XPS Investigation on Improving Hydrogen Sorption Kinetics of the KSiH3 System by Using Zr-Based Catalysts
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Structural and Morphological Characterization by XRD/SEM
3.2. Hydrogen Sorption Studies by Differential Scanning Calorimetry (DSC) and Thermogravimetric (TG) Analysis
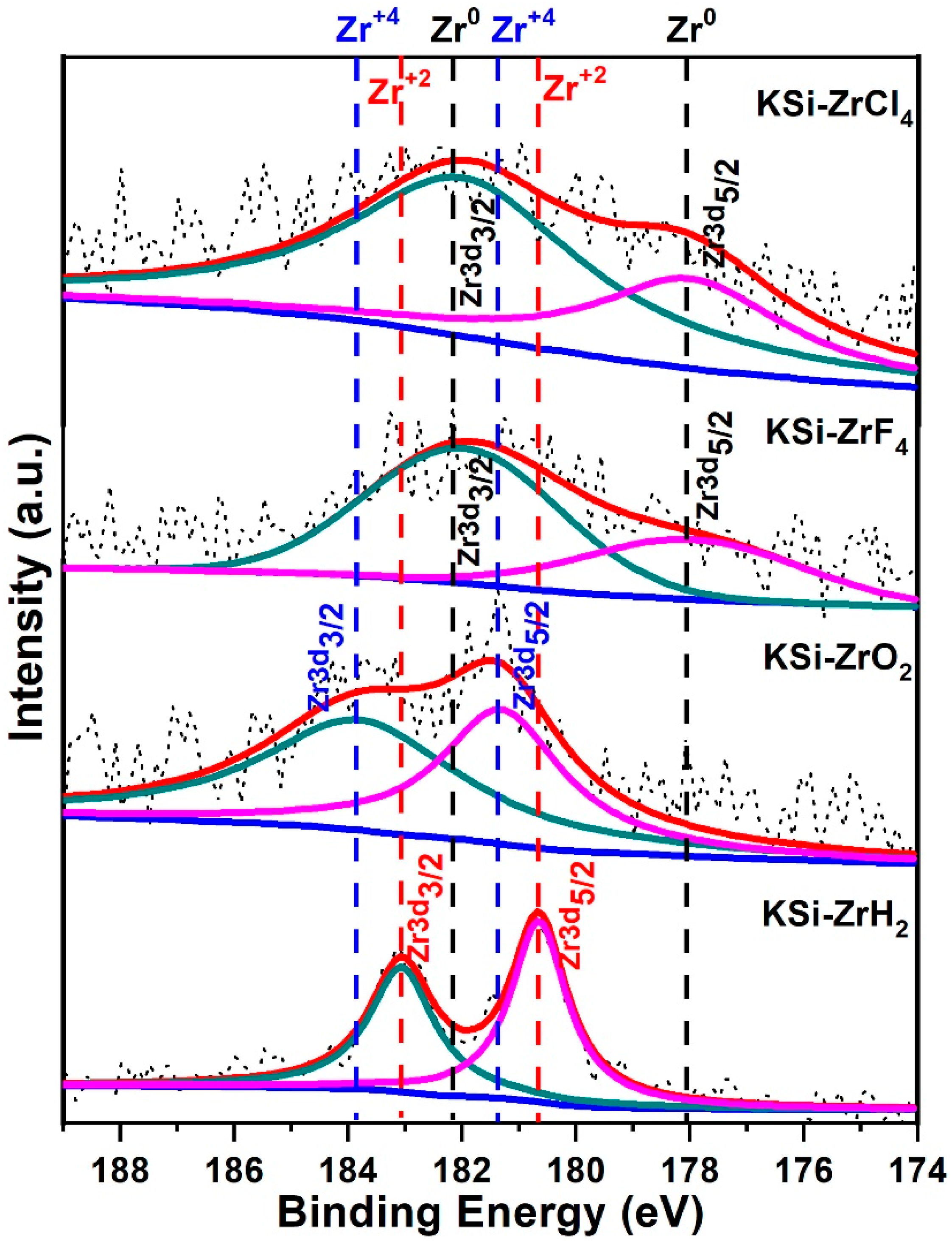
3.3. XPS Investigation for Mechanism of Zr-Based Catalysts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jain, I.P.; Lal, C.; Jain, A. Hydrogen Storage in Mg: A Most Promising Material. Int. J. Hydrogen Energy 2010, 35, 5133–5144. [Google Scholar] [CrossRef]
- Jain, A.; Miyaoka, H.; Ichikawa, T. Destabilization of lithium hydride by the substitution of group 14 elements: A review. Int. J. Hydrogen Energy 2016, 41, 5969–5978. [Google Scholar] [CrossRef]
- Jain, I.P.; Jain, P.; Jain, A. Novel hydrogen storage materials: A review of lightweight complex hydrides. J. Alloys Compd. 2010, 503, 303–339. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, S.; Qu, H.; Yu, X.F.; Peng, P. Alkali metal silanides α-MSiH3: A family of complex hydrides for solid-state hydrogen storage. Int. J. Hydrogen Energy 2017, 42, 12405–12413. [Google Scholar] [CrossRef]
- Available online: https://www.sciencedirect.com/science/article/abs/pii/S0360319915306728 (accessed on 16 March 2022).
- Chotard, J.-N.; Tang, W.S.; Raybaud, P.; Janot, R. Potassium Silanide (KSiH3): A Reversible Hydrogen Storage Material. Chem.-A Eur. J. 2011, 17, 12302–12309. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Ting, J.-M.; Agarwal, S.; Ichikawa, T.; Jain, A. The Catalytic Role of D-block Elements and Their Compounds for Improving Sorption Kinetics of Hydride Materials: A Review. Reactions 2021, 2, 333–364. [Google Scholar] [CrossRef]
- Jain, A.; Ichikawa, T.; Yamaguchi, S.; Miyaoka, H.; Kojima, Y. Catalytic modification in dehydrogenation properties of KSiH 3. Phys. Chem. Chem. Phys. 2014, 16, 26163–26167. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Miyaoka, H.; Ichikawa, T.; Kojima, Y. Tailoring the absorption-desorption properties of KSiH 3 compound using nano-metals (Ni, Co, Nb) as catalyst. J. Alloys Compd. 2015, 645, S144–S147. [Google Scholar] [CrossRef]
- Janot, R.; Tang, W.S.; Clémençon, D.; Chotard, J.-N. Catalyzed KSiH3 as a reversible hydrogen storage material. J. Mater. Chem. A 2016, 4, 19045–19052. [Google Scholar] [CrossRef]
- Sharma, S.; Guo, F.; Ichikawa, T.; Kojima, Y.; Agarwal, S.; Jain, A. Jain, Iron based catalyst for the improvement of the sorption properties of KSiH3. Int. J. Hydrogen Energy 2020, 45, 33681–33686. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, R.; Ichikawa, T.; Jain, A.; Agarwal, S. Milling induced surface modification of V-based catalyst to improve sorption kinetics of KSiH3: An XPS investigation. Int. J. Hydrogen Energy, 2022, in press. [CrossRef]
- Sharma, S.; Guo, F.; Ichikawa, T.; Kojima, Y.; Jain, A.; Agarwal, S. Enhancement in hydrogenation dehydrogenation kinetics of KSiH3 by the addition of Ti-based catalysts. Mater. Lett. 2021, 11, 100086. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, Z.; Zhu, X.; Yao, Z.; Sun, Z.; Ji, L.; Yan, N.; Xiao, B.; Chen, L. A striking catalytic effect of facile synthesized ZrMn2 nanoparticles on the de/rehydrogenation properties of MgH2. J. Alloys Compd. 2019, 805, 295–302. [Google Scholar] [CrossRef]
- Pighin, S.A.; Capurso, G.; Russo, S.L.; Peretti, H.A. Hydrogen sorption kinetics of magnesium hydride enhanced by the addition of Zr8Ni21 alloy. J. Alloys Compd. 2012, 530, 111–115. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Al-Matrouk, H.; Behbehani, M.; Shaban, E.; Alkandary, A.; Aldakheel, F.; Al-Saidi, M. Amorphous-versus big Cube-Zr2Ni for improving the kinetics of hydrogenation/dehydrogenation behaviors for MgH2 powders. Mater. Chem. Phys. 2018, 203, 17–26. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Y.; Xiao, X.; Lu, Y.; Zhang, M.; Zheng, J.; Chen, L. Highly efficient ZrH2 nanocatalyst for the superior hydrogenation kinetics of magnesium hydride under moderate conditions: Investigation and mechanistic insights. Appl. Surf. Sci. 2021, 541, 148375. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, Z.; Cai, Z.; Yan, N.; Lu, X.; Zhu, X.; Chen, L. Enhanced hydrogen storage properties of MgH2 by the synergetic catalysis of Zr0.4Ti0.6Co nanosheets and carbon nanotubes. Appl. Surf. Sci. 2020, 504, 144465. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, Z.; Yao, Z.; Ji, L.; Sun, Z.; Yan, N.; Zhang, B.; Xiao, B.; Du, J.; Zhu, X. Two-dimensional ZrCo nanosheets as highly effective catalyst for hydrogen storage in MgH2. J. Alloys Compd. 2019, 7, 5626–5634. [Google Scholar] [CrossRef]
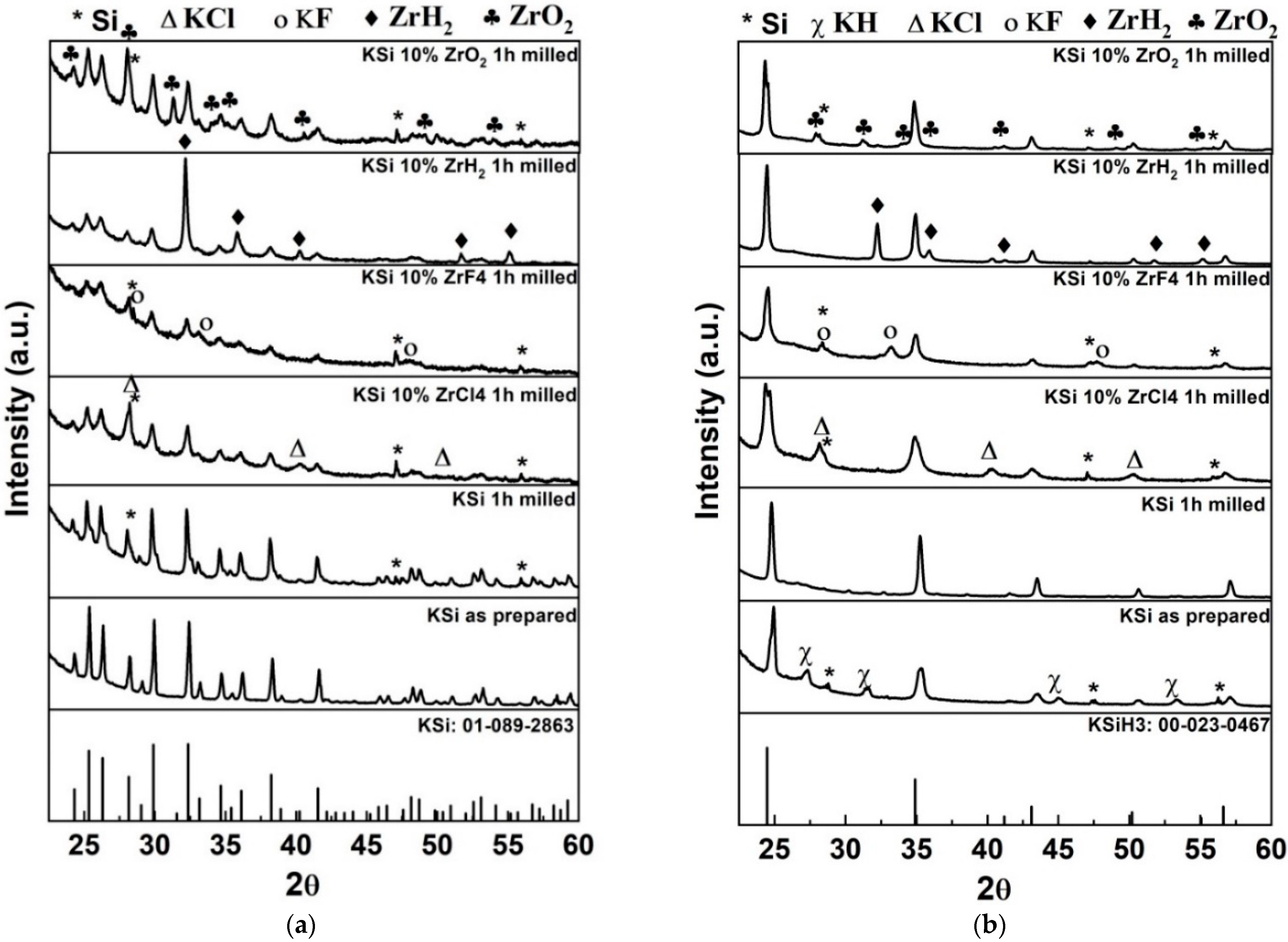
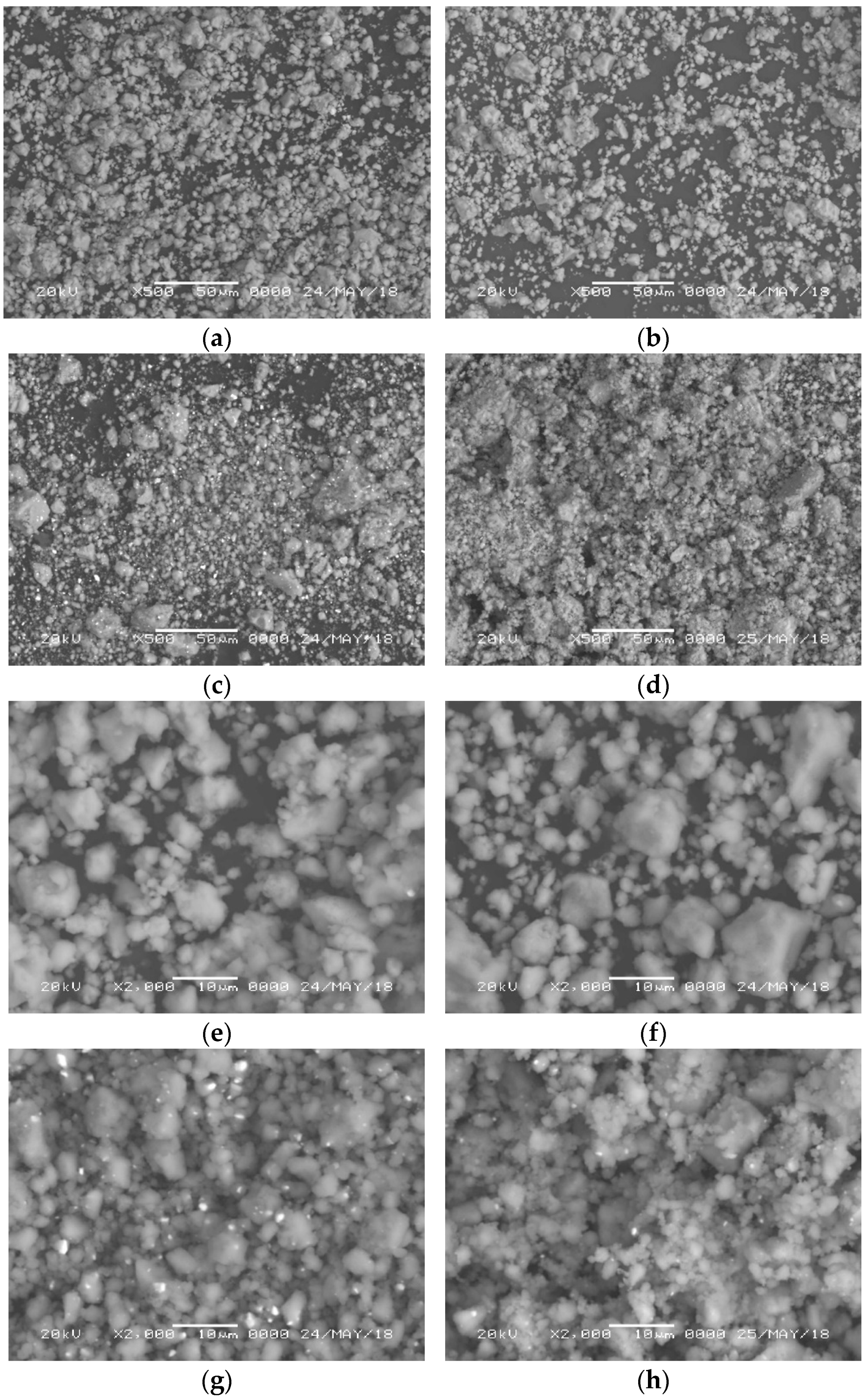
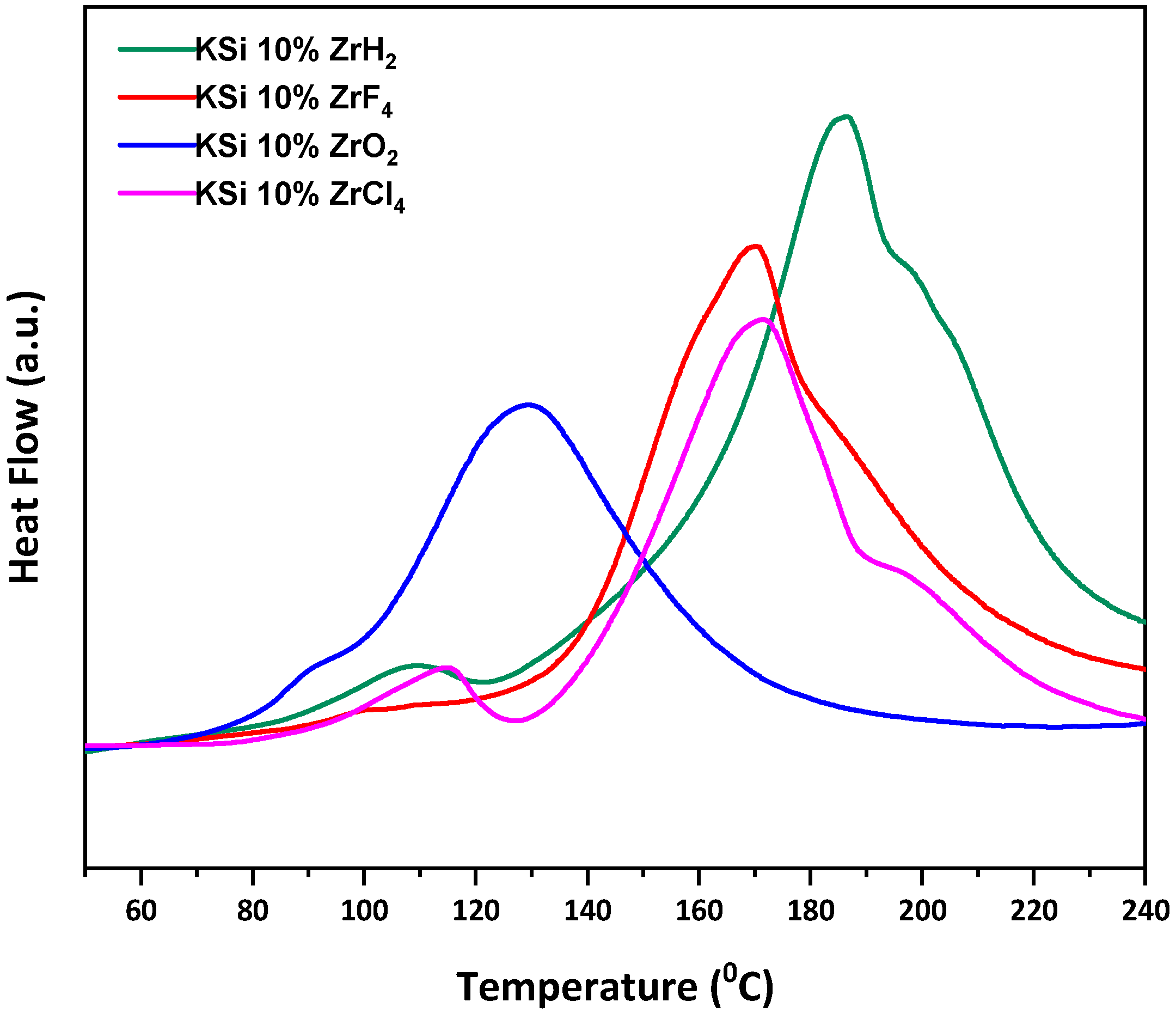
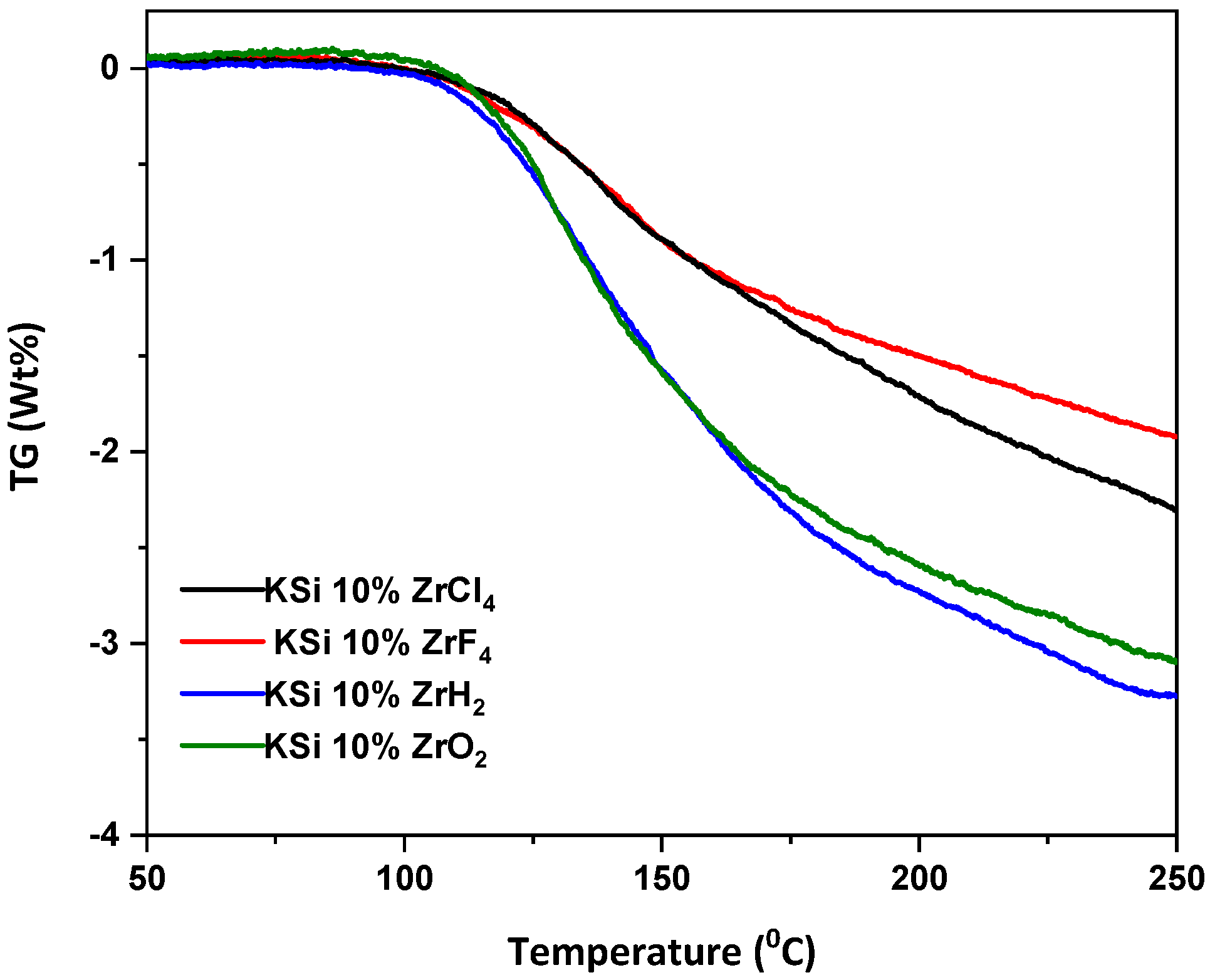
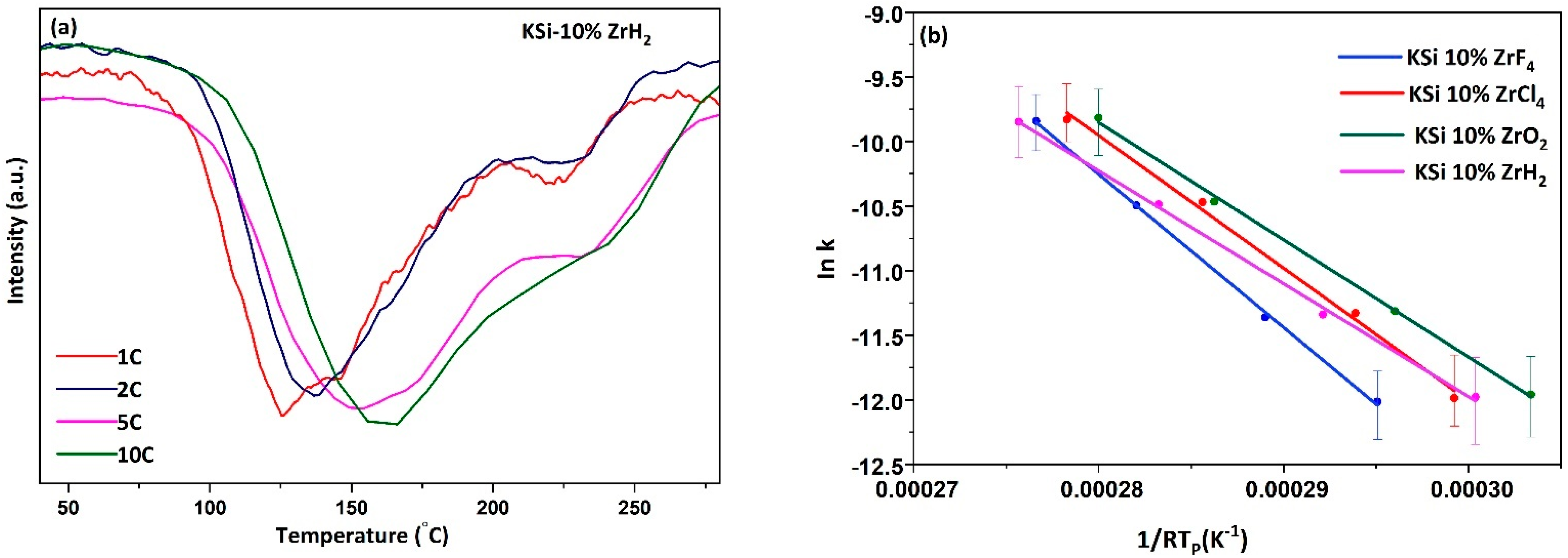
| Sample | Activation Energy (KJ mol−1) |
|---|---|
| KSi-ZrCl4 | 102.7 |
| KSi-ZrF4 | 118.6 |
| KSi-ZrO2 | 90.8 |
| KSi-ZrH2 | 87.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiwari, A.; Agarwal, S.; Shrivastava, K.; Ichikawa, T.; Jain, A.; Singh, R. XPS Investigation on Improving Hydrogen Sorption Kinetics of the KSiH3 System by Using Zr-Based Catalysts. Materials 2022, 15, 7454. https://doi.org/10.3390/ma15217454
Tiwari A, Agarwal S, Shrivastava K, Ichikawa T, Jain A, Singh R. XPS Investigation on Improving Hydrogen Sorption Kinetics of the KSiH3 System by Using Zr-Based Catalysts. Materials. 2022; 15(21):7454. https://doi.org/10.3390/ma15217454
Chicago/Turabian StyleTiwari, Anish, Shivani Agarwal, Kriti Shrivastava, Takayuki Ichikawa, Ankur Jain, and Rini Singh. 2022. "XPS Investigation on Improving Hydrogen Sorption Kinetics of the KSiH3 System by Using Zr-Based Catalysts" Materials 15, no. 21: 7454. https://doi.org/10.3390/ma15217454
APA StyleTiwari, A., Agarwal, S., Shrivastava, K., Ichikawa, T., Jain, A., & Singh, R. (2022). XPS Investigation on Improving Hydrogen Sorption Kinetics of the KSiH3 System by Using Zr-Based Catalysts. Materials, 15(21), 7454. https://doi.org/10.3390/ma15217454








