Comprehensive Analysis of Two H13-Type Starting Materials Used for Laser Cladding and Aerosol Particles Formed in This Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Powders Used in the Laser Cladding Process
2.2. Additive Manufacturing
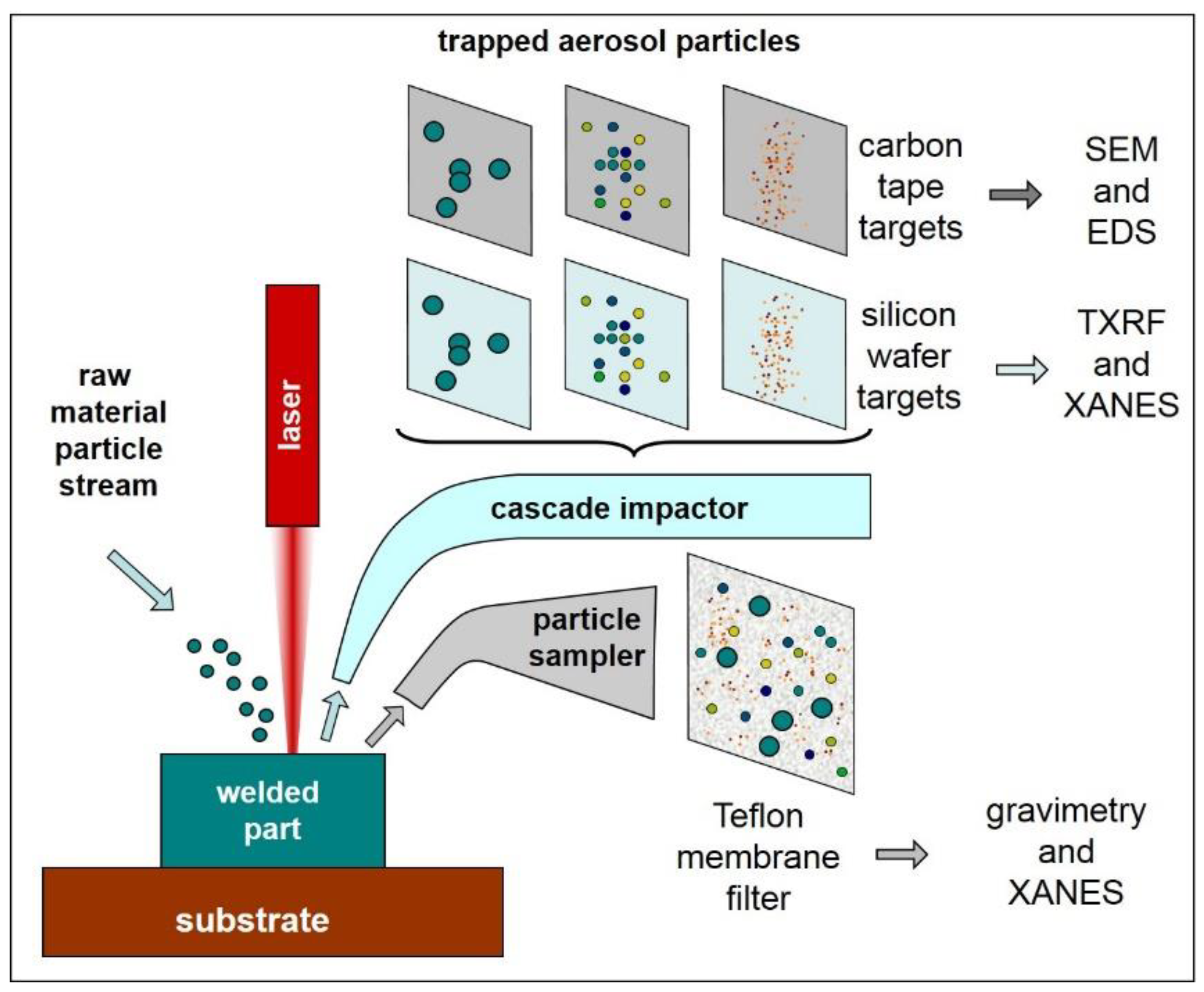
2.3. Aerosol Collection and Monitoring Devices
2.4. Scanning Electron Microscopy and Energy-Dispersive Analysis
2.5. Total-Reflection X-ray Fluorescence Analysis
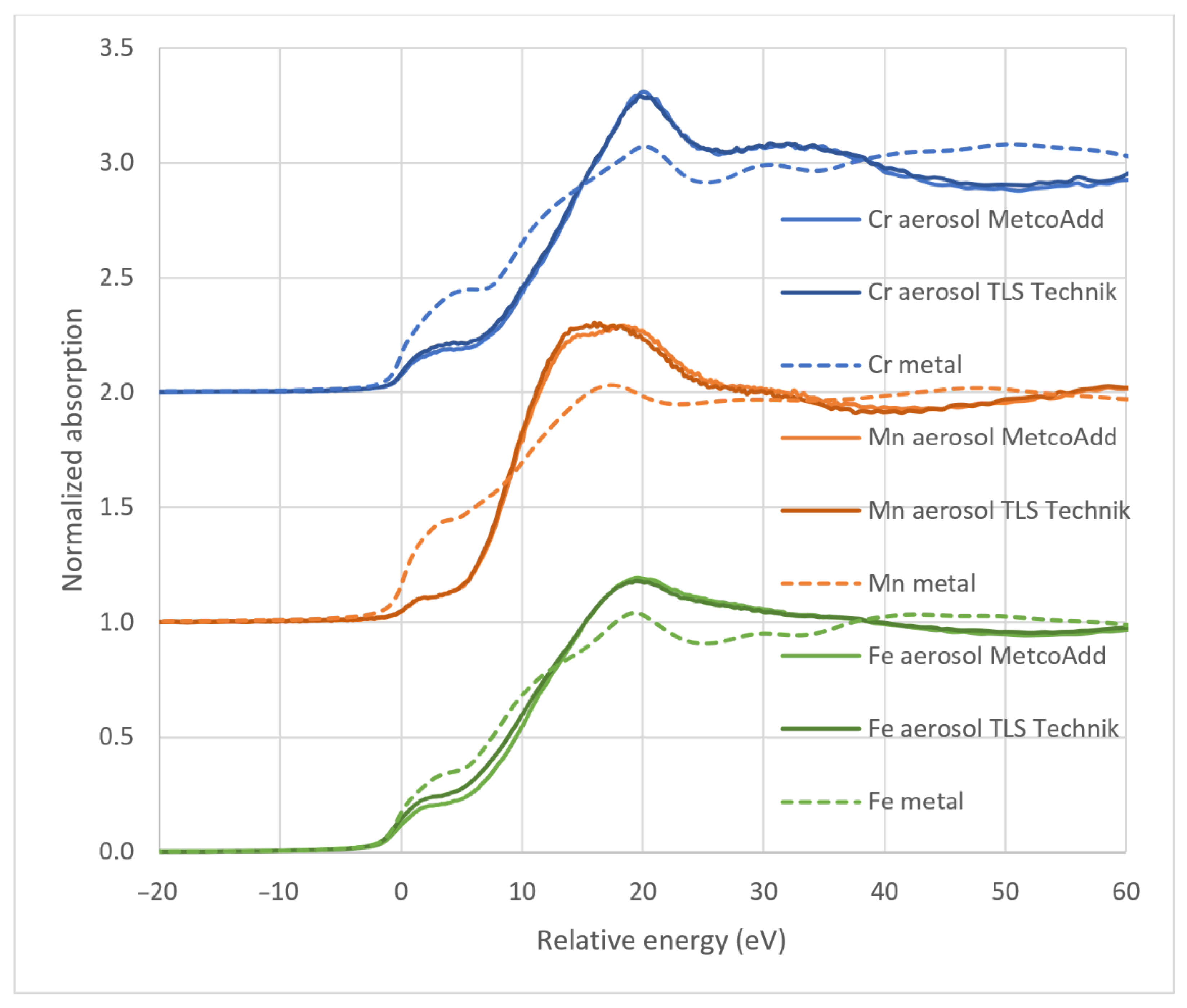
2.6. X-ray Absorption Near-Edge Structure Spectroscopy
3. Results and Discussion
3.1. Comparison of the Particle Size, Particle Morphology, and Composition of the As-Received Powder Samples
3.2. Shape, Size Distribution, and Composition of the Particles in the Fume Collected by the Cascade Impactor during the Laser Cladding Process
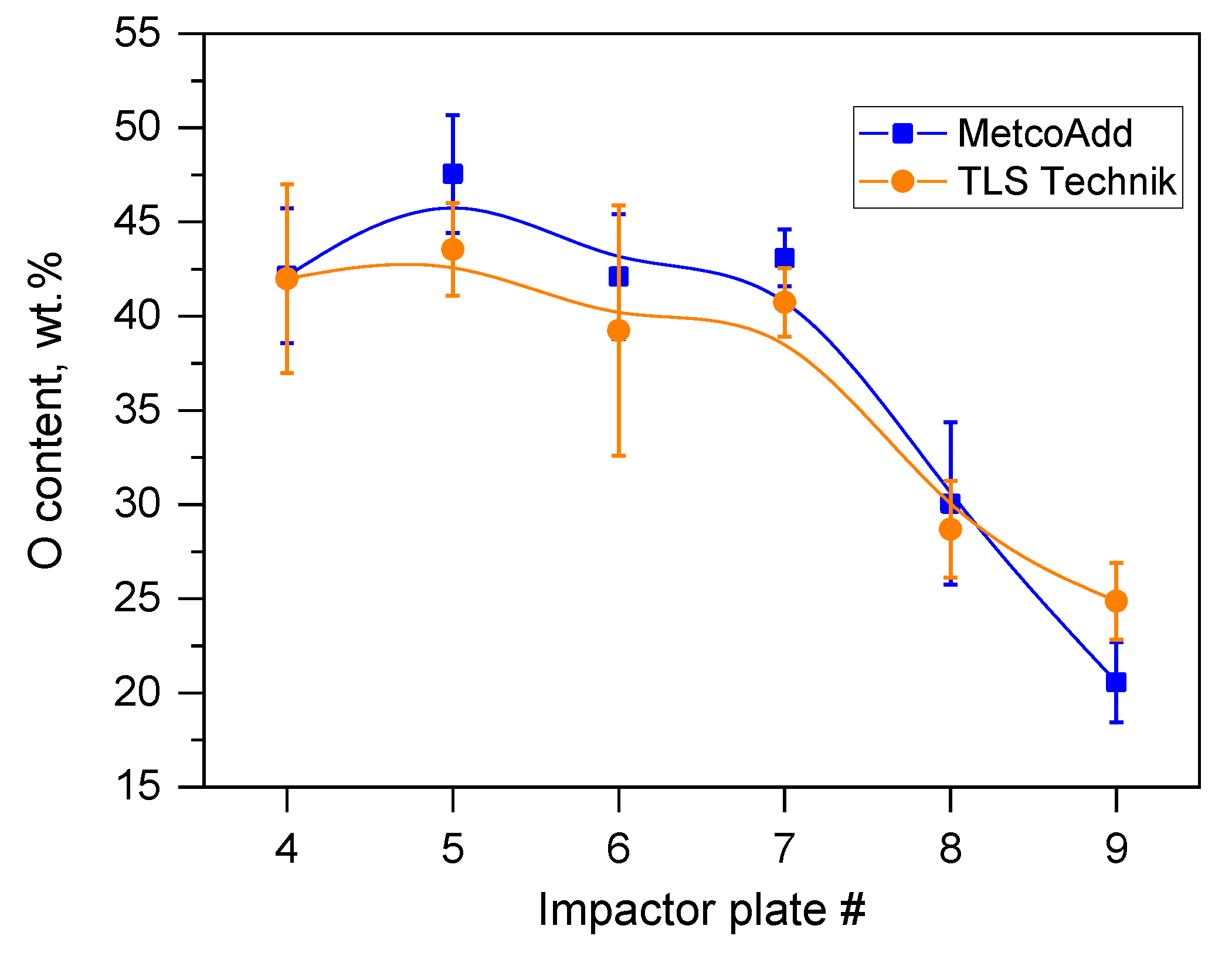
3.3. Oxidation State of Metals Assessed via XANES
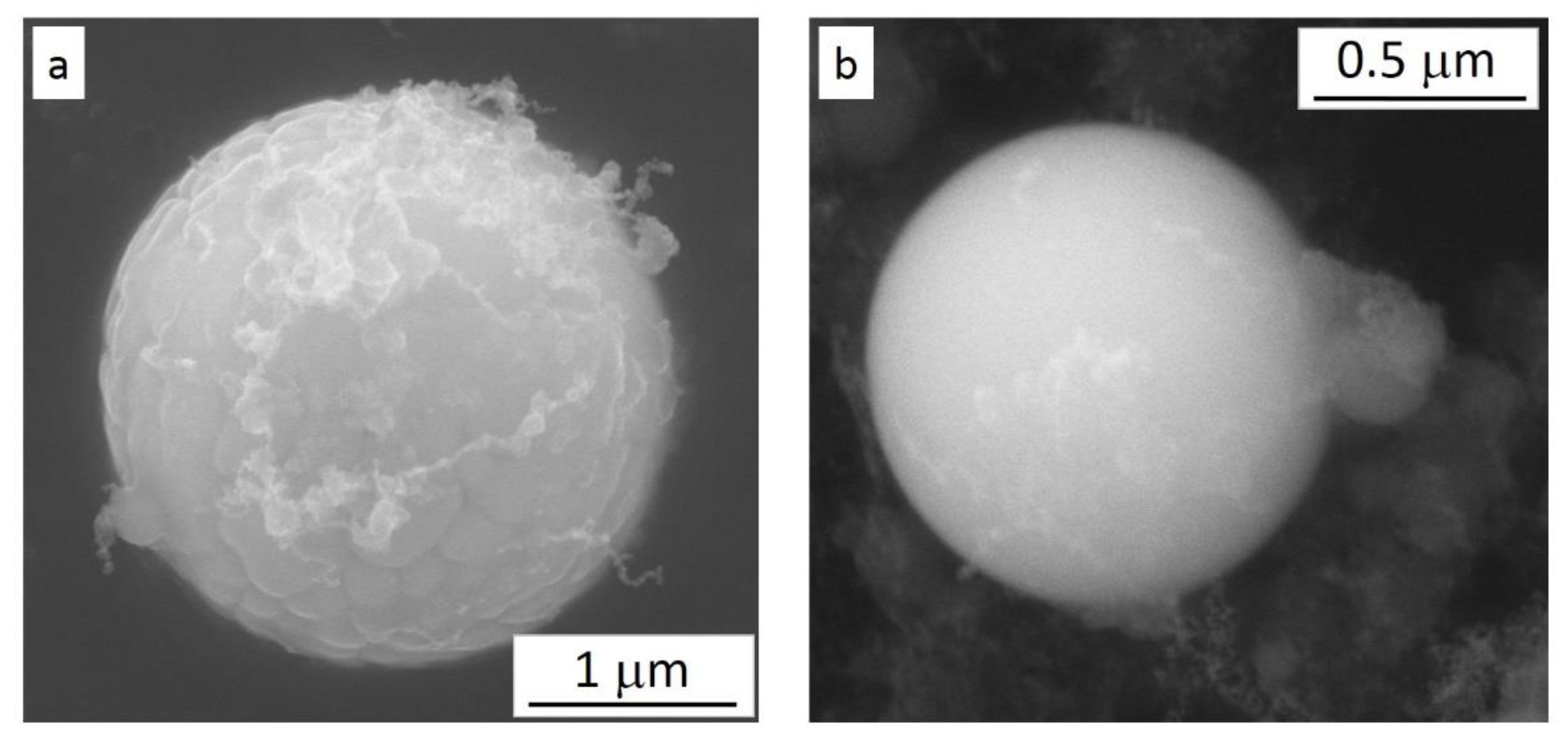
3.4. SEM Analysis of the Particulate Residues of the Starting Powders
4. Summary and Conclusions
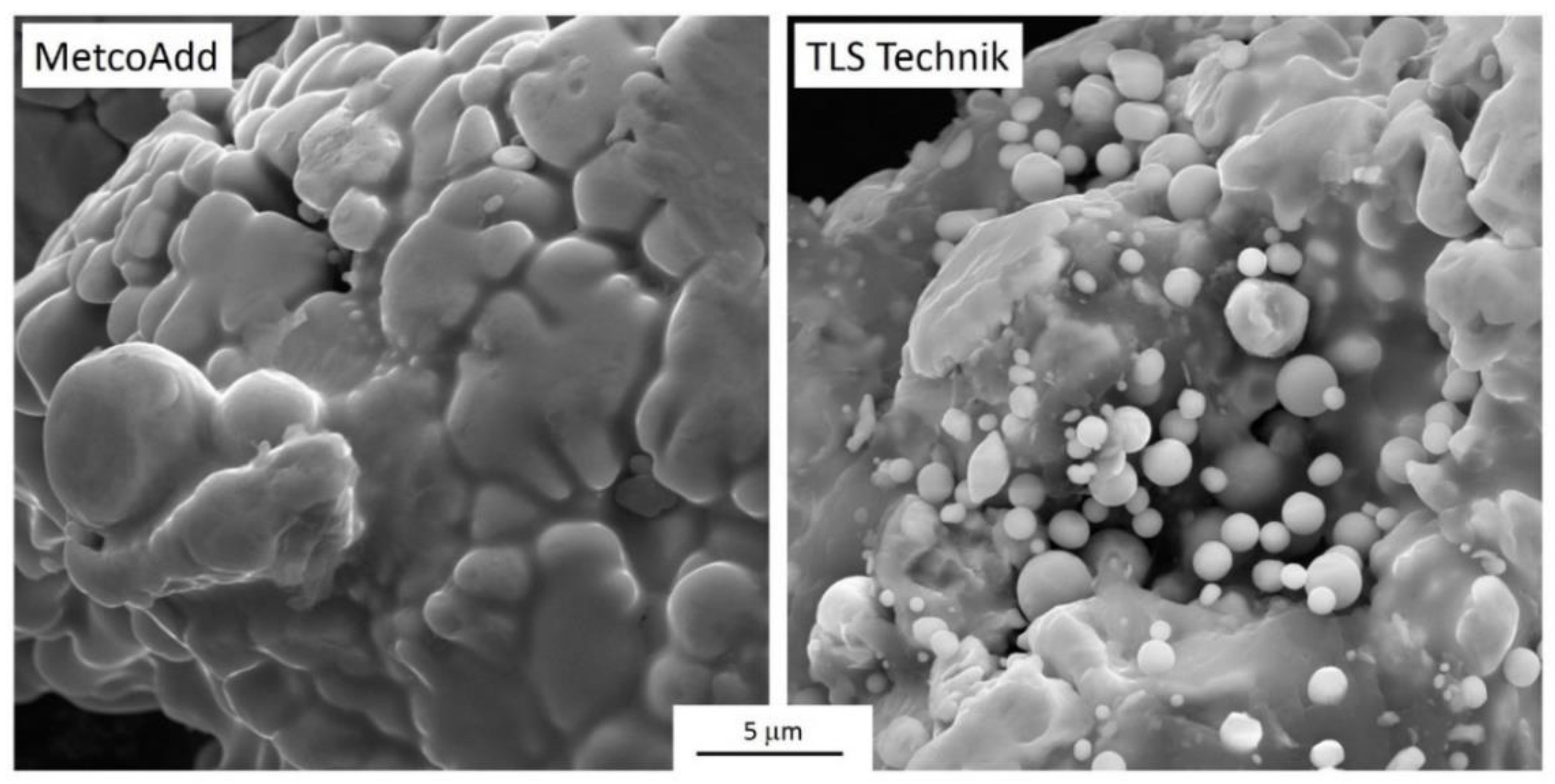
- The chemical composition of the powder particles conformed to the H13 specification and the manufacturers’ datasheets. The morphological analysis showed that the powder particles were mostly spherical, but also elongated and irregularly shaped ones were present. The powder particles from the two vendors carried different irregularity types. Smaller micrometer-sized satellite particles with the same chemical composition were also observed, particularly in one of the powders;
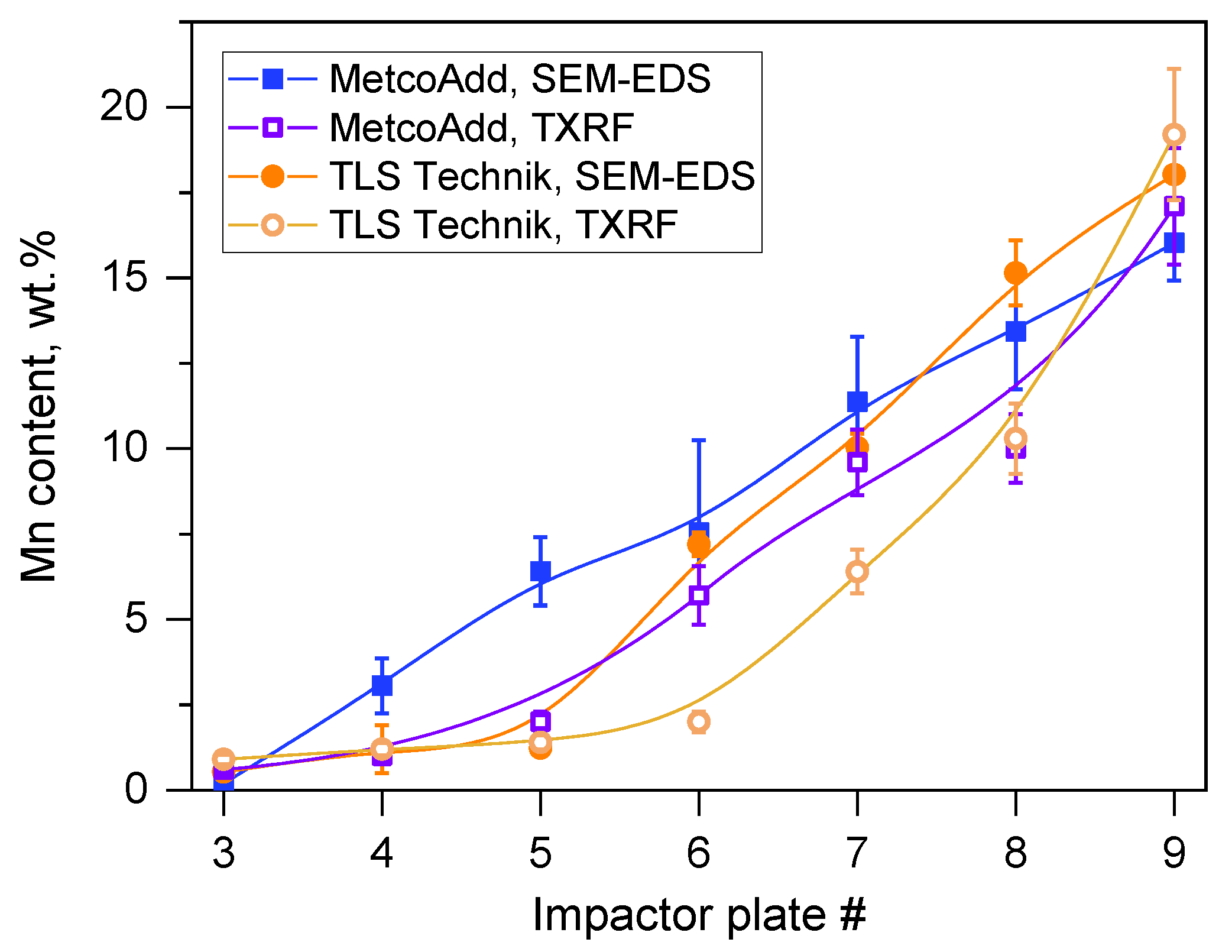
- We characterized the particles formed in the laser cladding process via SEM-EDS. In line with previous results [19], it was found that the composition of the aerosol product changed compared to the as-received metal powder. Metals with higher vapor pressure were enriched and became oxidized significantly in the finest fraction of the sampled aerosol;
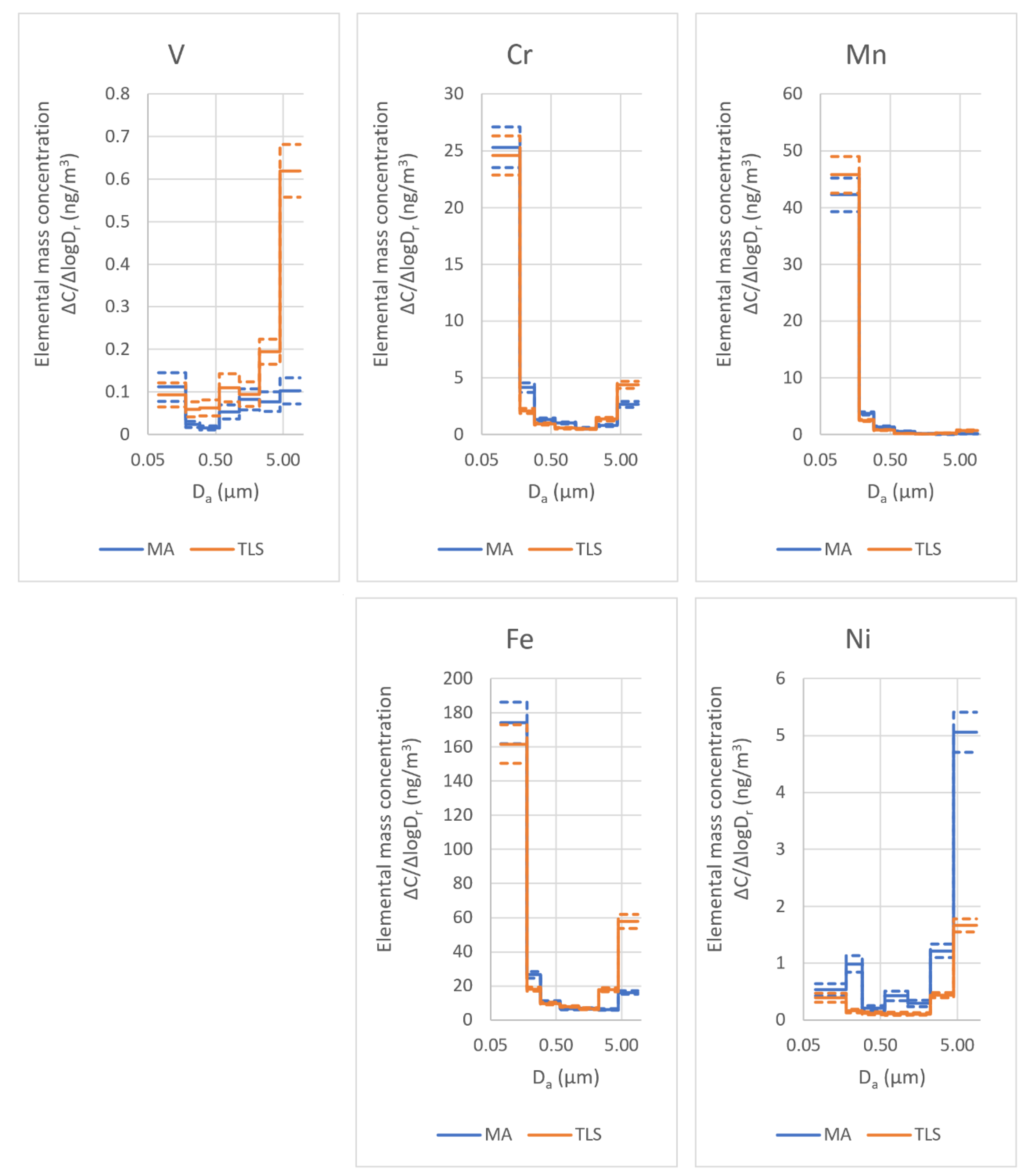
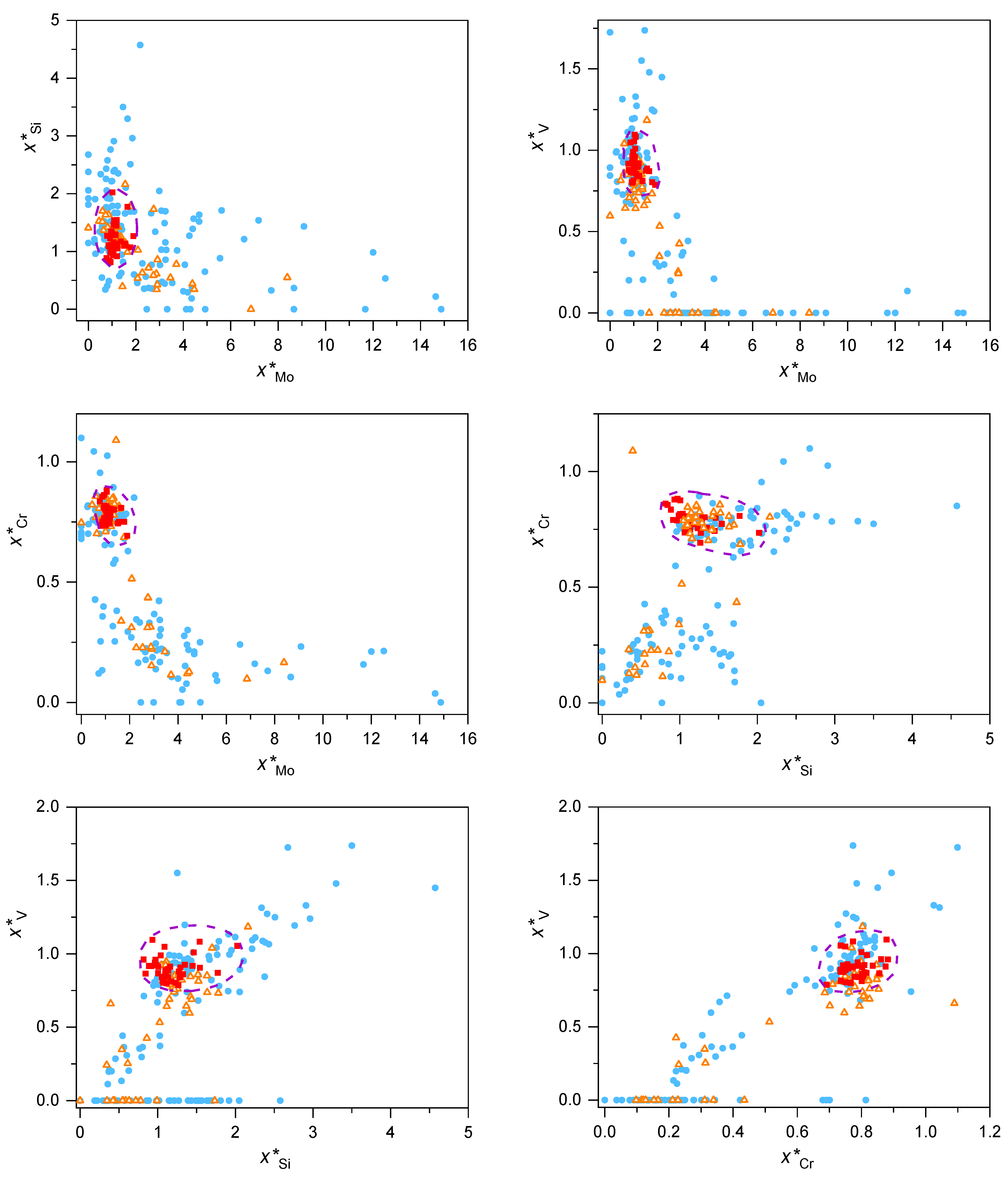
- The residues of the original particles, which were not incorporated into the welded part but were transferred with the aerosol, exhibited a composition change. The elements with relatively high vapor pressure (typically, V and Cr) escaped from the particle residues, while other elements with relatively low vapor pressure (e.g., Mo) accumulated. The concentration changes of the components were also correlated in the sense that a relatively high local Si content led to good retention of V and Cr, but a high local Mo content was linked to intense losses of V and Cr. The components missing from the residues of the metallic particles were found in the highly oxidized ultrafine aerosol;
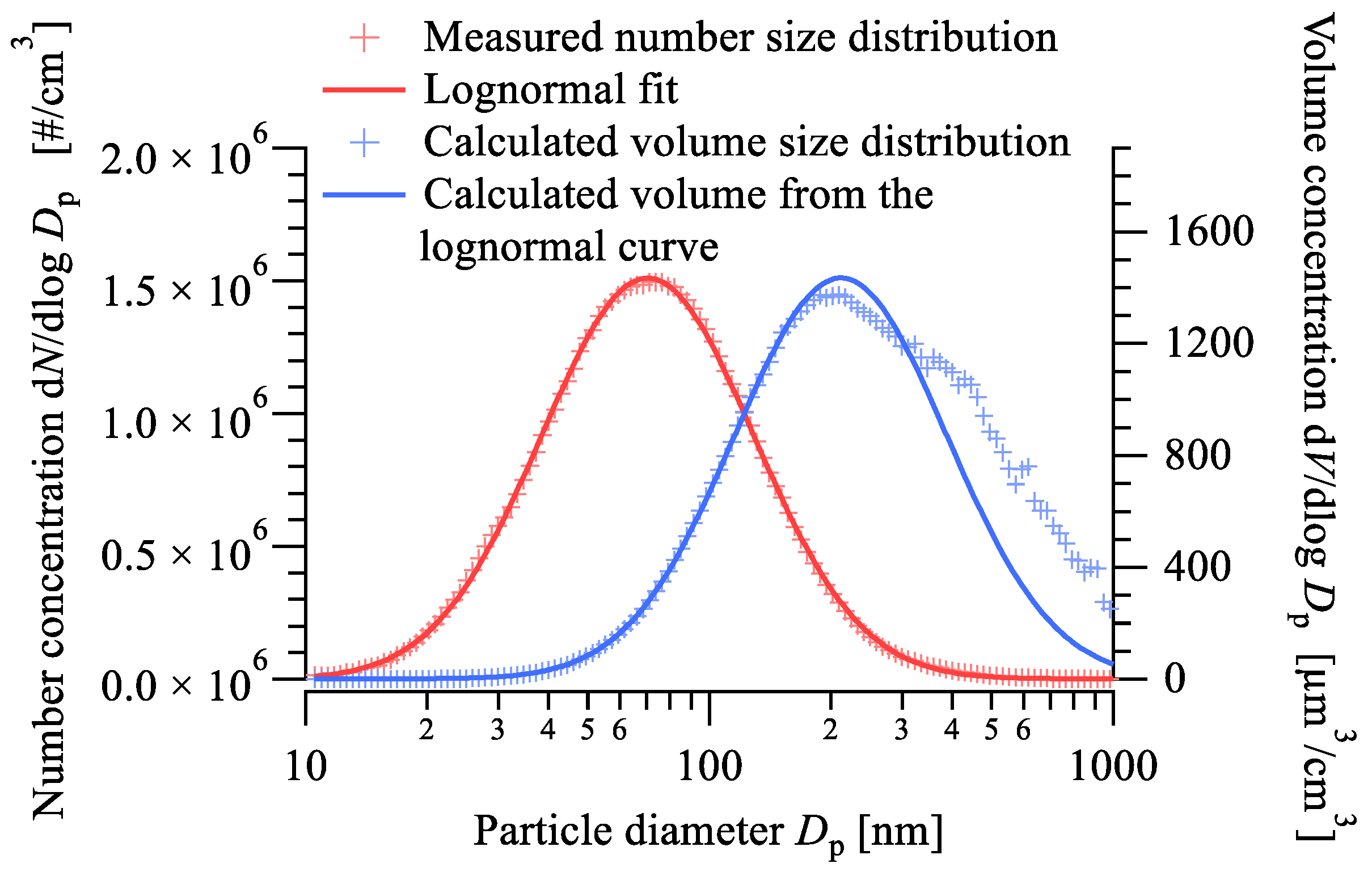
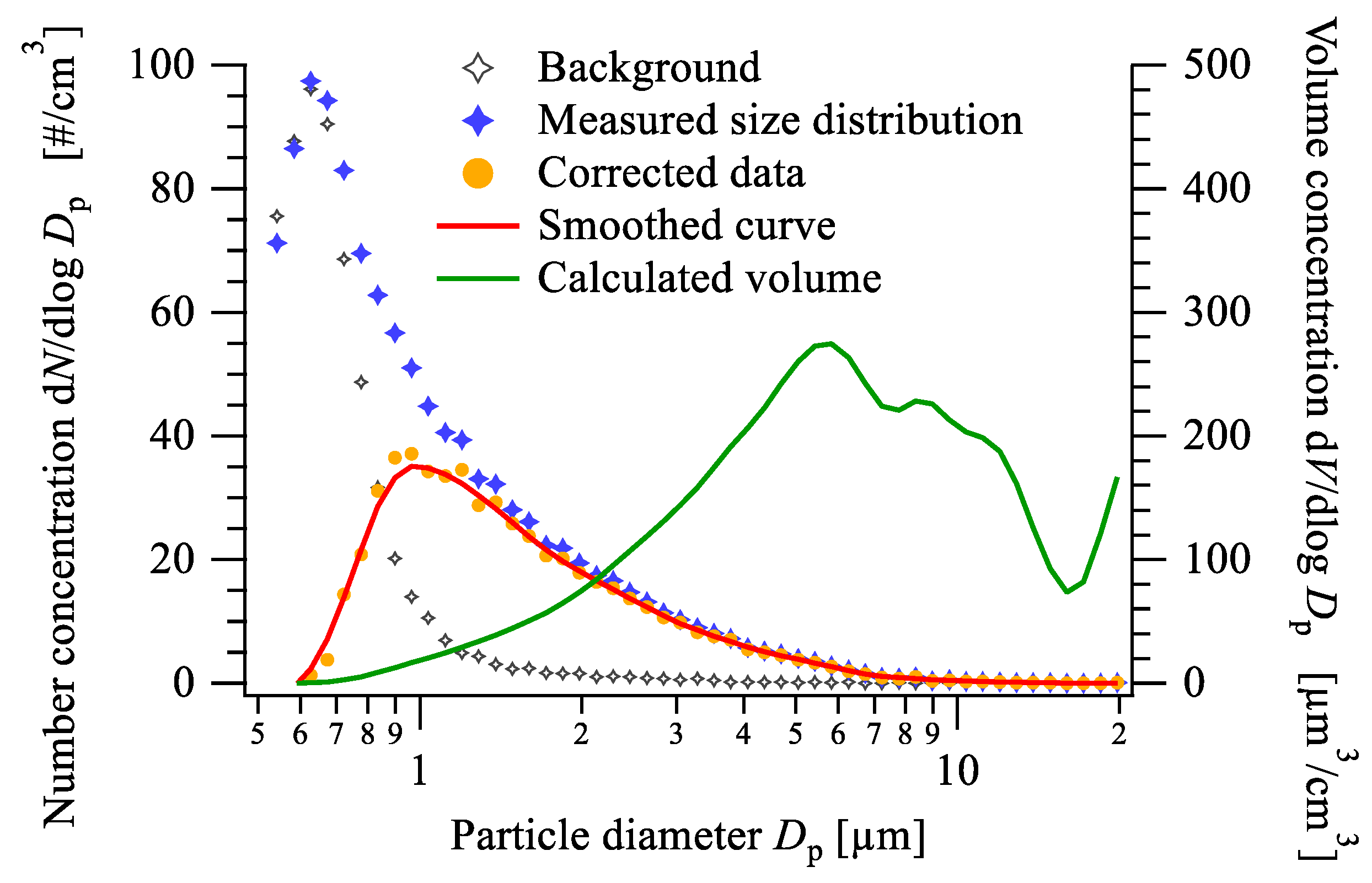
- The mass size distributions of the aerosol samples collected with the cascade impactor showed good agreement with the results of the electromobility and aerodynamic size measurements, which were conducted using a scanning mobility particle spectrometer and an aerodynamic particle sizer. Our results show that the majority of the newly formed aerosol particles fall into the ultrafine region. The number size distribution follows the lognormal distribution with a peak value at slightly below 70 nm, while the maximum of the volume size distribution is at 200 nm;
- The morphology and chemical composition of the collected size-fractionated aerosol samples were determined via scanning electron microscopy, energy-dispersive spectroscopy, and a total-reflection X-ray fluorescence analysis. The size distributions of the elemental concentrations could be calculated from air concentrations for each size fraction determined via TXRF. The calculated size distribution of the iron, which is the balance in the composition of the H13 types steels, showed significant conformity with the double peak volume size distributions calculated from the number size distributions measured by the SMPS and the APS;
- The bimodality of the mass size distribution was observed in the size distributions of the minor constituents, but to different extents, in line with the enrichment of the particular element. Strong evidence was found that the two modes consist of the newly formed aerosol particles at around 200 nm and the predominantly metallic satellite particles at around 5 µm, both in the respirable size range. Based on the literature data, it can also be assumed that both modes contribute to the adverse effects on the fabrication process, especially health-related concerns.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
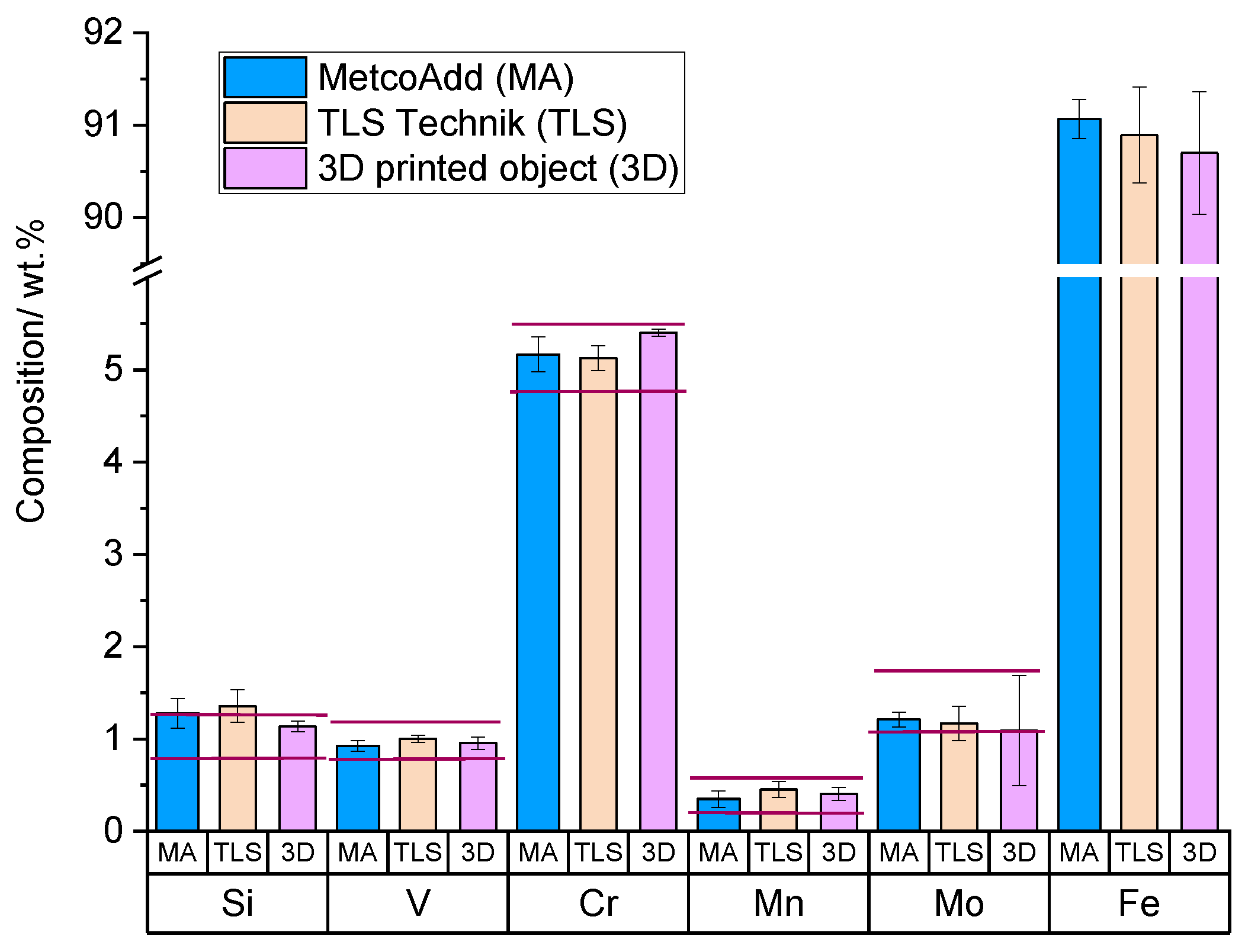
Appendix A. Compositions of the Raw Materials and the Welded Objects
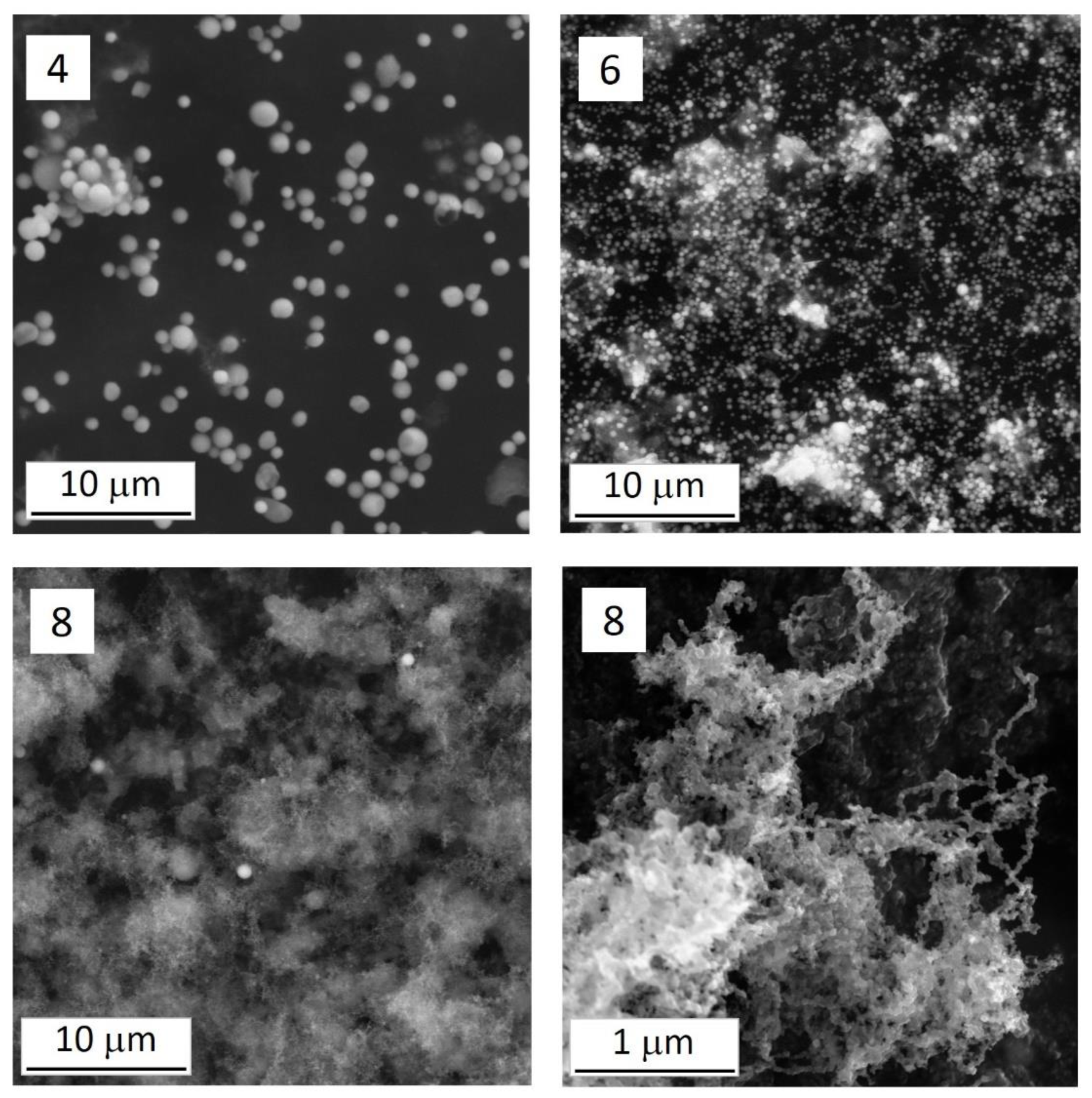
Appendix B. Morphological Description of the Raw Materials


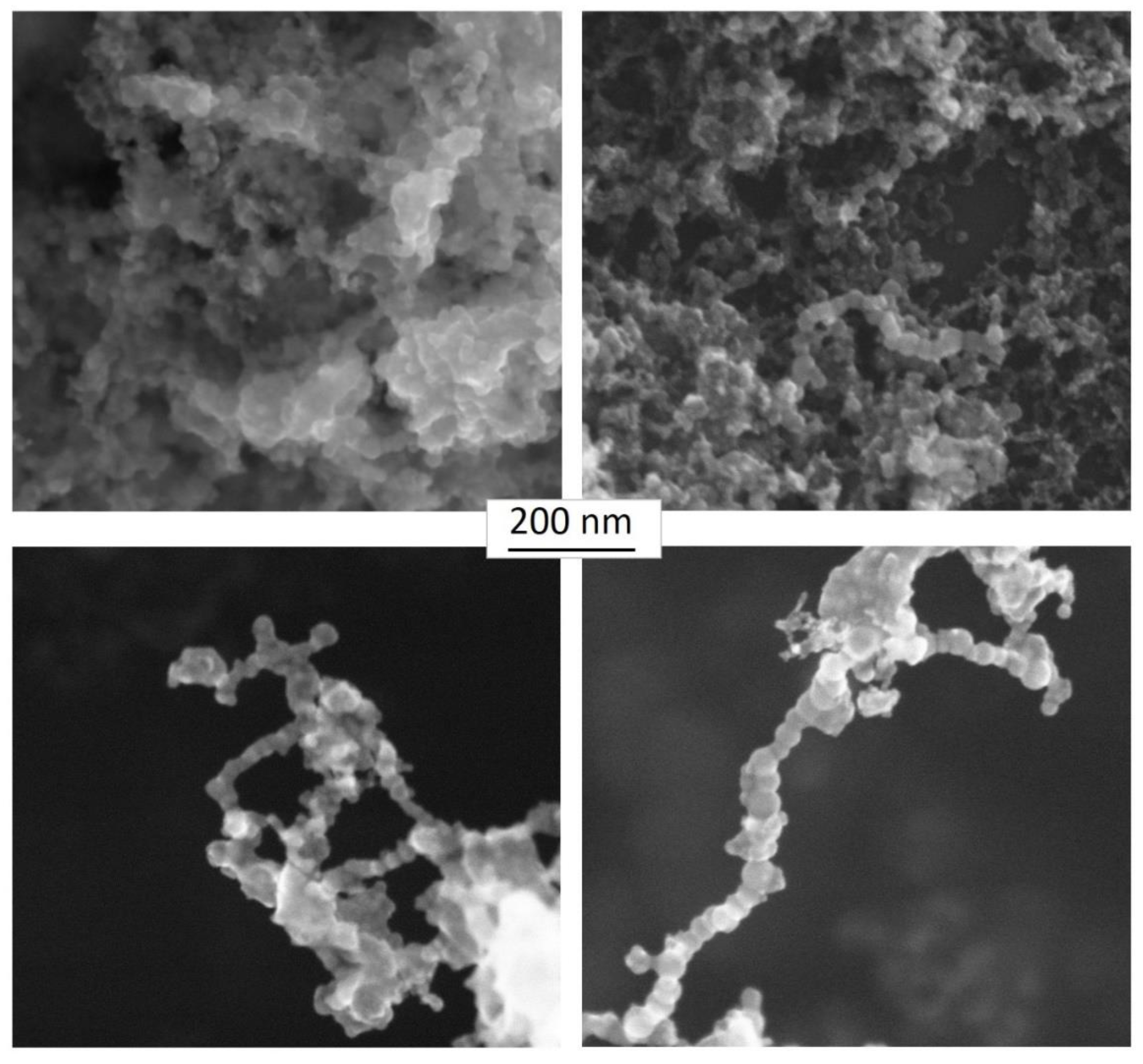
Appendix C. Morphology of the Fine-Grained Deposit on the Impactor Plates
Appendix D. Vapor Pressure and Oxidation Heat of the Elemental Components of the H13 Alloy
| Metal | Vapor Pressure /mbar | Oxidation State | Oxidation Heat /kJ moL−1 |
|---|---|---|---|
| V | 3.2 × 10−2 | +3 | −420 |
| Mn | 1.0 × 10–3 | +3 | −275 |
| Cr | 4.0 × 10–7 | +3 | −334 |
| Fe | 2.0 × 10–7 | +2 | −150 |
Appendix E. SEM-EDS Analysis of the Carbon Tape Used as the Impactor Plate

References
- Simonds, B.J.; Sowards, J.; Hadler, J.; Pfeif, E.; Wilthan, B.; Tanner, J.; Harris, C.; Williams, P.; Lehman, J. Time-Resolved Absorptance and Melt Pool Dynamics during Intense Laser Irradiation of a Metal. Phys. Rev. Appl. 2018, 10, 044061. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.A.; Calta, N.P.; Hammons, J.A.; Khairallah, S.A.; Nielsen, M.H.; Shuttlesworth, R.M.; Sinclair, N.; Matthews, M.J.; Jeffries, J.R.; Willey, T.M.; et al. Ultrafast dynamics of laser-metal interactions in additive manufacturing alloys captured by in situ X-ray imaging. Mater. Today Adv. 2019, 1, 100002. [Google Scholar] [CrossRef]
- Nozar, M.; Pokorna, V.; Zetkova, I. Potential health hazards of additive manufacturing. In Proceedings of the 30th International DAAAM Symposium “Intelligent Manufacturing & Automation”, Zadar, Croatia, 23–26 October 2019; pp. 654–662. [Google Scholar] [CrossRef]
- Chen, R.; Yin, H.; Cole, I.S.; Shen, S.; Zhou, X.; Wang, Y.; Tang, S. Exposure, assessment and health hazards of particulate matter in metal additive manufacturing: A review. Chemosphere 2020, 259, 127452. [Google Scholar] [CrossRef] [PubMed]
- Bau, S.; Rousset, D.; Payet, R.; Keller, F.X. Characterizing particle emissions from a direct energy deposition additive manufacturing process and associated occupational exposure to airborne particles. J. Occup. Environ. Hyg. 2020, 17, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Berlinger, B.; Náray, M.; Záray, G. Comparison of different sampling heads applied for investigation of welding fume. Microchem. J. 2007, 85, 25–30. [Google Scholar] [CrossRef]
- Stanislawska, M.; Halatek, T.; Cieslak, M.; Kaminska, I.; Kuras, R.; Janasik, B.; Wasowicz, W. Coarse, fine and ultrafine particles arising during welding—Analysis of occupational exposure. Microchem. J. 2017, 135, 1–9. [Google Scholar] [CrossRef]
- Thompson, S.M.; Bian, L.; Shamsaei, N.; Yadollahi, A. An overview of Direct Laser Deposition for additive manufacturing; Part I: Transport phenomena, modeling and diagnostics. Addit. Manuf. 2015, 8, 36–62. [Google Scholar] [CrossRef]
- Shamsaei, N.; Yadollahi, A.; Bian, L.; Thompson, S.M. An overview of Direct Laser Deposition for additive manufacturing; Part II: Mechanical behavior, process parameter optimization and control. Addit. Manuf. 2015, 8, 12–35. [Google Scholar] [CrossRef]
- Noskov, A.; Ervik, T.K.; Tsivilskiy, I.; Gilmutdinov, A.; Thomassen, Y. Characterization of ultrafine particles emitted during laser-based additive manufacturing of metal parts. Sci. Rep. 2020, 10, 20989. [Google Scholar] [CrossRef]
- Stefaniak, A.B.; Du Preez, S.; Du Plessis, J.L. Additive Manufacturing for Occupational Hygiene: A Comprehensive Review of Processes, Emissions, & Exposures. J. Toxicol. Environ. Health Part B Crit. Rev. 2021, 24, 173–222. [Google Scholar] [CrossRef]
- Andujar, P.; Simon-Deckers, A.; Galateau-Sallé, F.; Fayard, B.; Beaune, G.; Clin, B.; Billon-Galland, M.A.; Durupthy, O.; Pairon, J.C.; Doucet, J.; et al. Role of metal oxide nanoparticles in histopathological changes observed in the lung of welders. Part. Fibre Toxicol. 2014, 11, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ljunggren, S.A.; Karlsson, H.; Ståhlbom, B.; Krapi, B.; Fornander, L.; Karlsson, L.E.; Bergström, B.; Nordenberg, E.; Ervik, T.K.; Graff, P. Biomonitoring of Metal Exposure During Additive Manufacturing (3D Printing). Saf. Health Work. 2019, 10, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, N.; Eagar, T. Chemical Analysis of Welding Fume Particles. Airborne Particle Size is the Most Important Factor in Determining the Accuracy of a Method for Chemical Analysis. Weld. J. 2013, 84, 87s–93s. Available online: http://files.aws.org/wj/supplement/WJ_2005_06_s87.pdf (accessed on 24 August 2022).
- Sousa, M.; Arezes, P.; Silva, F. Nanomaterials exposure as an occupational risk in metal additive manufacturing. J. Phys. Conf. Ser. 2019, 1323, 012013. [Google Scholar] [CrossRef]
- McCarrick, S.; Wei, Z.; Moelijker, N.; Derr, R.; Persson, K.A.; Hendriks, G.; Odnevall Wallinder, I.; Hedberg, Y.; Karlsson, H.L. High variability in toxicity of welding fume nanoparticles from stainless steel in lung cells and reporter cell lines: The role of particle reactivity and solubility. Nanotoxicology 2019, 13, 1293–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa, M.; Arezes, P.; Silva, F. Occupational Exposure to Ultrafine Particles in Metal Additive Manufacturing: A Qualitative and Quantitative Risk Assessment. Int. J. Environ. Res. Public Health 2021, 18, 9788. [Google Scholar] [CrossRef]
- Alijagic, A.; Engwall, M.; Särndahl, E.; Karlsson, H.; Hedbrant, A.; Andersson, L.; Karlsson, P.; Dalemo, M.; Scherbak, N.; Färnlund, K.; et al. Particle Safety Assessment in Additive Manufacturing: From Exposure Risks to Advanced Toxicology Testing. Front. Toxicol. 2022, 4, 50. [Google Scholar] [CrossRef]
- Kugler, S.; Nagy, A.; Osán, J.; Péter, L.; Groma, V.; Pollastri, S.; Czitrovszky, A. Characterization of the ultrafine and fine particles formed during laser cladding with the Inconel 718 metal powder by means of X-ray spectroscopic techniques. Spectrochim. Acta Part B At. Spectrosc. 2021, 177, 106110. [Google Scholar] [CrossRef]
- Russo, R.E.; Mao, X.; Gonzalez, J.J.; Zorba, V.; Yoo, J. Laser ablation in analytical chemistry. Talanta 2002, 57, 425–451. [Google Scholar] [CrossRef]
- Koch, J.; Von Bohlen, A.; Hergenröder, R.; Niemax, K. Particle size distributions and compositions of aerosols produced by near-IR femto- and nanosecond laser ablation of brass. J. Anal. At. Spectrom. 2004, 19, 267–272. [Google Scholar] [CrossRef]
- Košler, J.; Wiedenbeck, M.; Wirth, R.; Hovorka, J.; Sylvester, P.; Míková, J. Chemical and phase composition of particles produced by laser ablation of silicate glass and zircon—Implications for elemental fractionation during ICP-MS analysis. J. Anal. At. Spectrom. 2005, 20, 402–409. [Google Scholar] [CrossRef]
- Liu, C. A Study of Particle Generation During Laser Ablation with Applications, Lawrence Berkeley National Laboratory. 2005. Available online: https://escholarship.org/uc/item/5rk6q24x (accessed on 12 July 2022).
- Yan, J.J.; Zheng, D.L.; Li, H.X.; Jia, X.; Sun, J.F.; Li, Y.L.; Qian, M.; Yan, M. Selective laser melting of H13: Microstructure and residual stress. J. Mater. Sci. 2017, 52, 12476–12485. [Google Scholar] [CrossRef]
- Yan, J.; Zhou, Y.; Gu, R.; Zhang, X.; Quach, W.M.; Yan, M. A comprehensive study of steel powders (316L, H13, P20 and 18Ni300) for their selective laser melting additive manufacturing. Metals 2019, 9, 86. [Google Scholar] [CrossRef] [Green Version]
- Anderson, I.E.; White, E.M.H.; Dehoff, R. Feedstock powder processing research needs for additive manufacturing development. Curr. Opin. Solid State Mater. Sci. 2018, 22, 8–15. [Google Scholar] [CrossRef]
- Whiting, J.; Fox, J. Characterization of Feedstock in the Powder Bed Fusion Process: Sources of Variation in Particle Size Distribution and the Factors that Influence them. In Proceedings of the Solid Freeform Fabrication Symposium, Austin, TX, USA, 8–11 August 2016; p. 12. [Google Scholar]
- Clayton, J.; Deffley, R. Optimising metal powders for additive manufacturing. Met. Powder Rep. 2014, 69, 14–17. [Google Scholar] [CrossRef]
- Ma, Y.; Evans, T.M.; Philips, N.; Cunningham, N. Numerical simulation of the effect of fine fraction on the flowability of powders in additive manufacturing. Powder Technol. 2020, 360, 608–621. [Google Scholar] [CrossRef]
- ASTM A681-08; Standard Specification for Tool Steels Alloy. ASTM: West Conshohocken, PA, USA, 2015.
- BS EN ISO 4957:2000; Tool Steels. British Standards Institution: London, UK, 2000.
- JIS-G4404; Alloy Tool Steel. JISC: Tokyo, Japan, 2000.
- May, K.R. An “ultimate” cascade impactor for aerosol assessment. J. Aerosol Sci. 1975, 6, 413–419. [Google Scholar] [CrossRef]
- DeCarlo, P.F.; Slowik, J.G.; Worsnop, D.R.; Davidovits, P.; Jimenez, J.L. Particle morphology and density characterization by combined mobility and aerodynamic diameter measurements. Part 1: Theory. Aerosol Sci. Technol. 2004, 38, 1185–1205. [Google Scholar] [CrossRef] [Green Version]
- Wobrauschek, P.; Prost, J.; Ingerle, D.; Osán, J.; Török, S.; Streli, C. Adaption of a TXRF WOBI-module with a low power X-ray tube. In Proceedings of the TXRF 2019—International Conference on Total Reflection X-Ray Fluorescence Analysis and Related Methods, Girona, Spain, 25–28 June 2019. [Google Scholar]
- Prost, J.; Zinkl, A.; Ingerle, D.; Wobrauschek, P.; Streli, C. Evaluation of a sample preparation procedure for total-reflection X-ray fluorescence analysis of directly collected airborne particulate matter samples. Spectrochim. Acta Part B At. Spectrosc. 2018, 147, 13–20. [Google Scholar] [CrossRef]
- Vekemans, B.; Janssens, K.; Vincze, L.; Adams, F.; van Espen, P. Analysis of X-ray spectra by iterative least squares (AXIL): New developments. X-Ray Spectrom. 1994, 23, 278–285. [Google Scholar] [CrossRef]
- Reinhardt, F.; Osán, J.; Török, S.; Pap, A.E.; Kolbe, M.; Beckhoff, B. Reference-free quantification of particle-like surface contaminations by grazing incidence X-ray fluorescence analysis. J. Anal. At. Spectrom. 2012, 27, 248–255. [Google Scholar] [CrossRef]
- Osán, J.; Börcsök, E.; Czömpöly, O.; Dian, C.; Groma, V.; Stabile, L.; Török, S. Experimental evaluation of the in-the-field capabilities of total-reflection X-ray fluorescence analysis to trace fine and ultrafine aerosol particles in populated areas. Spectrochim. Acta Part B At. Spectrosc. 2020, 167, 105852. [Google Scholar] [CrossRef]
- Di Cicco, A.; Aquilanti, G.; Minicucci, M.; Principi, E.; Novello, N.; Cognigni, A.; Olivi, L. Novel XAFS capabilities at ELETTRA synchrotron light source. J. Phys. Conf. Ser. 2009, 190, 012043. [Google Scholar] [CrossRef]
- Wrobel, P.M.; Bogovac, M.; Sghaier, H.; Leani, J.J.; Migliori, A.; Padilla-Alvarez, R.; Czyzycki, M.; Osan, J.; Kaiser, R.B.; Karydas, A.G. LabVIEW interface with Tango control system for a multi-technique X-ray spectrometry IAEA beamline end-station at Elettra Sincrotrone Trieste. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2016, 833, 105–109. [Google Scholar] [CrossRef] [Green Version]
- Karydas, A.G.; Czyzycki, M.; Leani, J.J.; Migliori, A.; Osan, J.; Bogovac, M.; Wrobel, P.; Vakula, N.; Padilla-Alvarez, R.; Menk, R.H.; et al. An IAEA multi-technique X-ray spectrometry endstation at Elettra Sincrotrone Trieste: Benchmarking results and interdisciplinary applications. J. Synchrotron Radiat. 2018, 28, 189–203. [Google Scholar] [CrossRef] [Green Version]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [Green Version]
- Huggins, F.E.; Shah, N.; Huffman, G.P.; Robertson, J.D. XAFS spectroscopic characterization of elements in combustion ash and fine particulate matter. Fuel Process. Technol. 2000, 65, 203–218. [Google Scholar] [CrossRef]
- Smoluchowski, M. Mathematical Theory of the Kinetics of the Coagulation of Colloidal Solutions. Z. Phys. Chem. 1917, 19, 129–135. [Google Scholar]
- Reist, P.C. Introduction to Aerosol Science; Macmillan Pub., Co.: New York, NY, USA, 1984. [Google Scholar]
- Hinds, W.C.; Zhu, Y. Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles, 3rd ed.; Wiley: Hoboken, NJ, USA, 2022. [Google Scholar]
- Wang, H.C. Particle density correction for the aerodynamic particle sizer. Aerosol Sci. Technol. 1987, 6, 191–198. [Google Scholar] [CrossRef]
- Mellin, P.; Shvab, R.; Strondl, A.; Randelius, M.; Brodin, H.; Hryha, E.; Nyborg, L. COPGLOW and XPS investigation of recycled metal powder for selective laser melting. Powder Metall. 2017, 60, 223–231. [Google Scholar] [CrossRef]

| Grade: Standard: | H13 ASTM A681 [30] | 1.2344/X40CrMoV5-1 EN ISO 4957: 2000 [31] | SKD61 JIS G4404 [32] |
|---|---|---|---|
| C | 0.32–0.45 | 0.35–0.42 | 0.35–0.42 |
| Si | 0.8–1.25 | 0.8–1.2 | 0.8–1.2 |
| P | <0.03 | <0.03 | <0.03 |
| S | <0.03 | <0.02 | <0.02 |
| V | 0.8–1.2 | 0.85–1.15 | 0.8–1.15 |
| Cr | 4.75–5.5 | 4.8–5.5 | 4.8–5.5 |
| Mn | 0.2–0.6 | 0.25–0.5 | 0.25–0.5 |
| Mo | 1.1–1.75 | 1.0–1.5 | 1.1–1.5 |
| Fe | Balance | Balance | Balance |
| Impactor Plate Number | Cut-Off Size of the Particles (µm) |
|---|---|
| 1 | 17.9 |
| 2 | 8.9 |
| 3 | 4.5 |
| 4 | 2.25 |
| 5 | 1.13 |
| 6 | 0.57 |
| 7 | 0.29 |
| 8 | 0.18 |
| 9 | 0.07 |
| Sample → ↓ Element | MetcoAdd, Total Aerosol | TLS Technik, Total Aerosol | TLS Technik, Stage 8 | TLS Technik, Stage 3 |
|---|---|---|---|---|
| Cr0 fraction | 0.29 | 0.36 | 0.40 | 0.82 |
| Cr3+ fraction | 0.71 | 0.64 | 0.60 | 0.18 |
| Cr6+ fraction | – | – | – | – |
| Cr mean oxidation number | +2.1 | +1.9 | +1.8 | +0.5 |
| Mn0 fraction | – | – | 0.01 | – |
| Mn2+ fraction | 0.57 | 0.63 | 0.85 | – |
| Mn3+ fraction | 0.43 | 0.37 | 0.14 | – |
| Mn mean oxidation number | +2.4 | +2.4 | +2.1 | – |
| Fe0 fraction | 0.56 | 0.77 | 0.74 | 0.92 |
| Fe2+ fraction | – | 0.10 | 0.10 | 0.07 |
| Fe3+ fraction | 0.44 | 0.13 | 0.16 | 0.01 |
| Fe mean oxidation number | +1.3 | +1.1 | +0.7 | +0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Péter, L.; Osán, J.; Kugler, S.; Groma, V.; Pollastri, S.; Nagy, A. Comprehensive Analysis of Two H13-Type Starting Materials Used for Laser Cladding and Aerosol Particles Formed in This Process. Materials 2022, 15, 7367. https://doi.org/10.3390/ma15207367
Péter L, Osán J, Kugler S, Groma V, Pollastri S, Nagy A. Comprehensive Analysis of Two H13-Type Starting Materials Used for Laser Cladding and Aerosol Particles Formed in This Process. Materials. 2022; 15(20):7367. https://doi.org/10.3390/ma15207367
Chicago/Turabian StylePéter, László, János Osán, Szilvia Kugler, Veronika Groma, Simone Pollastri, and Attila Nagy. 2022. "Comprehensive Analysis of Two H13-Type Starting Materials Used for Laser Cladding and Aerosol Particles Formed in This Process" Materials 15, no. 20: 7367. https://doi.org/10.3390/ma15207367







