Plasma Functionalization of Multi-Walled Carbon Nanotubes for Ammonia Gas Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of MWCNTs
2.2. Plasma Treatment
2.3. Testing of Ammonia Gas Sensors
2.4. Characterization of Samples
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arjmand, M.; Mahmoodi, M.; Gelves, G.A.; Park, S.; Sundararaj, U. Electrical and electromagnetic interference shielding properties of flow-induced oriented carbon nanotubes in polycarbonate. Carbon N. Y. 2011, 49, 3430–3440. [Google Scholar] [CrossRef]
- Bannov, A.G.; Nazarenko, O.B.; Maksimovskii, E.A.; Popov, M.V.; Berdyugina, I.I.S. Thermal behavior and flammability of epoxy composites based on multi-walled carbon nanotubes and expanded graphite: A comparative study. Appl. Sci. 2020, 10, 6928. [Google Scholar] [CrossRef]
- Guo, J.; Wang, J.; Xuan, C.; Wu, Z.; Lei, W.; Zhu, J.; Xiao, W.; Wang, D. Molybdenum carbides embedded on carbon nanotubes for efficient hydrogen evolution reaction. J. Electroanal. Chem. 2017, 801, 7–13. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Bannov, A.G.; Popov, M.V.; Yun, J.-W.; Nguyen, A.D.; Kim, Y.S. High-temperature-treated multiwall carbon nanotubes for hydrogen evolution reaction. Int. J. Hydrogen Energy 2018, 43, 6526–6531. [Google Scholar] [CrossRef]
- Pekarek, J.; Vrba, R.; Prasek, J.; Jasek, O.; Majzlikova, P.; Pekarkova, J.; Zajickova, L. MEMS Carbon Nanotubes Field Emission Pressure Sensor With Simplified Design: Performance and Field Emission Properties Study. IEEE Sens. J. 2015, 15, 1430–1436. [Google Scholar] [CrossRef]
- Wang, J. Carbon-nanotube based electrochemical biosensors: A review. Electroanalysis 2005, 17, 7–14. [Google Scholar] [CrossRef]
- Fam, D.W.H.; Tok, A.I.Y.; Palaniappan, A.; Nopphawan, P.; Lohani, A.; Mhaisalkar, S.G. Selective sensing of hydrogen sulphide using silver nanoparticle decorated carbon nanotubes. Sens. Actuators B Chem. 2009, 138, 189–192. [Google Scholar] [CrossRef]
- Mittal, M.; Kumar, A. Carbon nanotube (CNT) gas sensors for emissions from fossil fuel burning. Sens. Actuators B Chem. 2014, 203, 349–362. [Google Scholar] [CrossRef]
- Rigoni, F.; Tognolini, S.; Borghetti, P.; Drera, G.; Pagliara, S.; Goldoni, A.; Sangaletti, L. Enhancing the sensitivity of chemiresistor gas sensors based on pristine carbon nanotubes to detect low-ppb ammonia concentrations in the environment. Analyst 2013, 138, 7392–7399. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, P.; Eliáš, M.; Michalička, J.; Hegemann, D.; Pytlíček, Z.; Nečas, D.; Zajíčková, L. Atomic layer deposition of titanium dioxide on multi-walled carbon nanotubes for ammonia gas sensing. Surf. Coat. Technol. 2019, 370, 235–243. [Google Scholar] [CrossRef]
- Kumar, S.; Pavelyev, V.; Mishra, P.; Tripathi, N. A review on chemiresistive gas sensors based on carbon nanotubes: Device and technology transformation. Sens. Actuators A Phys. 2018, 283, 174–186. [Google Scholar] [CrossRef]
- Mendes, R.G.; Wróbel, P.S.; Bachmatiuk, A.; Sun, J.; Gemming, T.; Liu, Z.; Rümmeli, M.H. Carbon nanostructures as a multi-functional platform for sensing applications. Chemosensors 2018, 6, 60. [Google Scholar] [CrossRef] [Green Version]
- Si, P.; Mortensen, J.; Komolov, A.; Denborg, J.; Møller, P.J. Polymer coated quartz crystal microbalance sensors for detection of volatile organic compounds in gas mixtures. Anal. Chim. Acta 2007, 597, 223–230. [Google Scholar] [CrossRef]
- Ionescu, R.; Espinosa, E.H.; Sotter, E.; Llobet, E.; Vilanova, X.; Correig, X.; Felten, A.; Bittencourt, C.; Van Lier, G.; Charlier, J.C.; et al. Oxygen functionalisation of MWNT and their use as gas sensitive thick-film layers. Sens. Actuators B Chem. 2006, 113, 36–46. [Google Scholar] [CrossRef]
- Kim, T.; Kim, S.S.; Min, N.; Pak, J.J.; Lee, C.; Kim, S.S. NH3 sensitive chemiresistor sensors Using plasma functionalized multiwall carbon nanotubes/conducting polymer composites. In Proceedings of the IEEE Sensors 2008 Conference, Lecce, Italy, 26–29 October 2008; pp. 208–211. [Google Scholar]
- Nguyen, L.Q.; Phan, P.Q.; Duong, H.N.; Nguyen, C.D.; Nguyen, L.H. Enhancement of NH3 gas sensitivity at room temperature by carbon nanotube-based sensor coated with Co nanoparticles. Sensors 2013, 13, 1754–1762. [Google Scholar] [CrossRef] [Green Version]
- Rigoni, F.; Drera, G.; Pagliara, S.; Goldoni, A.; Sangaletti, L. High sensitivity, moisture selective, ammonia gas sensors based on single-walled carbon nanotubes functionalized with indium tin oxide nanoparticles. Carbon N. Y. 2014, 80, 356–363. [Google Scholar] [CrossRef]
- Kim, J.H.; Song, M.J.; Kim, K.B.; Jin, J.H.; Min, N.K. Evaluation of surface cleaning procedures in terms of gas sensing properties of spray-deposited CNT film: Thermal- and O2 plasma treatments. Sensors 2017, 17, 73. [Google Scholar] [CrossRef] [Green Version]
- Bannov, A.G.; Prášek, J.; Jašek, O.; Zajíčková, L. Investigation of Pristine Graphite Oxide as Room-Temperature Chemiresistive Ammonia Gas Sensing Material. Sensors 2017, 17, 320. [Google Scholar] [CrossRef] [PubMed]
- Synek, P.; Jašek, O.; Zajíčková, L. Study of microwave torch plasmachemical synthesis of iron oxide nanoparticles focused on theanalysis of phase composition. Plasma Chem. Plasma Process. 2014, 34, 327–341. [Google Scholar] [CrossRef]
- Bannov, A.G.; Jašek, O.; Manakhov, A.; Márik, M.; Nečas, D.; Zajíčková, L. High-Performance Ammonia Gas Sensors Based on Plasma Treated Carbon Nanostructures. IEEE Sens. J. 2017, 17, 1964–1970. [Google Scholar] [CrossRef]
- Manakhov, A.; Michlíček, M.; Nečas, D.; Polčák, J.; Makhneva, E.; Eliáš, M.; Zajíčková, L. Carboxyl-rich coatings deposited by atmospheric plasma co-polymerization of maleic anhydride and acetylene. Surf. Coat. Technol. 2015, 295, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Qiao, Y.J.; Zhang, H.; Hu, Y.X.; Li, W.P.; Liu, W.J.; Shang, H.M.; Qu, M.Z.; Peng, G.C.; Xie, Z.W. A chain-like compound of Si@CNT nanostructures and MOF-derived porous carbon as an anode for Li-ion batteries. Int. J. Miner. Metall. Mater. 2021, 28, 1611–1620. [Google Scholar] [CrossRef]
- Ferrari, A.; Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys. Rev. B 2001, 64, 075414. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron-phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Avile, F. Evaluation of mild acid oxidation treatments for MWCNT functionalization. Carbon N. Y. 2009, 7, 5–10. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Wang, J.; Cui, S. Sulfonitric treatment of Multiwalled carbon nanotubes and their dispersibility in water. Materials 2018, 11, 2442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bannov, A.G.; Popov, M.V.; Brester, A.E.; Kurmashov, P.B. Recent Advances in Ammonia Gas Sensors Based on Carbon Nanomaterials. Micromachines 2021, 12, 186. [Google Scholar] [CrossRef]
- Sharma, S.; Hussain, S.; Sengupta, K.; Islam, S.S. Development of MWCNTs/alumina composite-based sensor for trace level ammonia gas sensing. Appl. Phys. A Mater. Sci. Process. 2013, 111, 965–974. [Google Scholar] [CrossRef]
- Xia, W.; Hagen, V.; Kundu, S.; Wang, Y.; Somsen, C.; Eggeler, G.; Sun, G.; Grundmeier, G.; Stratmann, M.; Muhler, M. Controlled etching of carbon nanotubes by iron-catalyzed steam gasification. Adv. Mater. 2007, 19, 3648–3652. [Google Scholar] [CrossRef]
- Vikramaditya, T.; Sumithra, K. Effect of substitutionally boron-doped single-walled semiconducting zigzag carbon nanotubes on ammonia adsorption. J. Comput. Chem. 2014, 35, 586–594. [Google Scholar] [CrossRef]
- Kwak, D.; Lei, Y.; Maric, R. Ammonia gas sensors: A comprehensive review. Talanta 2019, 204, 713–730. [Google Scholar] [CrossRef] [PubMed]

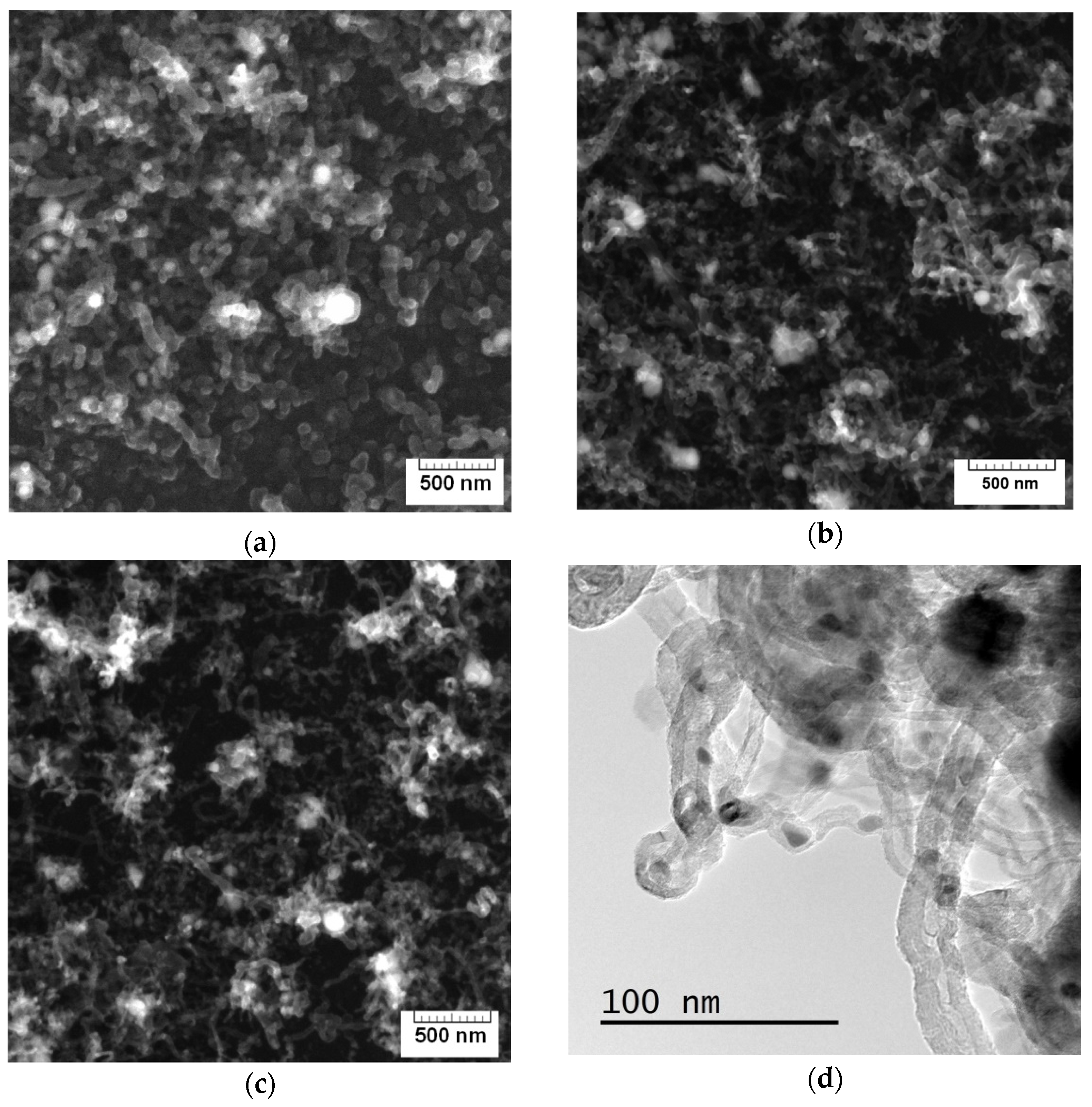

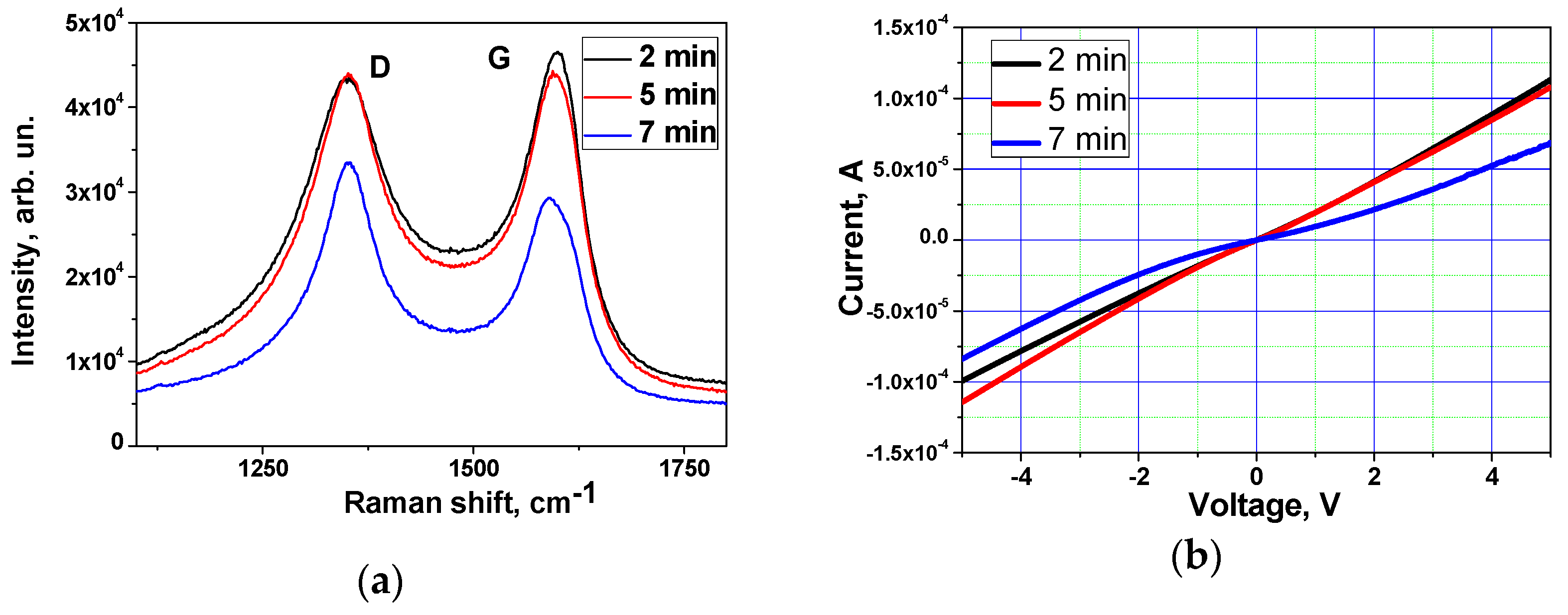

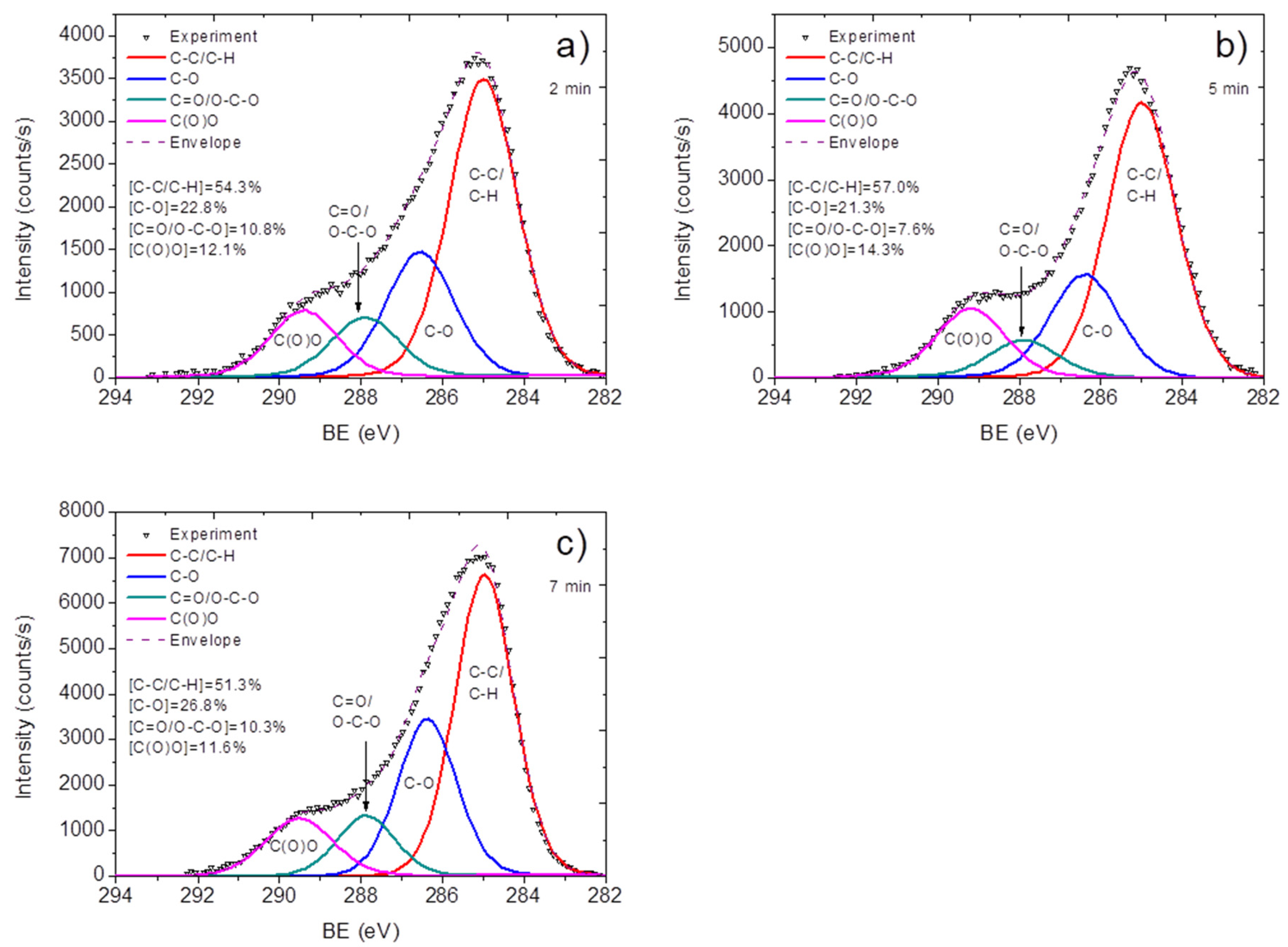
| Sample | D Peak Position, cm−1 | D Peak FWHM, cm−1 | G Peak Position, cm−1 | G Peak FWHM, cm−1 | I(D)/I(G) |
|---|---|---|---|---|---|
| Non-treated | 1348 | 131 | 1590 | 69 | 0.86 |
| 2 min | 1353 | 130 | 1591 | 65 | 0.87 |
| 5 min | 1353 | 119 | 1592 | 70 | 0.95 |
| 7 min | 1353 | 90 | 1589 | 59 | 1.08 |
| Duration of Treatment | Resistance of Sensor, kΩ | Sensor Response before Functionalization, % | Sensor Response after Functionalization, % | ||||
|---|---|---|---|---|---|---|---|
| 100 ppm | 250 ppm | 500 ppm | 100 ppm | 250 ppm | 500 ppm | ||
| 2 min | 52.7 | 1.7 | 2.3 | 2.9 | |||
| 5 min | 58.6 | 0.4 | 0.6 | 0.7 | 1.8 | 2.6 | 3.6 |
| 7 min | 110 | 0.7 | 1.0 | 1.3 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bannov, A.G.; Manakhov, A.M.; Shtansky, D.V. Plasma Functionalization of Multi-Walled Carbon Nanotubes for Ammonia Gas Sensors. Materials 2022, 15, 7262. https://doi.org/10.3390/ma15207262
Bannov AG, Manakhov AM, Shtansky DV. Plasma Functionalization of Multi-Walled Carbon Nanotubes for Ammonia Gas Sensors. Materials. 2022; 15(20):7262. https://doi.org/10.3390/ma15207262
Chicago/Turabian StyleBannov, Alexander G., Anton M. Manakhov, and Dmitry V. Shtansky. 2022. "Plasma Functionalization of Multi-Walled Carbon Nanotubes for Ammonia Gas Sensors" Materials 15, no. 20: 7262. https://doi.org/10.3390/ma15207262







