Mechanochemically Assisted Coal Fly Ash Conversion into Zeolite
Abstract
:1. Introduction
2. Materials and Methods
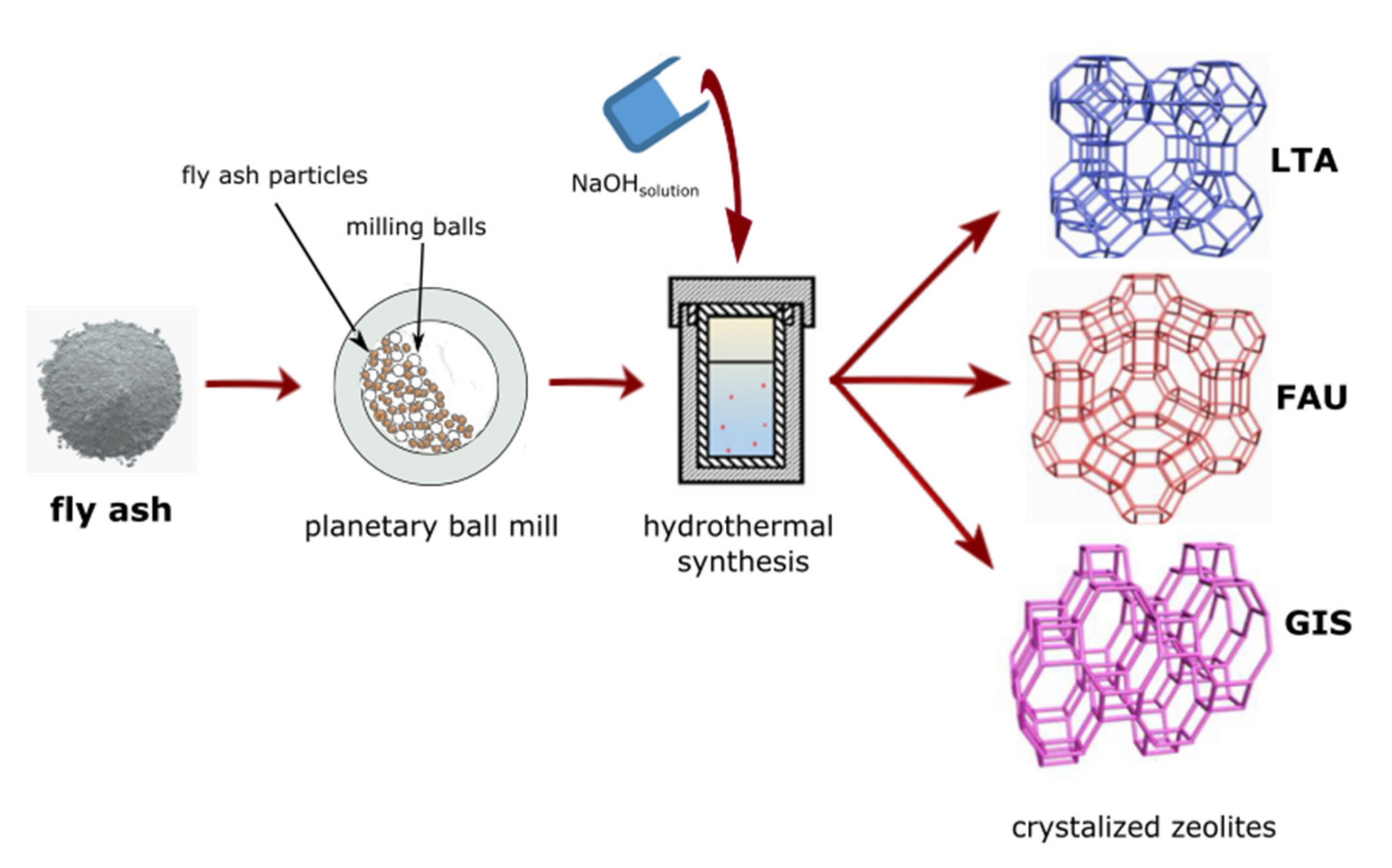
2.1. Mechanical Activation of Fly Ash
2.2. Conversion of Milled FA into Zeolite Type Materials
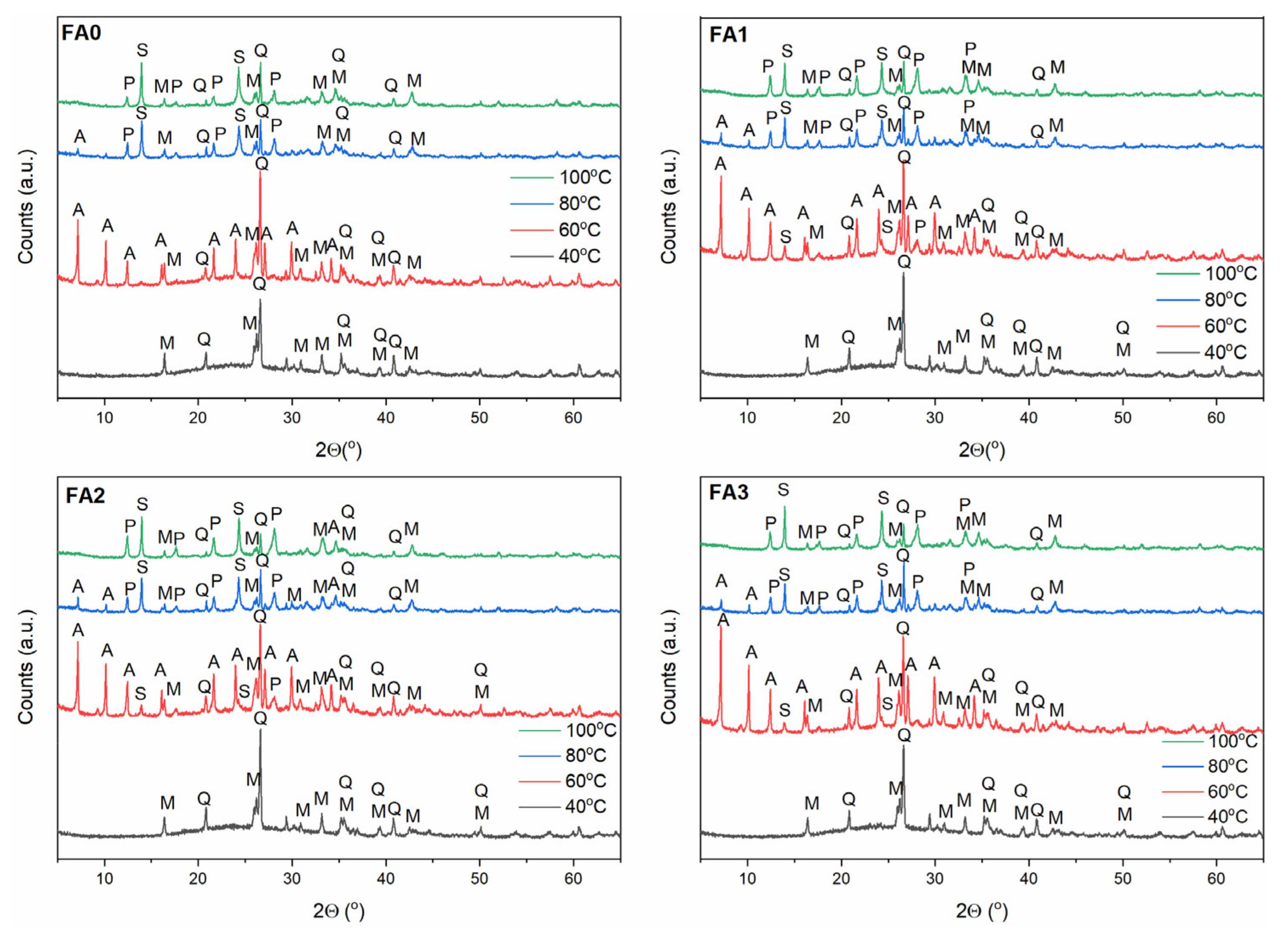
- Synthesis of Na-A phase: 40, 60, 80, 100 °C; 3 M NaOH solution.
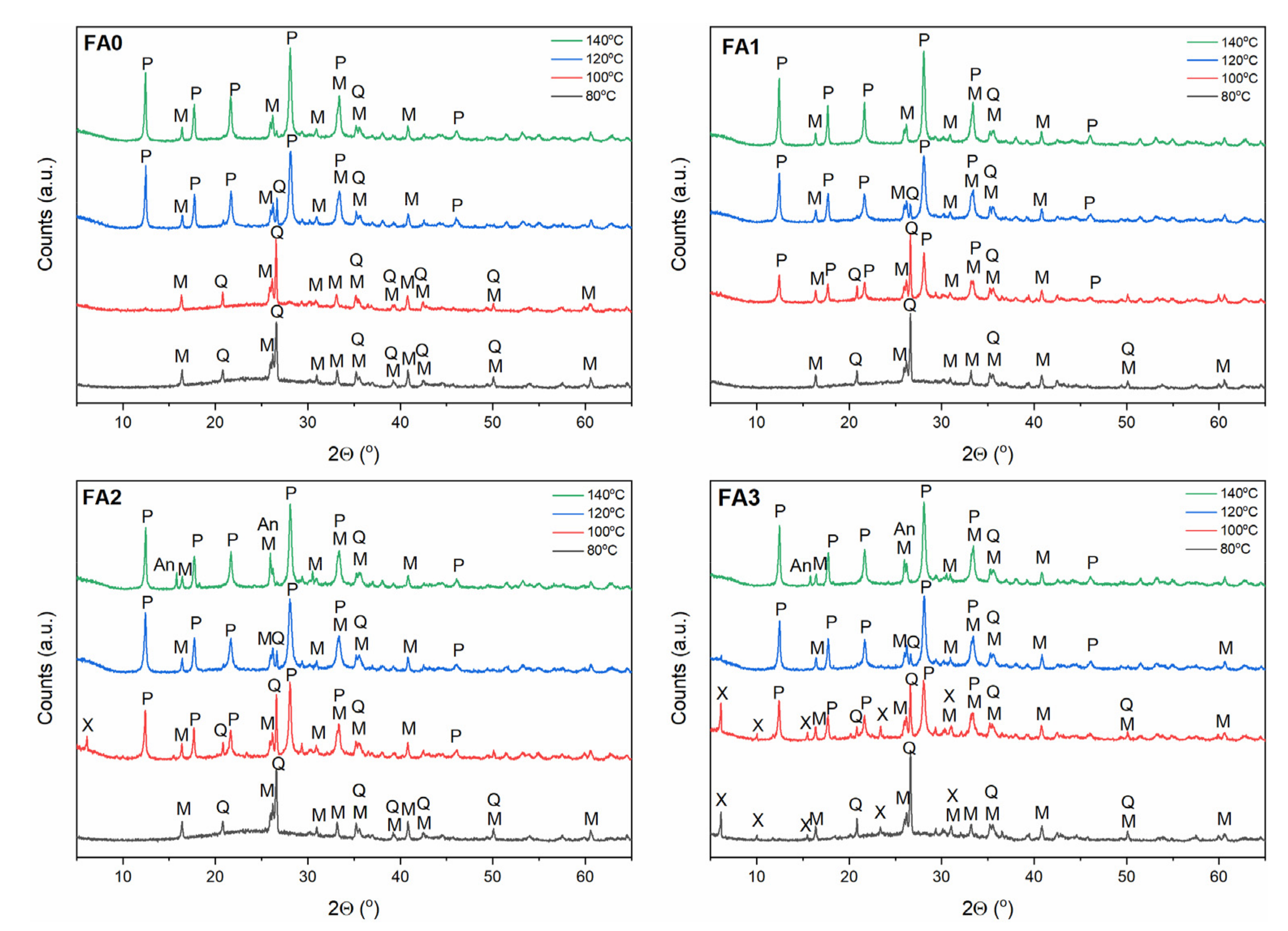
- Synthesis of Na-P1 phase: 80, 100, 120, 140 °C; 2 M NaOH solution.
- Synthesis of Na-X phase: 60, 80, 100, 120 °C; 3 M NaOH solution.
2.3. Materials Characterization
3. Results
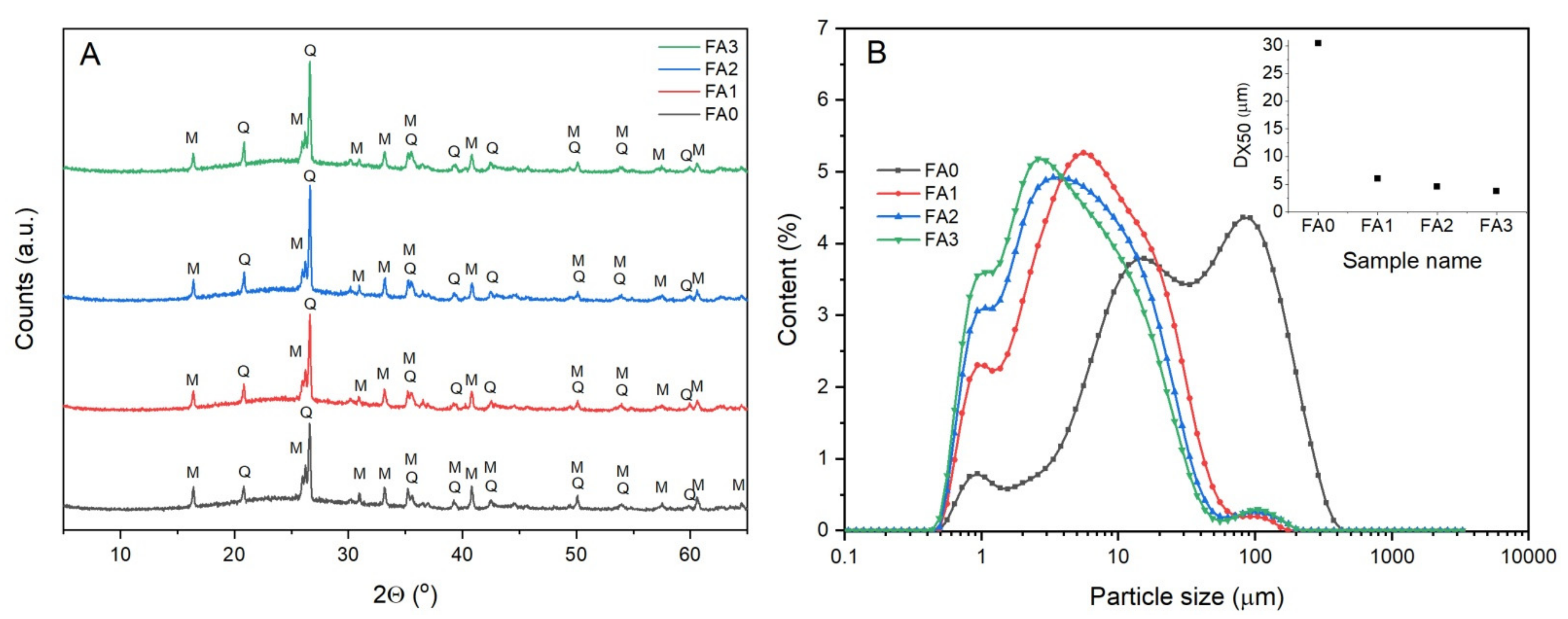
3.1. FA Characterization
3.2. Effect of Ball-Milling and Crystallization Temperature
3.3. Effect of Ball-Milling and Solution Conditioning
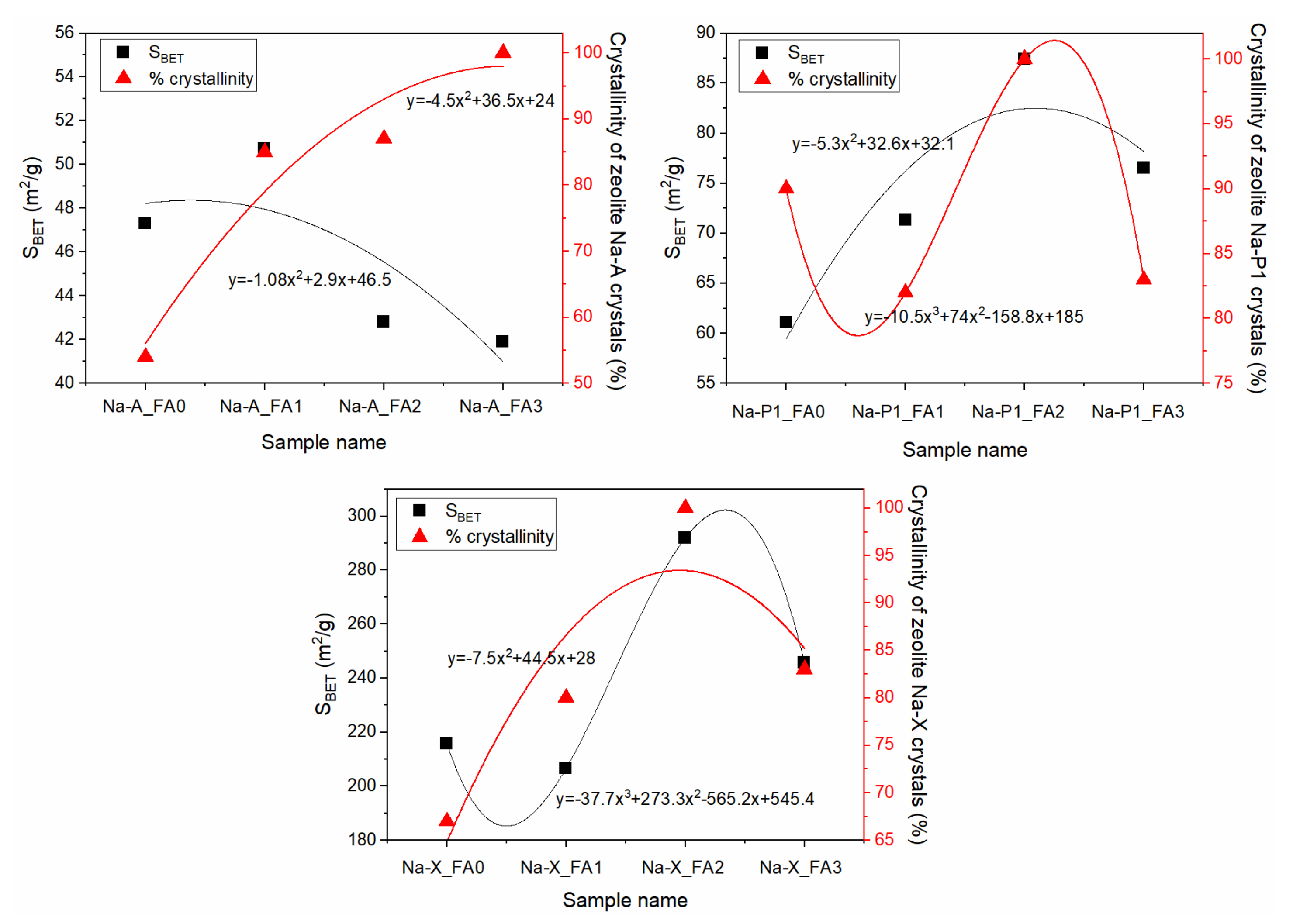
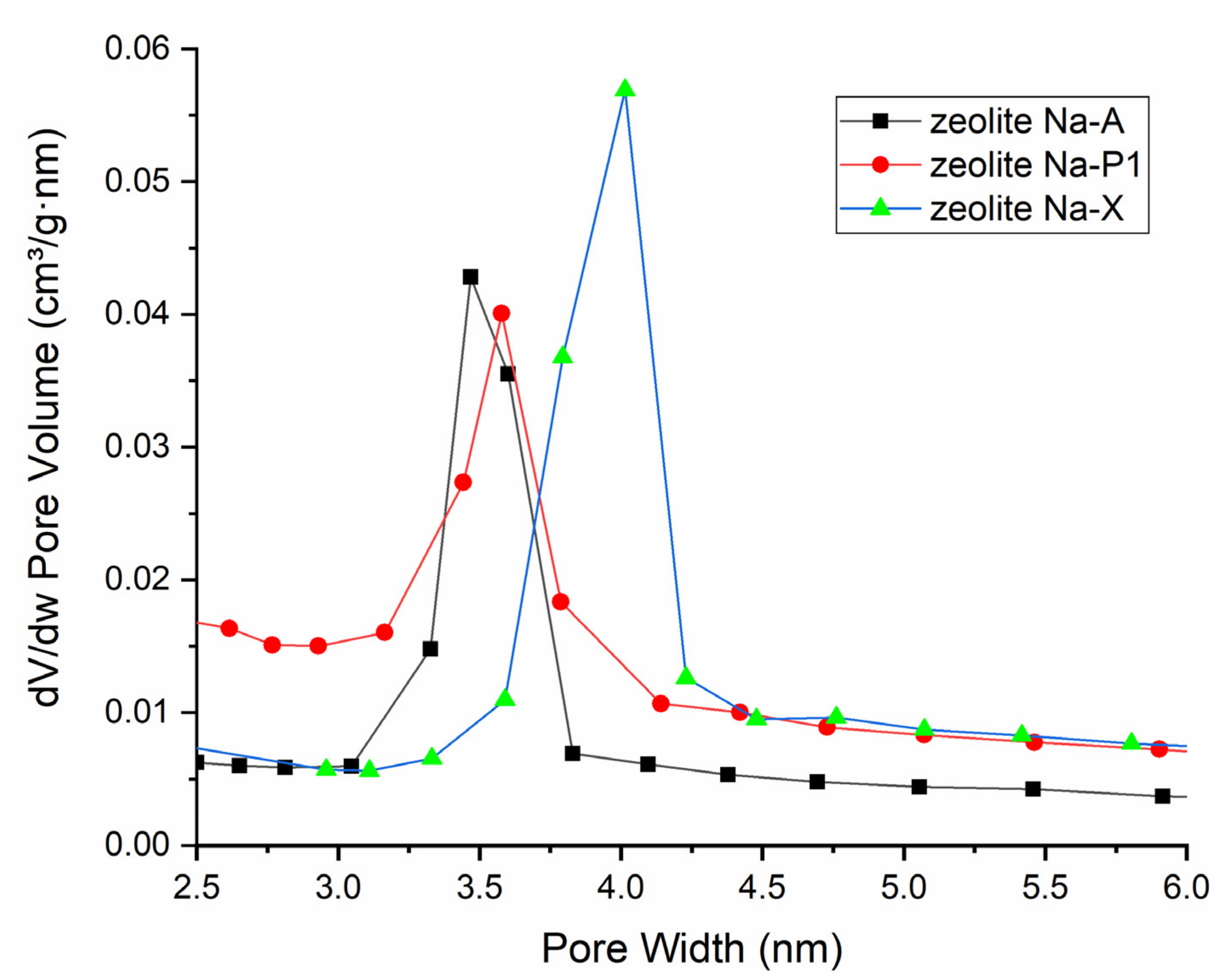
3.4. Characterization of FA-Derived Zeolitic Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Szczesniak, B.; Borysiuk, S.; Choma, J.; Jaroniec, M. Mechanochemical Synthesis of Highly Porous Materials. Mater. Horizons 2020, 7, 1457–1473. [Google Scholar] [CrossRef]
- Horie, K.; Barón, M.; Fox, R.B.; He, J.; Hess, M.; Kahovec, J.; Kitayama, T.; Kubisa, P.; Maréchal, E.; Mormann, W.; et al. International union of pure and applied chemistry macromolecular division commission on macromolecular nomenclature * subcommittee on macromolecular terminology * definitions of terms relating to reactions of polymers and to functional polymeric materials (iupac recommendations 2003). Pure Appl. Chem. 2004, 76, 889–906. [Google Scholar]
- Nada, M.H.; Larsen, S.C.; Gillan, E.G. Mechanochemically-Assisted Solvent-Free and Template-Free Synthesis of Zeolites ZSM-5 and Mordenite. Nanoscale Adv. 2019, 1, 3918–3928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.; Wu, Y.H.; Ma, S.H.; Zheng, S.L.; Chu, P.K. An Eco-Friendly and Cleaner Process for Preparing Architectural Ceramics from Coal Fly Ash: Pre-Activation of Coal Fly Ash by a Mechanochemical Method. J. Clean. Prod. 2019, 214, 419–428. [Google Scholar] [CrossRef]
- Rutkowska, G.; Wichowski, P.; Fronczyk, J.; Franus, M.; Chalecki, M. Use of Fly Ashes from Municipal Sewage Sludge Combustion in Production of Ash Concretes. Constr. Build. Mater. 2018, 188, 874–883. [Google Scholar] [CrossRef]
- Woszuk, A.; Bandura, L.; Franus, W. Fly Ash as Low Cost and Environmentally Friendly Filler and Its Effect on the Properties of Mix Asphalt. J. Clean. Prod. 2019, 235, 493–502. [Google Scholar] [CrossRef]
- Pan, F.; Lu, X.; Wang, Y.; Chen, S.; Wang, T.; Yan, Y. Synthesis and Crystallization Kinetics of ZSM-5 without Organic Template from Coal-Series Kaolinite. Microporous Mesoporous Mater. 2014, 184, 134–140. [Google Scholar] [CrossRef]
- Pashkova, V.; Mlekodaj, K.; Klein, P.; Brabec, L.; Zouzelka, R.; Rathousky, J.; Tokarova, V.; Dedecek, J. Mechanochemical Pretreatment for Efficient Solvent-Free Synthesis of SSZ-13 Zeolite. Chem. A Eur. J. 2019, 25, 12068–12073. [Google Scholar] [CrossRef]
- Ren, L.; Wu, Q.; Yang, C.; Zhu, L.; Li, C.; Zhang, P.; Zhang, H.; Meng, X.; Xiao, F.S. Solvent-Free Synthesis of Zeolites from Solid Raw Materials. J. Am. Chem. Soc. 2012, 134, 15173–15176. [Google Scholar] [CrossRef]
- Belviso, C.; Perchiazzi, N.; Cavalcante, F. Zeolite from Fly Ash: An Investigation on Metastable Behavior of the Newly Formed Minerals in a Medium-High-Temperature Range. Ind. Eng. Chem. Res. 2019, 58, 20472–20480. [Google Scholar] [CrossRef]
- Franus, W.; Wdowin, M.; Franus, M. Synthesis and Characterization of Zeolites Prepared from Industrial Fly Ash. Environ. Monit. Assess. 2014, 186, 5721–5729. [Google Scholar] [CrossRef]
- Wasielewski, R.; Wojtaszek, M.; Plis, A. Investigation of Fly Ash from Co-Combustion of Alternative Fuel (SRF) with Hard Coal in a Stoker Boiler. Arch. Environ. Prot. 2020, 46, 58–67. [Google Scholar] [CrossRef]
- Li, Z.; Xu, G.; Shi, X. Reactivity of Coal Fly Ash Used in Cementitious Binder Systems: A State-of-the-Art Overview. Fuel 2021, 301, 121031. [Google Scholar] [CrossRef]
- Kato, K.; Xin, Y.; Hitomi, T.; Shirai, T. Surface Modification of Fly Ash by Mechano-Chemical Treatment. Ceram. Int. 2019, 45, 849–853. [Google Scholar] [CrossRef]
- Nath, S.K.; Kumar, S. Reaction Kinetics of Fly Ash Geopolymerization: Role of Particle Size Controlled by Using Ball Mill. Adv. Powder Technol. 2019, 30, 1079–1088. [Google Scholar] [CrossRef]
- Zhuang, X.Y.; Chen, L.; Komarneni, S.; Zhou, C.H.; Tong, D.S.; Yang, H.M.; Yu, W.H.; Wang, H. Fly Ash-Based Geopolymer: Clean Production, Properties and Applications. J. Clean. Prod. 2016, 125, 253–267. [Google Scholar] [CrossRef]
- Abbas, S.; Saleem, M.A.; Kazmi, S.M.S.; Munir, M.J. Production of Sustainable Clay Bricks Using Waste Fly Ash: Mechanical and Durability Properties. J. Build. Eng. 2017, 14, 7–14. [Google Scholar] [CrossRef]
- Franus, M.; Panek, R.; Madej, J.; Franus, W. The Properties of Fly Ash Derived Lightweight Aggregates Obtained Using Microwave Radiation. Constr. Build. Mater. 2019, 227, 116677. [Google Scholar] [CrossRef]
- Kaczmarek, Z.; Mocek-Płóciniak, A.; Gajewski, P.; Mendyk, Ł.; Bocianowski, J. Physical and Soil Water Properties of Technosols Developed from Lignite Fly Ash. Arch. Environ. Prot. 2021, 47, 95–102. [Google Scholar] [CrossRef]
- Mushtaq, F.; Zahid, M.; Bhatti, I.A.; Nasir, S.; Hussain, T. Possible Applications of Coal Fly Ash in Wastewater Treatment. J. Environ. Manag. 2019, 240, 27–46. [Google Scholar] [CrossRef]
- Król, M. Natural vs. Synthetic Zeolites. Crystals 2020, 10, 622. [Google Scholar] [CrossRef]
- Ameh, A.E.; Fatoba, O.O.; Musyoka, N.M.; Petrik, L.F. Influence of Aluminium Source on the Crystal Structure and Framework Coordination of Al and Si in Fly Ash-Based Zeolite NaA. Powder Technol. 2017, 306, 17–25. [Google Scholar] [CrossRef]
- Deng, L.; Xu, Q.; Wu, H. Synthesis of Zeolite-like Material by Hydrothermal and Fusion Methods Using Municipal Solid Waste Fly Ash. Procedia Environ. Sci. 2016, 31, 662–667. [Google Scholar] [CrossRef] [Green Version]
- Längauer, D.; Čablík, V.; Hredzák, S.; Zubrik, A.; Matik, M.; Danková, Z. Preparation of Synthetic Zeolites from Coal Fly Ash by Hydrothermal Synthesis. Materials 2021, 14, 1267. [Google Scholar] [CrossRef]
- Boycheva, S.; Zgureva, D.; Lazarova, H.; Popova, M. Comparative Studies of Carbon Capture onto Coal Fly Ash Zeolites Na-X and Na–Ca-X. Chemosphere 2021, 271, 129505. [Google Scholar] [CrossRef]
- Popova, M.; Boycheva, S.; Lazarova, H.; Zgureva, D.; Lázár, K.; Szegedi, Á. VOC Oxidation and CO2 Adsorption on Dual Adsorption/Catalytic System Based on Fly Ash Zeolites. Catal. Today 2020, 357, 518–525. [Google Scholar] [CrossRef]
- Brochocka, A.; Nowak, A.; Panek, R.; Kozikowski, P.; Franus, W. Effective Removal of Odors from Air with Polymer Nonwoven Structures Doped by Porous Materials to Use in Respiratory Protective Devices. Arch. Environ. Prot. 2021, 47, 3–19. [Google Scholar] [CrossRef]
- Milojević-Rakić, M.; Popadić, D.; Janošević Ležaić, A.; Jevremović, A.; Nedić Vasiljević, B.; Uskoković-Marković, S.; Bajuk-Bogdanović, D. MFI, BEA and FAU Zeolite Scavenging Role in Neonicotinoids and Radical Species Elimination. Environ. Sci. Process. Impacts 2022, 24, 265–276. [Google Scholar] [CrossRef]
- Database of Zeolite Structures. Available online: http://www.iza-structure.org/databases/ (accessed on 28 September 2022).
- Franus, W. Characterization of X-Type Zeolite Prepared from Coal Fly Ash. J. Environ. Stud. 2012, 21, 337–343. [Google Scholar]
- Wdowin, M.; Franus, M.; Panek, R.; Badura, L.; Franus, W. The Conversion Technology of Fly Ash into Zeolites. Clean Technol. Environ. Policy 2014, 16, 1217–1223. [Google Scholar] [CrossRef] [Green Version]
- Kunecki, P.; Panek, R.; Wdowin, M.; Bień, T.; Franus, W. Influence of the Fly Ash Fraction after Grinding Process on the Hydrothermal Synthesis Efficiency of Na-A, Na-P1, Na-X and Sodalite Zeolite Types. Int. J. Coal Sci. Technol. 2021, 8, 291–311. [Google Scholar] [CrossRef]
- Marjanović, N.; Komljenović, M.; Baščarević, Z.; Nikolić, V. Improving Reactivity of Fly Ash and Properties of Ensuing Geopolymers through Mechanical Activation. Constr. Build. Mater. 2014, 57, 151–162. [Google Scholar] [CrossRef]
- Yankwa Djobo, J.N.; Elimbi, A.; Tchakouté, H.K.; Kumar, S. Mechanical Activation of Volcanic Ash for Geopolymer Synthesis: Effect on Reaction Kinetics, Gel Characteristics, Physical and Mechanical Properties. RSC Adv. 2016, 6, 39106–39117. [Google Scholar] [CrossRef]
- Mainganye, D.; Ojumu, T.; Petrik, L. Synthesis of Zeolites Na-P1 from South African Coal Fly Ash: Effect of Impeller Design and Agitation. Materials 2013, 6, 2074–2089. [Google Scholar] [CrossRef] [Green Version]
- Chu, Y.S.; Davaabal, B.; Kim, D.S.; Seo, S.K.; Kim, Y.; Ruescher, C.; Temuujin, J. Reactivity of Fly Ashes Milled in Different Milling Devices. Rev. Adv. Mater. Sci. 2019, 58, 179–188. [Google Scholar] [CrossRef]
- Maldonado, M.; Oleksiak, M.D.; Chinta, S.; Rimer, J.D. Controlling Crystal Polymorphism in Organic-Free Synthesis of Na-Zeolites. J. Am. Chem. Soc. 2013, 135, 2641–2652. [Google Scholar] [CrossRef]
- Pan, F.; Lu, X.; Wang, Y.; Chen, S.; Wang, T.; Yan, Y. Organic Template-Free Synthesis of ZSM-5 Zeolite from Coal-Series Kaolinite. Mater. Lett. 2014, 115, 5–8. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Lettino, A.; Fiore, S. Zeolite Synthesised from Fused Coal Fly Ash at Low Temperature Using Seawater for Crystallization. Coal Combust. Gasif. Prod. 2009, 1, 7–13. [Google Scholar] [CrossRef]
- Maia, A.Á.B.; Dias, R.N.; Angélica, R.S.; Neves, R.F. Influence of an Aging Step on the Synthesis of Zeolite NaA from Brazilian Amazon Kaolin Waste. J. Mater. Res. Technol. 2019, 8, 2924–2929. [Google Scholar] [CrossRef]
- Musyoka, N.M.; Petrik, L.F.; Hums, E.; Baser, H.; Schwieger, W. In Situ Ultrasonic Monitoring of Zeolite A Crystallization from Coal Fly Ash. Catal. Today 2012, 190, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Xiao, L.; Qu, R.; Liu, S.; Ye, D.; Song, H.; Wu, W.; Zheng, C.; Wu, X.; Gao, X. Synthesis and Characterization of a Single Phase Zeolite A Using Coal Fly Ash. RSC Adv. 2018, 8, 42200–42209. [Google Scholar] [CrossRef] [Green Version]
- Selim, M.M.; EL-Mekkawi, D.M.; Aboelenin, R.M.M.; Sayed Ahmed, S.A.; Mohamed, G.M. Preparation and Characterization of Na-A Zeolite from Aluminum Scrub and Commercial Sodium Silicate for the Removal of Cd2+ from Water. J. Assoc. Arab Univ. Basic Appl. Sci. 2017, 24, 19–25. [Google Scholar] [CrossRef]

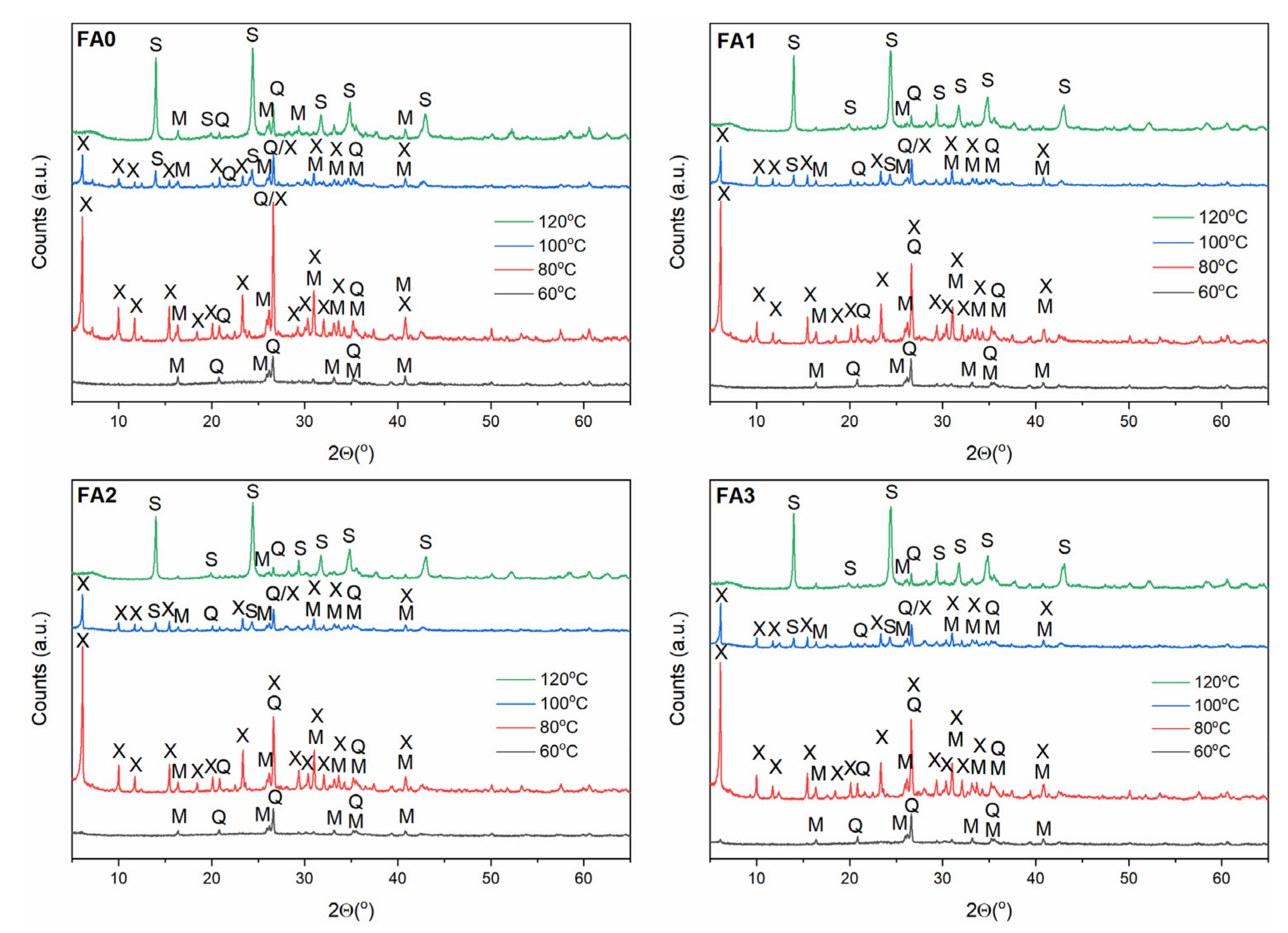

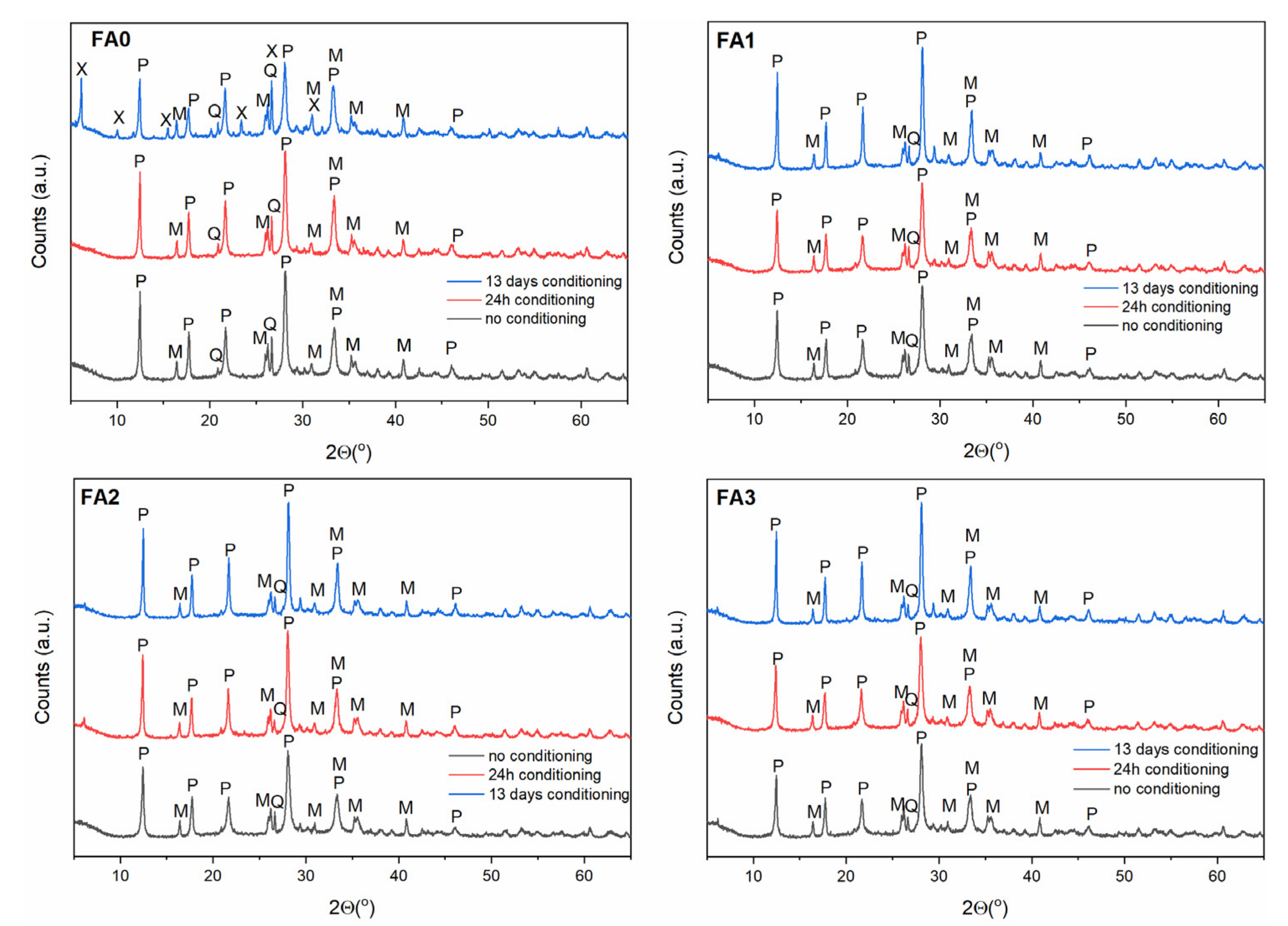
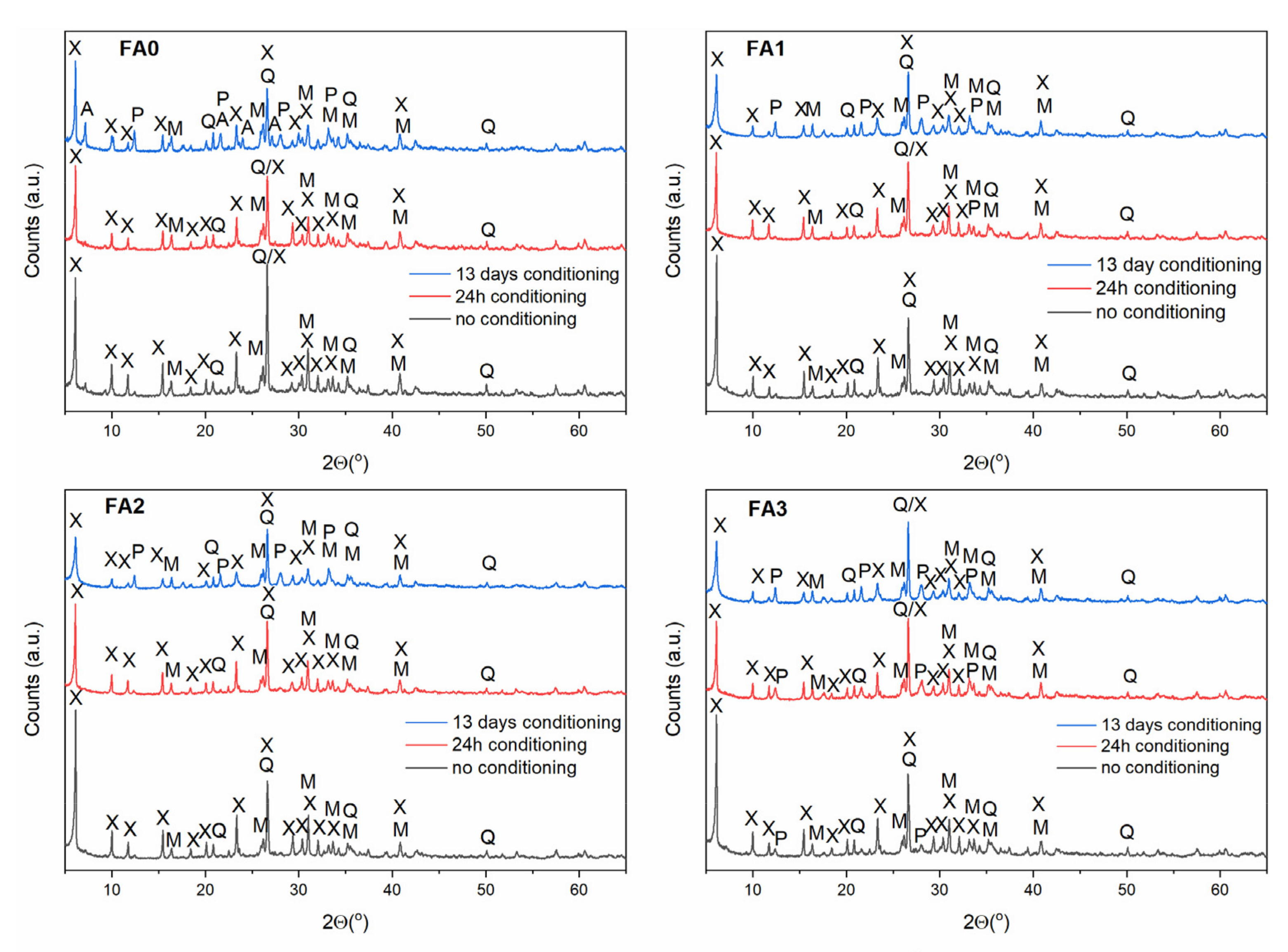

| Component | Content (%) |
|---|---|
| Na2O | 0.49 |
| MgO | 0.95 |
| Al2O3 | 25.80 |
| SiO2 | 51.64 |
| P2O5 | 1.76 |
| SO3 | 0.66 |
| K2O | 3.02 |
| CaO | 3.05 |
| TiO2 | 1.85 |
| Fe2O3 | 7.22 |
| Loss on ignition (LOI) | 3.29 |
| FA Features | FA0 | FA1 | FA2 | FA3 |
|---|---|---|---|---|
| Dx90 (µm) | 146.0 | 23.9 | 19.6 | 17.6 |
| Dx50 (µm) | 30.5 | 6.0 | 4.6 | 3.8 |
| Dx10 (µm) | 4.4 | 1.3 | 1.1 | 1.0 |
| SBET (m2/g) | 3.88 | 5.56 | 6.75 | 5.24 |
| Sample | Crystallinity [%] | SBET [m2/g] | Dav [nm] |
|---|---|---|---|
| NaA | 100 | 42 | 5.71 |
| NaP1 | 100 | 87 | 7.16 |
| NaX | 100 | 292 | 9.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabias-Blicharz, E.; Panek, R.; Franus, M.; Franus, W. Mechanochemically Assisted Coal Fly Ash Conversion into Zeolite. Materials 2022, 15, 7174. https://doi.org/10.3390/ma15207174
Grabias-Blicharz E, Panek R, Franus M, Franus W. Mechanochemically Assisted Coal Fly Ash Conversion into Zeolite. Materials. 2022; 15(20):7174. https://doi.org/10.3390/ma15207174
Chicago/Turabian StyleGrabias-Blicharz, Ewelina, Rafał Panek, Małgorzata Franus, and Wojciech Franus. 2022. "Mechanochemically Assisted Coal Fly Ash Conversion into Zeolite" Materials 15, no. 20: 7174. https://doi.org/10.3390/ma15207174
APA StyleGrabias-Blicharz, E., Panek, R., Franus, M., & Franus, W. (2022). Mechanochemically Assisted Coal Fly Ash Conversion into Zeolite. Materials, 15(20), 7174. https://doi.org/10.3390/ma15207174







