-27& (accessed on 1 October 2022).
- Liza, S.; Haseeb, A.S.M.A.; Abbas, A.A.; Masjuki, H.H. Failure Analysis of Retrieved UHMWPE Tibial Insert in Total Knee Replacement. Eng. Fail. Anal. 2011, 18, 1415–1423. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Rimnac, C.M.; Pruitt, L.; Jewett, C.W.; Goldberg, V.; Edidin, A.A. The Relationship between the Clinical Performance and Large Deformation Mechanical Behavior of Retrieved UHMWPE Tibial Inserts. Biomaterials 2000, 21, 283–291. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Foulds, J.R.; Jewett, C.W.; Srivastav, S.; Edidin, A.A. Validation of a Small Punch Testing Technique to Characterize the Mechanical Behaviour of Ultra-High-Molecular-Weight Polyethylene. Biomaterials 1997, 18, 1659–1663. [Google Scholar] [CrossRef]
- Edidin, A.A.; Kurtz, S.M. Influence of Mechanical Behavior on the Wear of 4 Clinically Relevant Polymeric Biomaterials in a Hip Simulator. J. Arthroplast. 2000, 15, 321–331. [Google Scholar] [CrossRef]
- Edidin, A.A. Development and Application of the Small Punch Test to UHMWPE. In UHMWPE Biomaterials Handbook; Elsevier: Amsterdam, The Netherlands, 2015; pp. 739–752. [Google Scholar]
- Cho, C.-H.; Murakami, T.; Sawae, Y. Influence of Microscopic Surface Asperities on the Wear of Ultra-High Molecular Weight Polyethylene in a Knee Prosthesis. Proc. Inst. Mech. Eng. H 2010, 224, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Medel, F.J.; Kurtz, S.M.; Parvizi, J.; Klein, G.R.; Kraay, M.J.; Rimnac, C.M. In Vivo Oxidation Contributes to Delamination but Not Pitting in Polyethylene Components for Total Knee Arthroplasty. J. Arthroplast. 2011, 26, 802–810. [Google Scholar] [CrossRef]
- Berry, D.J.; Currier, B.H.; Mayor, M.B.; Collier, J.P. Gamma-Irradiation Sterilization in an Inert Environment: A Partial Solution. Clin. Orthop. Relat. Res. 2012, 470, 1805–1813. [Google Scholar] [CrossRef]
- Cerquiglini, A.; Henckel, J.; Hothi, H.; Moser, L.B.; Eskelinen, A.; Hirschmann, M.T.; Hart, A.J. Retrieval Analysis of Contemporary Antioxidant Polyethylene: Multiple Material and Design Changes May Decrease Implant Performance. Knee Surg. Sport. Traumatol. Arthrosc. 2019, 27, 2111–2119. [Google Scholar] [CrossRef]
- Tone, S.; Hasegawa, M.; Naito, Y.; Pezzotti, G.; Sudo, A. Raman Spectroscopy Reveals Creep and Wear Rate of E-Beam-Sterilized Conventional UHMWPE Tibial Inserts. J. Mech. Behav. Biomed. Mater. 2020, 110, 103902. [Google Scholar] [CrossRef]
- Granquist, E.J.; Bouloux, G.; Dattilo, D.; Gonzalez, O.; Louis, P.J.; McCain, J.; Sinn, D.; Szymela, V.; Warner, M.; Quinn, P.D. Outcomes and Survivorship of Biomet Microfixation Total Joint Replacement System: Results From an FDA Postmarket Study. J. Oral. Maxillofac. Surg. 2020, 78, 1499–1508. [Google Scholar] [CrossRef]
- Hood, R.W.; Wright, T.M.; Burstein, A.H. Retrieval Analysis of Total Knee Prostheses: A Method and Its Application to 48 Total Condylar Prostheses. J. Biomed. Mater. Res. 1983, 17, 829–842. [Google Scholar] [CrossRef]
- EN 10371:2021-Metallic Materials-Small Punch Test Method. Available online: https://standards.iteh.ai/catalog/standards/cen/3bf5f9ce-4532-4a8b-ba9f-c7507d246312/en-10371-2021 (accessed on 27 September 2022).
- West, J.A.; Scudday, T.; Anderson, S.; Amin, N.H. Clinical Outcomes and Patient Satisfaction after Total Knee Arthroplasty: A Follow-up of the First 50 Cases by a Single Surgeon. J. Int. Med. Res. 2019, 47, 1667–1676. [Google Scholar] [CrossRef]
- Grecu, D.; Antoniac, I.; Trante, O.; Niculescu, M.; Lupescu, O. Failure Analysis of Retrieved Polyethylene Insert in Total Knee Replacement. Biomaterials 2016, 6, 12–15. [Google Scholar]
- Postler, A.; Lützner, C.; Beyer, F.; Tille, E.; Lützner, J. Analysis of Total Knee Arthroplasty Revision Causes. BMC Musculoskelet. Disord. 2018, 19, 55. [Google Scholar] [CrossRef]
- Kandahari, A.M.; Yang, X.; Laroche, K.A.; Dighe, A.S.; Pan, D.; Cui, Q. A Review of UHMWPE Wear-Induced Osteolysis: The Role for Early Detection of the Immune Response. Bone Res. 2016, 4, 16014. [Google Scholar] [CrossRef]
- Seon, J.-K.; Park, J.-K.; Shin, Y.-J.; Seo, H.-Y.; Lee, K.-B.; Song, E.-K. Comparisons of Kinematics and Range of Motion in High-Flexion Total Knee Arthroplasty: Cruciate Retaining vs. Substituting Designs. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 2016–2022. [Google Scholar] [CrossRef]
- Harato, K.; Bourne, R.B.; Victor, J.; Snyder, M.; Hart, J.; Ries, M.D. Midterm Comparison of Posterior Cruciate-Retaining versus -Substituting Total Knee Arthroplasty Using the Genesis II Prosthesis. Knee 2008, 15, 217–221. [Google Scholar] [CrossRef]
- Rose, R.M.; Nusbaum, H.J.; Schneider, H.; Ries, M.; Paul, I.; Crugnola, A.; Simon, S.R.; Radin, E.L. On the True Wear Rate of Ultra High-Molecular-Weight Polyethylene in the Total Hip Prosthesis. J. Bone Jt. Surg. Am. 1980, 62, 537–549. [Google Scholar] [CrossRef]
- Rostoker, W. The Appearances of Wear on Polyethylene--a Comparison of in Vivo and in Vitro Wear Surfaces. J. Biomed. Mater. Res. 1978, 12, 317–335. [Google Scholar] [CrossRef]
- Fabry, C.; Zietz, C.; Dammer, R.; Bader, R. 12 Patterns of Wear in Total Knee Replacement. In The Unhappy Total Knee Replacement: A Comprehensive Review and Management Guide; Hirschmann, M.T., Becker, R., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 135–145. ISBN 978-3-319-08099-4. [Google Scholar]
- Kim, K.T.; Lee, S.; Ko, D.O.; Seo, B.S.; Jung, W.S.; Chang, B.K. Causes of Failure after Total Knee Arthroplasty in Osteoarthritis Patients 55 Years of Age or Younger. Knee Surg. Relat. Res. 2014, 26, 13–19. [Google Scholar] [CrossRef]
- Hasandoost, L.; Rodriguez, O.; Alhalawani, A.; Zalzal, P.; Schemitsch, E.H.; Waldman, S.D.; Papini, M.; Towler, M.R. The Role of Poly(Methyl Methacrylate) in Management of Bone Loss and Infection in Revision Total Knee Arthroplasty: A Review. J. Funct. Biomater. 2020, 11, 25. [Google Scholar] [CrossRef]
- Sm, K.; Ha, G.; Jd, P. History and Systematic Review of Wear and Osteolysis Outcomes for First-Generation Highly Crosslinked Polyethylene. Clin. Orthop. Relat. Res. 2011, 469, 2262–2277. [Google Scholar] [CrossRef]
- Currier, B.H.; Currier, J.H.; Holdcroft, L.A.; Van Citters, D.W. Effectiveness of Anti-Oxidant Polyethylene: What Early Retrievals Can Tell Us. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 353–359. [Google Scholar] [CrossRef]
- Mathis, D.T.; Schmidli, J.; Hirschmann, M.T.; Amsler, F.; Henckel, J.; Hothi, H.; Hart, A. Comparative Retrieval Analysis of Antioxidant Polyethylene: Bonding of Vitamin-E Does Not Reduce in-Vivo Surface Damage. BMC Musculoskelet. Disord. 2021, 22, 1003. [Google Scholar] [CrossRef]
- Camacho, N.; Gonzalez, J.M.; Espinosa, D.; Mondragón, G.; Stafford, S. UHMWPE in Total Knee Arthroplasty: Successes & Failures. Rev. Colom. Mater. 2021, 16, 3–28. [Google Scholar] [CrossRef]
- Kunkel, S.T.; Moschetti, W.E.; Werth, P.; Fillingham, Y.; Jevsevar, D.; VanCitters, D.; Currier, J.; Currier, B.; Henderson, E. Tobacco Exposure Is Associated With Extremely Low Polyethylene Oxidation in Total Knee Arthroplasty Components. Arthroplast. Today 2021, 8, 243–246. [Google Scholar] [CrossRef]
- Christiner, T.; Pabbruwe, M.B.; Kop, A.M.; Parry, J.; Clark, G.; Collopy, D. Taper Corrosion and Adverse Local Tissue Reactions in Patients with a Modular Knee Prosthesis. JB JS Open Access 2018, 3, e0019. [Google Scholar] [CrossRef]
- Pourzal, R.; Cip, J.; Rad, E.; Laurent, M.P.; Berger, R.A.; Jacobs, J.J.; Wimmer, M.A. Joint Line Elevation and Tibial Slope Are Associated with Increased Polyethylene Wear in Cruciate-Retaining Total Knee Replacement. J. Orthop. Res. 2020, 38, 1596–1606. [Google Scholar] [CrossRef]
- Sisko, Z.W.; Teeter, M.G.; Lanting, B.A.; Howard, J.L.; McCalden, R.W.; Naudie, D.D.; MacDonald, S.J.; Vasarhelyi, E.M. Current Total Knee Designs: Does Baseplate Roughness or Locking Mechanism Design Affect Polyethylene Backside Wear? Clin. Orthop. Relat. Res. 2017, 475, 2970–2980. [Google Scholar] [CrossRef]
- Goldvasser, D.; Marchie, A.; Bragdon, L.K.; Bragdon, C.R.; Weidenhielm, L.; Malchau, H. Incidence of Osteolysis in Total Knee Arthroplasty: Comparison between Radiographic and Retrieval Analysis. J. Arthroplast. 2013, 28, 201–206. [Google Scholar] [CrossRef]
- Klutzny, M.; Singh, G.; Hameister, R.; Goldau, G.; Awiszus, F.; Feuerstein, B.; Stärke, C.; Lohmann, C.H. Screw Track Osteolysis in the Cementless Total Knee Replacement Design. J. Arthroplast. 2019, 34, 965–973. [Google Scholar] [CrossRef]
- Ansari, F.; Chang, J.; Huddleston, J.; Van Citters, D.; Ries, M.; Pruitt, L. Fractography and Oxidative Analysis of Gamma Inert Sterilized Posterior-Stabilized Tibial Insert Post Fractures: Report of Two Cases. Knee 2013, 20, 609–613. [Google Scholar] [CrossRef]
- UHMWPE Biomaterials Handbook-3rd Edition. Available online: https://www.elsevier.com/books/uhmwpe-biomaterials-handbook/kurtz/978-0-323-35401-1 (accessed on 1 June 2022).
- ASTM International-ASTM F2003-00-Standard Guide for Accelerated Aging of Ultra-High Molecular Weight Polyethylene | Engineering360. Available online: https://standards.globalspec.com/std/3803981/astm-f2003-00 (accessed on 9 June 2022).
- Azam, A.M.; Ali, A.; Khan, H.; Yasin, T.; Mehmood, M.S. Analysis of Degradation in UHMWPE a Comparative Study among the Various Commercial and Laboratory Grades UHMWPE. IOP Conf. Ser. Mater. Sci. Eng. 2016, 146, 012025. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Muratoglu, O.K.; Gsell, R.; Greer, K.; Shen, F.W.; Cooper, C.; Buchanan, F.J.; Spiegelberg, S.; Yau, S.S.; Edidin, A.A. Interlaboratory Validation of Oxidation-Index Measurement Methods for UHMWPE after Long-Term Shelf Aging. J. Biomed. Mater. Res. 2002, 63, 15–23. [Google Scholar] [CrossRef]
- Li, C.; Shi, Y.; Zhang, R.; Wang, G.; Jia, J. Effect of Surface Modifications on the Properties of UHMWPE Fibres and Their Composites. e-Polymers 2019, 19, 40–49. [Google Scholar] [CrossRef]
- Zerbi, G.; Gallino, G.; Del Fanti, N.; Baini, L. Structural Depth Profiling in Polyethylene Films by Multiple Internal Reflection Infra-Red Spectroscopy. Polymer 1989, 30, 2324–2327. [Google Scholar] [CrossRef]
- Standard Specification for Ultra-High Molecular Weight Polyethylene Powder Blended With Alpha-Tocopherol (Vitamin E) and Fabricated Forms for Surgical Implant Applications. Available online: https://www.astm.org/f2695-07.html (accessed on 25 September 2022).
- López-Cervantes, A.; Domínguez-López, I.; Barceinas-Sánchez, J.D.O.; García-García, A.L. Effects of Surface Texturing on the Performance of Biocompatible UHMWPE as a Bearing Material during in Vitro Lubricated Sliding/Rolling Motion. J. Mech. Behav. Biomed. Mater. 2013, 20, 45–53. [Google Scholar] [CrossRef]
- Baena, J.C.; Wu, J.; Peng, Z. Wear Performance of UHMWPE and Reinforced UHMWPE Composites in Arthroplasty Applications: A Review. Lubricants 2015, 3, 413–436. [Google Scholar] [CrossRef]
- Chang, B.-P.; Md. Akil, H.; Bt. Md. Nasir, R. Comparative Study of Micro- and Nano-ZnO Reinforced UHMWPE Composites under Dry Sliding Wear. Wear 2013, 297, 1120–1127. [Google Scholar] [CrossRef]
- Chang, B.P.; Akil, H.M.; Nasir, R.M. Mechanical and Tribological Properties of Zeolite-Reinforced UHMWPE Composite for Implant Application. Procedia Eng. 2013, 68, 88–94. [Google Scholar] [CrossRef]
- Plumlee, K.; Schwartz, C.J. Improved Wear Resistance of Orthopaedic UHMWPE by Reinforcement with Zirconium Particles. Wear 2009, 267, 710–717. [Google Scholar] [CrossRef]

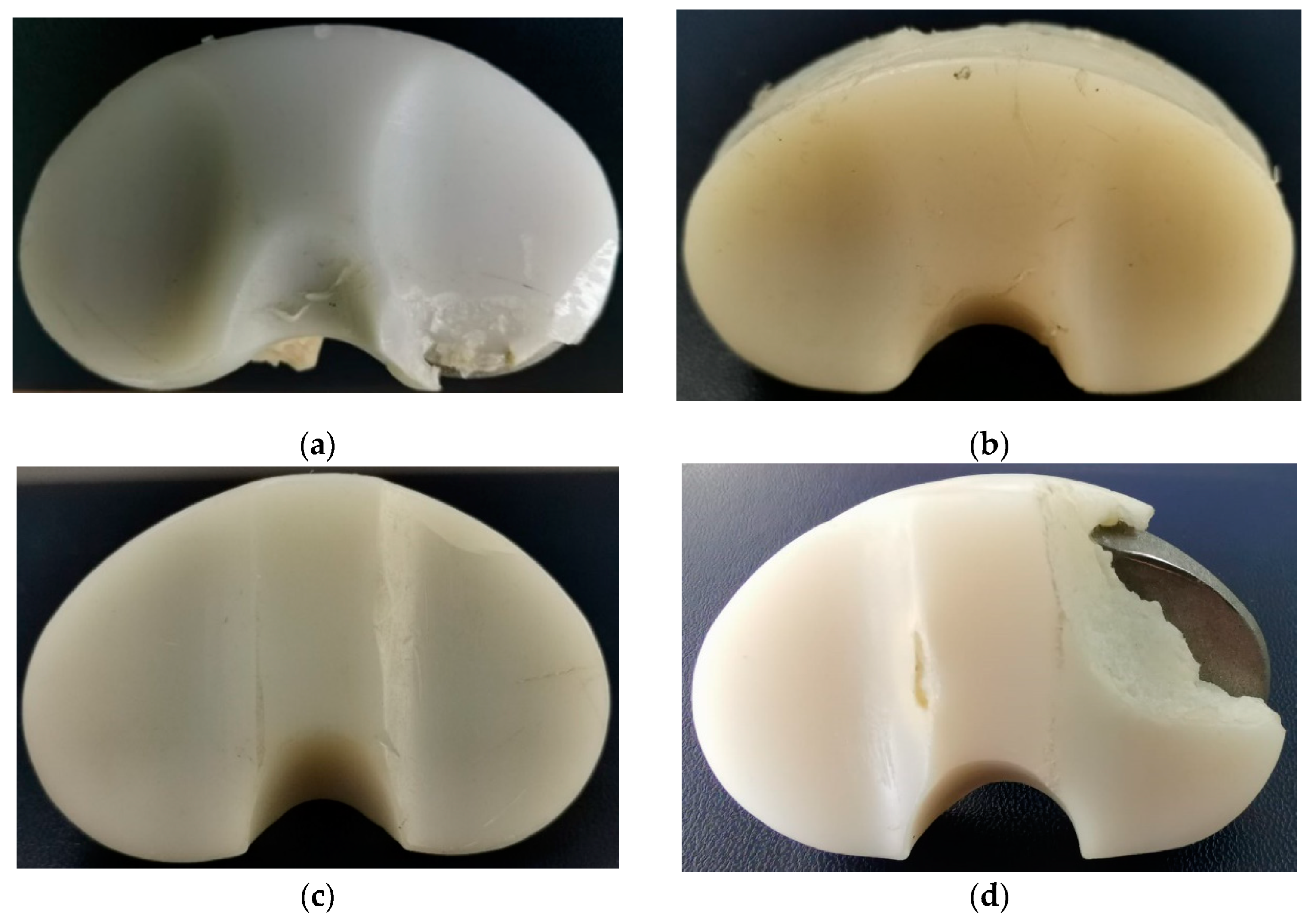
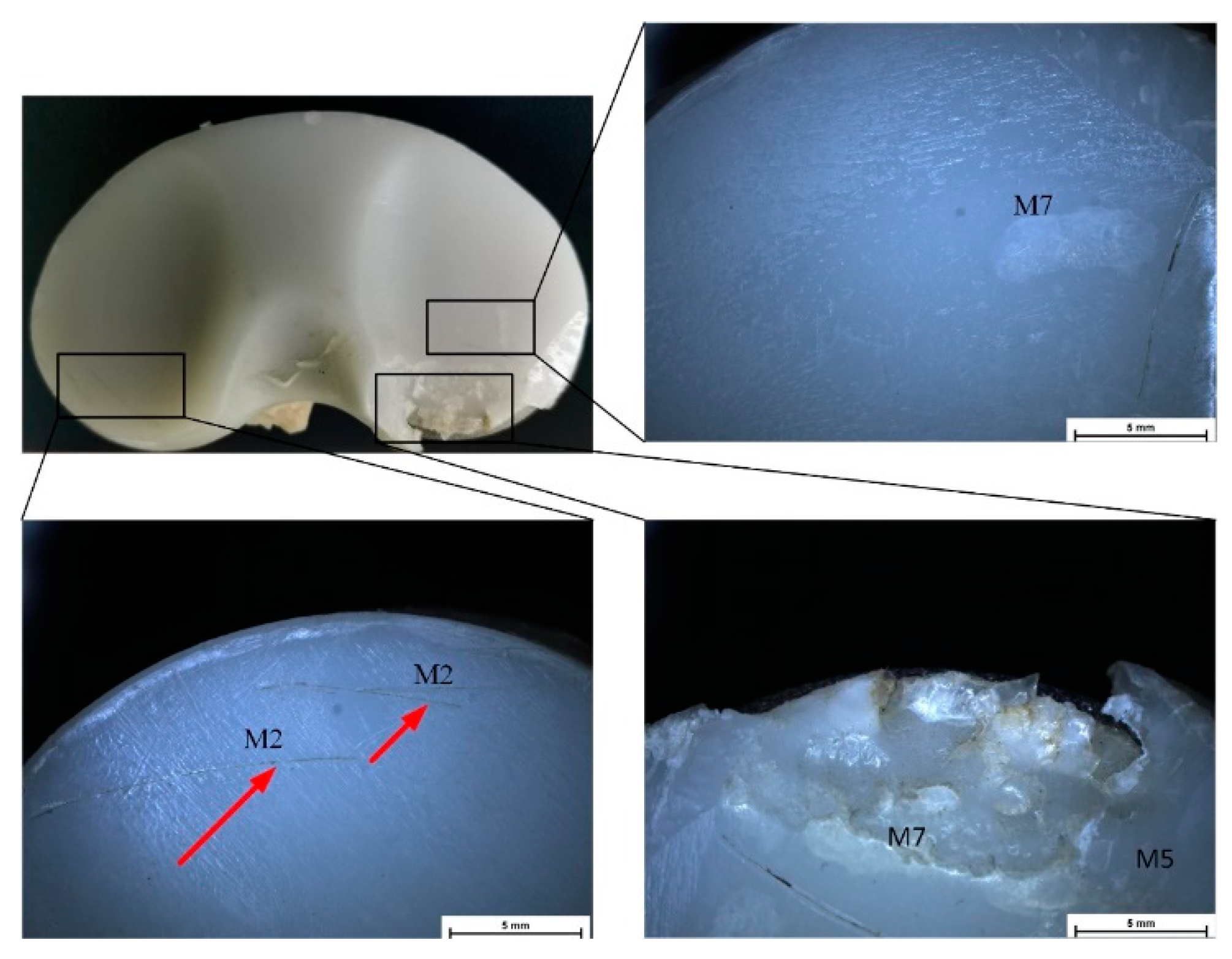
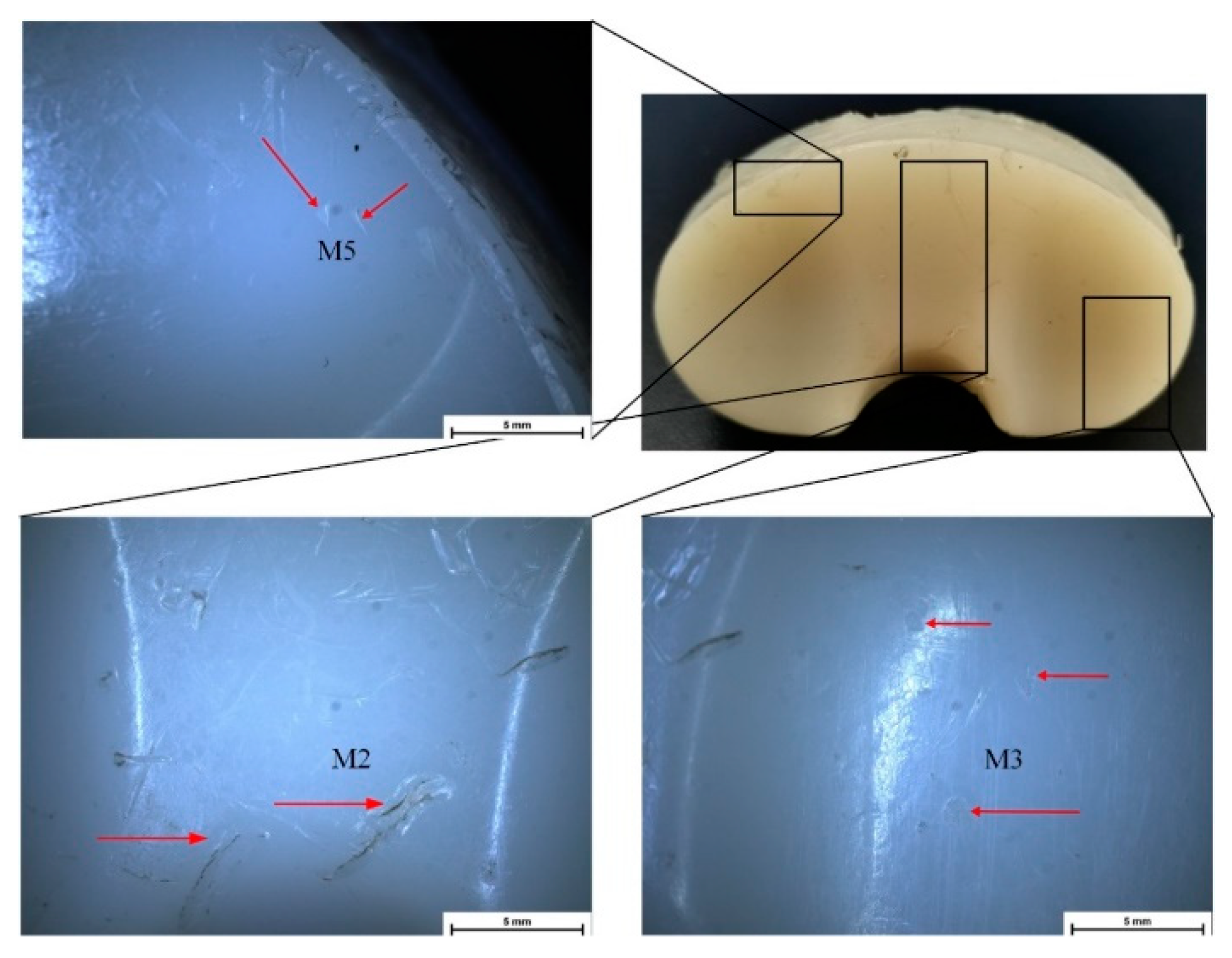



| UHMWPE Tibial Insert Data | Patient Clinical Data | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample ID | Hospital | Implant Life Span [Years] | Producer | Patient Age [Years] | Sex | Weight [kg]/Height [m] | KS | Pathologies | |
| Preoperative | Postoperative | ||||||||
| IT1 | Colentina | 5 | Zimmer | 54 | M | 75/1.7 | 55 | 85 | Diabetes, myelitis, vascular stroke, cervical spondylosis |
| IT2 | Colentina | 10 | Zimmer | 62 | F | 60/1.63 | 63 | 90 | Diabetes, osteoporosis, cardiac insufficiency |
| IT3 | Colentina | 15 | Zimmer | 69 | M | 85/1.73 | 71 | 94 | Schizophrenia |
| IT4 | Colentina | 10 | Zimmer | 65 | F | 70/1.6 | 73 | 96 | Cervical spondylotic myelopathy, osteoporosis |
| Sample ID | Deterioration Mode | M1 | M2 | M3 | M4 | M5 | M6 | M7 |
|---|---|---|---|---|---|---|---|---|
| IT1 | Zone | - | 3, 7 | - | 6 | 0, 7, 8, 9 | - | 7 |
| Grade | 0 | 1, 1 | 0 | 1 | 1, 2, 1, 1 | 0 | 1 | |
| Hood score | 9 | |||||||
| IT2 | Zone | - | 1, 3, 8, 9 | 1, 6, 7 | - | 1 | - | 1 |
| Grade | 0 | 1, 1, 1, 1 | 1, 1, 1 | 0 | 1 | 0 | 1 | |
| Hood score | 9 | |||||||
| IT3 | Zone | 5 | 3, 4, 5, 6, 7 | 3, 4 | - | - | - | 4 |
| Grade | 1 | 1, 1, 1, 2, 2 | 1, 1 | 0 | 0 | 0 | 1 | |
| Hood score | 10 | |||||||
| IT4 | Zone | 2, 3 | 1, 9 | 1,9 | 2 | 2, 5, 6, 7, 9 | - | 4, 5, 6, 9 |
| Grade | 1, 1 | 1, 1 | 2, 1 | 2 | 2, 3, 3, 3, 1 | 0 | 3, 3, 3, 2 | |
| Hood score | 32 | |||||||
| Sample ID | C-H Asymmetric Stretch in CH2 [cm−1] | C-H Symmetric Stretch in CH2 [cm−1] | CH2 Bend [cm−1] | CH2 Bend [cm−1] | C=O Stretch [cm−1] | C-O-C Stretch [cm−1] | OH Stretch [cm−1] | Reference of 1370 cm−1 Used for OI Computation [cm−1] |
|---|---|---|---|---|---|---|---|---|
| IT1 | 2914.8 | 2845.4 | 1465 | 720.2 | 1738 | 1166 | 3300 | 1369 |
| IT2 | 2912.9 | 2846 | 1465 | 720 | 1720 | 1167 | 3300 | 1365 |
| IT3 | 2913 | 2845 | 1464.7 | 721 | 1728 | 1166 | 3300 | 1369 |
| IT4 | 2913.9 | 2844 | 1464.6 | 720 | 1735 | 1165 | 3300 | 1369.2 |
| Control | 2914 | 2845 | 1463.7 | 720 | 1738 | 1160 | - | 1366 |
| Sample ID | AC=O [cm2] | Aref [cm2] | OI |
|---|---|---|---|
| IT1 | 63.44 | 12.04 | 5.26 |
| IT2 | 45.219 | 4.683 | 9.66 |
| IT3 | 118.079 | 17.11 | 6.9 |
| IT4 | 185.91 | 18.99 | 9.78 |
| Control | 7.67 | 5.76 | 1.33 |
| Sample ID | X [%] |
|---|---|
| IT1 | 56 |
| IT2 | 62 |
| IT3 | 67 |
| IT4 | 15 |
| Control | - |
| Sample ID | Young’s Modulus [MPa] | Initial Peak Load [N] | Ultimate Load [N] | Ultimate Displacement [mm] | Work to Failure [mJ] |
|---|---|---|---|---|---|
| IT1 | 1100 ± 175 | 68 ± 1.5 | 51 ± 2.6 | 3.8 ± 0.07 | 188.92 ± 6 |
| IT2 | 1267± 170 | 60 ± 3.1 | 44 ± 4.3 | 3.7 ± 0.03 | 163 ± 3 |
| IT3 | 1160 ± 180 | 63 ± 1.8 | 48 ± 2.9 | 3.77 ± 0.2 | 178.4 ± 6 |
| IT4 | 1300 ± 180 | 58 ± 10 | 40.5 ± 3 | 3.4 ± 0.04 | 139.88 ± 2 |
| Control | 987 ± 175 | 75 ± 2.5 | 52 ± 1.8 | 4 ± 0.5 | 206.06 ± 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manescu, V.; Antoniac, I.; Antoniac, A.; Paltanea, G.; Miculescu, M.; Bita, A.-I.; Laptoiu, S.; Niculescu, M.; Stere, A.; Paun, C.; et al. Failure Analysis of Ultra-High Molecular Weight Polyethylene Tibial Insert in Total Knee Arthroplasty. Materials 2022, 15, 7102. https://doi.org/10.3390/ma15207102
Manescu V, Antoniac I, Antoniac A, Paltanea G, Miculescu M, Bita A-I, Laptoiu S, Niculescu M, Stere A, Paun C, et al. Failure Analysis of Ultra-High Molecular Weight Polyethylene Tibial Insert in Total Knee Arthroplasty. Materials. 2022; 15(20):7102. https://doi.org/10.3390/ma15207102
Chicago/Turabian StyleManescu (Paltanea), Veronica, Iulian Antoniac, Aurora Antoniac, Gheorghe Paltanea, Marian Miculescu, Ana-Iulia Bita, Stefan Laptoiu, Marius Niculescu, Alexandru Stere, Costel Paun, and et al. 2022. "Failure Analysis of Ultra-High Molecular Weight Polyethylene Tibial Insert in Total Knee Arthroplasty" Materials 15, no. 20: 7102. https://doi.org/10.3390/ma15207102
APA StyleManescu, V., Antoniac, I., Antoniac, A., Paltanea, G., Miculescu, M., Bita, A.-I., Laptoiu, S., Niculescu, M., Stere, A., Paun, C., & Cristea, M. B. (2022). Failure Analysis of Ultra-High Molecular Weight Polyethylene Tibial Insert in Total Knee Arthroplasty. Materials, 15(20), 7102. https://doi.org/10.3390/ma15207102



.png)






