Epithelial Biological Response to Machined Titanium vs. PVD Zirconium-Coated Titanium: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Physical Vapor Deposition (PVD)
- I.
- Temperature 80 °C;
- II.
- Pressure of preparation to the process 2.5 × 10−3;
- III.
- Pressure of deposition 2.5 × 101;
- IV.
- Time of deposition 180 s;
- V.
- Thickness of the film 70 µm.
2.2. Scanning Electron Microscopy (SEM) and Energy-Dispersive Spectroscopy (EDS)
2.3. Cell Cultures and Incubation with Discs
2.4. Cell Viability Analysis
2.5. Fluorescence (DAPI)
2.6. Western Blot
2.7. Statistical Analysis
3. Results
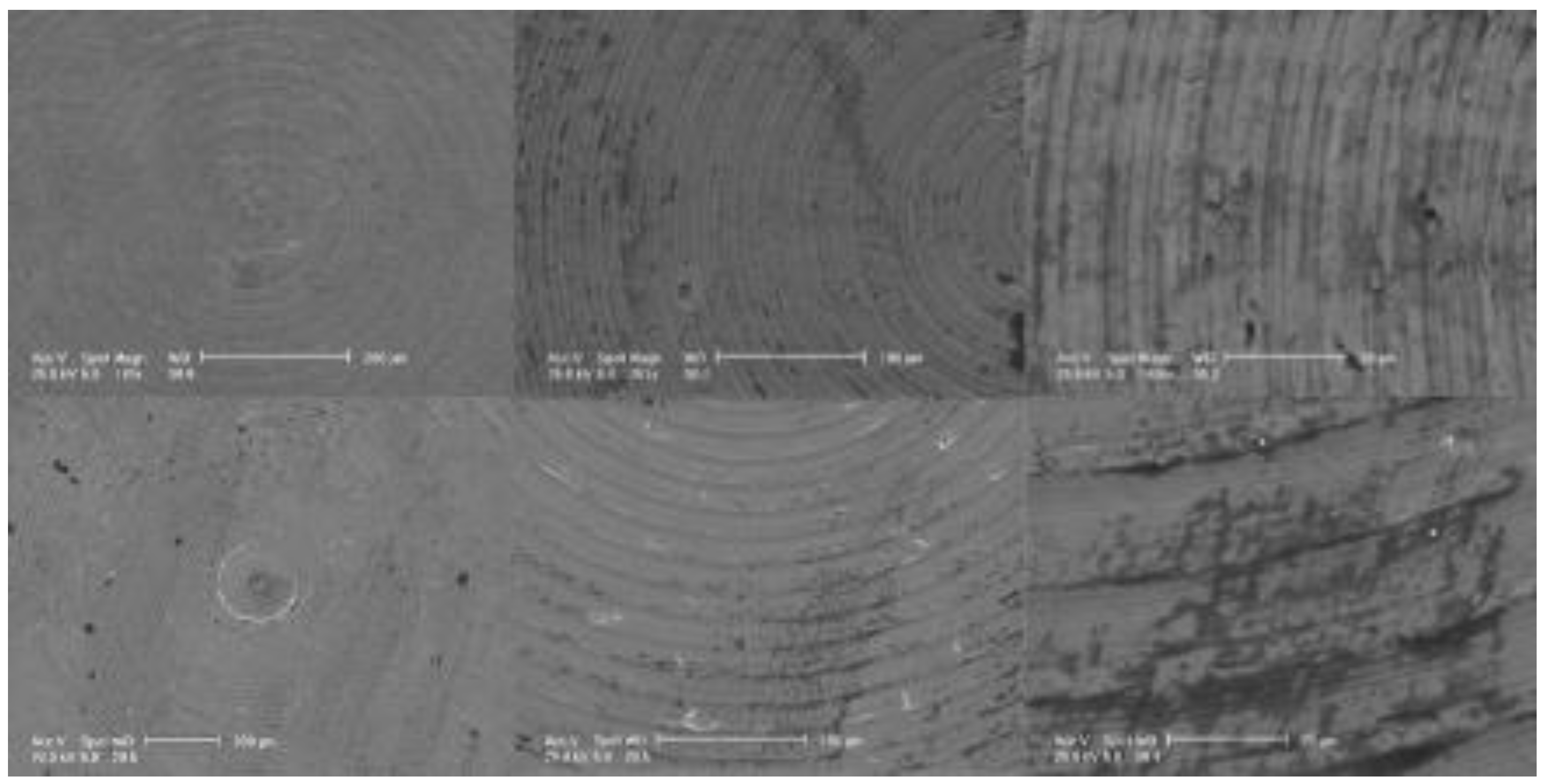
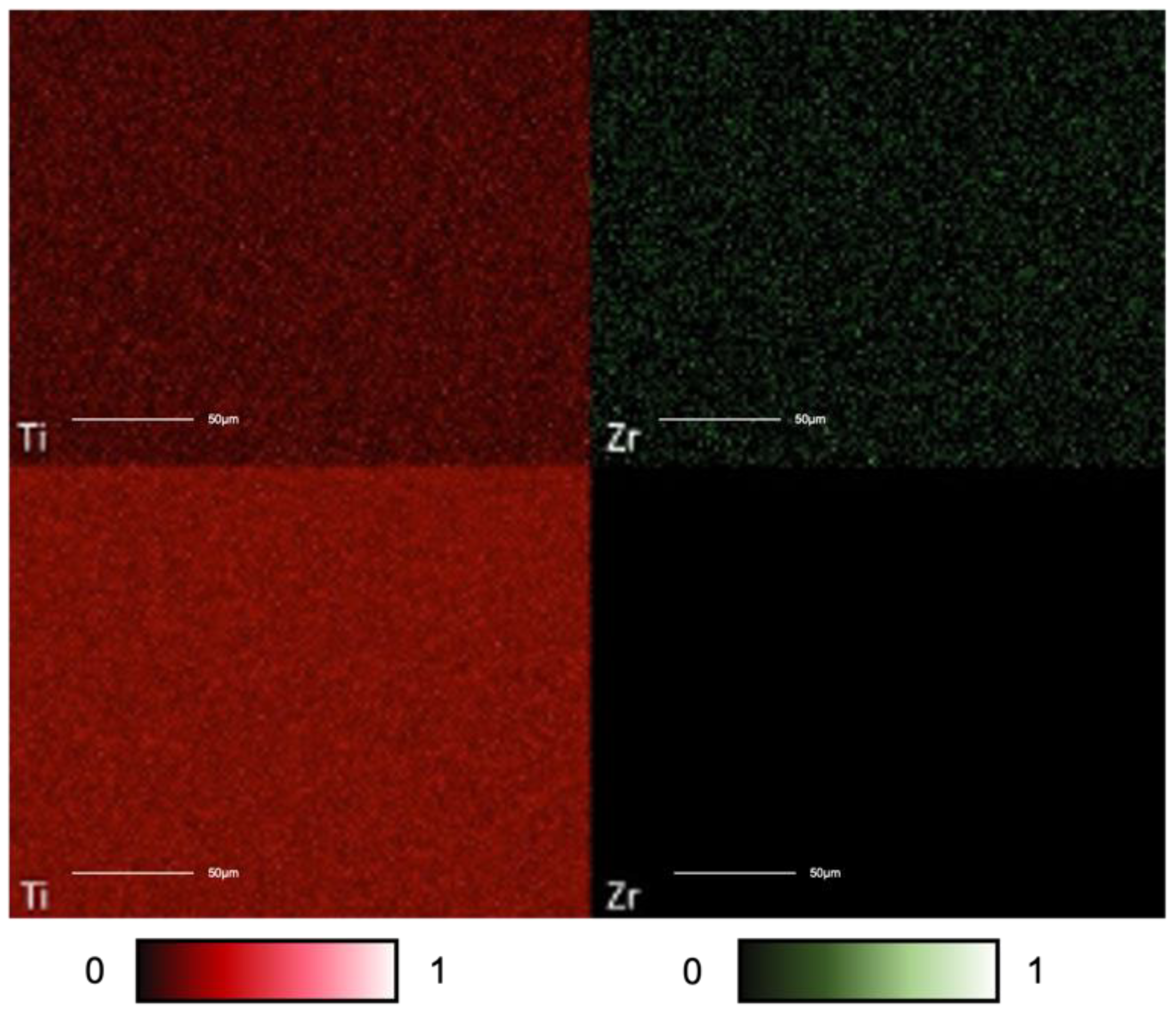
3.1. SEM and EDS Analysis
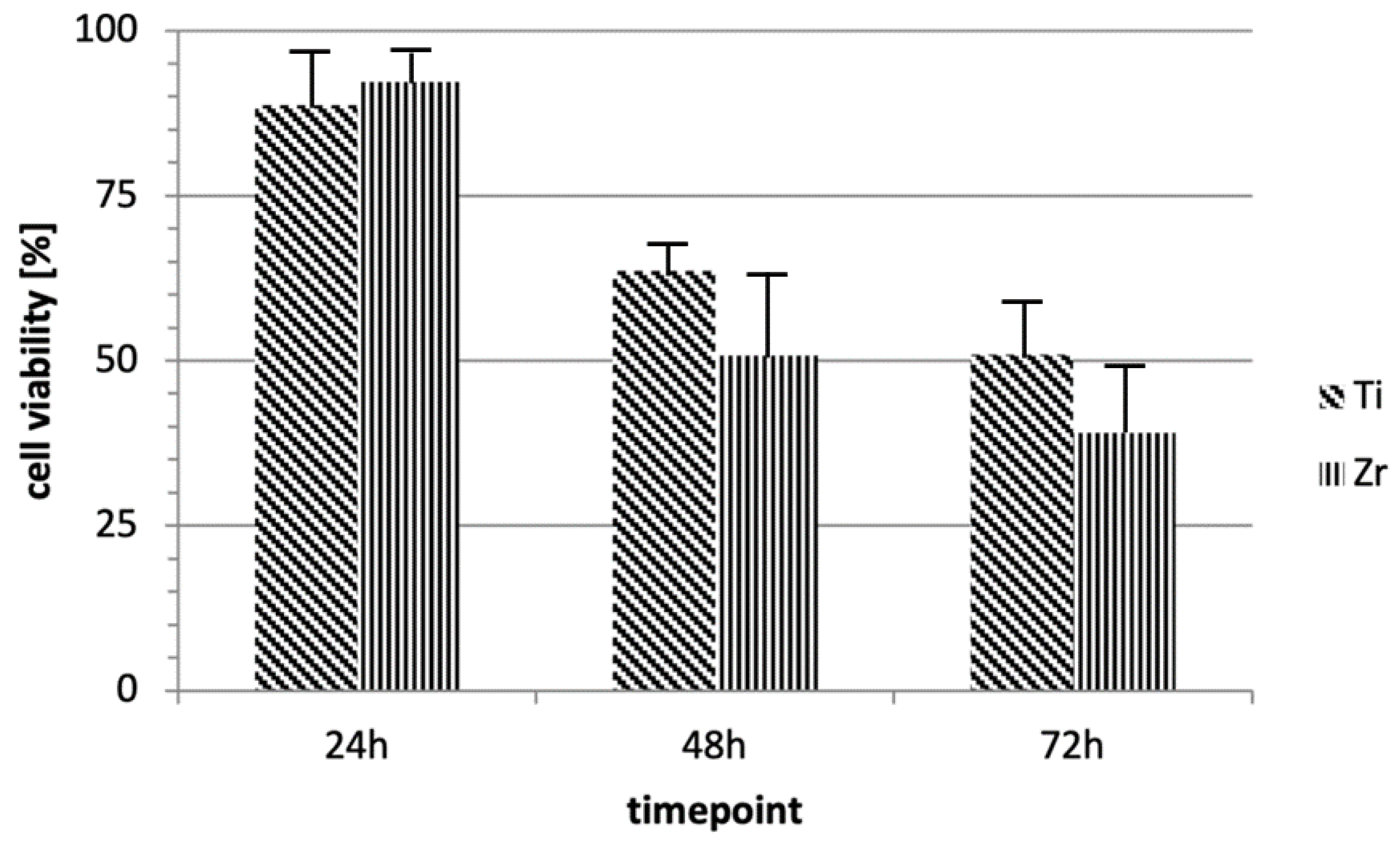
3.2. Cellular Vitality
3.3. DAPI
3.4. Western Blot
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jung, R.E.; Zembic, A.; Pjetursson, B.E.; Zwahlen, M.; Thoma, D.S. Systematic Review of the Survival Rate and the Incidence of Biological, Technical, and Aesthetic Complications of Single Crowns on Implants Reported in Longitudinal Studies with a Mean Follow-up of 5 Years. Clin. Oral Implant. Res. 2012, 23 (Suppl. S6), 2–21. [Google Scholar] [CrossRef] [PubMed]
- Pjetursson, B.E.; Thoma, D.; Jung, R.; Zwahlen, M.; Zembic, A. A Systematic Review of the Survival and Complication Rates of Implant-Supported Fixed Dental Prostheses (FDPs) after a Mean Observation Period of at Least 5 Years. Clin. Oral Implant. Res. 2012, 23 (Suppl. S6), 22–38. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Valente, N.A.; Strasding, M.; Zwahlen, M.; Liu, S.; Sailer, I. A Systematic Review of the Survival and Complication Rates of Zirconia-Ceramic and Metal-Ceramic Single Crowns. Clin. Oral Implant. Res. 2018, 29 (Suppl. S16), 199–214. [Google Scholar] [CrossRef] [PubMed]
- Bambini, F.; Memè, L.; Procaccini, M.; Rossi, B.; Lo Muzio, L. Bone Scintigraphy and SPECT in the Evaluation of the Osseointegrative Response to Immediate Prosthetic Loading of Endosseous Implants: A Pilot Study. Int. J. Oral Maxillofac. Implant. 2004, 19, 80–86. [Google Scholar]
- Quaranta, A.; D’Isidoro, O.; Bambini, F.; Putignano, A. Potential Bone to Implant Contact Area of Short Versus Standard Implants: An In Vitro Micro-Computed Tomography Analysis. Implant. Dent. 2016, 25, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Bambini, F.; Lo Muzio, L.; Procaccini, M. Retrospective Analysis of the Influence of Abutment Structure Design on the Success of Implant Unit. A 3-Year Controlled Follow-up Study. Clin. Oral Implant. Res. 2001, 12, 319–324. [Google Scholar] [CrossRef]
- Brito, C.; Tenenbaum, H.C.; Wong, B.K.C.; Schmitt, C.; Nogueira-Filho, G. Is Keratinized Mucosa Indispensable to Maintain Peri-Implant Health? A Systematic Review of the Literature. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 643–650. [Google Scholar] [CrossRef]
- Bambini, F.; Orilisi, G.; Quaranta, A.; Memè, L. Biological Oriented Immediate Loading: A New Mathematical Implant Vertical Insertion Protocol, Five-Year Follow-Up Study. Materials 2021, 14, 387. [Google Scholar] [CrossRef]
- Bambini, F.; Giannetti, L.; Memè, L.; Pellecchia, M.; Selvaggio, R. Comparative Analysis of Direct and Indirect Implant Impression Techniques an in Vitro Study. An in Vitro Study. Minerva Stomatol. 2005, 54, 395–402. [Google Scholar] [PubMed]
- Araujo, M.G.; Lindhe, J. Peri-Implant Health. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S230–S236. [Google Scholar] [CrossRef]
- Grassi, A.; Memè, L.; Orsini, G.; Licini, C.; Orilisi, G.; Bambini, F. A New Surgical Protocol For Horizontal Ridge Augmentation: Simplified Apposition Technique. Human Histologic and Radiographic Analysis After 2.5 Years of Follow-Up, A Case Report. Arch. Clin. Med. Case Rep. 2020, 4, 239–252. [Google Scholar] [CrossRef]
- Giorgini, E.; Sabbatini, S.; Conti, C.; Rubini, C.; Rocchetti, R.; Fioroni, M.; Memè, L.; Orilisi, G. Fourier Transform Infrared Imaging Analysis of Dental Pulp Inflammatory Diseases. Oral Dis. 2017, 23, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Lindhe, J.; Berglundh, T. The Interface between the Mucosa and the Implant. Periodontol. 2000 1998, 17, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Ivanovski, S.; Lee, R. Comparison of Peri-Implant and Periodontal Marginal Soft Tissues in Health and Disease. Periodontol. 2000 2018, 76, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, C.; Tessarolo, F.; Caola, I.; Wennström, J.; Nollo, G.; Berglundh, T. Morphogenesis of Peri-Implant Mucosa Revisited: An Experimental Study in Humans. Clin. Oral Implant. Res. 2014, 25, 997–1003. [Google Scholar] [CrossRef]
- Berglundh, T.; Lindhe, J. Dimension of the Periimplant Mucosa. Biological Width Revisited. J. Clin. Periodontol. 1996, 23, 971–973. [Google Scholar] [CrossRef]
- Berglundh, T.; Lindhe, J.; Ericsson, I.; Marinello, C.P.; Liljenberg, B.; Thomsen, P. The Soft Tissue Barrier at Implants and Teeth. Clin. Oral Implant. Res. 1991, 2, 81–90. [Google Scholar] [CrossRef]
- Schroeder, H.E.; Listgarten, M.A. The Gingival Tissues: The Architecture of Periodontal Protection. Periodontol. 2000 1997, 13, 91–120. [Google Scholar] [CrossRef]
- Berglundh, T.; Lindhe, J.; Jonsson, K.; Ericsson, I. The Topography of the Vascular Systems in the Periodontal and Peri-Implant Tissues in the Dog. J. Clin. Periodontol. 1994, 21, 189–193. [Google Scholar] [CrossRef]
- Moon, I.S.; Berglundh, T.; Abrahamsson, I.; Linder, E.; Lindhe, J. The Barrier between the Keratinized Mucosa and the Dental Implant. An Experimental Study in the Dog. J. Clin. Periodontol. 1999, 26, 658–663. [Google Scholar] [CrossRef]
- Buser, D.; Weber, H.P.; Donath, K.; Fiorellini, J.P.; Paquette, D.W.; Williams, R.C. Soft Tissue Reactions to Non-Submerged Unloaded Titanium Implants in Beagle Dogs. J. Periodontol. 1992, 63, 225–235. [Google Scholar] [CrossRef]
- Lindhe, J.; Berglundh, T.; Ericsson, I.; Liljenberg, B.; Marinello, C. Experimental Breakdown of Peri-Implant and Periodontal Tissues. A Study in the Beagle Dog. Clin. Oral Implant. Res. 1992, 3, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-T.; Huang, Y.-W.; Zhu, L.; Weltman, R. Prevalences of Peri-Implantitis and Peri-Implant Mucositis: Systematic Review and Meta-Analysis. J. Dent. 2017, 62, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.S.; Gil, A.; Hämmerle, C.H.F.; Jung, R.E. Management and Prevention of Soft Tissue Complications in Implant Dentistry. Periodontol. 2000 2022, 88, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Meme, L.; Santarelli, A.; Marzo, G.; Emanuelli, M.; Nocini, P.F.; Bertossi, D.; Putignano, A.; Dioguardi, M.; Lo Muzio, L.; Bambini, F. Novel Hydroxyapatite Biomaterial Covalently Linked to Raloxifene. Int. J. Immunopathol. Pharmacol. 2014, 27, 437–444. [Google Scholar] [CrossRef]
- Santarelli, A.; Mascitti, M.; Orsini, G.; Memè, L.; Rocchetti, R.; Tiriduzzi, P.; Sampalmieri, F.; Putignano, A.; Procaccini, M.; Lo Muzio, L.; et al. Osteopontin, Osteocalcin and OB-Cadherin Expression in Synthetic Nanohydroxyapatite vs. Bovine Hydroxyapatite Cultured Osteoblastic-like Cells. J. Biol. Regul. Homeost Agents 2014, 28, 523–529. [Google Scholar]
- Bambini, F.; Greci, L.; Memè, L.; Santarelli, A.; Carinci, F.; Pezzetti, F.; Procaccini, M.; Lo Muzio, L. Raloxifene Covalently Bonded to Titanium Implants by Interfacing with (3-Aminopropyl)-Triethoxysilane Affects Osteoblast-like Cell Gene Expression. Int. J. Immunopathol. Pharmacol. 2006, 19, 905–914. [Google Scholar] [CrossRef]
- Muzio, L.L.; Santarelli, A.; Orsini, G.; Memè, L.; Mattioli-Belmonte, M.; De Florio, I.; Gatto, R.; Gallusi, G.; Nocini, P.F.; Bertossi, D.; et al. MG63 and MC3T3-E1 Osteoblastic Cell Lines Response to Raloxifene. Eur. J. Inflamm. 2013, 11, 797–804. [Google Scholar] [CrossRef]
- Memè, L.; Strappa, E.M.; Monterubbianesi, R.; Bambini, F.; Mummolo, S. SEM and FT-MIR Analysis of Human Demineralized Dentin Matrix: An In Vitro Study. Appl. Sci. 2022, 12, 1480. [Google Scholar] [CrossRef]
- Bambini, F.; Santarelli, A.; Putignano, A.; Procaccini, M.; Orsini, G.; Memè, L.; Sartini, D.; Emanuelli, M.; Lo Muzio, L. Use of Supercharged Cover Screw as Static Magnetic Field Generator for Bone Healing, 1st Part: In Vitro Enhancement of Osteoblast-like Cell Differentiation. J. Biol. Regul. Homeost Agents 2017, 31, 215–220. [Google Scholar]
- Bambini, F.; Santarelli, A.; Putignano, A.; Procaccini, M.; Orsini, G.; Di Iorio, D.; Memè, L.; Sartini, D.; Emanuelli, M.; Lo Muzio, L. Use of Supercharged Cover Screw as Static Magnetic Field Generator for Bone Healing, 2nd Part: In Vivo Enhancement of Bone Regeneration in Rabbits. J. Biol. Regul. Homeost Agents 2017, 31, 481–485. [Google Scholar] [PubMed]
- Nevins, M.; Kim, D.M.; Jun, S.-H.; Guze, K.; Schupbach, P.; Nevins, M.L. Histologic Evidence of a Connective Tissue Attachment to Laser Microgrooved Abutments: A Canine Study. Int. J. Periodontics Restor. Dent. 2010, 30, 245–255. [Google Scholar]
- Koidou, V.P.; Argyris, P.P.; Skoe, E.P.; Siqueira, J.M.; Chen, X.; Zhang, L.; Hinrichs, J.E.; Costalonga, M.; Aparicio, C. Peptide Coatings Enhance Keratinocyte Attachment towards Improving Peri-Implant Mucosal Seal. Biomater. Sci. 2018, 6, 1936–1945. [Google Scholar] [CrossRef] [PubMed]
- Groessner-Schreiber, B.; Hannig, M.; Dück, A.; Griepentrog, M.; Wenderoth, D.F. Do Different Implant Surfaces Exposed in the Oral Cavity of Humans Show Different Biofilm Compositions and Activities? Eur. J. Oral Sci. 2004, 112, 516–522. [Google Scholar] [CrossRef]
- Chang, Y.-Y.; Huang, H.-L.; Lai, C.-H.; Hsu, J.-T.; Shieh, T.-M.; Wu, A.Y.-J.; Chen, C.-L. Analyses of Antibacterial Activity and Cell Compatibility of Titanium Coated with a Zr–C–N Film. PLoS ONE 2013, 8, e56771. [Google Scholar] [CrossRef] [PubMed]
- Campagna, R.; Bacchetti, T.; Salvolini, E.; Pozzi, V.; Molinelli, E.; Brisigotti, V.; Sartini, D.; Campanati, A.; Ferretti, G.; Offidani, A.; et al. Paraoxonase-2 Silencing Enhances Sensitivity of A375 Melanoma Cells to Treatment with Cisplatin. Antioxidants 2020, 9, 1238. [Google Scholar] [CrossRef]
- Seta, R.; Mascitti, M.; Campagna, R.; Sartini, D.; Fumarola, S.; Santarelli, A.; Giuliani, M.; Cecati, M.; Muzio, L.L.; Emanuelli, M. Overexpression of Nicotinamide N-Methyltransferase in HSC-2 OSCC Cell Line: Effect on Apoptosis and Cell Proliferation. Clin Oral Investig 2019, 23, 829–838. [Google Scholar] [CrossRef]
- Fumarola, S.; Cecati, M.; Sartini, D.; Ferretti, G.; Milanese, G.; Galosi, A.B.; Pozzi, V.; Campagna, R.; Morresi, C.; Emanuelli, M.; et al. Bladder Cancer Chemosensitivity Is Affected by Paraoxonase-2 Expression. Antioxidants 2020, 9, 175. [Google Scholar] [CrossRef]
- Campagna, R.; Salvolini, E.; Pompei, V.; Pozzi, V.; Salvucci, A.; Molinelli, E.; Brisigotti, V.; Sartini, D.; Campanati, A.; Offidani, A.; et al. Nicotinamide N-Methyltransferase Gene Silencing Enhances Chemosensitivity of Melanoma Cell Lines. Pigment. Cell Melanoma Res. 2021, 34, 1039–1048. [Google Scholar] [CrossRef]
- Nicholson, J.W. Titanium Alloys for Dental Implants: A Review. Prosthesis 2020, 2, 100–116. [Google Scholar] [CrossRef]
- Bambini, F.; Pellecchia, M.; Memè, L.; Santarelli, A.; Emanuelli, M.; Procaccini, M.; Muzio, L.L. Anti-Inflammatory Cytokines in Peri-Implant Soft Tissues: A Preliminary Study on Humans Using CDNA Microarray Technology. Eur. J. Inflamm. 2007, 5, 121–127. [Google Scholar] [CrossRef]
- Piconi, C.; Maccauro, G. Zirconia as a Ceramic Biomaterial. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef]
- Ji, M.-K.; Park, S.-W.; Lee, K.; Kang, I.-C.; Yun, K.-D.; Kim, H.-S.; Lim, H.-P. Evaluation of Antibacterial Activity and Osteoblast-like Cell Viability of TiN, ZrN and (Ti1-XZrx)N Coating on Titanium. J. Adv. Prosthodont. 2015, 7, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-L.; Chang, Y.-Y.; Weng, J.-C.; Chen, Y.-C.; Lai, C.-H.; Shieh, T.-M. Anti-Bacterial Performance of Zirconia Coatings on Titanium Implants. Thin Solid Film. 2013, 528, 151–156. [Google Scholar] [CrossRef]
- Grössner-Schreiber, B.; Herzog, M.; Hedderich, J.; Dück, A.; Hannig, M.; Griepentrog, M. Focal Adhesion Contact Formation by Fibroblasts Cultured on Surface-Modified Dental Implants: An in Vitro Study. Clin. Oral Implant. Res. 2006, 17, 736–745. [Google Scholar] [CrossRef]
- Brunello, G.; Brun, P.; Gardin, C.; Ferroni, L.; Bressan, E.; Meneghello, R.; Zavan, B.; Sivolella, S. Biocompatibility and Antibacterial Properties of Zirconium Nitride Coating on Titanium Abutments: An in Vitro Study. PLoS ONE 2018, 13, e0199591. [Google Scholar] [CrossRef]
- Sollazzo, V.; Pezzetti, F.; Scarano, A.; Piattelli, A.; Bignozzi, C.A.; Massari, L.; Brunelli, G.; Carinci, F. Zirconium Oxide Coating Improves Implant Osseointegration in Vivo. Dent. Mater. 2008, 24, 357–361. [Google Scholar] [CrossRef]
- Sollazzo, V.; Palmieri, A.; Pezzetti, F.; Bignozzi, C.A.; Argazzi, R.; Massari, L.; Brunelli, G.; Carinci, F. Genetic Effect of Zirconium Oxide Coating on Osteoblast-like Cells. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 84, 550–558. [Google Scholar] [CrossRef]
- Rizzi, M.; Gatti, G.; Migliario, M.; Marchese, L.; Rocchetti, V.; Renò, F. Effect of Zirconium Nitride Physical Vapor Deposition Coating on Preosteoblast Cell Adhesion and Proliferation onto Titanium Screws. J. Prosthet. Dent. 2014, 112, 1103–1110. [Google Scholar] [CrossRef]
- Kaluđerović, M.R.; Schreckenbach, J.P.; Graf, H.-L. Zirconia Coated Titanium for Implants and Their Interactions with Osteoblast Cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 44, 254–261. [Google Scholar] [CrossRef]
- Kaluđerović, M.R.; Mändl, S.; Kohlweyer, H.; Graf, H.-L. Physical Vapour Deposition of Zirconia on Titanium: Fabrication, Characterization and Interaction with Human Osteoblast Cells. J. Mater. Sci. Mater. Med. 2015, 26, 267. [Google Scholar] [CrossRef] [PubMed]
- Groessner-Schreiber, B.; Neubert, A.; Müller, W.-D.; Hopp, M.; Griepentrog, M.; Lange, K.-P. Fibroblast Growth on Surface-Modified Dental Implants: An in Vitro Study. J. Biomed. Mater. Res. A 2003, 64, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Franková, J.; Pivodová, V.; Růžička, F.; Tománková, K.; Šafářová, K.; Vrbková, J.; Ulrichová, J. Comparing Biocompatibility of Gingival Fibroblasts and Bacterial Strains on a Different Modified Titanium Discs. J. Biomed. Mater. Res. A 2013, 101, 2915–2924. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Memè, L.; Sartini, D.; Pozzi, V.; Emanuelli, M.; Strappa, E.M.; Bittarello, P.; Bambini, F.; Gallusi, G. Epithelial Biological Response to Machined Titanium vs. PVD Zirconium-Coated Titanium: An In Vitro Study. Materials 2022, 15, 7250. https://doi.org/10.3390/ma15207250
Memè L, Sartini D, Pozzi V, Emanuelli M, Strappa EM, Bittarello P, Bambini F, Gallusi G. Epithelial Biological Response to Machined Titanium vs. PVD Zirconium-Coated Titanium: An In Vitro Study. Materials. 2022; 15(20):7250. https://doi.org/10.3390/ma15207250
Chicago/Turabian StyleMemè, Lucia, Davide Sartini, Valentina Pozzi, Monica Emanuelli, Enrico M. Strappa, Paolo Bittarello, Fabrizio Bambini, and Gianni Gallusi. 2022. "Epithelial Biological Response to Machined Titanium vs. PVD Zirconium-Coated Titanium: An In Vitro Study" Materials 15, no. 20: 7250. https://doi.org/10.3390/ma15207250
APA StyleMemè, L., Sartini, D., Pozzi, V., Emanuelli, M., Strappa, E. M., Bittarello, P., Bambini, F., & Gallusi, G. (2022). Epithelial Biological Response to Machined Titanium vs. PVD Zirconium-Coated Titanium: An In Vitro Study. Materials, 15(20), 7250. https://doi.org/10.3390/ma15207250







