Chemical State of Potassium on the Surface of Iron Oxides: Effects of Potassium Precursor Concentration and Calcination Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Potassium-Doped Iron Oxides
2.2. Characterization of Materials
3. Results and Discussion
3.1. Powder X-ray Diffraction Analysis
3.2. Thermal Analysis
3.3. Elemental Composition
3.4. Raman Spectroscopy Analysis
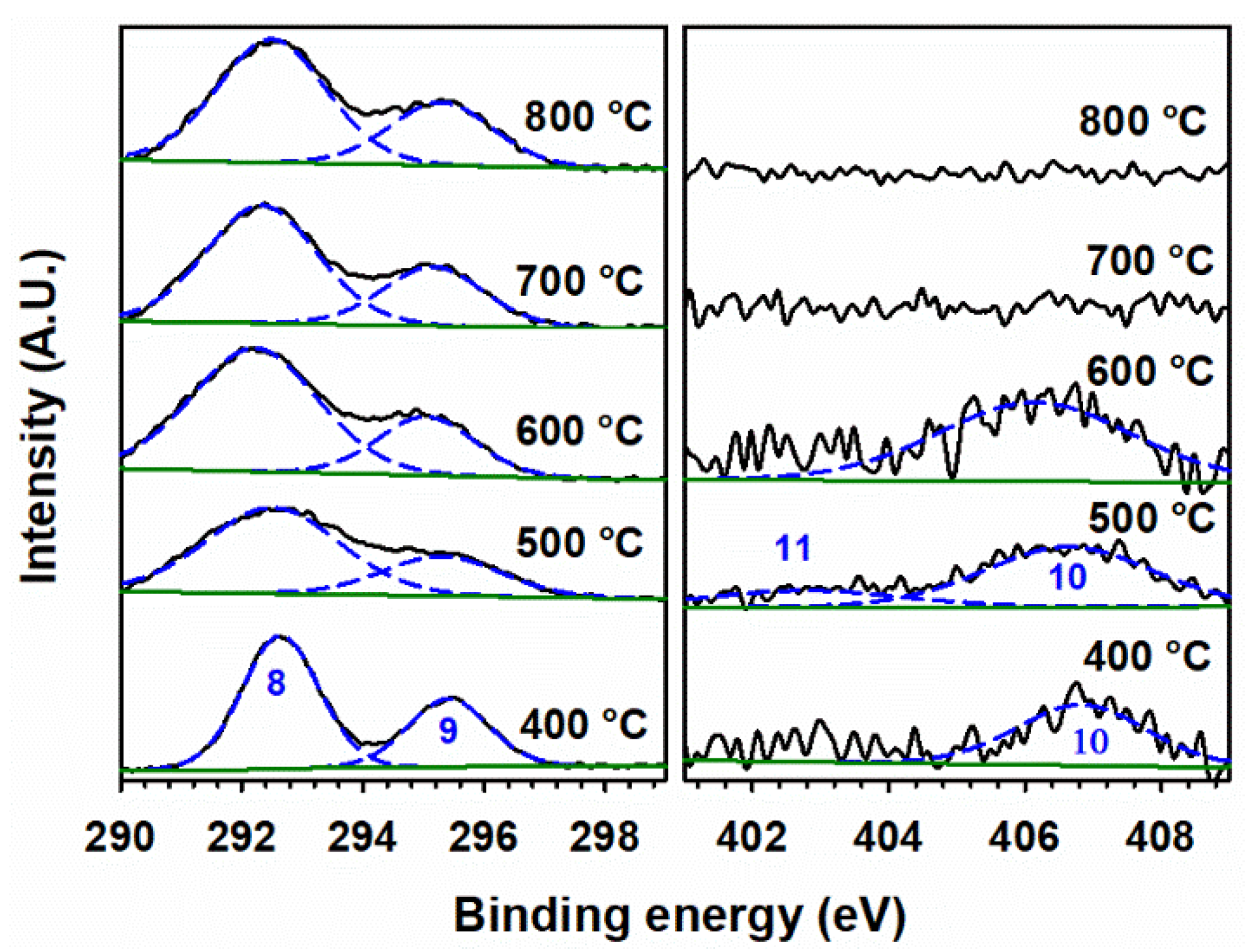
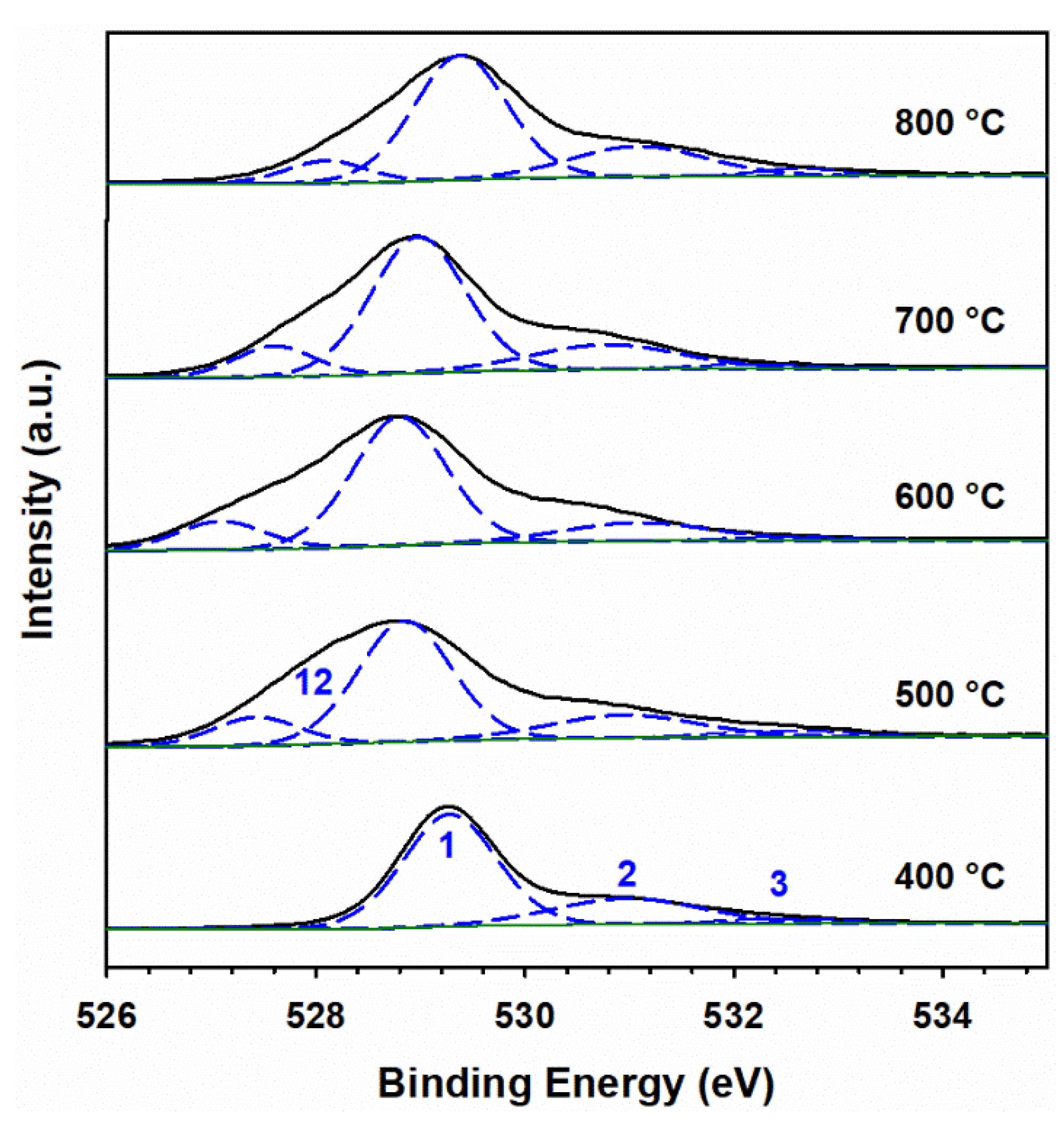
3.5. XPS Analysis
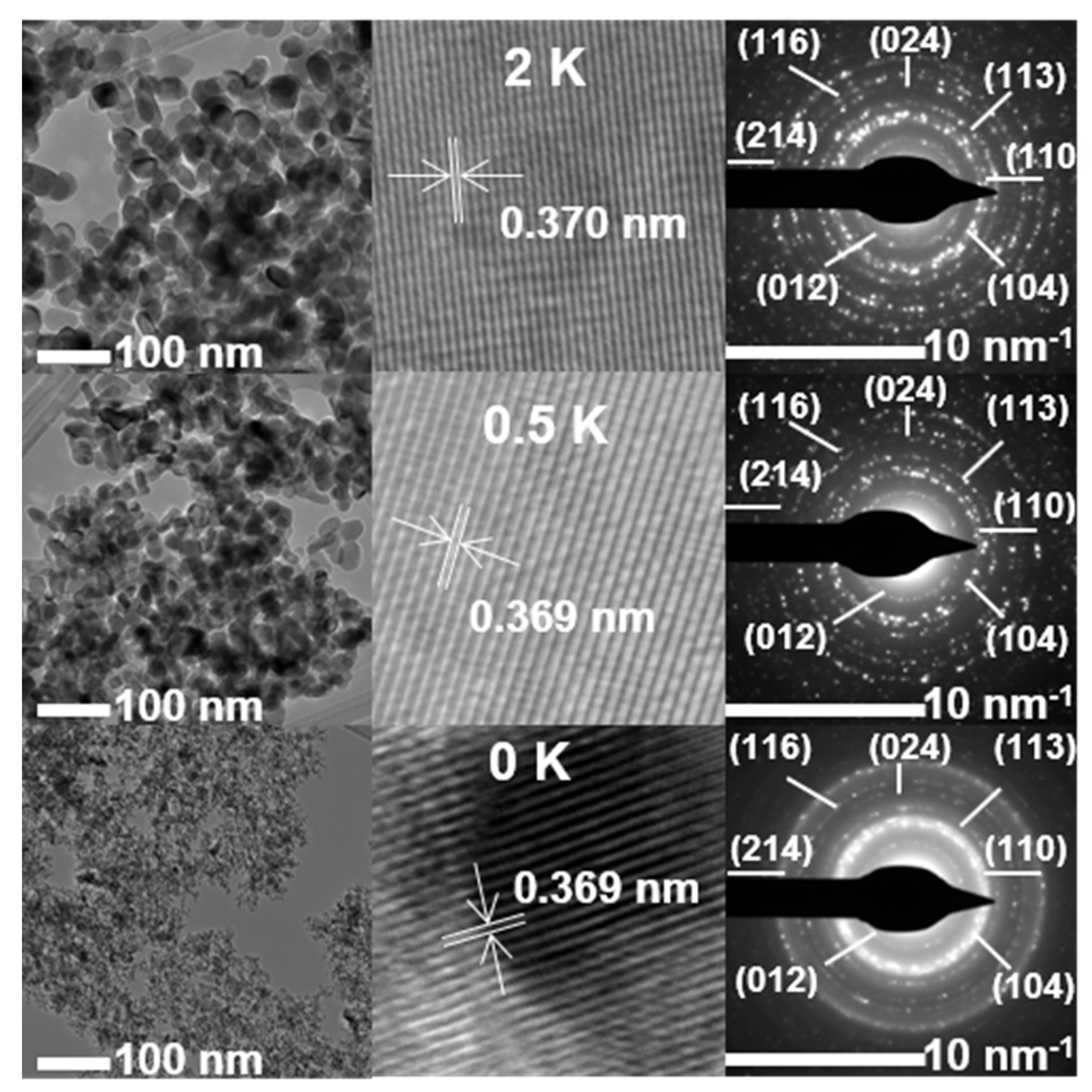
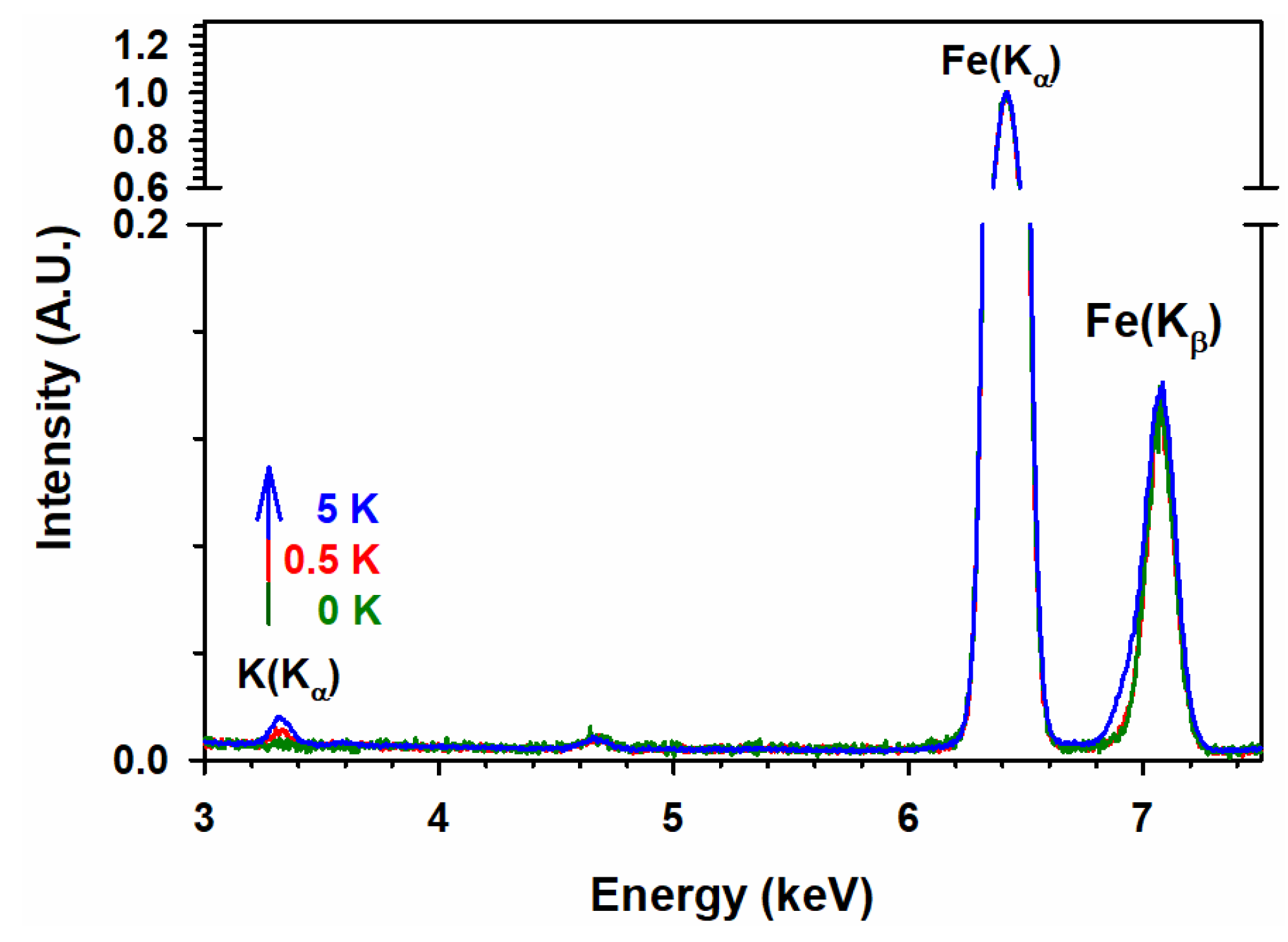
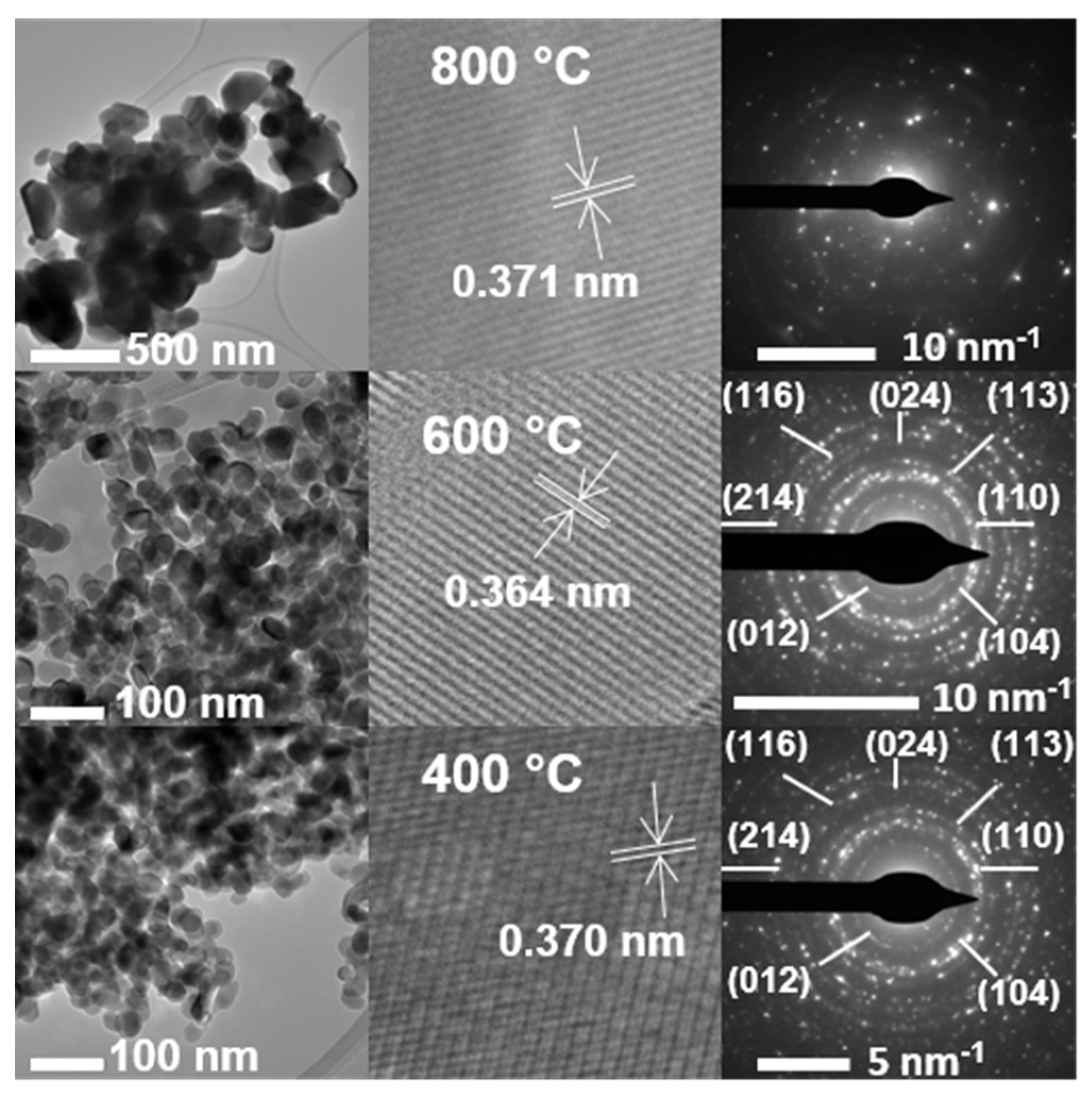
3.6. TEM and EDS Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Fischer, F.; Tropsch, H. The Synthesis of Petroleum at Atmospheric Pressures from Gasification Products of Coal. Brennst. Chem. 1926, 7, 97–104. [Google Scholar]
- Fischer, F.; Tropsch, H. The Preparation of Synthetic oil Mixtures (Synthol) from Carbon Monoxide and Hydrogen. Brennst. Chem. 1923, 4, 276–285. [Google Scholar]
- Govender, N.; de Croon, M.; Schouten, J.C. Reactivity of Surface Carbonaceous Intermediates on an Iron-based Fischer–Tropsch Catalyst. Appl. Catal. A Gen. 2010, 373, 81–89. [Google Scholar] [CrossRef]
- Benziger, J.; Madix, R. The effects of Carbon, Oxygen, Sulfur and Potassium Adlayers on CO and H2 Adsorption on Fe(100). Surf. Sci. 1980, 94, 119–153. [Google Scholar] [CrossRef]
- Dry, M.; Shingles, T.; Boshoff, L.; Oosthuizen, G.J. Heats of Chemisorption on Promoted Iron Surfaces and the Role of Alkali in Fischer-Tropsch Synthesis. J. Catal. 1969, 15, 190–199. [Google Scholar] [CrossRef]
- Amelse, J.; Butt, J.; Schwartz, L. Carburization of Supported Iron Synthesis Catalysts. J. Phys. Chem. 1978, 82, 558–563. [Google Scholar] [CrossRef]
- Bukur, D.B.; Mukesh, D.; Patel, S.A. Promoter Effects on Precipitated Iron Catalysts for Fischer-Tropsch Synthesis. Ind. Eng. Chem. Res. 1990, 29, 194–204. [Google Scholar] [CrossRef]
- Arakawa, H.; Bell, A.T. Effects of Potassium Promotion on the Activity and Selectivity of Iron Fischer-Tropsch Catalysts. Ind. Eng. Chem. Process Des. Dev. 1983, 22, 97–103. [Google Scholar] [CrossRef]
- Yang, Y.; Xiang, H.; Xu, Y.; Bai, L.; Li, Y. Effect of Potassium Promoter on Precipitated Iron-Manganese Catalyst for Fischer–Tropsch Synthesis. Appl. Catal. A Gen. 2004, 266, 181–194. [Google Scholar] [CrossRef]
- Storch, H.H. The Fischer-Tropsch and Related Processes for Synthesis of Hydrocarbons by Hydrogenation of Carbon Monoxide. In Advances in Catalysis; Frankenburg, W.G., Komarewsky, V.I., Rideal, E.K., Eds.; Academic Press: New York, NY, USA, 1948; Volume 1, pp. 115–156. [Google Scholar]
- Cho, J.; Jeong, M.; Bae, J. Fischer-Tropsch Synthesis on Potassium-Modified FeO Nanoparticles. Res. Chem. Intermed. 2016, 42, 335–350. [Google Scholar] [CrossRef]
- Liu, B.; Li, W.; Zheng, J.; Lin, Q.; Zhang, X.; Zhang, J.; Jiang, F.; Xu, Y.; Liu, X. CO2 Formation Mechanism in Fischer–Tropsch Synthesis Over Iron-based Catalysts: A Combined Experimental and Theoretical Study. Catal. Sci. Technol. 2018, 8, 5288–5301. [Google Scholar] [CrossRef]
- Pendyala, V.R.R.; Graham, U.M.; Jacobs, G.; Hamdeh, H.H.; Davis, B.H. Fischer–Tropsch Synthesis: Deactivation as a Function of Potassium Promoter Loading for Precipitated Iron Catalyst. Catal. Lett. 2014, 144, 1704–1716. [Google Scholar] [CrossRef]
- Asano, K.; Ohnishi, C.; Iwamoto, S.; Shioya, Y.; Inoue, M. Potassium-doped Co3O4 Catalyst for Direct Decomposition of N2O. Appl. Catal. B Environ. 2008, 78, 242–249. [Google Scholar] [CrossRef]
- Tang, X.; Gao, F.; Xiang, Y.; Yi, H.; Zhao, S.; Liu, X.; Li, Y. Effect of Potassium-Precursor Promoters on Catalytic Oxidation Activity of Mn-CoOx Catalysts for NO Removal. Ind. Eng. Chem. Res. 2015, 54, 9116–9123. [Google Scholar] [CrossRef]
- Solís-Casados, D.; Escobar, J.; Orozco, I.G.; Klimova, T. Effect of Potassium Content on the Performance of CoMo/Al2O3-MgO-K2O(x) Catalysts in Hydrodesulfurization of Dibenzothiophene. Ind. Eng. Chem. Res. 2011, 50, 2755–2761. [Google Scholar] [CrossRef]
- Tian, S.; Wang, S.; Wu, Y.; Gao, J.; Wang, P.; Xie, H.; Yang, G.; Han, Y.; Tan, Y. The Role of Potassium Promoter in Isobutanol Synthesis over Zn–Cr Based Catalysts. Catal. Sci. Technol. 2016, 6, 4105–4115. [Google Scholar] [CrossRef]
- Cheng, Y.; Lin, J.; Wu, T.; Xie, S.; Pei, Y.; Yan, S.; Qiao, M.; Zong, B. Mg and K Dual-Decorated Fe-on-Reduced Graphene Oxide for Selective Catalyzing CO Hydrogenation to Light Olefins with Mitigated CO2 Emission and Enhanced Activity. Appl. Catal. B Environ. 2017, 204, 475–485. [Google Scholar] [CrossRef]
- Satthawong, R.; Koizumi, N.; Song, C.; Prasassarakich, P. Light Olefin Synthesis from CO2 Hydrogenation Over K-Promoted Fe–Co Bimetallic Catalysts. Catal. Today 2015, 251, 34–40. [Google Scholar] [CrossRef]
- Lohitharn, N.; Goodwin, J.G. Effect of K Promotion of Fe and FeMn Fischer–Tropsch Synthesis Catalysts: Analysis at the Site Level Using SSITKA. J. Catal. 2008, 260, 7–16. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, M.; Liu, B.; Xu, Y.; Liu, X. Insights Into the Influence of Support and Potassium or Sulfur Promoter on Iron-Based Fischer–Tropsch Synthesis: Understanding the Control of Catalytic Activity, Selectivity to Lower Olefins, and Catalyst Deactivation. Catal. Sci. Technol. 2017, 7, 1245–1265. [Google Scholar] [CrossRef]
- Maniak, G.; Stelmachowski, P.; Kotarba, A.; Sojka, Z.; Rico-Pérez, V.; Bueno-López, A. Rationales for the Selection of the Best Precursor for Potassium Doping of Cobalt Spinel Based de N2O Catalyst. Appl. Catal. B Environ. 2013, 136-137, 302–307. [Google Scholar] [CrossRef]
- Mavlyankariev, S.; Ahlers, S.; Kondratenko, V.; Linke, D.; Kondratenko, E. Effect of Support and Promoter on Activity and Selectivity of Gold Nanoparticles in Propanol Synthesis from CO2, C2H4 and H2. ACS Catal. 2016, 6, 3317–3325. [Google Scholar] [CrossRef]
- Shi, Z.; Yang, H.; Gao, P.; Li, X.; Zhong, L.; Wang, H.; Liu, H.; Wei, W.; Sun, Y. Direct Conversion of CO2 to Long-Chain Hydrocarbon Fuels over K–Promoted CoCu/TiO2 Catalysts. Catal. Today 2018, 311, 65–73. [Google Scholar] [CrossRef]
- Li, J.; Cheng, X.; Zhang, C.; Dong, W.; Yang, Y.; Li, Y. Comparative Study of Iron-Based Fischer–Tropsch Synthesis Catalysts Promoted with Strontium or Potassium. Catal. Lett. 2016, 146, 2574–2584. [Google Scholar] [CrossRef]
- Priyanka; Srivastava, V.C. Photocatalytic Oxidation of Dye Bearing Wastewater by Iron Doped Zinc Oxide. Ind. Eng. Chem. Res. 2013, 52, 17790–17799. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, G.; Lu, Y.; Zhang, H. Redox performance of Fe2O3/Al2O3 oxygen carrier calcined at different temperature in chemical looping process. Fuel 2022, 310, 122381. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Q.; Wu, J.; Zhang, Z.; Wei, P.; Liu, Y. Effect of Fe2O3–TiO2-Sm2O3 composite additive on sintering behavior and thermal properties of Al2O3 ceramics for thermal storage. Ceram. Int. 2022, 48, 11356–11364. [Google Scholar] [CrossRef]
- Niu, M.-S.; Yang, H.-H.; Zhou, H.; Yi, X.; Zhou, X.; Zhan, J.; Liu, Y. Synergy of the successive modification of cryptomelane MnO2 by potassium insertion and nitrogen doping for catalytic formaldehyde oxidation. Chem. Eng. J. 2022, 431, 133928. [Google Scholar] [CrossRef]
- Kharlamova, T.S.; Verkhov, V.A.; Kulchakovskaya, E.V.; Svetlichnyi, V.A.; Cadete Santos Aires, F.J.; Bargiela, P.; Vodyankina, O.V. Effect of metal-doping (Me = Fe, Ce, Sn) on phase composition, structural peculiarities, and CO oxidation catalytic activity of cryptomelane-type MnO2. J. Alloys Compd. 2022, 917, 165504. [Google Scholar] [CrossRef]
- Lyu, Z.; Niu, S.; Han, K.; Lu, C.; Li, Y. Theoretical insights into the poisoning effect of Na and K on α-Fe2O3 catalyst for selective catalytic reduction of NO with NH3. App. Catal. A Gen. 2021, 610, 117968. [Google Scholar] [CrossRef]
- Gu, M.; Dai, S.; Qiu, R.; Ford, M.E.; Cao, C.; Wachs, I.E.; Zhu, M. Structure–Activity Relationships of Copper- and Potassium-Modified Iron Oxide Catalysts during Reverse Water–Gas Shift Reaction. ACS Catal. 2021, 11, 12609–12619. [Google Scholar] [CrossRef]
- Tian, P.; Gu, M.; Qiu, R.; Yang, Z.; Xuan, F.; Zhu, M. Tunable Carbon Dioxide Activation Pathway over Iron Oxide Catalysts: Effects of Potassium. Ind. Eng. Chem. Res. 2021, 60, 8705–8713. [Google Scholar] [CrossRef]
- Barrios, A.J.; Peron, D.V.; Chakkingal, A.; Dugulan, A.I.; Moldovan, S.; Nakouri, K.; Thuriot-Roukos, J.; Wojcieszak, R.; Thybaut, J.W.; Virginie, M.; et al. Efficient Promoters and Reaction Paths in the CO2 Hydrogenation to Light Olefins over Zirconia-Supported Iron Catalysts. ACS Catal. 2022, 12, 3211–3225. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, H.; Zheng, H.; Zhang, L.; Liu, B.; Song, W.; Liu, J.; Zhu, W.; Li, H.; Zhao, Z. Insight into the Potassium Poisoning Effect for Selective Catalytic Reduction of NOx with NH3 over Fe/Beta. ACS Catal. 2021, 11, 14727–14739. [Google Scholar] [CrossRef]
- Al Maksoud, W.; Rai, R.K.; Morlanés, N.; Harb, M.; Ahmad, R.; Ould-Chikh, S.; Anjum, D.; Hedhili, M.N.; Al-Sabban, B.E.; Albahily, K.; et al. Active and stable Fe-based catalyst, mechanism, and key role of alkali promoters in ammonia synthesis. J. Catal. 2021, 394, 353–365. [Google Scholar] [CrossRef]
- Chang, H.; Lin, Q.; Cheng, M.; Zhang, K.; Feng, B.; Chai, J.; Lv, Y.; Men, Z. Effects of Potassium Loading over Iron–Silica Interaction, Phase Evolution and Catalytic Behavior of Precipitated Iron-Based Catalysts for Fischer-Tropsch Synthesis. Catalysts 2022, 12, 916. [Google Scholar] [CrossRef]
- Folke, J.; Dembélé, K.; Girgsdies, F.; Song, H.; Eckert, R.; Reitmeier, S.; Reitzmann, A.; Schlögl, R.; Lunkenbein, T.; Ruland, H. Promoter effect on the reduction behavior of wuestite-based catalysts for ammonia synthesis. Catal. Today 2022, 387, 12–22. [Google Scholar] [CrossRef]
- Xu, M.; Cao, C.; Xu, J. Understanding kinetically interplaying reverse water-gas shift and Fischer-Tropsch synthesis during CO2 hydrogenation over Fe-based catalysts. Appl. Catal. A Gen. 2022, 641, 118682. [Google Scholar] [CrossRef]
- DeLaHunt, J.S.; Lindeman, T.G. Review of the safety of potassium and potassium oxides, including deactivation by introduction into water. J. Chem. Health Saf. 2007, 14, 21–32. [Google Scholar] [CrossRef]
- Wang, R.; Xu, C.; Sun, J.; Gao, L. Three-Dimensional Fe2O3 Nanocubes/Nitrogen-doped Graphene Aerogels: Nucleation Mechanism and Lithium Storage Properties. Sci. Rep. 2014, 4, 7171. [Google Scholar] [CrossRef] [Green Version]
- Markus, Y. (Ed.) Ion Properties; Marcel Dekker: New York, NY, USA, 1997. [Google Scholar]
- Kramer, C.M.; Munir, Z.A.; Volponi, J.V. Differential Scanning Calorimetry of Sodium and Potassium Nitrates and Nitrites. Thermochim. Acta 1982, 55, 11–17. [Google Scholar] [CrossRef]
- Freeman, E.S. The Kinetics of the Thermal Decomposition of Potassium Nitrate and of the Reaction between Potassium Nitrite and Oxygen. J. Am. Chem. Soc. 1957, 79, 838–842. [Google Scholar] [CrossRef]
- Stern, K.H. High Temperature Properties and Decomposition of Inorganic Salts part 3, Nitrates and Nitrites. J. Phys. Chem. Ref. Data 1972, 1, 747–772. [Google Scholar] [CrossRef] [Green Version]
- Oza, T.M. Thermal Decomposition of Sodium and Potassium Nitrites. J. Indian Chem. Soc. 1945, 22, 173–180. [Google Scholar]
- Wang, Y.; Huang, W.Y.; Chun, Y.; Xia, J.R.; Zhu, J.H. Dispersion of Potassium Nitrate and the Resulting Strong Basicity on Zirconia. Chem. Mater. 2001, 13, 670–677. [Google Scholar] [CrossRef]
- Bartholomew, R.F. A Study of the Equilibrium KNO3(l) ⇄ KNO2(l) + 1/2O2(g) over the Temperature Range 550–750°. J. Phys. Chem. 1966, 70, 3442–3446. [Google Scholar] [CrossRef]
- Chernyshova, I.V.; Hochella Jr, M.F.; Madden, A.S. Size-dependent Structural Transformations of Hematite Nanoparticles. 1. Phase Transition. Phys. Chem. Chem. Phys. 2007, 9, 1736–1750. [Google Scholar] [CrossRef]
- Jubb, A.M.; Allen, H.C. Vibrational Spectroscopic Characterization of Hematite, Maghemite, and Magnetite Thin Films Produced by Vapor Deposition. ACS Appl. Mater. Interfaces 2010, 2, 2804–2812. [Google Scholar] [CrossRef]
- Bersani, D.; Lottici, P.P.; Montenero, A. Micro-Raman Investigation of Iron Oxide Films and Powders Produced by Sol–Gel Syntheses. J. Raman Spectrosc. 1999, 30, 355–360. [Google Scholar] [CrossRef]
- Chourpa, I.; Douziech-Eyrolles, L.; Ngaboni-Okassa, L.; Fouquenet, J.; Cohen-Jonathan, S.; Soucé, M.; Marchais, H.; Dubois, P. Molecular Composition of Iron Oxide Nanoparticles, Precursors for Magnetic Drug Targeting, as Characterized by Confocal Raman Microspectroscopy. Analyst 2005, 130, 1395–1403. [Google Scholar] [CrossRef]
- Brooker, M.H.; Irish, D.E. Infrared and Raman Spectral Studies of KNO2–KNO3 Solutions. Can. J. Chem. 1968, 46, 229–233. [Google Scholar] [CrossRef]
- Kraushofer, F.; Jakub, Z.; Bichler, M.; Hulva, J.; Drmota, P.; Weinold, M.; Schmid, M.; Setvin, M.; Diebold, U.; Blaha, P.; et al. Atomic-Scale Structure of the Hematite α-Fe2O3(12) “R-Cut” Surface. J. Phys. Chem. C 2018, 122, 1657–1669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, T.; Hayes, P. Effect of Curve Fitting Parameters on Quantitative Analysis of Fe0.94O And Fe2O3 using XPS. J. Electron Spectrosc. Relat. Phenom. 2006, 152, 6–11. [Google Scholar] [CrossRef]
- Jolm, F.M.; William, F.S.; Peter, E.S.; Kennetlf, D.B. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Nanayakkara, C.E.; Jayaweera, P.M.; Rubasinghege, G.; Baltrusaitis, J.; Grassian, V.H. Surface Photochemistry of Adsorbed Nitrate: The Role of Adsorbed Water in the Formation of Reduced Nitrogen Species on α-Fe2O3 Particle Surfaces. J. Phys. Chem. A 2014, 118, 158–166. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Rioult, M.; Stanescu, D.; Fonda, E.; Barbier, A.; Magnan, H. Oxygen Vacancies Engineering of Iron Oxides Films for Solar Water Splitting. J. Phys. Chem. C 2016, 120, 7482–7490. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Fang, W.; Ren, J.; Li, Y.; Yao, H.; Wang, J.; Li, Z. Enhanced Photoreduction CO2 Activity over Direct Z-Scheme α-Fe2O3/Cu2O Heterostructures under Visible Light Irradiation. ACS Appl. Mater. Interfaces 2015, 7, 8631–8639. [Google Scholar] [CrossRef]
- Newbury, D.E.; Ritchie, N.W.M. Is Scanning Electron Microscopy/Energy Dispersive X-ray Spectrometry (SEM/EDS) Quantitative? Scanning 2013, 35, 141–168. [Google Scholar] [CrossRef]
- Parish, C.M.; Brewer, L.N. Key Parameters Affecting Quantitative Analysis of STEM-EDS Spectrum Images. Microsc. Microanal. 2010, 16, 259–272. [Google Scholar] [CrossRef]
- Morrison, C.; Sun, H.; Yao, Y.; Loomis, R.A.; Buhro, W.E. Methods for the ICP-OES Analysis of Semiconductor Materials. Chem. Mater. 2020, 32, 1760–1768. [Google Scholar] [CrossRef]
- Li, C.; Yan, N.; Ye, Y.; Lv, Z.; He, X.; Huang, J.; Zhang, N. Thermal analysis and stability of boron/potassium nitrate pyrotechnic composition at 180 °C. Appl. Sci. 2019, 9, 3630. [Google Scholar] [CrossRef] [Green Version]
- Bykova, E.; Dubrovinsky, L.; Dubrovinskaia, N.; Bykov, M.; McCammon, C.; Ovsyannikov, S.V.; Liermann, H.P.; Kupenko, I.; Chumakov, A.I.; Rüffer, R.; et al. Structural Complexity of Simple Fe2O3 at High Pressures and Temperatures. Nat. Commun. 2016, 7, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
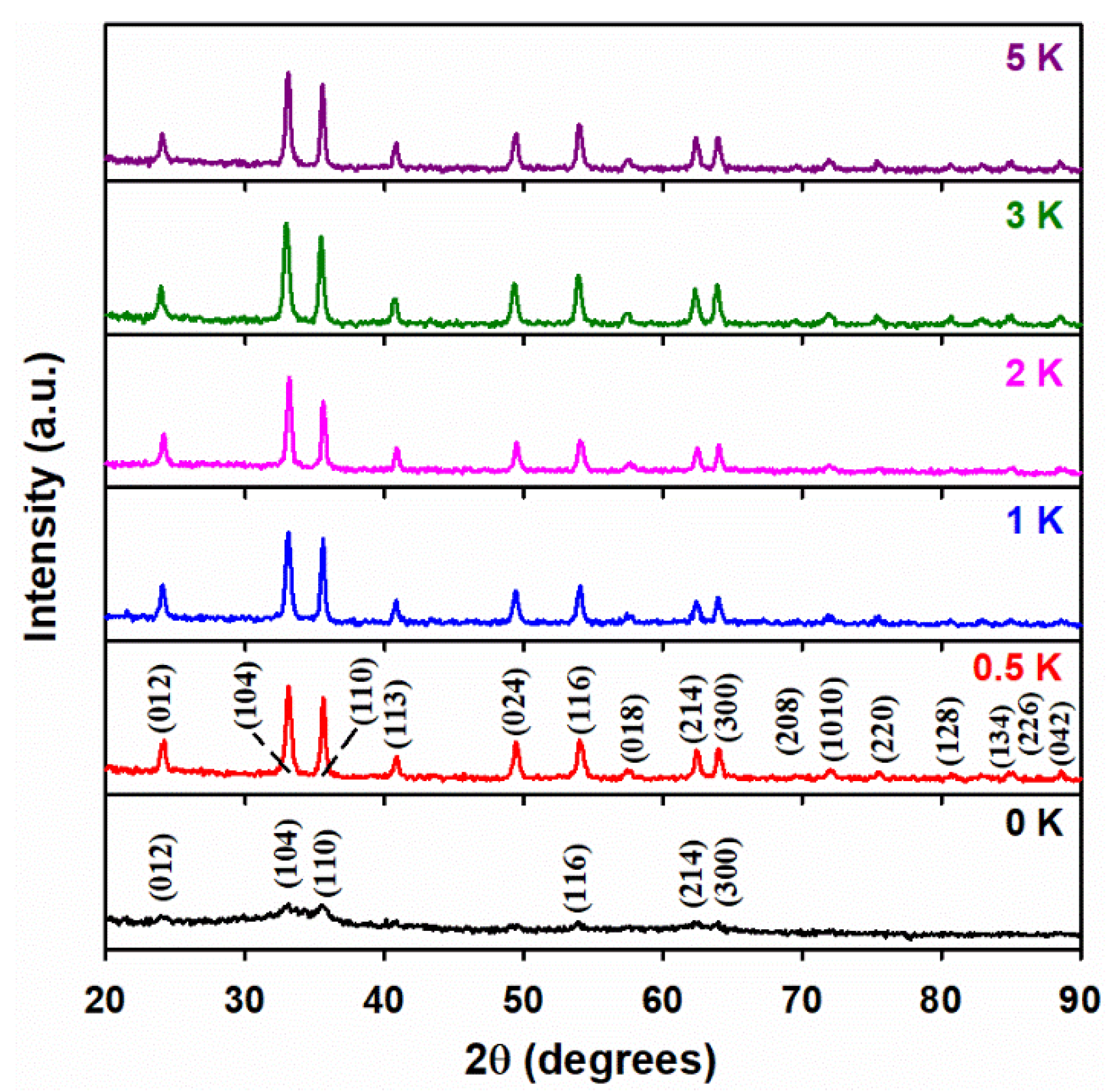


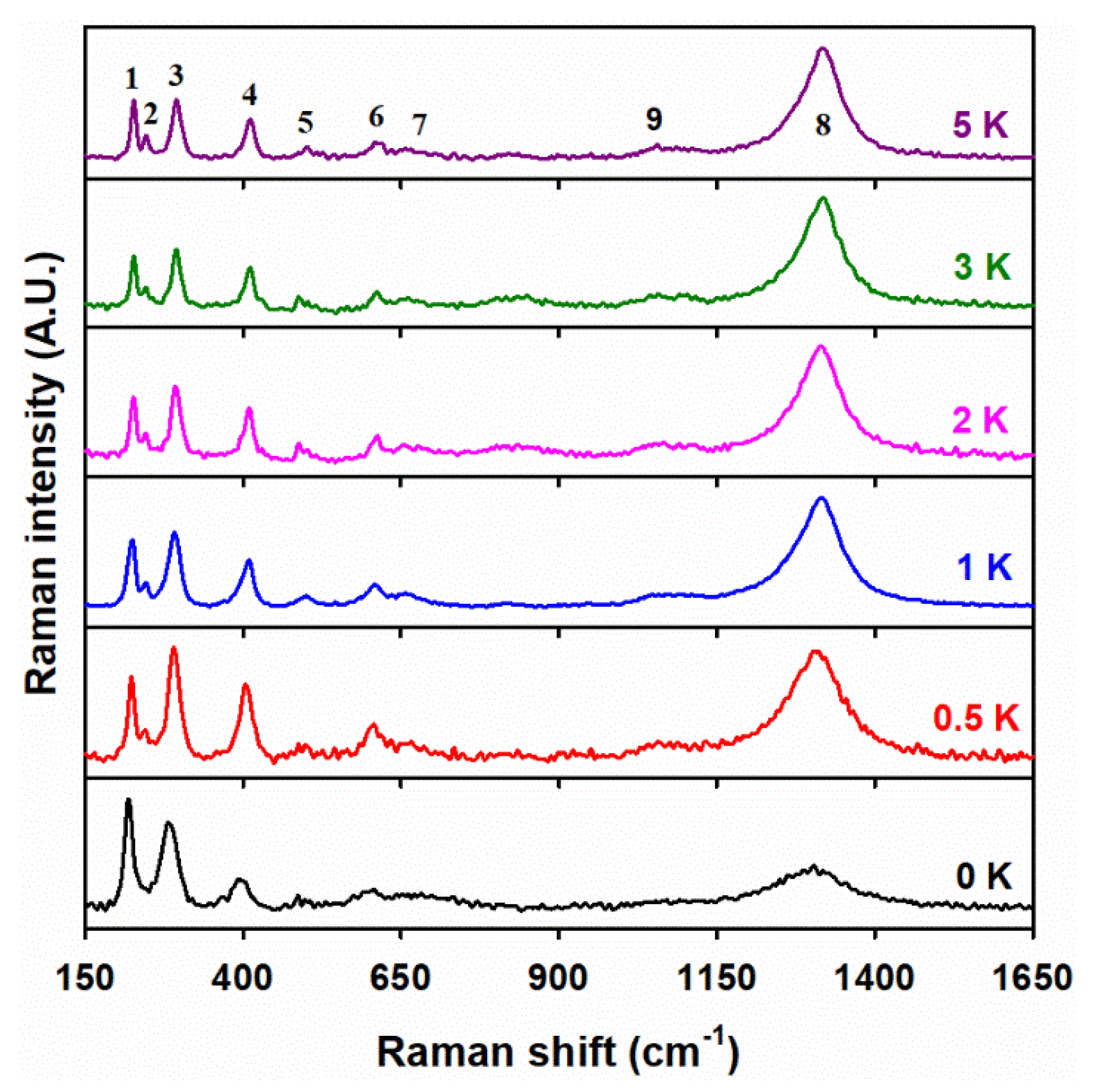
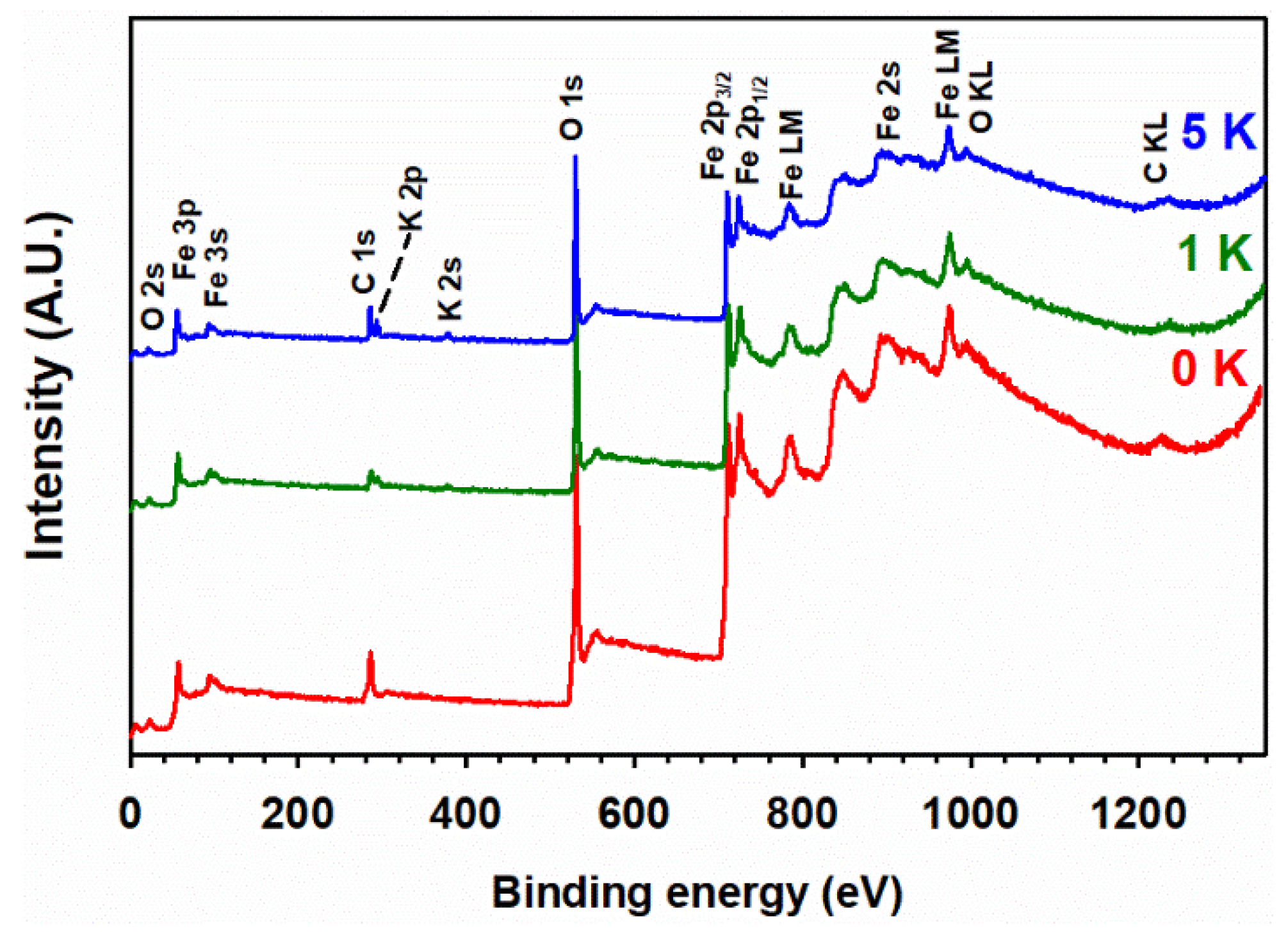

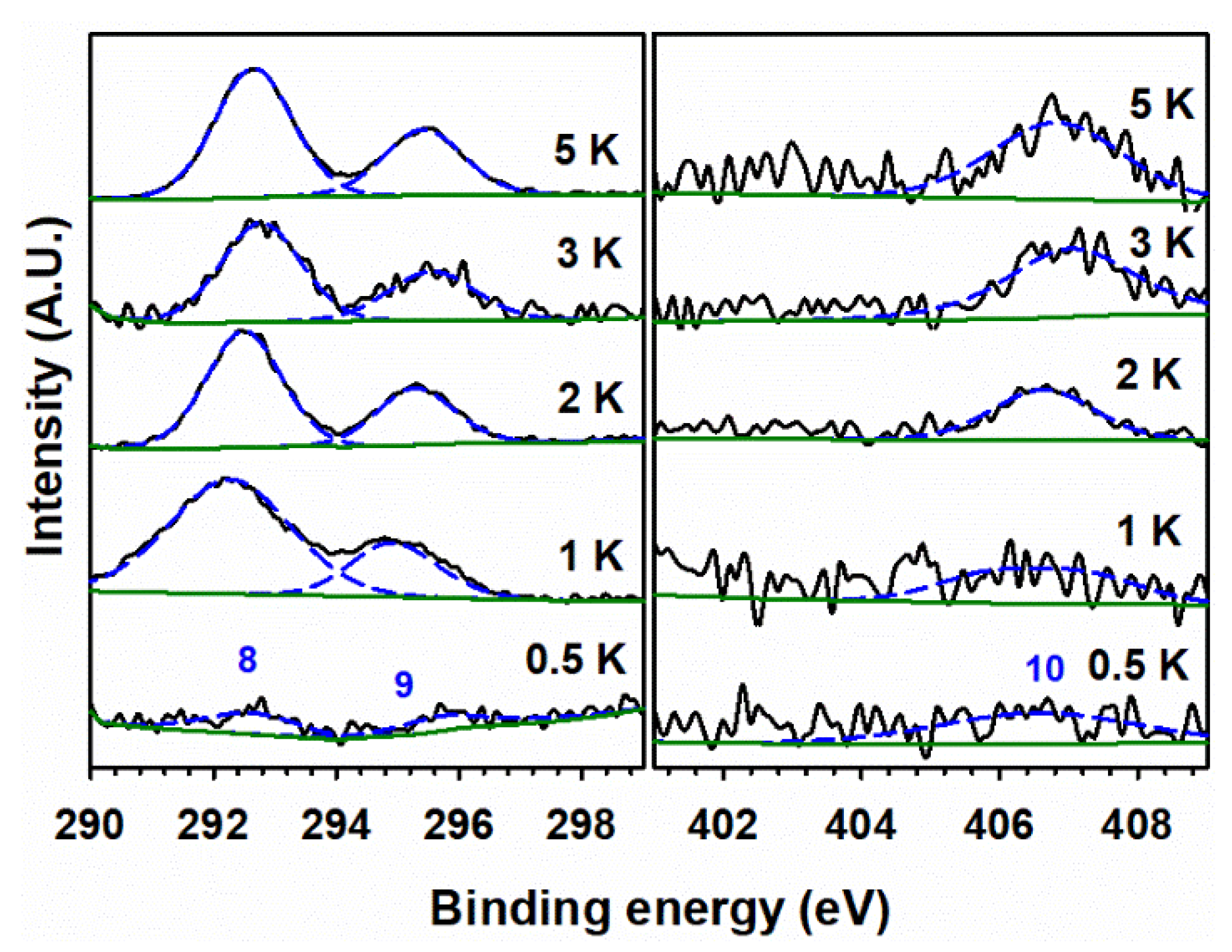
| X i | T (°C) | D (nm) ii | a (Å) iii | c (Å) iii |
|---|---|---|---|---|
| 0 | 400 | 4.15 | 5.059 | 13.746 |
| ½ | 400 | 16.1 | 5.042 | 13.782 |
| 1 | 400 | 18.1 | 5.047 | 13.787 |
| 2 | 400 | 19.3 | 5.040 | 13.761 |
| 3 | 400 | 17.1 | 5.063 | 13.844 |
| 5 | 400 | 20.0 | 5.044 | 13.794 |
| 5 | 500 | 20.0 | 5.051 | 13.807 |
| 5 | 600 | 21.2 | 5.058 | 13.829 |
| 5 | 700 | 23.6 | 5.046 | 13.780 |
| 5 | 800 | 36.6 | 5.067 | 13.841 |
| Nominal iv | Experimental v |
|---|---|
| 100 Fe:0 K | 100 Fe:0 K |
| 100 Fe:½ K | 100 Fe:0.548(2) K |
| 100 Fe:1 K | 100 Fe:1.275(3) K |
| 100 Fe:2 K | 100 Fe:2.750(5) K |
| 100 Fe:3 K | 100 Fe:3.209(13) K |
| 100 Fe:5 K | 100 Fe:6.413(56) K |
| Material | T (°C) | Diameter (nm) vi | Xvi |
|---|---|---|---|
| 100 Fe:0 K | 400 | 2.56 | 0.00 |
| 100 Fe:½ K | 400 | 15.4 | 0.48 |
| 100 Fe:2 K | 400 | 17.5 | 0.85 |
| 100 Fe:5 K | 400 | 17.6 | 0.93 |
| 100 Fe:5 K | 600 | 20.0 | 2.80 |
| 100 Fe:5 K | 800 | 135.3 | 1.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoque, M.A.; Guzman, M.I.; Selegue, J.P.; Gnanamani, M.K. Chemical State of Potassium on the Surface of Iron Oxides: Effects of Potassium Precursor Concentration and Calcination Temperature. Materials 2022, 15, 7378. https://doi.org/10.3390/ma15207378
Hoque MA, Guzman MI, Selegue JP, Gnanamani MK. Chemical State of Potassium on the Surface of Iron Oxides: Effects of Potassium Precursor Concentration and Calcination Temperature. Materials. 2022; 15(20):7378. https://doi.org/10.3390/ma15207378
Chicago/Turabian StyleHoque, Md. Ariful, Marcelo I. Guzman, John P. Selegue, and Muthu Kumaran Gnanamani. 2022. "Chemical State of Potassium on the Surface of Iron Oxides: Effects of Potassium Precursor Concentration and Calcination Temperature" Materials 15, no. 20: 7378. https://doi.org/10.3390/ma15207378
APA StyleHoque, M. A., Guzman, M. I., Selegue, J. P., & Gnanamani, M. K. (2022). Chemical State of Potassium on the Surface of Iron Oxides: Effects of Potassium Precursor Concentration and Calcination Temperature. Materials, 15(20), 7378. https://doi.org/10.3390/ma15207378








