Irradiation Damage Independent Deuterium Retention in WMoTaNbV
Abstract
1. Introduction
2. Experimental
2.1. Sample Preparation
2.2. Positron Lifetime Spectroscopy
2.3. D Implantation
2.4. Elastic Recoil Detection Analysis (ERDA)
2.5. Secondary Ion Mass Spectrometry (SIMS)
2.6. Thermal Desorption Measurements
3. Results
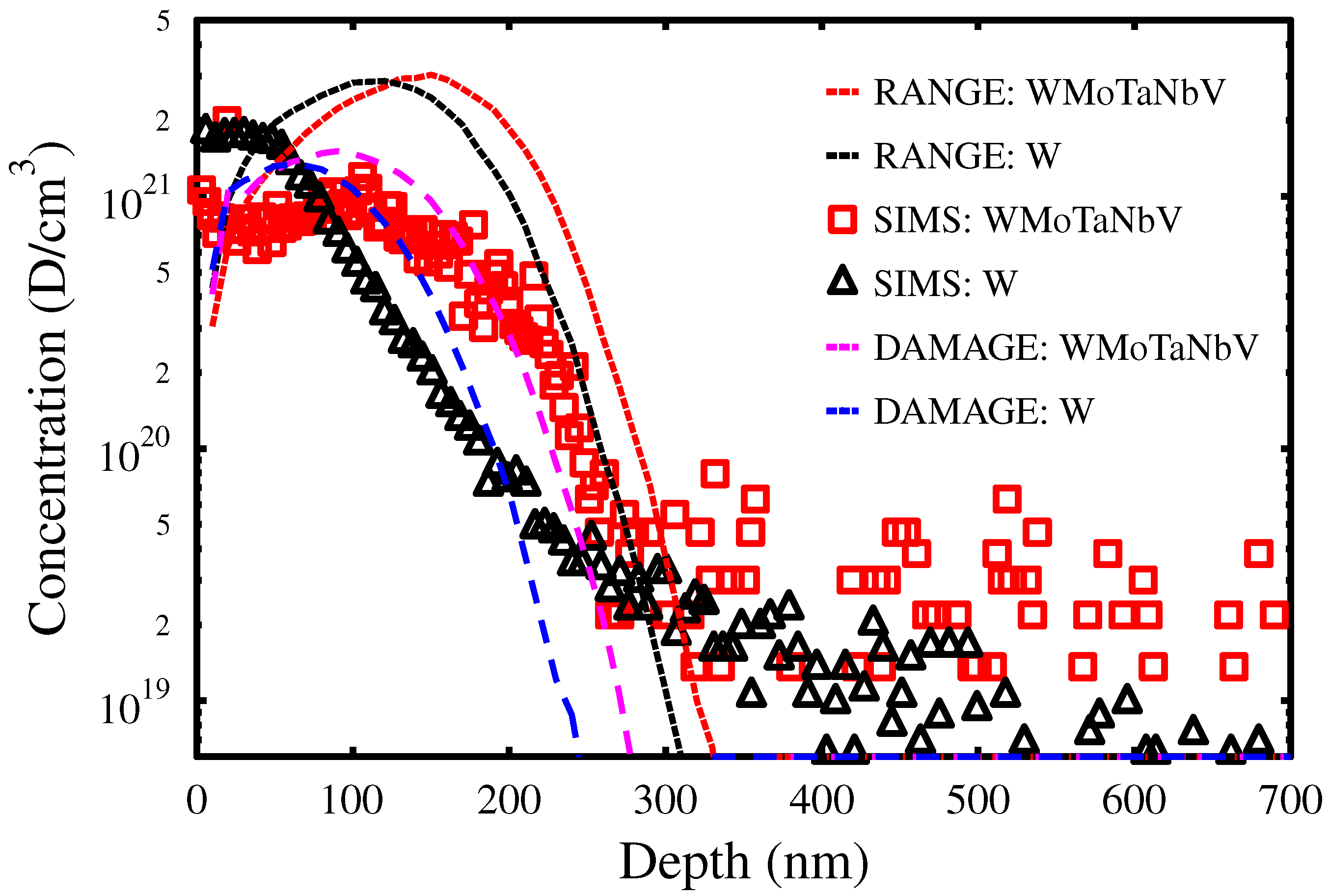
3.1. D Concentration Profiles
3.2. Vacancy Defects and Irradiation Damage
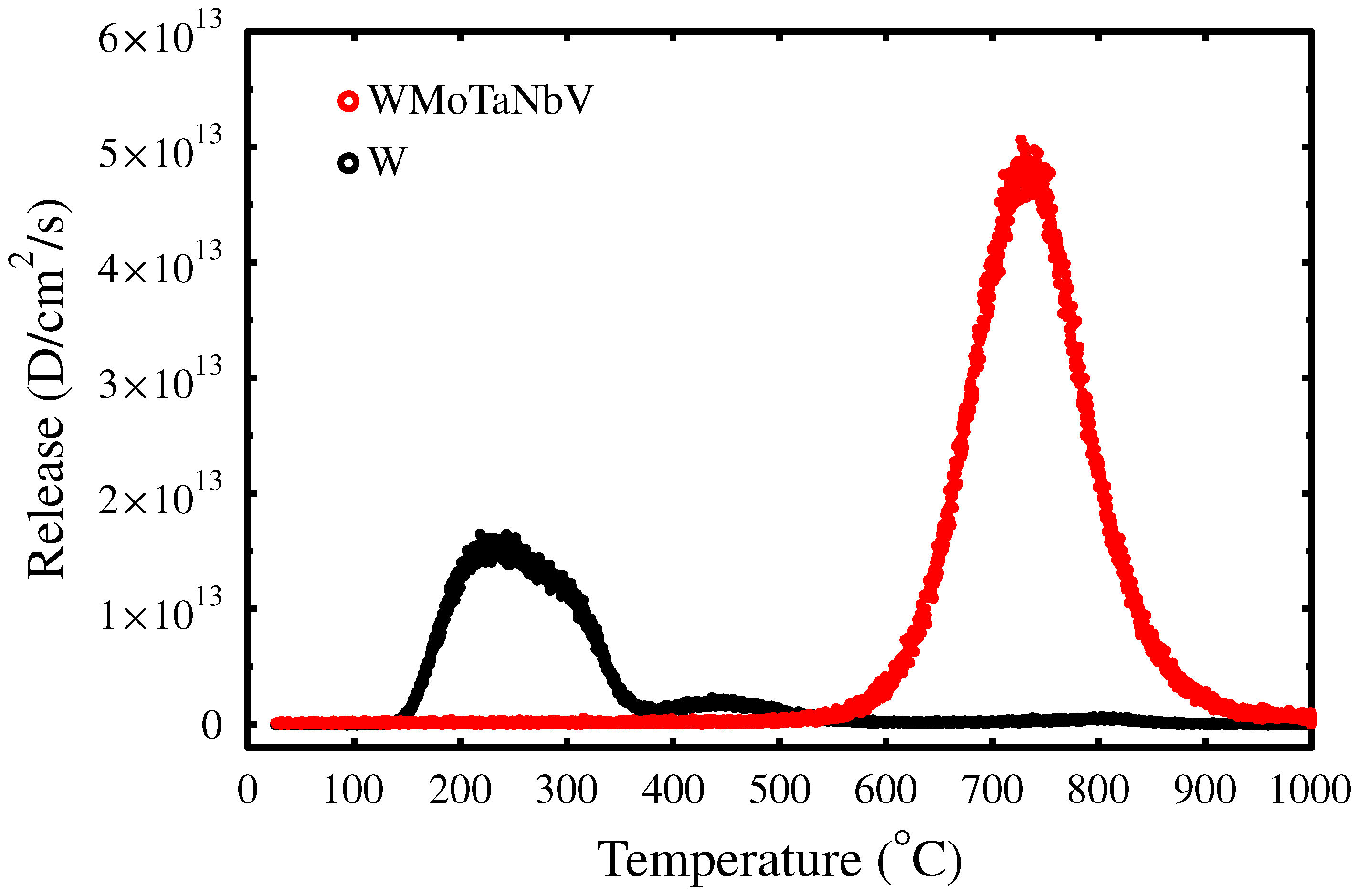
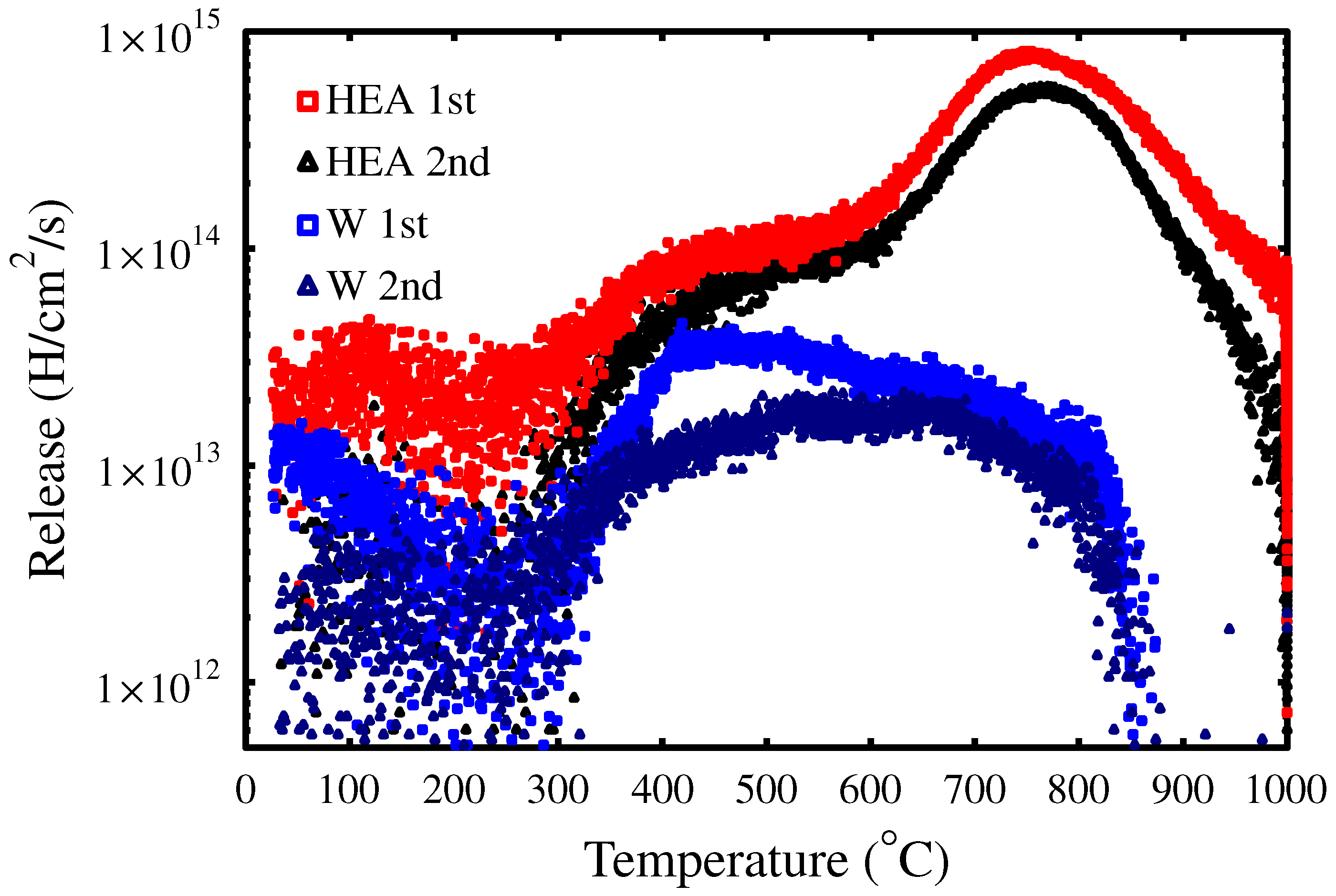
3.3. Trapping Dynamics
3.4. H Trapping to Irradiation-Induced Defects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HEA | high entropy alloy |
| ERDA | elastic recoil detection analysis |
| SIMS | secondary ion mass spectrometry |
| TDS | thermal desorption spectrometry |
References
- Stork, D.; Agostini, P.; Boutard, J.-L.; Buckthorpe, D.; Diegele, E.; Dudarev, S.L.; English, C.; Federici, G.; Gilbert, M.R.; Gonzalez, S.; et al. Developing Structural, High-heat flux and Plasma Facing Materials for a near-term DEMO Fusion Power Plant: The EU Assessment. J. Nucl. Mater. 2014, 455, 277. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375377, 213. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.P.; Liaw, P.K. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Long, Y.; Su, K.; Zhang, J.; Liang, X.; Peng, H.; Li, X. Enhanced Strength of a Mechanical Alloyed NbMoTaWVTi Refractory High Entropy Alloy. Materials 2018, 11, 669. [Google Scholar] [CrossRef]
- High Entropy Materials Center of National Tsing Hua University. Available online: http://hemc.mse.nthu.edu.tw/en/ (accessed on 15 February 2022).
- Tseng, K.K.; Juan, C.-C.; Tso, S.; Chen, H.-C.; Tsai, C.-W.; Yeh, J.-W. Effects of Mo, Nb, Ta, Ti, and Zr on Mechanical Properties of Equiatomic Hf-Mo-Nb-Ta-Ti-Zr Alloys. Entropy 2018, 21, 15. [Google Scholar] [CrossRef]
- Tuomisto, F.; Makkonen, I. Defect identification in semiconductors with positron annihilation: Experiment and theory. Rev. Mod. Phys. 2013, 85, 1583. [Google Scholar] [CrossRef]
- Ziegler, J.F. SRIM-2011 Software Package. Available online: http://www.srim.org (accessed on 1 January 2022).
- Baron-Wiechec, A.; Heinola, K.; Likonen, J.; Alves, E.; Catarino, N.; Coad, J.P.; Corregidor, V.; Jepu, I.; Matthews, G.F.; Widdowson, A.; et al. Thermal desorption spectrometry of beryllium plasma facing tiles exposed in the JET tokamak. Fusion Eng. Des. 2018, 133, 135–141. [Google Scholar] [CrossRef]
- Konobeyev, A.Y.; Fischer, U.; Korovin, Y.A.; Simakov, S.P. Evaluation of effective threshold displacement energies and other data required for the calculation of advanced atomic displacement cross-sections. J. Energy Technol. 2017, 3, 169–175. [Google Scholar] [CrossRef]
- Ahlgren, T.; Heinola, K.; Vörtler, K.; Keinonen, J. Simulation of irradiation induced deuterium trapping in tungsten. J. Nucl. Mater. 2012, 427, 152–161. [Google Scholar] [CrossRef]
- Heikinheimo, J.; Mizohata, K.; Räisänen, J.; Ahlgren, T.; Jalkanen, P.; Lahtinen, A.; Catarino, N.; Alves, E.; Tuomisto, F. Direct observation of mono-vacancy and self-interstitial recovery in tungsten. APL Mater. 2019, 7, 021103. [Google Scholar] [CrossRef]
- Troev, T.; Popov, E.; Staikov, P.; Nankov, N.; Yoshiie, T. Positron simulations of defects in tungsten containing hydrogen and helium. Nucl. Instrum. 2009, 267, 535–541. [Google Scholar] [CrossRef]
- Lhuillier, P.E.; Barthe, M.F.; Desgardin, P.; Egger, W.; Sperr, P. Positron annihilation studies on the nature and thermal behaviour of irradiation induced defects in tungsten. Phys. Status Solidi C 2009, 6, 2329–2332. [Google Scholar] [CrossRef]
- Tompkins, F.C. Chemisorption of Gases on Metals; Academic Press: London, UK, 1978. [Google Scholar]
- Kissinger, H.E. Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Bur. Stand. 1956, 57, 217–221. [Google Scholar] [CrossRef]
- Ahlgren, T.; Heinola, K. Improvements to the Sink Strength Theory Used in Multi-Scale Rate Equation Simulations of Defects in Solids. Materials 2020, 13, 2621. [Google Scholar] [CrossRef]
- Bukonte, L.; Ahlgren, T.; Heinola, K. Modelling of monovacancy diffusion in W over wide temperature range. J. Appl. Phys. 2014, 115, 123504. [Google Scholar] [CrossRef]
- Rasch, K.-D.; Siegel, R.W.; Schultz, H. Quenching and recovery investigations of vacancies in tungsten. Philos. Mag. A 1980, 41, 91–117. [Google Scholar] [CrossRef]
- Mundy, J.N.; Rothman, S.J.; Lam, N.Q.; Hoff, H.A.; Nowicki, L.J. Self-diffusion in tungsten. Phys. Rev. B 1978, 18, 6566. [Google Scholar] [CrossRef]
- Heinola, K.; Ahlgren, T.; Norlund, K.; Keinonen, J. Hydrogen interaction with point defects in tungsten. Phys. Rev. B 2010, 82, 094102. [Google Scholar] [CrossRef]
- You, Y.-W.; Kong, X.-S.; Wu, X.-B.; Xu, Y.-C.; Fang, Q.F.; Chen, J.L.; Luo, G.-N.; Liu, C.S.; Pan, B.C.; Wang, Z. Dissolving, trapping and detrapping mechanisms of hydrogen in bcc and fcc transition metals. AIP Adv. 2013, 3, 012118. [Google Scholar] [CrossRef]
- Sun, L.; Jin, S.; Li, X.-C.; Zhang, Y.; Lu, G.-H. Hydrogen behaviors in molybdenum and tungsten and a generic vacancy trapping mechanism for H bubble formation. J. Nucl. Mater. 2013, 434, 395–401. [Google Scholar] [CrossRef]
- Heinola, K.; Ahlgren, T. Hydrogen retention to impurities in tungsten: A multi-scale study. J. Nucl. Mater. 2013, 438, S1001–S1004. [Google Scholar] [CrossRef]
- Li, Z.; Tasan, C.C.; Springer, H.; Gault, B.; Raabe, D. Interstitial atoms enable joint twinning and transformation induced plasticity in strong and ductile high- entropy alloys. Sci. Rep. 2017, 7, 40704. [Google Scholar] [CrossRef]
- Lu, E.; Zhao, J.; Makkonen, I.; Mizohata, K.; Li, Z.; Hua, M.; Djurabekova, F.; Tuomisto, F. Enhancement of vacancy diffusion by C and N interstitials in the equiatomic FeMnNiCoCr high entropy alloy. Acta Mater. 2021, 215, 117093. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Thomas, S.L.; Patala, S. Vacancy diffusion in multi-principal element alloys: The role of chemical disorder in the ordered lattice. Acta Mater. 2020, 196, 144–153. [Google Scholar] [CrossRef]

| ERDA | TDS | |||||
|---|---|---|---|---|---|---|
| Impl. Dose * | Abs. Reten. | Rel. Reten. ** | Heating Rate | Abs. Reten. | Rel. Reten. ** | |
| W | 4.25 | 1.64 | 38.5% | 10 °C/min | 1.50 | 35.3% |
| HEA | 20 °C/min | 4.14 | 90.4% | |||
| HEA | 4.58 | 1.54 | 33.6% | 10 °C/min | 4.20 | 91.7% |
| HEA | 5 °C/min | 4.33 | 94.5% |
| W (eV) [22] | Mo (eV) [24] | Ta (eV) [23] | Nb (eV) [23] | V (eV) [23] | |
|---|---|---|---|---|---|
| 1H | 1.60 | 1.25 | 0.38 | 0.41 | 0.44 |
| 2H | 1.57 | 1.25 | 0.43 | 0.47 | 0.53 |
| 3H | 1.39 | 0.97 | 0.33 | 0.30 | 0.43 |
| 4H | 1.28 | 0.89 | 0.26 | 0.25 | 0.34 |
| 5H | 1.17 | 0.75 | 0.35 | 0.24 | 0.33 |
| 6H | 0.64 | 0.54 | 0.13 | 0.09 | 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liski, A.; Vuoriheimo, T.; Jalkanen, P.; Mizohata, K.; Lu, E.; Likonen, J.; Heino, J.; Heinola, K.; Zayachuk, Y.; Widdowson, A.; et al. Irradiation Damage Independent Deuterium Retention in WMoTaNbV. Materials 2022, 15, 7296. https://doi.org/10.3390/ma15207296
Liski A, Vuoriheimo T, Jalkanen P, Mizohata K, Lu E, Likonen J, Heino J, Heinola K, Zayachuk Y, Widdowson A, et al. Irradiation Damage Independent Deuterium Retention in WMoTaNbV. Materials. 2022; 15(20):7296. https://doi.org/10.3390/ma15207296
Chicago/Turabian StyleLiski, Anna, Tomi Vuoriheimo, Pasi Jalkanen, Kenichiro Mizohata, Eryang Lu, Jari Likonen, Jouni Heino, Kalle Heinola, Yevhen Zayachuk, Anna Widdowson, and et al. 2022. "Irradiation Damage Independent Deuterium Retention in WMoTaNbV" Materials 15, no. 20: 7296. https://doi.org/10.3390/ma15207296
APA StyleLiski, A., Vuoriheimo, T., Jalkanen, P., Mizohata, K., Lu, E., Likonen, J., Heino, J., Heinola, K., Zayachuk, Y., Widdowson, A., Tseng, K.-K., Tsai, C.-W., Yeh, J.-W., Tuomisto, F., & Ahlgren, T. (2022). Irradiation Damage Independent Deuterium Retention in WMoTaNbV. Materials, 15(20), 7296. https://doi.org/10.3390/ma15207296










