Catalytic Hydrogen Evolution from H2S Cracking over CrxZnS Catalyst in a Cylindrical Single-Layered Dielectric Barrier Discharge Plasma Reactor
Abstract
:1. Introduction
2. Experimental Part
2.1. Chemicals
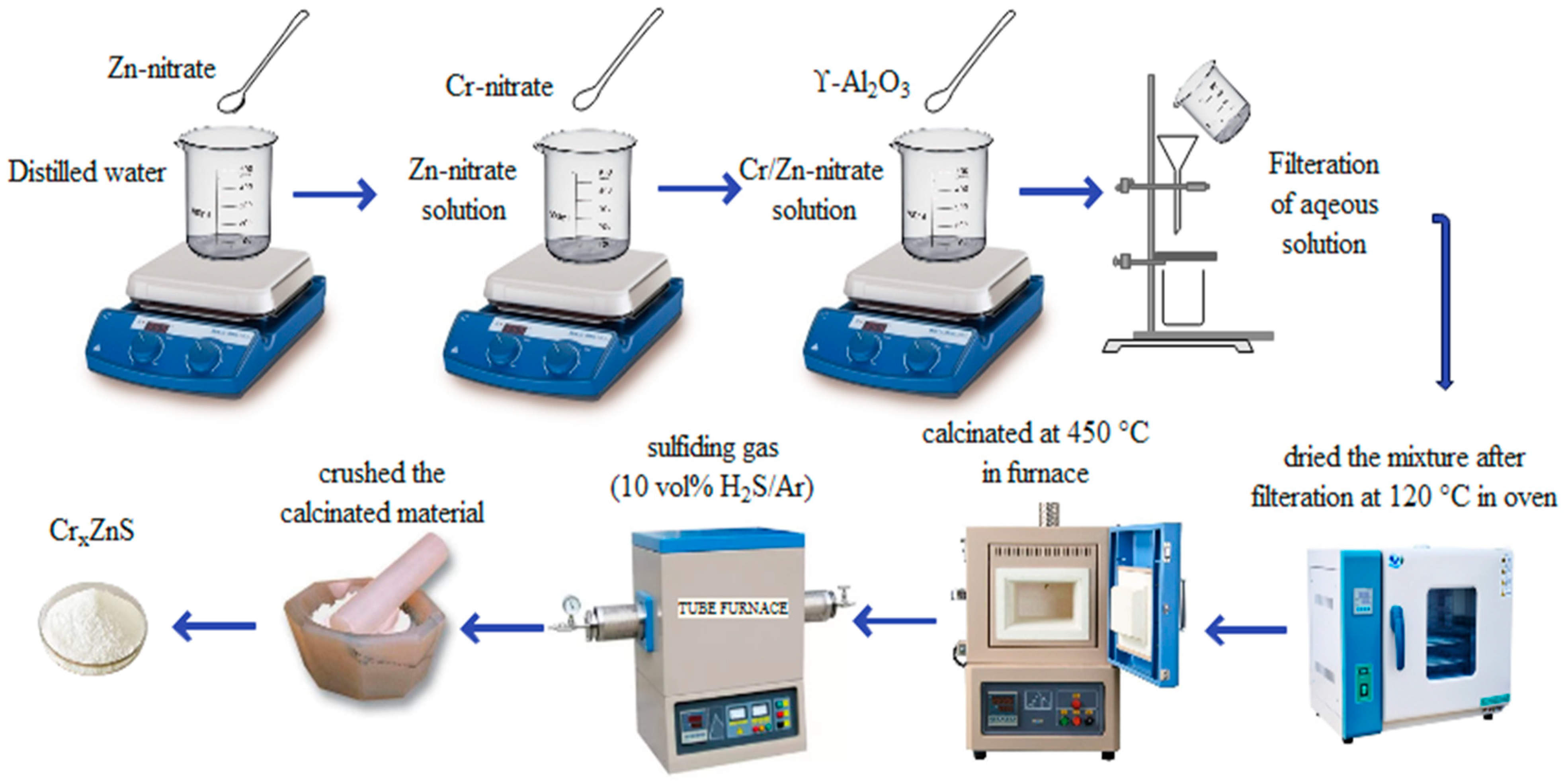
2.2. Preparation of Photocatalyst
2.3. DBD Plasma-Assisted Hydrogen Evolution
3. Results and Discussion
3.1. FTIR Analysis of Catalyst
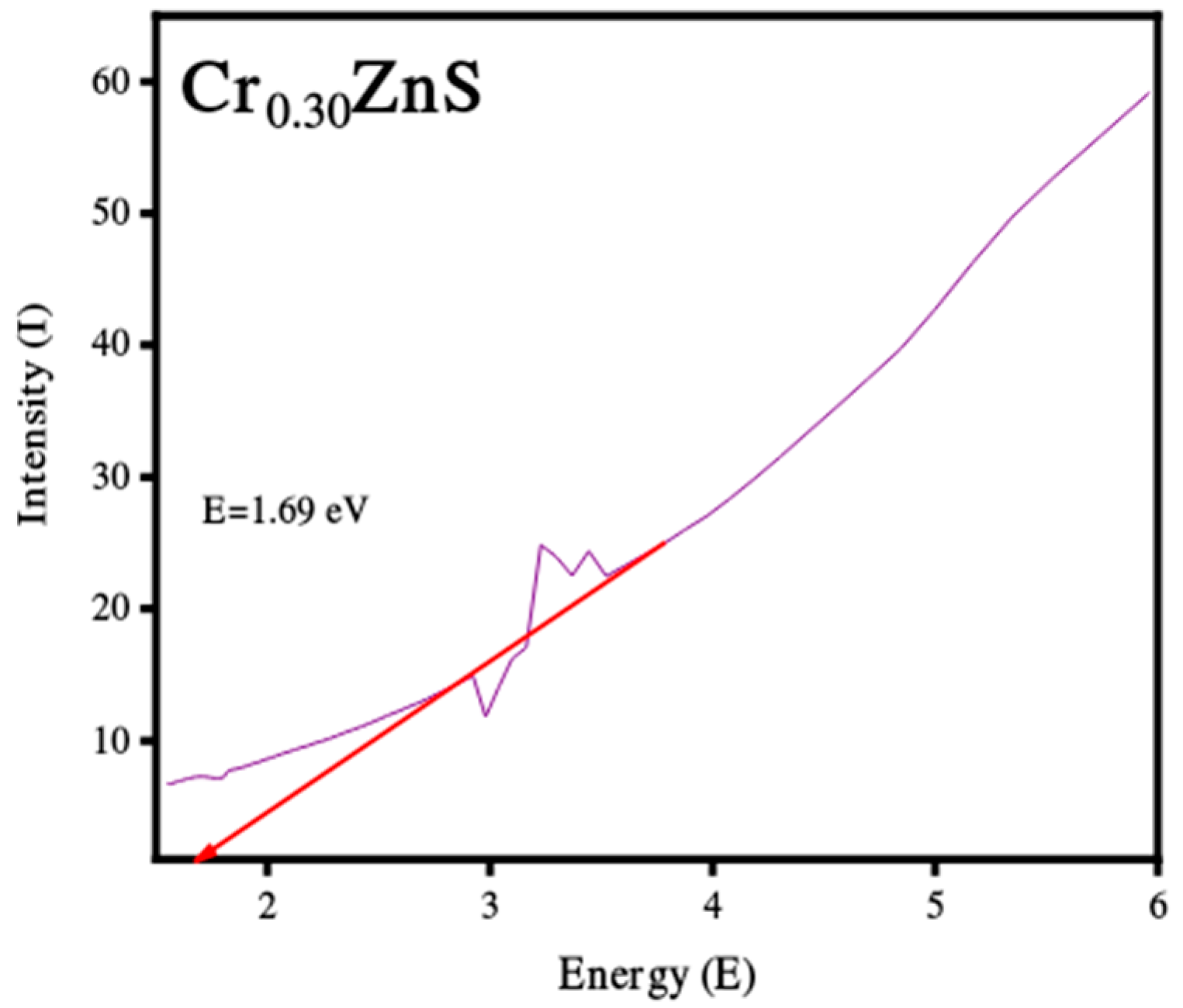
3.2. UV-Visible Analysis
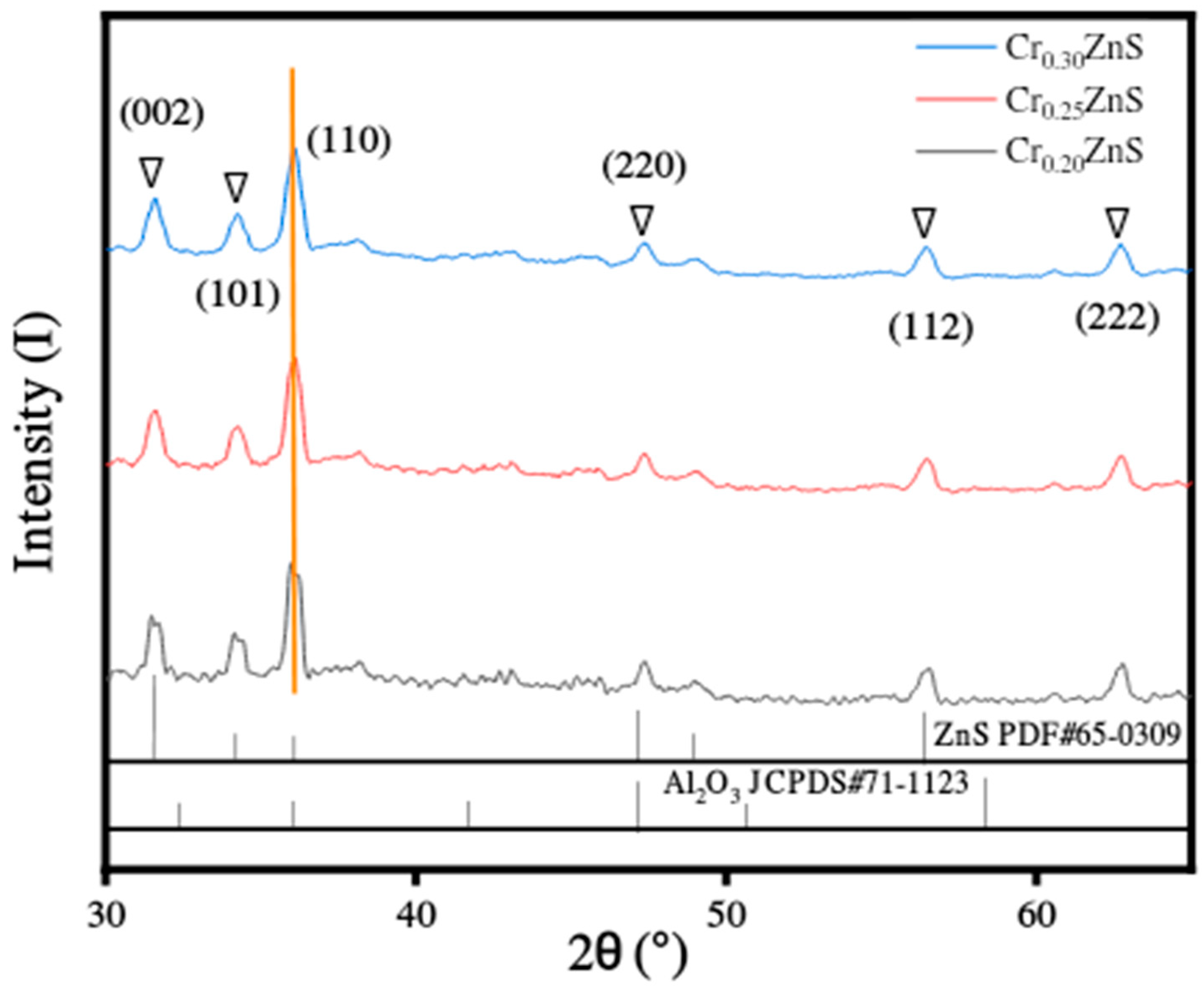
3.3. X-ray Diffraction Analysis
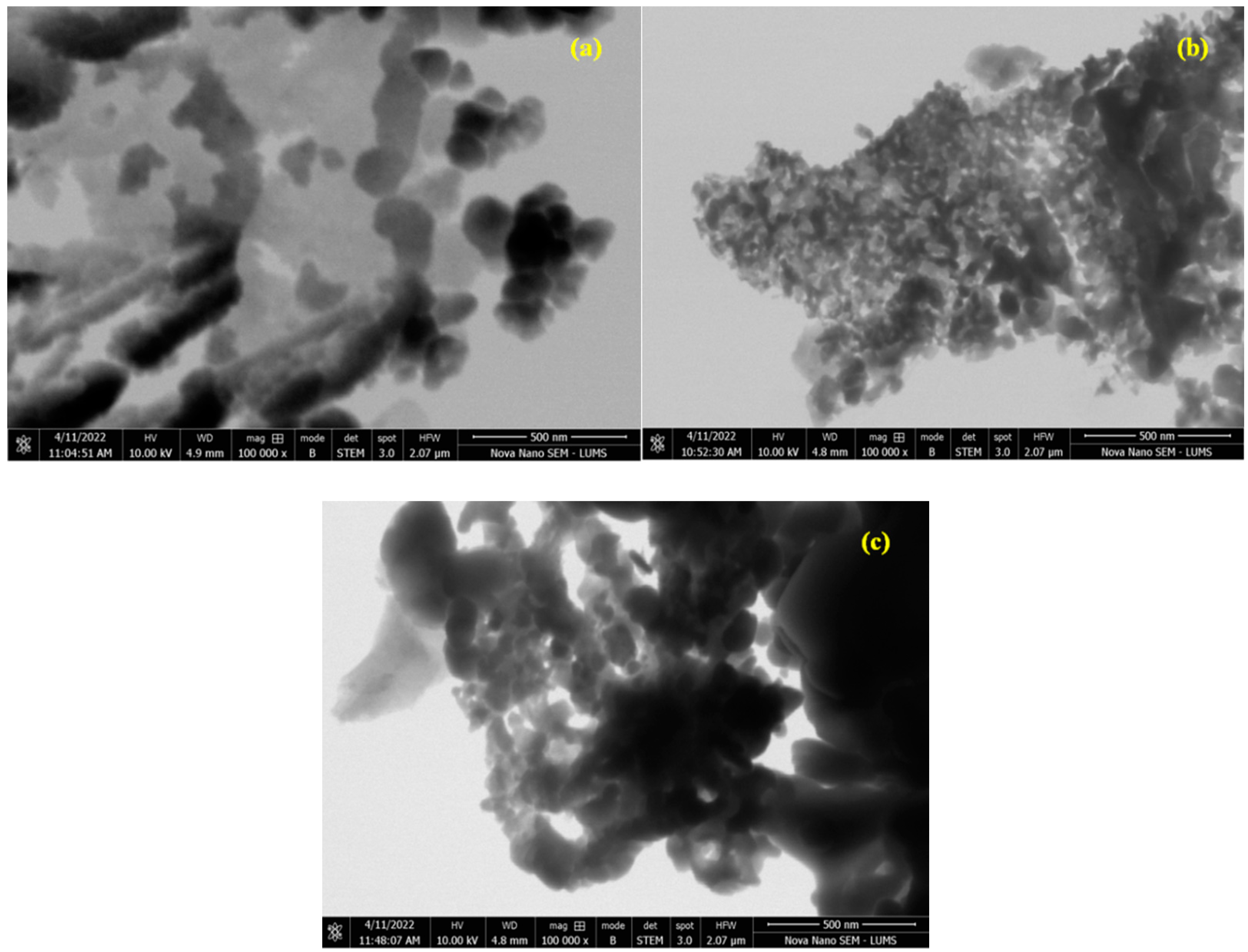
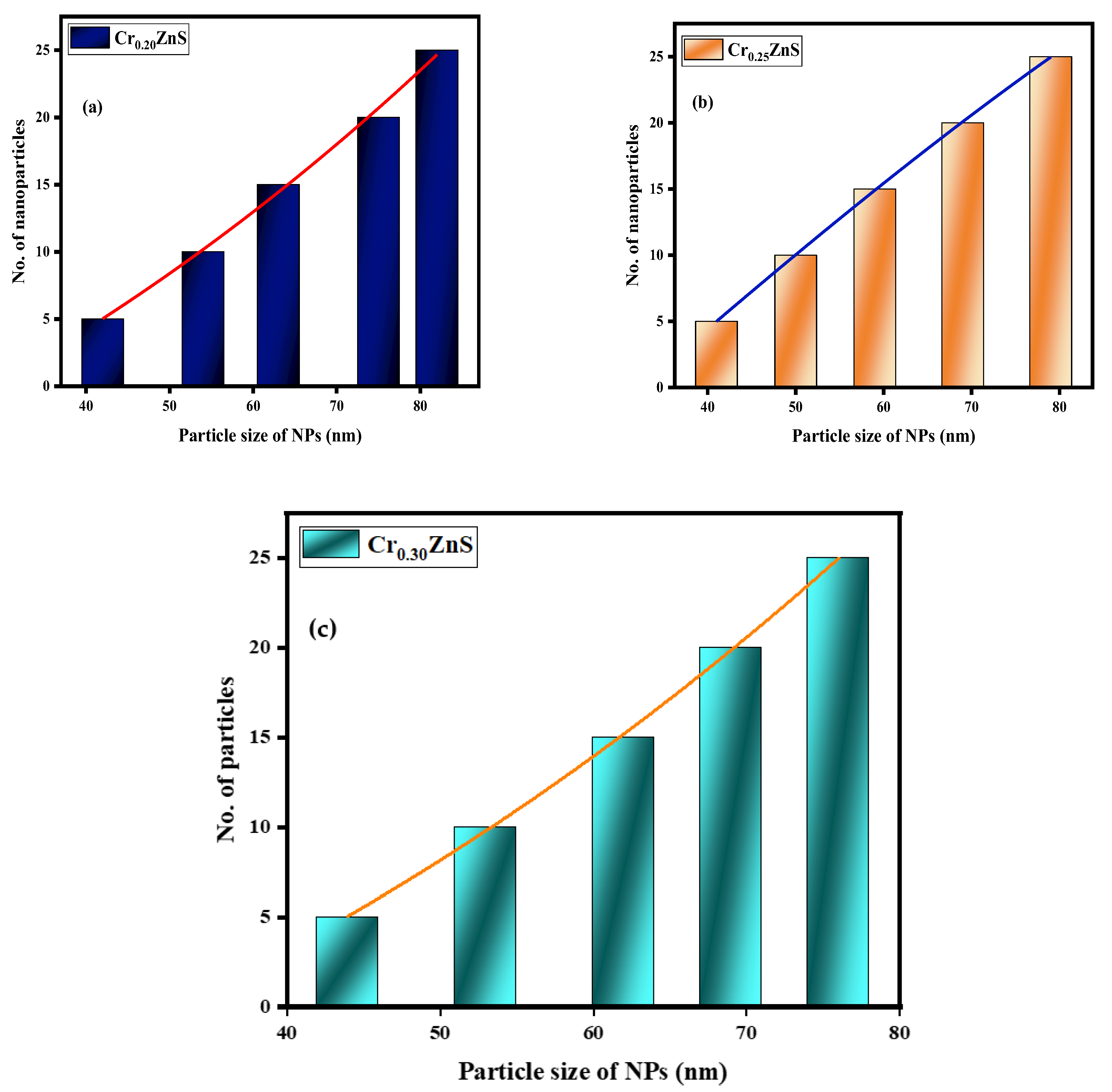
3.4. STEM Morphology Analysis
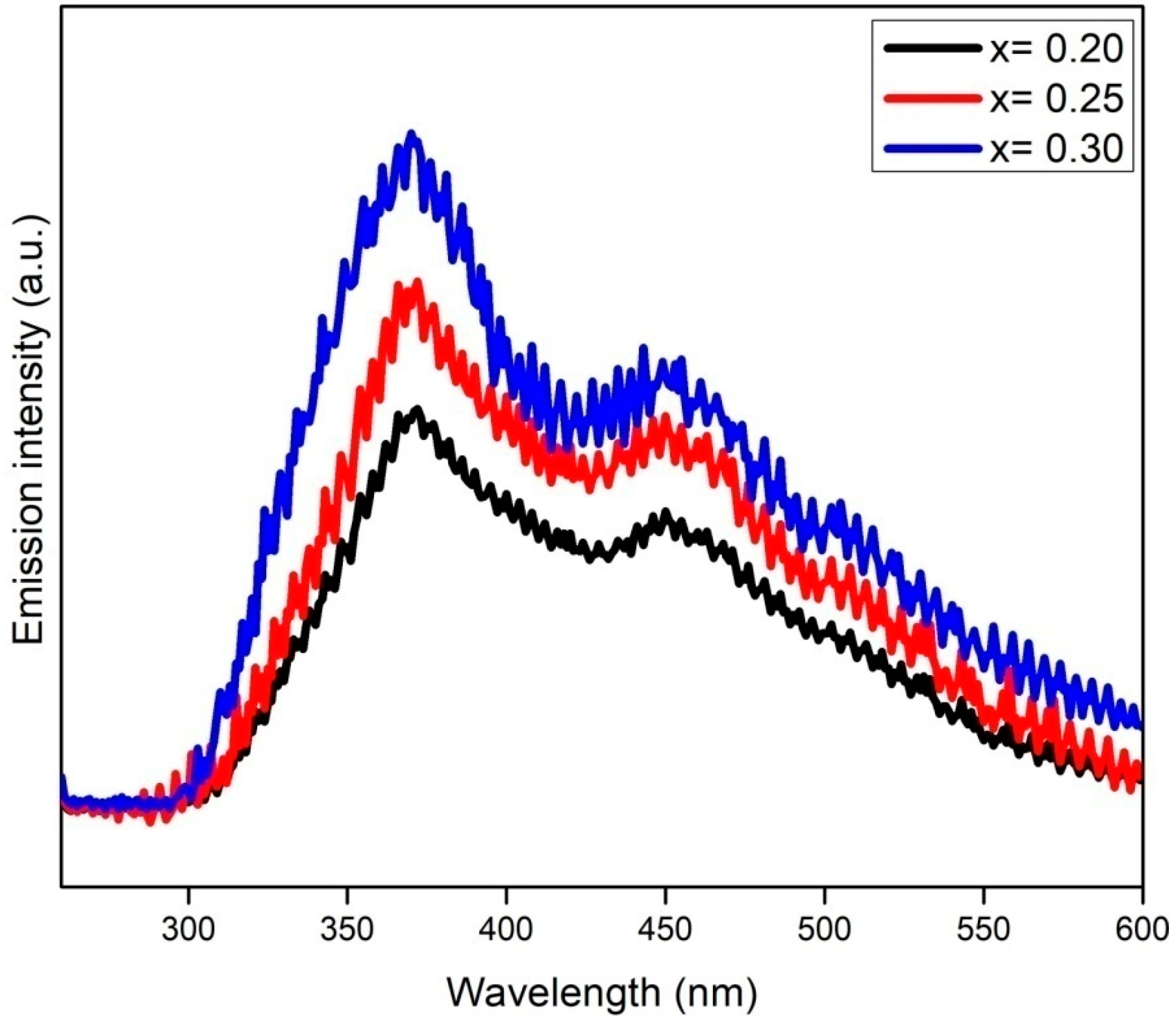
3.5. Photoluminescence Analysis
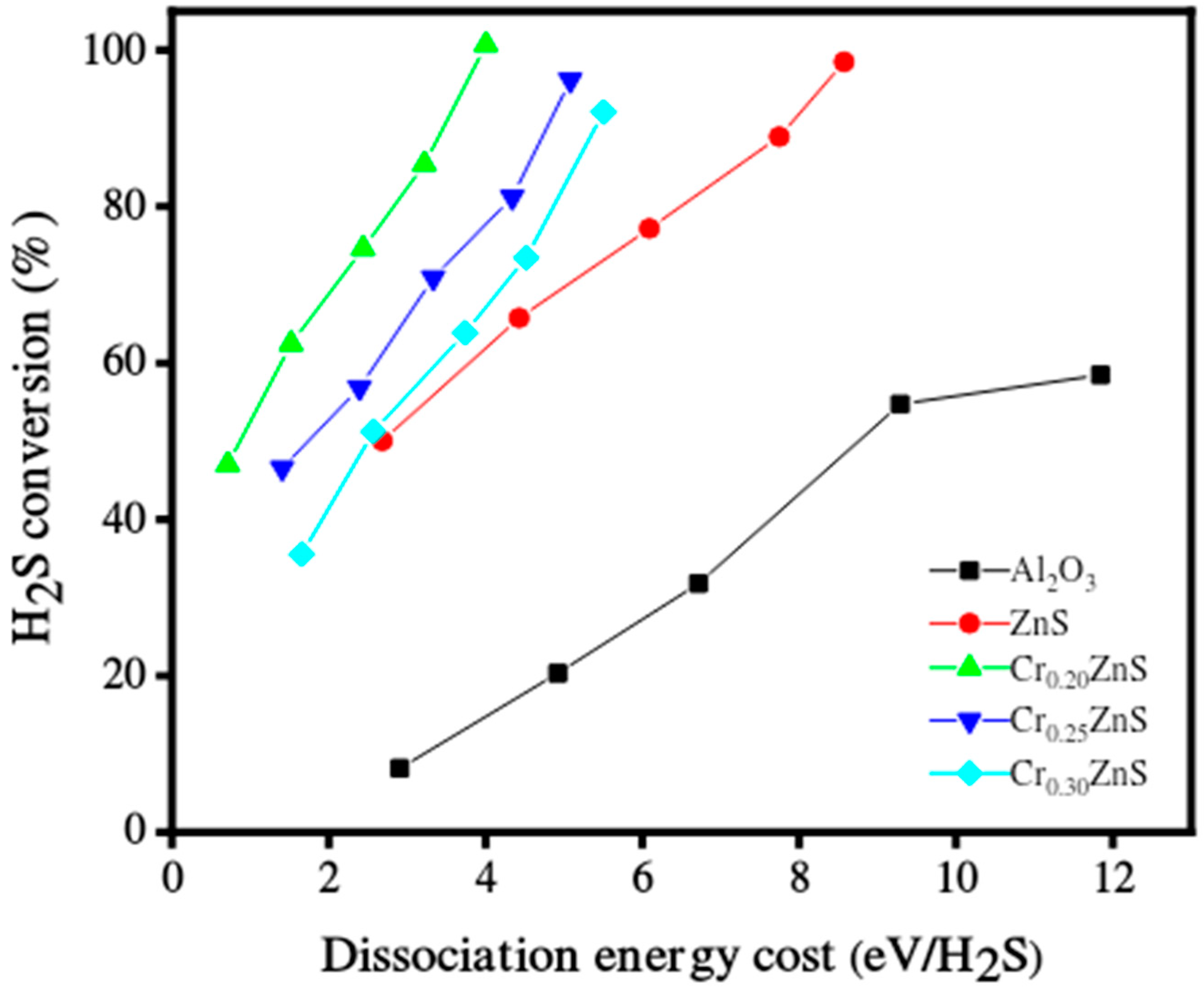
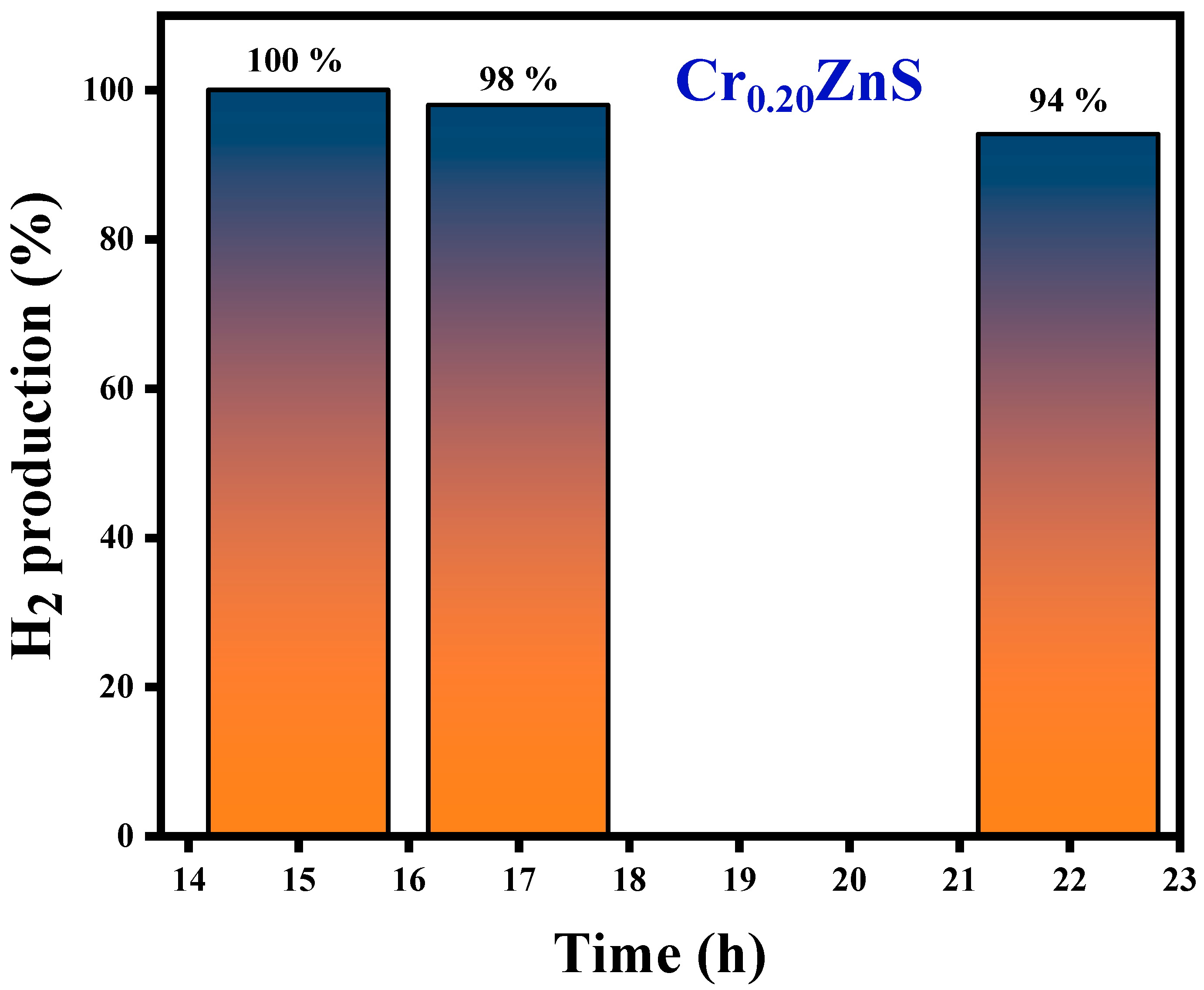
3.6. Hydrogen Evolution Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thangam, N.; Suganya Devi, B.; Lalitha Muthu, A.C. Hydrogen production from Hydrogen Sulfide Waste stream using Ru/Cd0.6Zn0.4S Photocatalyst. Int. J. New Technol. Res. 2015, 1, 4–10. [Google Scholar]
- Liao, J.; Shao, Y.; Feng, Y.; Zhang, J.; Song, C.; Zeng, W.; Tang, J.; Dong, H.; Liu, Q.; Li, H. Interfacial charge transfer induced dual-active-sites of heterostructured Cu0.8Ni0.2WO4 nanoparticles in ammonia borane methanolysis for fast hydrogen production. Appl. Catal. B Environ. 2022, 320, 121973. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Y.; Liao, Q.; Zhang, W.; Huang, Z.; Chen, X.; Shao, Y.; Dong, H.; Liu, Q.; Li, H. Modulation the electronic structure of hollow structured CuO-NiCo2O4 nanosphere for enhanced catalytic activity towards methanolysis of ammonia borane. Fuel 2022, 332, 126045. [Google Scholar] [CrossRef]
- Reddy, D.A.; Murali, G.; Vijayalakshmi, R.P.; Reddy, B.K. Room-temperature ferromagnetism in EDTA capped Cr-doped ZnS nanoparticles. Appl. Phys. A 2011, 105, 119–124. [Google Scholar] [CrossRef]
- Deshmukh, G.M.; Shete, A.; Pawar, D.M. Oxidative absorption of hydrogen sulfide using an iron-chelate based process: Chelate degradation. J. Chem. Technol. Biotechnol. 2013, 88, 432–436. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; Li, X.; Wang, A.; Song, C.; Hu, Y. Hydrogen production via decomposition of hydrogen sulfide by synergy of non-thermal plasma and semiconductor catalysis. Int. J. Hydrogen Energy 2013, 38, 14415–14423. [Google Scholar] [CrossRef]
- Zaman, J.; Chakma, A. Production of hydrogen and sulfur from hydrogen sulfide. Fuel Processing Technol. 1995, 41, 159–198. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, X.; Shao, Y.; Chen, X.; Wang, H.; Li, J.; Wu, M.; Dong, H.; Liu, Q.; Li, H. Modulating the Acidic Properties of Mesoporous Mox–Ni0.8Cu0.2O Nanowires for Enhanced Catalytic Performance toward the Methanolysis of Ammonia Borane for Hydrogen Production. ACS Appl. Mater. Interfaces 2022, 14, 27979–27993. [Google Scholar] [CrossRef]
- Duan, L.B.; Zhao, X.R.; Liu, J.M.; Wang, T.; Rao, G.H. Room-temperature ferromagnetism in lightly Cr-doped ZnO nanoparticles. Appl. Phys. A 2010, 99, 679–683. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, X.; Mu, X.; Li, Y.; Fang, K. Highly selective conversion of H2S–CO2 to syngas by combination of non-thermal plasma and MoS2/Al2O3. J. CO2 Util. 2020, 37, 45–54. [Google Scholar] [CrossRef]
- Eyasu, A.; Yadav, O.P.; Bachheti, R.K. Photocatalytic degradation of methyl orange dye using Cr-doped ZnS nanoparticles under visible radiation. Int. J. Chem. Tech. Res. 2013, 5, 1452–1461. [Google Scholar]
- Poornaprakash, B.; Naveen Kumar, K.; Chalapathi, U.; Reddeppa, M.; Poojitha, P.T.; Park, S.H. Chromium doped ZnS nanoparticles: Chemical, structural, luminescence and magnetic studies. J. Mater. Sci. Mater. Electron. 2016, 27, 6474–6479. [Google Scholar] [CrossRef]
- Barnhart, J. Occurrences, uses, and properties of chromium. Regul. Toxicol. Pharmacol. 1997, 26, S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Bodke, M.R.; Purushotham, Y.; Dole, B.N. Crystallographic and optical studies on Cr doped ZnS nanocrystals. Cerâmica 2014, 60, 425–428. [Google Scholar] [CrossRef] [Green Version]
- Linga Reddy, E.; Karuppiah, J.; Renken, A.; Kiwi-Minsker, L.; Subrahmanyam, C. Kinetics of the decomposition of hydrogen sulfide in a dielectric barrier discharge reactor. Chem. Eng. Technol. 2012, 35, 2030–2034. [Google Scholar] [CrossRef]
- Kogelschatz, U.; Eliasson, B.; Egli, W. Dielectric-barrier discharges. Principle and applications. Le J. De Phys. IV 1997, 7, C4–C47. [Google Scholar] [CrossRef]
- Bodke, M. Synthesis and characterization of chromium doped zinc sulfide nanoparticles. Open Access Libr. J. 2015, 2, 1. [Google Scholar] [CrossRef]
- Palma, V.; Cortese, M.; Renda, S.; Ruocco, C.; Martino, M.; Meloni, E. A review about the recent advances in selected nonthermal plasma assisted solid–gas phase chemical processes. Nanomaterials 2020, 10, 1596. [Google Scholar] [CrossRef]
- Bhat, V.S.; Tilakraj, T.S.; Patil, M.K.; Pujari, V.; Inamdar, S.R. One-Pot Synthesis of Biocompatible Glycine Protected Chromium Doped ZnS Nanoparticles and their Optical Properties. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2022; Volume 1221, p. 012029. [Google Scholar]
- Dake, D.V.; Raskar, N.D.; Mane, V.A.; Sonpir, R.B.; Stathatos, E.; Asokan, K.; Babu, P.D.; Dole, B.N. Exploring the role of defects on diverse properties of Cr-substituted ZnS nanostructures for photocatalytic applications. Appl. Phys. A 2020, 126, 1–15. [Google Scholar] [CrossRef]
- Amaranatha Reddy, D.; Murali, G.; Vijayalakshmi, R.P.; Reddy, B.K.; Sreedhar, B. Effect of Cr doping on the structural and optical properties of ZnS nanoparticles. Cryst. Res. Technol. 2011, 46, 731–736. [Google Scholar] [CrossRef]
- Mohammed, B.A.; Ahmed, L.M. Improvement the Photo Catalytic Properties of ZnS nanoparticle with Loaded Manganese and Chromium by Co-Precipitation Method. J. Glob. Pharma Technol. 2018, 10, 129–138. [Google Scholar]
- Batra, V.; Kotru, S.; Varagas, M.; Ramana, C.V. Optical constants and band gap determination of Pb0.95La0.05 Zr0.54Ti0.46O3 thin films using spectroscopic ellipsometry and UV–visible spectroscopy. Opt. Mater. 2015, 49, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Kaur, P.; Kumar, S.; Singh, A.; Rao, S.M. Improved magnetism in Cr doped ZnS nanoparticles with nitrogen co-doping synthesized using chemical co-precipitation technique. J. Mater. Sci. Mater. Electron. 2015, 26, 9158–9163. [Google Scholar] [CrossRef]
- Ramasamy, V.; Praba, K.; Murugadoss, G. Synthesis and study of optical properties of transition metals doped ZnS nanoparticles. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2012, 96, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.A.; Divya, A.; Murali, G.; Vijayalakshmi, R.P.; Reddy, B.K. Synthesis and optical properties of Cr doped ZnS nanoparticles capped by 2-mercaptoethanol. Phys. B Condens. Matter 2011, 406, 1944–1949. [Google Scholar] [CrossRef]
- Choi, B.; Shim, H.; Allabergenov, B. Red photoluminescence and blue-shift caused by phase transformation in multilayer films of titanium dioxide and zinc sulfide. Opt. Mater. Express 2015, 5, 2156–2163. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, S.W.; Loo, S.C.J.; Xue, C. Nanoparticle heterojunctions in ZnS–ZnO hybrid nanowires for visible-light-driven photocatalytic hydrogen generation. Cryst. Eng. Comm. 2013, 15, 5688–5693. [Google Scholar] [CrossRef]
- Irfan, M.; Shukrullah, S.; Naz, M.Y.; Ahmad, I.; Shoukat, B.; Legutko, S.; Alsaiari, M.A. Si/SiO2/Al2O3 Supported Growth of CNT Forest for the Production of La/ZnO/CNT Photocatalyst for Hydrogen Production. Materials 2022, 15, 3226. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Ahmad, I.; Shukrullah, S.; Hussain, H.; Atif, M.; Legutko, S.; Petru, J.; Hatala, M.; Naz, M.Y.; Rahman, S. Construction of 0D/2D Schottky Heterojunctions of ZnO and Ti3C2 Nanosheets with the Enriched Transfer of Interfacial Charges for Photocatalytic Hydrogen Evolution. Materials 2022, 15, 4557. [Google Scholar] [CrossRef]
- Ahmad, I.; Shukrullah, S.; Naz, M.Y.; Ahmad, M.; Ahmed, E.; Liu, Y.; Hussain, A.; Iqbal, S.; Ullah, S. Recent advances and challenges in 2D/2D heterojunction photocatalysts for solar fuels applications. Adv. Colloid Interface Sci. 2022, 304, 102661. [Google Scholar] [CrossRef]
- Liang, W.J.; Fang, H.-P.; Li, J.; Zheng, F.; Li, J.X.; Jin, Y.Q. Performance of non-thermal DBD plasma reactor during the removal of hydrogen sulfide. J. Electrost. 2011, 69, 206–213. [Google Scholar] [CrossRef]
- Xing, C.; Zhang, Y.; Yan, W.; Guo, L. Band structure-controlled solid solution of Cd1-x ZnxS photocatalyst for hydrogen production by water splitting. Int. J. Hydrogen Energy 2006, 31, 2018–2024. [Google Scholar] [CrossRef]
- Reddy, E.L.; Biju, V.M.; Subrahmanyam, C. Production of hydrogen and sulfur from hydrogen sulfide assisted by nonthermal plasma. Appl. Energy 2012, 95, 87–92. [Google Scholar] [CrossRef]
- Hu, J.S.; Ren, L.L.; Guo, Y.G.; Liang, H.P.; Cao, A.M.; Wan, L.J.; Bai, C.L. Mass production and high photocatalytic activity of ZnS nanoporous nanoparticles. Angew. Chem. Int. Ed. 2005, 44, 1269–1273. [Google Scholar] [CrossRef]
- Chivers, T.; Lau, C. The use of thermal diffusion column reactors for the production of hydrogen and sulfur from the thermal decomposition of hydrogen sulfide over transition metal sulfides. Int. J. Hydrogen Energy 1987, 12, 561–569. [Google Scholar] [CrossRef]
- Kalamaras, C.M.; Efstathiou, A.M. Hydrogen production technologies: Current state and future developments. In Conference Papers in Science; Hindawi: London, UK, 2013; Volume 2013. [Google Scholar]
- Zhao, L.; Wang, Y.; Wang, A.; Li, X.; Song, C.; Hu, Y. Cr–doped ZnS semiconductor catalyst with high catalytic activity for hydrogen production from hydrogen sulfide in non-thermal plasma. Catal. Today 2019, 337, 83–89. [Google Scholar] [CrossRef]

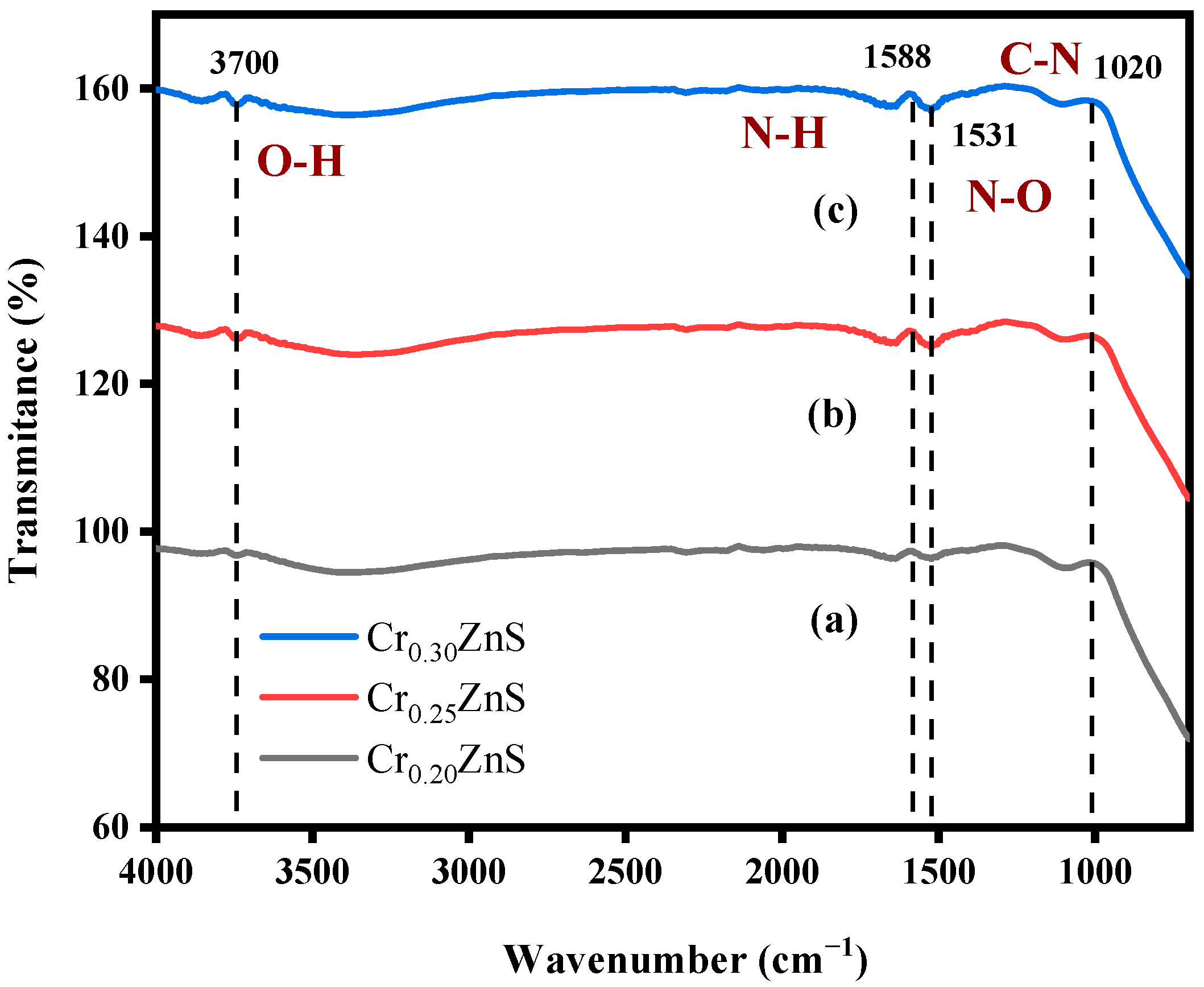


| FTIR peaks | Spectrumregion | Appearance | Bonding force | Group | Compoundclass |
|---|---|---|---|---|---|
| 3700 | GFR | medium, sharp | intermolecular | O―H stretching | alcohol |
| 1588 | GFR | medium | - | N―H bending | amines |
| 1531 | GFR | strong | - | N―O stretching | nitro- compound |
| 1020 | FPR | medium | - | C―N stretching | amines |
| Catalyst | FWHM | Grain size (nm) | Band gap (eV) |
|---|---|---|---|
| Cr0.20ZnS | 0.477 | 18.30 | 2.68 |
| Cr0.25ZnS | 0.488 | 17.89 | 2.48 |
| Cr0.30ZnS | 0.499 | 17.49 | 1.69 |
| Catalyst | H2S conversion (%) | SIE (J/L) | Energy consumption (eV) |
|---|---|---|---|
| Cr0.20ZnS | 100 | 14.66 | 0.120 |
| Cr0.25ZnS | 96 | 14.66 | 0.124 |
| Cr0.30ZnS | 90 | 14.66 | 0.138 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afzal, S.; Hussain, H.; Naz, M.Y.; Shukrullah, S.; Ahmad, I.; Irfan, M.; Mursal, S.N.F.; Legutko, S.; Kruszelnicka, I.; Ginter-Kramarczyk, D. Catalytic Hydrogen Evolution from H2S Cracking over CrxZnS Catalyst in a Cylindrical Single-Layered Dielectric Barrier Discharge Plasma Reactor. Materials 2022, 15, 7426. https://doi.org/10.3390/ma15217426
Afzal S, Hussain H, Naz MY, Shukrullah S, Ahmad I, Irfan M, Mursal SNF, Legutko S, Kruszelnicka I, Ginter-Kramarczyk D. Catalytic Hydrogen Evolution from H2S Cracking over CrxZnS Catalyst in a Cylindrical Single-Layered Dielectric Barrier Discharge Plasma Reactor. Materials. 2022; 15(21):7426. https://doi.org/10.3390/ma15217426
Chicago/Turabian StyleAfzal, Saba, Humaira Hussain, Muhammad Yasin Naz, Shazia Shukrullah, Irshad Ahmad, Muhammad Irfan, Salim Nasar Faraj Mursal, Stanislaw Legutko, Izabela Kruszelnicka, and Dobrochna Ginter-Kramarczyk. 2022. "Catalytic Hydrogen Evolution from H2S Cracking over CrxZnS Catalyst in a Cylindrical Single-Layered Dielectric Barrier Discharge Plasma Reactor" Materials 15, no. 21: 7426. https://doi.org/10.3390/ma15217426
APA StyleAfzal, S., Hussain, H., Naz, M. Y., Shukrullah, S., Ahmad, I., Irfan, M., Mursal, S. N. F., Legutko, S., Kruszelnicka, I., & Ginter-Kramarczyk, D. (2022). Catalytic Hydrogen Evolution from H2S Cracking over CrxZnS Catalyst in a Cylindrical Single-Layered Dielectric Barrier Discharge Plasma Reactor. Materials, 15(21), 7426. https://doi.org/10.3390/ma15217426








