Minimization of Adverse Effects Associated with Dental Alloys
Abstract
1. Introduction
2. The Main Effects of Dental Alloys on the Human Organism
3. Clinical Manifestations of Adverse Effects Associated with Dental Alloys
3.1. Local Manifestations
- mouth burning without any visible lesions of the oral mucosa is commonly associated with galvanic current between different dental alloys. According to different authors, in patients with metal structures in the mouth, a burning sensation was observed with a frequency of 17% to 33% [39,44,45,46]. However, it should be kept in mind that burning mouth syndrome may develop in patients without metallic appliances and may be associated with a number of other systemic and local factors, such as vitamin deficiency, hormonal changes associated with menopause, local infections of the oral cavity, xerostomia, denture-related lesions, allergies, medications, and systemic diseases, including diabetes mellitus [47].
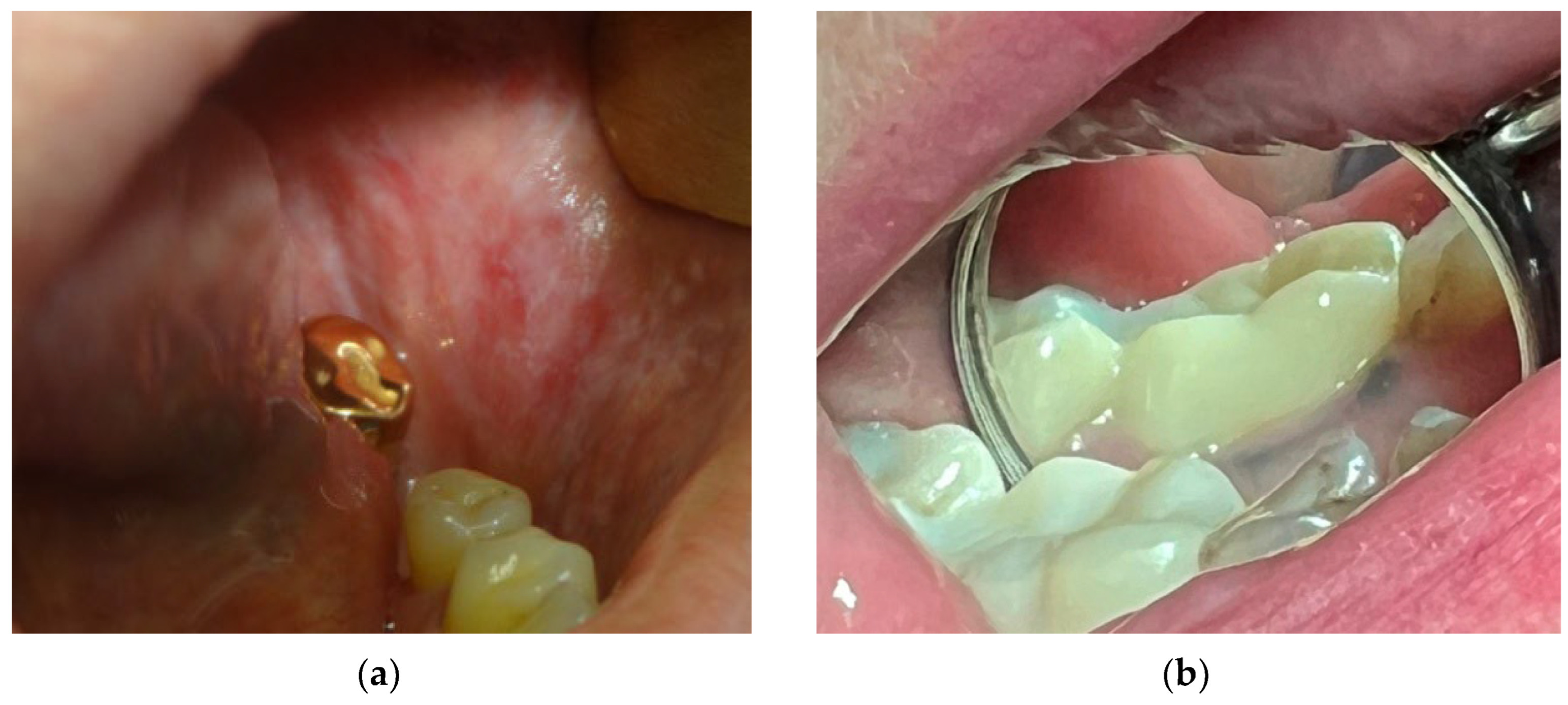
- oral lichen planus and lichenoid reaction (Figure 3a) manifest as multiple white papules, merging into the characteristic Wickham rete [48]. According to different authors, these phenomena are observed with a frequency of 12% to 78% among patients with metal structures in the mouth [44,49,50]. The condition may develop due to chronic irritation by galvanic current or as a delayed-type hypersensitivity reaction. Lichenoid reactions of the oral cavity are histologically or clinically indistinguishable from lichen planus, even though the latter may present within skin lesions and is not necessarily localized in direct contact with a metal structure. Both lichenoid reactions and lichen planus are precancerous [51].
- pigmentation of oral mucosa (Figure 3b) appears as a dark spot on the mucosa near to the metal structure, and it most often occurs upon contact with amalgam and silver-containing alloys. Moreover, metal particles can deposit on the oral mucosa during the placement or removal of amalgam fillings and appear as dark pigmented lesions [52]. In the presence of a galvanic couple, the pigmentation processes can be enhanced [53]. However, it must be taken into account that any dark pigmented lesion can be not only a benign discoloration but also potentially represent melanoma [54,55,56,57].
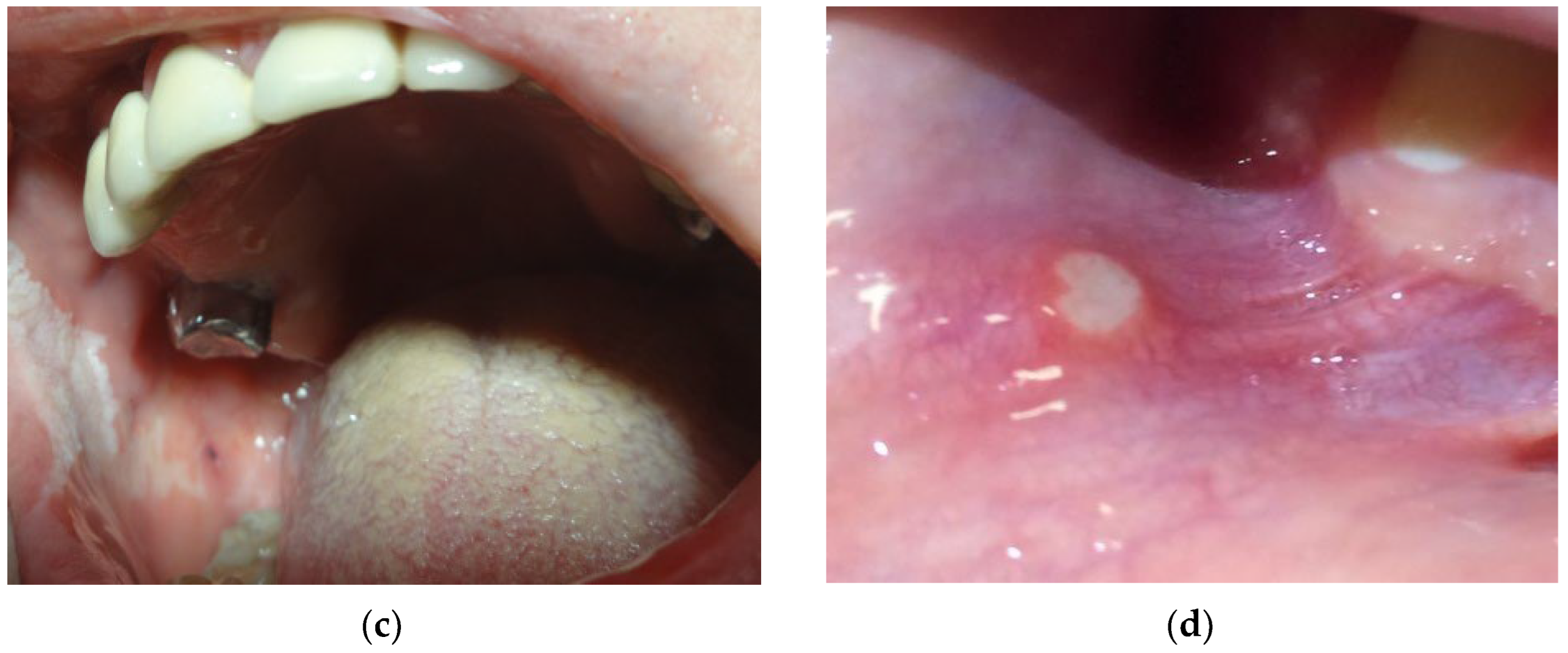
- leukoplakia (Figure 3c) is characterized by the emergence of increased keratinization areas on the mucous membrane. The prevalence of leukoplakia ranges from 0.5 to 3.4% and occurs most often in people older than 50 years [58]. It should be noted that the frequency of malignant transformation of leukoplakia ranges from 0.1 to 17% [59]. According to the observations of Gönen Z.B. et al., hyperkeratotic lesions may occur due to a hypersensitivity reaction to amalgam [58].
3.2. Systemic Adverse Effects of Dental Alloys
3.3. Associations between Dental Alloys and Systemic Diseases
4. Factors Affecting the Risk of Dental Alloys Side Effects
4.1. Corrosion Resistance and Biocompatability of Common Dental Alloys
4.2. Composition and pH of the Saliva
4.3. Oral Microbiota
4.4. Oral Care Products
4.5. Dietary Behaviors
4.6. Bad Habits
4.7. Systemic Diseases and Conditions
5. Approaches to Reduce the Risk of Adverse Events Associated with Dental Alloys
5.1. Industrial Methods to Reduce Corrosion of Dental Alloys
5.2. Clinical Recommendations for Treatment Planning
5.3. Recommendations for Patients with Metallic Prosthetic or Orthodontic Appliances
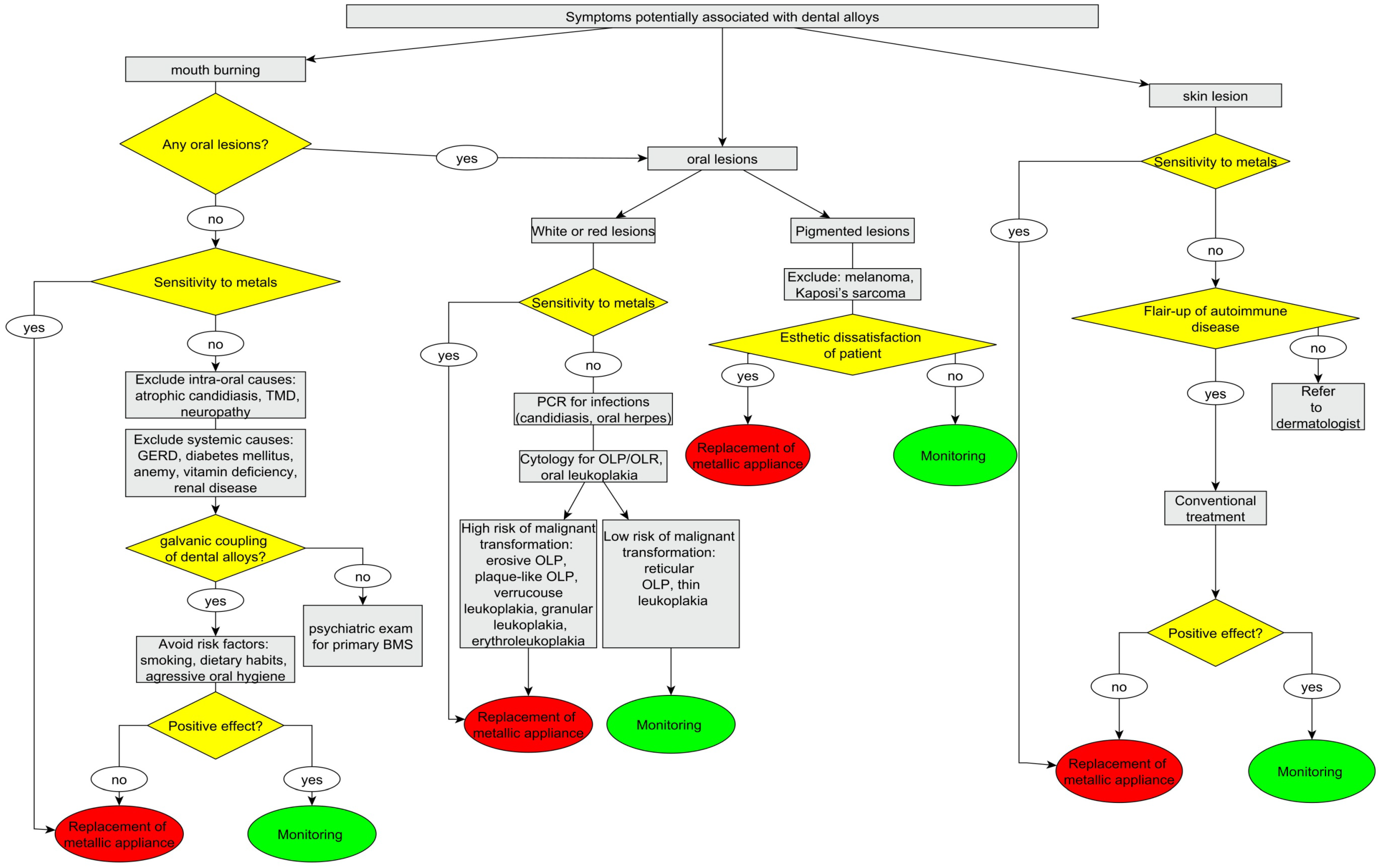
5.4. Recommendations for Patients with Oral and/or Systemic Symptoms, Potentially Associated with Dental Alloys
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zitzmann, N.U.; Hagmann, E.; Weiger, R. What Is the Prevalence of Various Types of Prosthetic Dental Restorations in Europe? Clin. Oral Implants Res. 2007, 18 (Suppl. 3), 20–33. [Google Scholar] [CrossRef] [PubMed]
- Elani, H.W.; Starr, J.R.; Da Silva, J.D.; Gallucci, G.O. Trends in Dental Implant Use in the U.S. 1999–2016, and Projections to 2026. J. Dent. Res. 2018, 97, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Borghei, S.; Broadbent, J.; Stevens, R.; Chaudhry, K.; Subramani, K. Orthodontists’ Preference on Type of Rigid Fixed Functional Appliance for Skeletal Class II Correction: A Survey Study. J. Clin. Exp. Dent. 2020, 12, e958–e963. [Google Scholar] [CrossRef]
- Marek, M. Interactions between Dental Amalgams and the Oral Environment. Adv. Dent. Res. 1992, 6, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.D.; Broughton, S.; Drangsholt, M. Oral Lichen Planus and Dental Materials: A Case-Control Study. Contact Dermat. 2003, 48, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.F. T Lymphocyte-Mediated Immune Responses to Chemical Haptens and Metal Ions: Implications for Allergic and Autoimmune Disease. Int. Arch. Allergy Immunol. 2004, 134, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, U.; Felicita, A.S.; Mahendra, L.; Kanji, M.A.; Varadarajan, S.; Raj, A.T.; Feroz, S.M.A.; Mehta, D.; Baeshen, H.A.; Patil, S. Assessing the Potential Association Between Microbes and Corrosion of Intra-Oral Metallic Alloy-Based Dental Appliances Through a Systematic Review of the Literature. Front. Bioeng. Biotechnol. 2021, 9, 631103. [Google Scholar] [CrossRef]
- Kaličanin, B.; Ajduković, Z. Influence of Saliva Medium on Freeing Heavy Metal Ion from Fixed Dentures. Sci. Total Environ. 2008, 397, 41–45. [Google Scholar] [CrossRef]
- Mathew, M.T.; Kerwell, S.; Lundberg, H.J.; Sukotjo, C.; Mercuri, L.G. Tribocorrosion and Oral and Maxillofacial Surgical Devices. Br. J. Oral Maxillofac. Surg. 2014, 52, 396–400. [Google Scholar] [CrossRef]
- Revathi, A.; Borrás, A.D.; Muñoz, A.I.; Richard, C.; Manivasagam, G. Degradation Mechanisms and Future Challenges of Titanium and Its Alloys for Dental Implant Applications in Oral Environment. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 1354–1368. [Google Scholar] [CrossRef]
- Dikopova, N.Z.; Volkov, A.G.; Arakelyan, M.G.; Makarenko, N.V.; Soxova, I.A.; Doroshina, V.J.; Arzukanyan, A.V.; Margaryan, E.G. The Study of the Electrochemical Potentials of Metal Structures in the Oral Cavity in Diseases of the Oral Mucosa. New Armen. Med. J. 2020, 14, 54–58. [Google Scholar]
- Mikulewicz, M.; Chojnacka, K.; Woźniak, B.; Downarowicz, P. Release of Metal Ions from Orthodontic Appliances: An in Vitro Study. Biol. Trace Elem. Res. 2012, 146, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Nimeri, G.; Curry, J.; Berzins, D.; Liu, D.; Ahuja, B.; Lobner, D. Cytotoxic Evaluation of Two Orthodontic Silver Solder Materials on Human Periodontal Ligament Fibroblast Cells and the Effects of Antioxidant and Antiapoptotic Reagents. Angle Orthod. 2021, 91, 349–355. [Google Scholar] [CrossRef]
- Ortiz, A.J.; Fernández, E.; Vicente, A.; Calvo, J.L.; Ortiz, C. Metallic Ions Released from Stainless Steel, Nickel-Free, and Titanium Orthodontic Alloys: Toxicity and DNA Damage. Am. J. Orthod. Dentofacial Orthop. 2011, 140, e115–e122. [Google Scholar] [CrossRef]
- McGinley, E.L.; Fleming, G.J.P.; Moran, G.P. Development of a Discriminatory Biocompatibility Testing Model for Non-Precious Dental Casting Alloys. Dent. Mater. 2011, 27, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Jiang, L.; Lin, H.; Cheng, H. Cell Death Affected by Dental Alloys: Modes and Mechanisms. Dent. Mater. J. 2017, 36, 82–87. [Google Scholar] [CrossRef]
- Kovač, V.; Poljšak, B.; Primožič, J.; Jamnik, P. Are Metal Ions That Make up Orthodontic Alloys Cytotoxic, and Do They Induce Oxidative Stress in a Yeast Cell Model? Int. J. Mol. Sci. 2020, 21, 7993. [Google Scholar] [CrossRef]
- Rincic Mlinaric, M.; Durgo, K.; Katic, V.; Spalj, S. Cytotoxicity and Oxidative Stress Induced by Nickel and Titanium Ions from Dental Alloys on Cells of Gastrointestinal Tract. Toxicol. Appl. Pharmacol. 2019, 383, 114784. [Google Scholar] [CrossRef]
- Sardaro, N.; della Vella, F.; Incalza, M.A.; Stasio, D.D.I.; Lucchese, A.; Contaldo, M.; Laudadio, C.; Petruzzi, M. Oxidative Stress and Oral Mucosal Diseases: An Overview. In Vivo 2019, 33, 289–296. [Google Scholar] [CrossRef]
- Imirzalioglu, P.; Alaaddinoglu, E.; Yilmaz, Z.; Oduncuoglu, B.; Yilmaz, B.; Rosenstiel, S. Influence of Recasting Different Types of Dental Alloys on Gingival Fibroblast Cytotoxicity. J. Prosthet. Dent. 2012, 107, 24–33. [Google Scholar] [CrossRef]
- Noumbissi, S.; Scarano, A.; Gupta, S. A Literature Review Study on Atomic Ions Dissolution of Titanium and Its Alloys in Implant Dentistry. Materials 2019, 12, 368. [Google Scholar] [CrossRef] [PubMed]
- Kheder, W.; Al Kawas, S.; Khalaf, K.; Samsudin, A.R. Impact of Tribocorrosion and Titanium Particles Release on Dental Implant Complications—A Narrative Review. Jpn. Dent. Sci. Rev. 2021, 57, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Loyola-Rodríguez, J.P.; Lastra-Corso, I.; García-Cortés, J.O.; Loyola-Leyva, A.; Domínguez-Pérez, R.A.; Avila-Arizmendi, D.; Contreras-Palma, G.; González-Calixto, C. In Vitro Determination of Genotoxicity Induced by Brackets Alloys in Cultures of Human Gingival Fibroblasts. J. Toxicol. 2020, 2020, 1467456. [Google Scholar] [CrossRef]
- Faccioni, F.; Franceschetti, P.; Cerpelloni, M.; Fracasso, M.E. In Vivo Study on Metal Release from Fixed Orthodontic Appliances and DNA Damage in Oral Mucosa Cells. Am. J. Orthod. Dentofac. Orthop. 2003, 124, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Baričević, M.; Ratkaj, I.; Mladinić, M.; Želježić, D.; Kraljević, S.P.; Lončar, B.; Stipetić, M.M. In Vivo Assessment of DNA Damage Induced in Oral Mucosa Cells by Fixed and Removable Metal Prosthodontic Appliances. Clin. Oral Investig. 2012, 16, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Alp, G.; Çakmak, G.; Sert, M.; Burgaz, Y. Corrosion Potential in Artificial Saliva and Possible Genotoxic and Cytotoxic Damage in Buccal Epithelial Cells of Patients Who Underwent Ni-Cr Based Porcelain-Fused-to-Metal Fixed Dental Prostheses. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2018, 827, 19–26. [Google Scholar] [CrossRef]
- Tomakidi, P.; Koke, U.; Kern, R.; Erdinger, L.; Krüger, H.; Kohl, A.; Komposch, G. Assessment of Acute Cyto- and Genotoxicity of Corrosion Eluates Obtained from Orthodontic Materials Using Monolayer Cultures of Immortalized Human Gingival Keratinocytes. J. Orofac. Orthop. 2000, 61, 2–19. [Google Scholar] [CrossRef]
- Angelieri, F.; Carlin, V.; Martins, R.A.; Ribeiro, D.A. Biomonitoring of Mutagenicity and Cytotoxicity in Patients Undergoing Fixed Orthodontic Therapy. Am. J. Orthod. Dentofacial Orthop. 2011, 139 (Suppl. 4), e399–e404. [Google Scholar] [CrossRef]
- Velasco-Ortega, E.; Jos, A.; Cameán, A.M.; Pato-Mourelo, J.; Segura-Egea, J.J. In Vitro Evaluation of Cytotoxicity and Genotoxicity of a Commercial Titanium Alloy for Dental Implantology. Mutat. Res. 2010, 702, 17–23. [Google Scholar] [CrossRef]
- Kitagawa, M.; Murakami, S.; Akashi, Y.; Oka, H.; Shintani, T.; Ogawa, I.; Inoue, T.; Kurihara, H. Current Status of Dental Metal Allergy in Japan. J. Prosthodont. Res. 2019, 63, 309–312. [Google Scholar] [CrossRef]
- Podzimek, S.; Tomka, M.; Sommerova, P.; Lyuya-Mi, Y.; Bartova, J.; Prochazkova, J. Immune Markers in Oral Discomfort Patients before and after Elimination of Oral Galvanism. Neuroendocrinol. Lett. 2013, 34, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Venclíková, Z.; Benada, O.; Bártová, J.; Joska, L.; Mrklas, L.; Procházková, J.; Stejskal, V.; Podzimek, Š. In Vivo Effects of Dental Casting Alloys. Neuro Endocrinol. Lett. 2006, 27 (Suppl. 1), 61–68. [Google Scholar] [PubMed]
- Kerosuo, H.M.; Dahl, J.E. Adverse Patient Reactions during Orthodontic Treatment with Fixed Appliances. Am. J. Orthod. Dentofac. Orthop. 2007, 132, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Balcheva, M.; Panov, V.; Krasteva, A.; Markova, M. Allergy to Dental Amalgam. J. Med. Dent. Pract. 2019, 6, 968–974. [Google Scholar] [CrossRef]
- de Morais, L.S.; Serra, G.G.; Albuquerque Palermo, E.F.; Andrade, L.R.; Müller, C.A.; Meyers, M.A.; Elias, C.N. Systemic Levels of Metallic Ions Released from Orthodontic Mini-Implants. Am. J. Orthod. Dentofacial Orthop. 2009, 135, 522–529. [Google Scholar] [CrossRef]
- El Sawy, A.A.; Shaarawy, M.A. Evaluation of Metal Ion Release from Ti6Al4V and Co-Cr-Mo Casting Alloys: In Vivo and In Vitro Study. J. Prosthodont. 2014, 23, 89–97. [Google Scholar] [CrossRef]
- Karnam, S.K.; Naveen Reddy, A.; Manjith, C.M. Comparison of Metal Ion Release from Different Bracket Archwire Combinations: An in Vitro Study. J. Contemp. Dent. Pract. 2012, 13, 376–381. [Google Scholar] [CrossRef]
- López-Alías, J.F.; Martinez-Gomis, J.; Anglada, J.M.; Peraire, M. Ion Release from Dental Casting Alloys as Assessed by a Continuous Flow System: Nutritional and Toxicological Implications. Dent. Mater. 2006, 22, 832–837. [Google Scholar] [CrossRef]
- Alnazzawi, A. Effect of Fixed Metallic Oral Appliances on Oral Health. J. Int. Soc. Prev. Community Dent. 2018, 8, 93. [Google Scholar] [CrossRef]
- Ayres, S. Sore Mouth Caused by Electrogalvanic Current. J. R. Soc. Med. 1984, 77, 708–709. [Google Scholar]
- Von Fraunhofer, J.A.; Staheli, P.J. The Measurement of Galvanic Corrosion Currents in Dental Amalgam. Corros. Sci. 1972, 12, 767–773. [Google Scholar] [CrossRef]
- Wartenberg, M.; Wirtz, N.; Grob, A.; Niedermeier, W.; Hescheler, J.; Peters, S.C.; Sauer, H. Direct Current Electrical Fields Induce Apoptosis in Oral Mucosa Cancer Cells by NADPH Oxidase-Derived Reactive Oxygen Species. Bioelectromagnetics 2008, 29, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Korraah, A.; Odenthal, M.; Kopp, M.; Vigneswaran, N.; Sacks, P.G.; Dienes, H.P.; Stützer, H.; Niedermeier, W. Induction of Apoptosis and Up-Regulation of Cellular Proliferation in Oral Leukoplakia Cell Lines inside Electric Field. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Koch, P.; Bahmer, F.A. Oral Lesions and Symptoms Related to Metals Used in Dental Restorations: A Clinical, Allergological, and Histologic Study. J. Am. Acad. Dermatol. 1999, 41, 422–430. [Google Scholar] [CrossRef]
- Coculescu, E.C.; Tovaru, S.; Coculescu, B.I. Epidemiological and Etiological Aspects of Burning Mouth Syndrome. J. Med. Life 2014, 7, 305–309. [Google Scholar]
- Zakrzewska, J.; Buchanan, J.A.G. Burning Mouth Syndrome. BMJ Clin. Evid. 2016, 2016, 1307. [Google Scholar]
- Maltsman-Tseikhin, A.; Moricca, P.; Niv, D. Burning Mouth Syndrome: Will Better Understanding Yield Better Management? Pain Pract. 2007, 7, 151–162. [Google Scholar] [CrossRef]
- Minciullo, P.L.; Paolino, G.; Vacca, M.; Gangemi, S.; Nettis, E. Unmet Diagnostic Needs in Contact Oral Mucosal Allergies. Clin. Mol. Allergy 2016, 14, 10. [Google Scholar] [CrossRef]
- McParland, H.; Warnakulasuriya, S. Oral Lichenoid Contact Lesions to Mercury and Dental Amalgam—A Review. J. Biomed. Biotechnol. 2012, 2016, 589569. [Google Scholar] [CrossRef]
- Thornhill, M.H.; Pemberton, M.N.; Simmons, R.K.; Theaker, E.D. Amalgam-Contact Hypersensitivity Lesions and Oral Lichen Planus. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 95, 291–299. [Google Scholar] [CrossRef]
- Schmidt-Westhausen, A.M. Oral Lichen Planus and Lichenoid Lesions: What’s New? Quintessence Int. 2020, 51, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Laimer, J.; Henn, R.; Helten, T.; Sprung, S.; Zelger, B.; Zelger, B.; Steiner, R.; Schnabl, D.; Offermanns, V.; Bruckmoser, E.; et al. Amalgam Tattoo versus Melanocytic Neoplasm—Differential Diagnosis of Dark Pigmented Oral Mucosa Lesions Using Infrared Spectroscopy. PLoS ONE 2018, 13, e0207026. [Google Scholar] [CrossRef] [PubMed]
- Sutow, E.J.; Maillet, W.A.; Hall, G.C. Corrosion Potential Variation of Aged Dental Amalgam Restorations over Time. Dent. Mater. 2006, 22, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Amano, H.; Tamura, A.; Yasuda, M.; Yamanaka, M.; Takeuchi, Y.; Sasaoka, K.; Yokoo, S.; Ishikawa, O. Amalgam Tattoo of the Oral Mucosa Mimics Malignant Melanoma. J. Dermatol. 2011, 38, 101–103. [Google Scholar] [CrossRef]
- Alawi, F. Pigmented Lesions of the Oral Cavity. An Update. Dent. Clin. N. Am. 2013, 57, 699–710. [Google Scholar] [CrossRef]
- Lambertini, M.; Patrizi, A.; Fanti, P.A.; Melotti, B.; Caliceti, U.; Magnoni, C.; Misciali, C.; Baraldi, C.; Ravaioli, G.M.; Dika, E. Oral Melanoma and Other Pigmentations: When to Biopsy? J. Eur. Acad. Dermatol. Venereol. 2018, 32, 209–214. [Google Scholar] [CrossRef]
- Vera-Kellet, C.; Del Barrio-Díaz, P. Oral Amalgam Tattoo Mimicking Melanoma. N. Engl. J. Med. 2016, 374, e21. [Google Scholar] [CrossRef]
- Gönen, Z.; Asan, C.Y.; Etöz, O.; Alkan, A. Oral Leukoplakia Associated with Amalgam Restorations. J. Oral Sci. 2016, 58, 445–448. [Google Scholar] [CrossRef]
- Holmstrup, P.; Vedtofte, P.; Reibel, J.; Stoltze, K. Long-Term Treatment Outcome of Oral Premalignant Lesions. Oral Oncol. 2006, 42, 461–474. [Google Scholar] [CrossRef]
- Pigatto, P.D.; Brambilla, L.; Ferrucci, S.M.; Ronchi, A.; Minoia, C.; Guzzi, G. Allergy and Adverse Reactions to Dental Amalgam: An Epidemiological Assessment. Contact Dermat. 2010, 63, 95. [Google Scholar]
- Muris, J.; Goossens, A.; Gonçalo, M.; Bircher, A.J.; Giménez-Arnau, A.; Foti, C.; Rustemeyer, T.; Feilzer, A.J.; Kleverlaan, C.J. Sensitization to Palladium and Nickel in Europe and the Relationship with Oral Disease and Dental Alloys. Contact Dermat. 2015, 72, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Valentine-Thon, E.; Müller, K.; Guzzi, G.; Kreisel, S.; Ohnsorge, P.; Sandkamp, M. LTT-MELISA® Is Clinically Relevant for Detecting and Monitoring Metal Sensitivity. Neuroendocrinol. Lett. 2006, 27 (Suppl. 1), 17–24. [Google Scholar] [PubMed]
- Vrbova, R.; Podzimek, S.; Himmlova, L.; Roubickova, A.; Janovska, M.; Janatova, T.; Bartos, M.; Vinsu, A. Titanium and Other Metal Hypersensitivity Diagnosed by MELISA® Test: Follow-Up Study. Biomed Res. Int. 2021, 2021, 5512091. [Google Scholar] [CrossRef] [PubMed]
- Pföhler, C.; Vogt, T.; Müller, C.S.L. Kontaktallergische Gastritis: Seltene Manifestation Einer Metallallergie. Hautarzt 2016, 67, 359–364. [Google Scholar] [CrossRef]
- Zigante, M.; Rincic Mlinaric, M.; Kastelan, M.; Perkovic, V.; Trinajstic Zrinski, M.; Spalj, S. Symptoms of Titanium and Nickel Allergic Sensitization in Orthodontic Treatment. Prog. Orthod. 2020, 21, 17. [Google Scholar] [CrossRef]
- Pamphlett, R.; Kum Jew, S. Inorganic Mercury in Human Astrocytes, Oligodendrocytes, Corticomotoneurons and the Locus Ceruleus: Implications for Multiple Sclerosis, Neurodegenerative Disorders and Gliomas. BioMetals 2018, 31, 807–819. [Google Scholar] [CrossRef]
- Casetta, I.; Invernizzi, M.; Granieri, E. Multiple Sclerosis and Dental Amalgam: Case-Control Study in Ferrara, Italy. Neuroepidemiology 2001, 20, 134–137. [Google Scholar] [CrossRef]
- Bates, M.N.; Fawcett, J.; Garrett, N.; Cutress, T.; Kjellstrom, T. Health Effects of Dental Amalgam Exposure: A Retrospective Cohort Study. Int. J. Epidemiol. 2004, 33, 894–902. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Chang, C.W.; Lee, H.L.; Chuang, C.C.; Chiu, H.C.; Li, W.Y.; Horng, J.T.; Fu, E. Association between History of Dental Amalgam Fillings and Risk of Parkinson’s Disease: A Population-Based Retrospective Cohort Study in Taiwan. PLoS ONE 2016, 11, e0166552. [Google Scholar] [CrossRef]
- Tseng, C.F.; Chen, K.H.; Yu, H.C.; Chang, Y.C. Association between Dental Amalgam Filling and Essential Tremor: A Nationwide Population-Based Case Control Study in Taiwan. Int. J. Environ. Res. Public Health 2020, 17, 780. [Google Scholar] [CrossRef]
- Tseng, C.F.; Chen, K.H.; Yu, H.C.; Huang, F.M.; Chang, Y.C. Dental Amalgam Fillings and Multiple Sclerosis: A Nationwide Population-Based Case-Control Study in Taiwan. Int. J. Environ. Res. Public Health 2020, 17, 2637. [Google Scholar] [CrossRef] [PubMed]
- Gallusi, G.; Libonati, A.; Piro, M.; Di Taranto, V.; Montemurro, E.; Campanella, V. Is Dental Amalgam a Higher Risk Factor Rather than Resin-Based Restorations for Systemic Conditions? A Systematic Review. Materials 2021, 14, 1980. [Google Scholar] [CrossRef] [PubMed]
- Geier, D.A.; Geier, M.R. Dental Amalgam Fillings and Mercury Vapor Safety Limits in American Adults. Hum. Exp. Toxicol. 2022, 41, 9603271221106341. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, V.; Hudecek, R.; Stejskal, J.; Sterzl, I. Diagnosis and Treatment of Metal-Induced Side-Effects. Neuroendocrinol. Lett. 2006, 27 (Suppl. 1), 7–16. [Google Scholar]
- Manousek, J.; Stejskal, V.; Kubena, P.; Jarkovsky, J.; Nemec, P.; Lokaj, P.; Dostalova, L.; Zadakova, A.; Pavlusova, M.; Benesova, K.; et al. Delayed-Type Hypersensitivity to Metals of Environmental Burden in Patients with Takotsubo Syndrome—Is There a Clinical Relevance? PLoS ONE 2016, 11, e0164786. [Google Scholar] [CrossRef]
- Sterzl, I.; Prochazkova, J.; Hrda, P.; Matucha, P.; Bartova, J.; Stejskal, V. Removal of Dental Amalgam Decreases Anti-TPO and Anti-Tg Autoantibodies in Patients with Autoimmune Thyroiditis. Neuroendocrinol. Lett. 2006, 27 (Suppl. 1), 25–30. [Google Scholar]
- Geier, D.A.; Geier, M.R. Dental Amalgams and the Incidence Rate of Arthritis among American Adults. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2021, 14, 11795441211016260. [Google Scholar] [CrossRef]
- Rachmawati, D.; Muris, J.; Scheper, R.J.; Rustemeyer, T.; Kleverlaan, C.J.; Feilzer, A.J.; Von Blomberg, B.M.E.; Van Hoogstraten, I.M.W. Continuing the Quest for Autoimmunity Due to Oral Metal Exposure. Autoimmunity 2015, 48, 494–501. [Google Scholar] [CrossRef]
- Kisakol, G. Dental Amalgam Implantation and Thyroid Autoimmunity. Bratisl. Lek. Listy 2014, 115, 22–24. [Google Scholar] [CrossRef]
- Chen, K.-H.; Yu, H.-C.; Chang, Y.-C. Analysis of Dental Amalgam Fillings on Primary Sjögren’s Syndrome. Medicine 2021, 100, e28031. [Google Scholar] [CrossRef]
- Muller, A.W.J.; Loon, L.A.J.; Davidson, C.L. Electrical Potentials of Restorations in Subjects without Oral Complaints. J. Oral Rehabil. 1990, 17, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Saǧlam, A.M.Ş.; Baysal, V.; Ceylan, A.M. Nickel and Cobalt Hypersensitive Reaction before and after Orthodontic Therapy in Children. J. Contemp. Dent. Pract. 2004, 5, 79–90. [Google Scholar] [CrossRef]
- Bilhan, H.; Bilgin, T.; Cakir, A.F.; Yuksel, B.; Von Fraunhofer, J.A. The Effect of Mucine, IgA, Urea, and Lysozyme on the Corrosion Behavior of Various Non-Precious Dental Alloys and Pure Titanium in Artificial Saliva. J. Biomater. Appl. 2007, 22, 197–221. [Google Scholar] [CrossRef] [PubMed]
- Mercieca, S.; Caligari Conti, M.; Buhagiar, J.; Camilleri, J. Assessment of Corrosion Resistance of Cast Cobalt- and Nickel-Chromium Dental Alloys in Acidic Environments. J. Appl. Biomater. Funct. Mater. 2018, 16, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Jirau-Colón, H.; González-Parrilla, L.; Martinez-Jiménez, J.; Adam, W.; Jiménez-Velez, B. Rethinking the Dental Amalgam Dilemma: An Integrated Toxicological Approach. Int. J. Environ. Res. Public Health 2019, 16, 1036. [Google Scholar] [CrossRef]
- Senkutvan, R.S.; Jacob, S.; Charles, A.; Vadgaonkar, V.; Jatol-Tekade, S.; Gangurde, P. Evaluation of Nickel Ion Release from Various Orthodontic Arch Wires: An in Vitro Study. J. Int. Soc. Prev. Community Dent. 2014, 4, 12. [Google Scholar] [CrossRef]
- Kassapidou, M.; Hjalmarsson, L.; Johansson, C.B.; Hammarström Johansson, P.; Morisbak, E.; Wennerberg, A.; Franke Stenport, V. Cobalt-Chromium Alloys Fabricated with Four Different Techniques: Ion Release, Toxicity of Released Elements and Surface Roughness. Dent. Mater. 2020, 36, e352–e363. [Google Scholar] [CrossRef]
- Sarraf, M.; Rezvani Ghomi, E.; Alipour, S.; Ramakrishna, S.; Liana Sukiman, N. A State-of-the-Art Review of the Fabrication and Characteristics of Titanium and Its Alloys for Biomedical Applications. Bio-Des. Manuf. 2022, 5, 371. [Google Scholar] [CrossRef]
- Nagay, B.E.; Cordeiro, J.M.; Barao, V.A.R. Insight into Corrosion of Dental Implants: From Biochemical Mechanisms to Designing Corrosion-Resistant Materials. Curr. Oral Heal. Rep. 2022, 9, 7. [Google Scholar] [CrossRef]
- Cordeiro, J.M.; Barão, V.A.R. Is There Scientific Evidence Favoring the Substitution of Commercially Pure Titanium with Titanium Alloys for the Manufacture of Dental Implants? Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 1201–1215. [Google Scholar] [CrossRef]
- Sedarat, C.; Harmand, M.F.; Naji, A.; Nowzari, H. In Vitro Kinetic Evaluation of Titanium Alloy Biodegradation. J. Periodontal Res. 2001, 36, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.C.; Tokuhara, C.K.; Rocha, L.A.; Oliveira, R.C.; Lisboa-Filho, P.N.; Costa Pessoa, J. Vanadium Ionic Species from Degradation of Ti-6Al-4V Metallic Implants: In Vitro Cytotoxicity and Speciation Evaluation. Mater. Sci. Eng. C 2019, 96, 730–739. [Google Scholar] [CrossRef] [PubMed]
- van Vuuren, L.J.; Odendaal, J.S.; Pistorius, P.C. Galvanic Corrosion of Dental Cobalt-Chromium Alloys and Dental Amalgam in Artificial Saliva. SADJ 2008, 63, 034–038. [Google Scholar]
- Ciszewski, A.; Baraniak, M.; Urbanek-Brychczyńska, M. Corrosion by Galvanic Coupling between Amalgam and Different Chromium-Based Alloys. Dent. Mater. 2007, 23, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Taher, N.M.; Al Jabab, A.S. Galvanic Corrosion Behavior of Implant Suprastructure Dental Alloys. Dent. Mater. 2003, 19, 54–59. [Google Scholar] [CrossRef]
- Iijima, M.; Endo, K.; Yuasa, T.; Ohno, H.; Hayashi, K.; Kakizaki, M.; Mizoguchi, I. Galvanic Corrosion Behavior of Orthodontic Archwire Alloys Coupled to Bracket Alloys. Angle Orthod. 2006, 76, 705–711. [Google Scholar] [CrossRef]
- Nayak, R.S.; Shafiuddin, B.; Pasha, A.; Vinay, K.; Narayan, A.; Shetty, S.V. Comparison of Galvanic Currents Generated Between Different Combinations of Orthodontic Brackets and Archwires Using Potentiostat: An In Vitro Study. J. Int. Oral Health 2015, 7, 29–35. [Google Scholar] [PubMed]
- Oh, K.T.; Kim, K.N. Electrochemical Properties of Suprastructures Galvanically Coupled to a Titanium Implant. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 70, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Soares, F.M.S.; Elias, C.N.; Monteiro, E.S.; Coimbra, M.E.R.; Santana, A.I.C. Galvanic Corrosion of Ti Dental Implants Coupled to CoCrMo Prosthetic Component. Int. J. Biomater. 2021, 2021, 1313343. [Google Scholar] [CrossRef] [PubMed]
- Mellado-Valero, A.; Muñoz, A.I.; Pina, V.G.; Sola-Ruiz, M.F. Electrochemical Behaviour and Galvanic Effects of Titanium Implants Coupled to Metallic Suprastructures in Artificial Saliva. Materials 2018, 11, 171. [Google Scholar] [CrossRef]
- Amine, M.; Merdma, W.; El Boussiri, K. Electrogalvanism in Oral Implantology: A Systematic Review. Int. J. Dent. 2022, 2022, 4575416. [Google Scholar] [CrossRef]
- Sikora, C.L.; Alfaro, M.F.; Yuan, J.C.C.; Barao, V.A.; Sukotjo, C.; Mathew, M.T. Wear and Corrosion Interactions at the Titanium/Zirconia Interface: Dental Implant Application. J. Prosthodont. 2018, 27, 842–852. [Google Scholar] [CrossRef] [PubMed]
- ISO 10271:2020; Dentistry—Corrosion Test Methods for Metallic Materials. ISO: Geneva, Switzerland, 2020. Available online: https://www.iso.org/standard/73445.html (accessed on 17 October 2022).
- Barão, V.A.R.; Ramachandran, R.A.; Matos, A.O.; Badhe, R.V.; Grandini, C.R.; Sukotjo, C.; Ozevin, D.; Mathew, M. Prediction of Tribocorrosion Processes in Titanium-Based Dental Implants Using Acoustic Emission Technique: Initial Outcome. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 123, 112000. [Google Scholar] [CrossRef] [PubMed]
- Elshahawy, W.M.; Watanabe, I.; Kramer, P. In Vitro Cytotoxicity Evaluation of Elemental Ions Released from Different Prosthodontic Materials. Dent. Mater. 2009, 25, 1551–1555. [Google Scholar] [CrossRef]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef] [PubMed]
- Fage, S.W.; Muris, J.; Jakobsen, S.S.; Thyssen, J.P. Titanium: A Review on Exposure, Release, Penetration, Allergy, Epidemiology, and Clinical Reactivity. Contact Dermat. 2016, 74, 323–345. [Google Scholar] [CrossRef]
- ISO 7405:2018; Dentistry—Evaluation of Biocompatibility of Medical Devices used in Dentistry. ISO: Geneva, Switzerland, 2018. Available online: https://www.iso.org/standard/71503.html (accessed on 17 October 2022).
- Klausner, M.; Handa, Y.; Aizawa, S. In Vitro Three-Dimensional Organotypic Culture Models of the Oral Mucosa. Vitr. Cell. Dev. Biol. Anim. 2021, 57, 148–159. [Google Scholar] [CrossRef]
- Rahimi, C.; Rahimi, B.; Padova, D.; Rooholghodos, S.A.; Bienek, D.R.; Luo, X.; Kaufman, G.; Raub, C.B. Oral Mucosa-on-a-Chip to Assess Layer-Specific Responses to Bacteria and Dental Materials. Biomicrofluidics 2018, 12, 054106. [Google Scholar] [CrossRef]
- Koning, J.J.; Rodrigues Neves, C.T.; Schimek, K.; Thon, M.; Spiekstra, S.W.; Waaijman, T.; de Gruijl, T.D.; Gibbs, S. A Multi-Organ-on-Chip Approach to Investigate How Oral Exposure to Metals Can Cause Systemic Toxicity Leading to Langerhans Cell Activation in Skin. Front. Toxicol. 2022, 3, 824825. [Google Scholar] [CrossRef]
- Lu, Y.P.; Huang, J.W.; Lee, I.N.; Weng, R.C.; Lin, M.Y.; Yang, J.T.; Lin, C.T. A Portable System to Monitor Saliva Conductivity for Dehydration Diagnosis and Kidney Healthcare. Sci. Rep. 2019, 9, 14771. [Google Scholar] [CrossRef]
- Spirk, C.; Hartl, S.; Pritz, E.; Gugatschka, M.; Kolb-Lenz, D.; Leitinger, G.; Roblegg, E. Comprehensive Investigation of Saliva Replacement Liquids for the Treatment of Xerostomia. Int. J. Pharm. 2019, 571, 118759. [Google Scholar] [CrossRef] [PubMed]
- Winter, C.; Keimel, R.; Gugatschka, M.; Kolb, D.; Leitinger, G.; Roblegg, E. Investigation of Changes in Saliva in Radiotherapy-Induced Head Neck Cancer Patients. Int. J. Environ. Res. Public Health 2021, 18, 1629. [Google Scholar] [CrossRef]
- Xu, F.; Laguna, L.; Sarkar, A. Aging-Related Changes in Quantity and Quality of Saliva: Where Do We Stand in Our Understanding? J. Texture Stud. 2019, 50, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Bel’skaya, L.V.; Kosenok, V.K.; Sarf, E.A. Chronophysiological Features of the Normal Mineral Composition of Human Saliva. Arch. Oral Biol. 2017, 82, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Saibaba, G.; Srinivasan, M.; Priya Aarthy, A.; Silambarasan, V.; Archunan, G. Ultrastructural and Physico-Chemical Characterization of Saliva during Menstrual Cycle in Perspective of Ovulation in Human. Drug Discov. Ther. 2017, 11, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Sridhar, S.; Aghyarian, S.; Watkins-Curry, P.; Chan, J.Y.; Pozzi, A.; Rodrigues, D.C. Corrosion Behavior of Zirconia in Acidulated Phosphate Fluoride. J. Appl. Oral Sci. 2016, 24, 52–60. [Google Scholar] [CrossRef]
- Koppolu, P.; Sirisha, S.; Penala, S.; Reddy, P.K.; Alotaibi, D.H.; Abusalim, G.S.; Lingam, A.S.; Mukhtar, A.H.; Barakat, A.; Almokhatieb, A.A. Correlation of Blood and Salivary PH Levels in Healthy, Gingivitis, and Periodontitis Patients before and after Non-Surgical Periodontal Therapy. Diagnostics 2022, 12, 97. [Google Scholar] [CrossRef]
- Ferrer, M.D.; Pérez, S.; Lopez, A.L.; Sanz, J.L.; Melo, M.; Llena, C.; Mira, A. Evaluation of Clinical, Biochemical and Microbiological Markers Related to Dental Caries. Int. J. Environ. Res. Public Health 2021, 18, 6049. [Google Scholar] [CrossRef]
- Borg, W.; Cassar, G.; Camilleri, L.; Attard, N.; Camilleri, J. Surface Microstructural Changes and Release of Ions from Dental Metal Alloy Removable Prostheses in Patients Suffering from Acid Reflux. J. Prosthodont. 2018, 27, 115–119. [Google Scholar] [CrossRef]
- Dosedělová, V.; Ďurč, P.; Dolina, J.; Konečný, Š.; Foret, F.; Kubáň, P. Analysis of Bicarbonate, Phosphate and Other Anions in Saliva by Capillary Electrophoresis with Capacitively Coupled Contactless Conductivity Detection in Diagnostics of Gastroesophageal Reflux Disease. Electrophoresis 2020, 41, 116–122. [Google Scholar] [CrossRef]
- Muralidharan, D.; Fareed, N.; Pradeep, P.V.; Margabandhu, S.; Ramalingam, K.; Ajith Kumar, B.V. Qualitative and Quantitative Changes in Saliva among Patients with Thyroid Dysfunction Prior to and Following the Treatment of the Dysfunction. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Marín Martínez, L.; Molino Pagán, D.; López Jornet, P. Trace Elements in Saliva as Markers of Type 2 Diabetes Mellitus. Biol. Trace Elem. Res. 2018, 186, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Quade, B.N.; Parker, M.D.; Occhipinti, R. The Therapeutic Importance of Acid-Base Balance. Biochem. Pharmacol. 2021, 183, 114278. [Google Scholar] [CrossRef] [PubMed]
- Rahiotis, C.; Petraki, V.; Mitrou, P. Changes in Saliva Characteristics and Carious Status Related to Metabolic Control in Patients with Type 2 Diabetes Mellitus. J. Dent. 2021, 108, 103629. [Google Scholar] [CrossRef]
- Geckili, O.; Bilhan, H.; Bilgin, T.; Von Fraunhofer, J.A. The Effect of Urea on the Corrosion Behavior of Different Dental Alloys. Indian J. Dent. Res. 2012, 23, 75. [Google Scholar] [CrossRef]
- Teixeira, H.; Branco, A.C.; Rodrigues, I.; Silva, D.; Cardoso, S.; Colaço, R.; Serro, A.P.; Figueiredo-Pina, C.G. Effect of Albumin, Urea, Lysozyme and Mucin on the Triboactivity of Ti6Al4V/Zirconia Pair Used in Dental Implants. J. Mech. Behav. Biomed. Mater. 2021, 118, 104451. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, L.-C.; Wu, B.; Yu, L.-Y.; Wang, X.-P.; Liu, Y. A Comparative Analysis of Metal Allergens Associated with Dental Alloy Prostheses and the Expression of HLA-DR in Gingival Tissue. Mol. Med. Rep. 2016, 13, 91–98. [Google Scholar] [CrossRef]
- McGinley, E.L.; Dowling, A.H.; Moran, G.P.; Fleming, G.J.P. Influence of S. Mutans on Base-Metal Dental Casting Alloy Toxicity. J. Dent. Res. 2013, 92, 92–97. [Google Scholar] [CrossRef]
- Mystkowska, J.; Niemirowicz-Laskowska, K.; Łysik, D.; Tokajuk, G.; Dąbrowski, J.R.; Bucki, R. The Role of Oral Cavity Biofilm on Metallic Biomaterial Surface Destruction–Corrosion and Friction Aspects. Int. J. Mol. Sci. 2018, 19, 743. [Google Scholar] [CrossRef]
- Mathew, M.T.; Barão, V.A.; Yuan, J.C.C.; Assunção, W.G.; Sukotjo, C.; Wimmer, M.A. What Is the Role of Lipopolysaccharide on the Tribocorrosive Behavior of Titanium? J. Mech. Behav. Biomed. Mater. 2012, 8, 71–85. [Google Scholar] [CrossRef]
- Figueiredo-Pina, C.G.; Guedes, M.; Sequeira, J.; Pinto, D.; Bernardo, N.; Carneiro, C. On the Influence of Streptococcus Salivarius on the Wear Response of Dental Implants: An in Vitro Study. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Fais, L.M.G.; Fernandes-Filho, R.B.; Pereira-Da-Silva, M.A.; Vaz, L.G.; Adabo, G.L. Titanium Surface Topography after Brushing with Fluoride and Fluoride-Free Toothpaste Simulating 10 Years of Use. J. Dent. 2012, 40, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.C.L.; de Bordin, A.R.V.; Pedrazzi, V.; Rodrigues, R.C.S.; Ribeiro, R.F. Effect of Whitening Toothpaste on Titanium and Titanium Alloy Surfaces. Braz. Oral Res. 2012, 26, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Nogués, L.; Martinez-Gomis, J.; Molina, C.; Peraire, M.; Salsench, J.; Sevilla, P.; Gil, F.J. Dental Casting Alloys Behaviour during Power Toothbrushing with Toothpastes with Various Abrasivities. Part I: Wear Behavior. J. Mater. Sci. Mater. Med. 2008, 19, 3041–3048. [Google Scholar] [CrossRef]
- Shuto, T.; Mine, Y.; Makihira, S.; Nikawa, H.; Wachi, T.; Kakimoto, K. Alterations to Titanium Surface Depending on the Fluorides and Abrasives in Toothpaste. Materials 2021, 15, 51. [Google Scholar] [CrossRef]
- Anwar, E.M.; Kheiralla, L.S.; Tammam, R.H. Effect of Fluoride on the Corrosion Behavior of Ti and Ti6Al4V Dental Implants Coupled with Different Superstructures. J. Oral Implantol. 2011, 37, 309–317. [Google Scholar] [CrossRef]
- Erdogan, A.T.; Nalbantgil, D.; Ulkur, F.; Sahin, F. Metal Ion Release from Silver Soldering and Laser Welding Caused by Different Types of Mouthwash. Angle Orthod. 2015, 85, 665–672. [Google Scholar] [CrossRef]
- Polychronis, G.; Al Jabbari, Y.S.; Eliades, T.; Zinelis, S. Galvanic Coupling of Steel and Gold Alloy Lingual Brackets with Orthodontic Wires: Is Corrosion a Concern? Angle Orthod. 2018, 88, 450–457. [Google Scholar] [CrossRef]
- Jafari, K.; Rahimzadeh, S.; Hekmatfar, S. Nickel Ion Release from Dental Alloys in Two Different Mouthwashes. J. Dent. Res. Dent. Clin. Dent. Prospect. 2019, 13, 19–23. [Google Scholar] [CrossRef]
- Rincic Mlinaric, M.; Karlovic, S.; Ciganj, Z.; Acev, D.P.; Pavlic, A.; Spalj, S. Oral Antiseptics and Nickel-Titanium Alloys: Mechanical and Chemical Effects of Interaction. Odontology 2019, 107, 150–157. [Google Scholar] [CrossRef]
- Pavlic, A.; Perissinotto, F.; Turco, G.; Contardo, L.; Stjepan, S. Do Chlorhexidine and Probiotics Solutions Provoke Corrosion of Orthodontic Mini-Implants? An In Vitro Study. Int. J. Oral Maxillofac. Implant. 2019, 33, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Pupim, D.; Peixoto, R.F.; MacEdo, A.P.; Palma-Dibb, R.G.; De Mattos, M.D.G.C.; Galo, R. Influence of the Commercial Mouthwashes on the Corrosion Behaviour of Dental Alloy. Mater. Res. 2022, 25, e20210385. [Google Scholar] [CrossRef]
- Shahabi, M.; Jahanbin, A.; Esmaily, H.; Sharifi, H.; Salari, S. Comparison of Some Dietary Habits on Corrosion Behavior of Stainless Steel Brackets: An in Vitro Study. J. Clin. Pediatr. Dent. 2011, 35, 429–432. [Google Scholar] [CrossRef]
- Rachmawati, D.; Cahyasari, D.A.; Febiantama, A.T.; Hidayati, L.; Kleverlaan, C.J. Brewed Robusta Coffee Increases Nickel Ion Release from Dental Alloys: An In Vitro Study. Materials 2021, 14, 7069. [Google Scholar] [CrossRef]
- Parenti, S.I.; Guicciardi, S.; Melandri, C.; Sprio, S.; Lafratta, E.; Tampieri, A.; Bonetti, G.A. Effect of Soft Drinks on the Physical and Chemical Features of Nickel-Titanium-Based Orthodontic Wires. Acta Odontol. Scand. 2012, 70, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhao, M.; Chen, W.; Xu, K.; Huang, F.; Qu, J.; Xu, Z.; Wang, X.; Wang, Y.; Zhu, Y.; et al. Mainstream Cigarette Smoke Induces Autophagy and Promotes Apoptosis in Oral Mucosal Epithelial Cells. Arch. Oral Biol. 2020, 111, 104646. [Google Scholar] [CrossRef] [PubMed]
- Balaji, S. Tobacco Smoking and Surgical Healing of Oral Tissues: A Review. Indian J. Dent. Res. 2008, 19, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, S.Y.; Degirmenci, N.; Tugrul, S.; Ozturan, O. Effects of Smoking on Healthy Oral Mucosa: A Red-Green-Blue (Photographic) Evaluation. Mater. Res. 2017, 13 (Suppl. 27), 9–13. [Google Scholar]
- Yagyuu, T.; Funayama, N.; Imada, M.; Kirita, T. Effect of Smoking Status and Programmed Death-Ligand 1 Expression on the Microenvironment and Malignant Transformation of Oral Leukoplakia: A Retrospective Cohort Study. PLoS ONE 2021, 16, e0250359. [Google Scholar] [CrossRef]
- dos Santos Maidana, M.; Varela Junior, A.S.; Corcini, C.D.; Pereira, J.R.; Pires, D.M.; Tavella, R.A.; Fernandes, C.L.F.; dos Santos, M.; Garcia, E.M.; da Silva Júnior, F.M.R. Oral Cytological Changes in Young Adults Related to Alcohol Consumption. Arch. Oral Biol. 2021, 126, 105127. [Google Scholar] [CrossRef]
- Vassoler, T.; Dogenski, L.C.; Sartori, V.K.; Presotto, J.S.; Cardoso, M.Z.; Zandoná, J.; Trentin, M.S.; Linden, M.S.; Palhano, H.S.; Vargas, J.E.; et al. Evaluation of the Genotoxicity of Tobacco and Alcohol in Oral Mucosa Cells: A Pilot Study. J. Contemp. Dent. Pract. 2021, 22, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Saluja, P.; Shetty, V.; Dave, A.; Arora, M.; Hans, V.; Madan, A. Comparative Evaluation of the Effect of Menstruation, Pregnancy and Menopause on Salivary Flow Rate, PH and Gustatory Function. J. Clin. Diagn. Res. 2014, 8, ZC81. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, D.R.; Komali, G.; Jayanthi, K.; Dinesh, D.; Saikavitha, T.V.; Dinesh, P. Evaluation of Salivary Flow Rate, PH and Buffer in Pre, Post & Post Menopausal Women on HRT. J. Clin. Diagn. Res. 2014, 8, 233–236. [Google Scholar] [CrossRef]
- Ciesielska, A.; Kusiak, A.; Ossowska, A.; Grzybowska, M.E. Changes in the Oral Cavity in Menopausal Women—A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 253. [Google Scholar] [CrossRef]
- Okada, S.; Iwata, K.; Katagiri, A. Pathognomonic Hypersensitivity of the Oral Mucosa and Tongue Induced by Diabetes Mellitus Accompanied by Saliva Reduction in Rats. J. Oral Facial Pain Headache 2021, 35, 54–61. [Google Scholar] [CrossRef]
- Marín-Martínez, L.; Molino-Pagán, D.; López-Jornet, P. Trace Elements in Saliva and Plasma of Patients with Type 2 Diabetes: Association to Metabolic Control and Complications. Diabetes Res. Clin. Pract. 2019, 157, 107871. [Google Scholar] [CrossRef]
- Femiano, F.; Gombos, F.; Esposito, V.; Nunziata, M.; Scully, C. Burning Mouth Syndrome (BMS): Evaluation of Thyroid and Taste. Med. Oral Patol. Oral Cir. Bucal 2006, 11, 22–25. [Google Scholar]
- Femiano, F.; Lanza, A.; Buonaiuto, C.; Gombos, F.; Nunziata, M.; Cuccurullo, L.; Cirillo, N. Burning Mouth Syndrome and Burning Mouth in Hypothyroidism: Proposal for a Diagnostic and Therapeutic Protocol. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 105, e22–e27. [Google Scholar] [CrossRef]
- Stejskal, V.; Reynolds, T.; Bjørklund, G. Increased Frequency of Delayed Type Hypersensitivity to Metals in Patients with Connective Tissue Disease. J. Trace Elem. Med. Biol. 2015, 31, 230–236. [Google Scholar] [CrossRef]
- Louise McGinley, E.; Coleman, D.C.; Moran, G.P.; Fleming, G.J.P. Effects of Surface Finishing Conditions on the Biocompatibility of a Nickel-Chromium Dental Casting Alloy. Dent. Mater. 2011, 27, 637–650. [Google Scholar] [CrossRef]
- Moslehifard, E.; Ghaffari, T.; Mohammadian-Navid, S.; Ghafari-Nia, M.; Farmani, A.; Nasirpouri, F. Effect of Chemical Passivation on Corrosion Behavior and Ion Release of a Commercial Chromium-Cobalt Alloy. J. Dent. Res. Dent. Clin. Dent. Prospect. 2020, 14, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Verdeguer, P.; Gil, J.; Punset, M.; Manero, J.M.; Nart, J.; Vilarrasa, J.; Ruperez, E. Citric Acid in the Passivation of Titanium Dental Implants: Corrosion Resistance and Bactericide Behavior. Materials 2022, 15, 545. [Google Scholar] [CrossRef] [PubMed]
- Prando, D.; Brenna, A.; Diamanti, M.V.; Beretta, S.; Bolzoni, F.; Ormellese, M.; Pedeferri, M.P. Corrosion of Titanium: Part 2: Effects of Surface Treatments. J. Appl. Biomater. Funct. Mater. 2018, 16, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Xuereb, M.; Camilleri, J.; Attard, N. Systematic Review of Current Dental Implant Coating Materials and Novel Coating Techniques. Int. J. Prosthodont. 2015, 28, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Qiao, Y.; Liu, X. Surface Modification of Biomaterials Using Plasma Immersion Ion Implantation and Deposition. Interface Focus 2012, 2, 325–336. [Google Scholar] [CrossRef]
- Li, Q.; Li, L.; Zhao, M.; Dong, L.; Wu, J.; Li, D. Biological Actions of Cu/Zn Coimplanted TiN on Ti-6Al-4V Alloy. Biointerphases 2019, 14, 051008. [Google Scholar] [CrossRef]
- Shi, Q.; Qian, Z.; Liu, D.; Liu, H. Surface Modification of Dental Titanium Implant by Layer-by-Layer Electrostatic Self-Assembly. Front. Physiol. 2017, 8, 574. [Google Scholar] [CrossRef]
- Mistry, S.; Roy, R.; Kundu, B.; Datta, S.; Kumar, M.; Chanda, A.; Kundu, D. Clinical Outcome of Hydroxyapatite Coated, Bioactive Glass Coated, and Machined Ti6Al4V Threaded Dental Implant in Human Jaws: A Short-Term Comparative Study. Implant Dent. 2016, 25, 252–260. [Google Scholar] [CrossRef]
- Velloso, G.; Moraschini, V.; dos Santos Porto Barboza, E. Hydrophilic Modification of Sandblasted and Acid-Etched Implants Improves Stability during Early Healing: A Human Double-Blind Randomized Controlled Trial. Int. J. Oral Maxillofac. Surg. 2019, 48, 684–690. [Google Scholar] [CrossRef]
- Kunrath, M.F.; Muradás, T.C.; Penha, N.; Campos, M.M. Innovative Surfaces and Alloys for Dental Implants: What about Biointerface-Safety Concerns? Dent. Mater. 2021, 37, 1447–1462. [Google Scholar] [CrossRef]
- Raap, U.; Stiesch, M.; Kapp, A. Contact Allergy to Dental Materials. JDDG J. Dtsch. Dermatol. Ges. 2012, 10, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Sutow, E.J.; Maillet, W.A.; Taylor, J.C.; Hall, G.C. In Vivo Galvanic Currents of Intermittently Contacting Dental Amalgam and Other Metallic Restorations. Dent. Mater. 2004, 20, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Procházková, J.; Podzimek, Š.; Tomka, M.; Kučerová, H.; Mihaljevič, M.; Hána, K.; Mikšovský, M.; Šterzl, I.; Vinšová, J. Metal Alloys in the Oral Cavity as a Cause of Oral Discomfort in Sensitive Patients. Neuroendocrinol. Lett. 2006, 27 (Suppl. 1), 53–58. [Google Scholar] [PubMed]
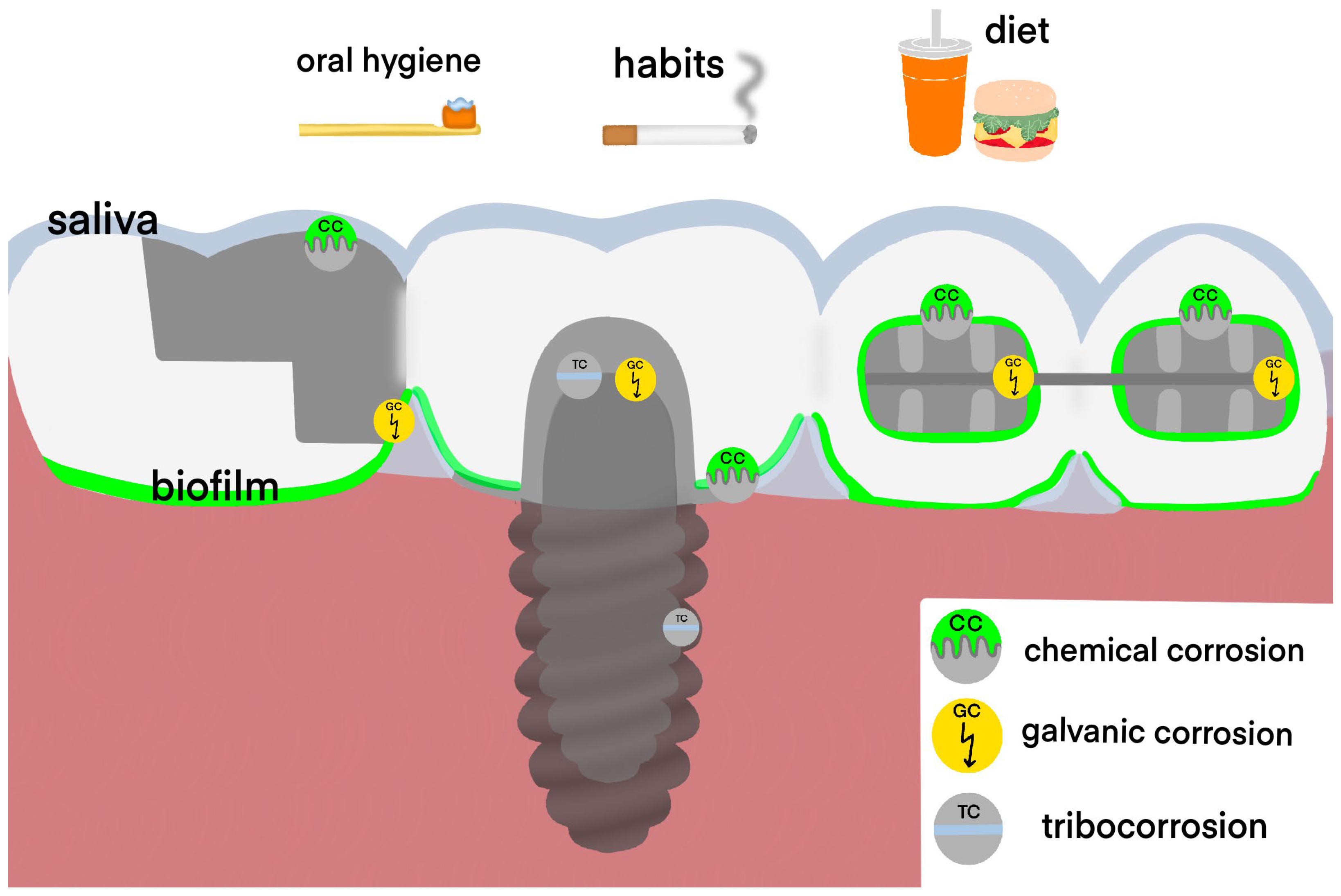
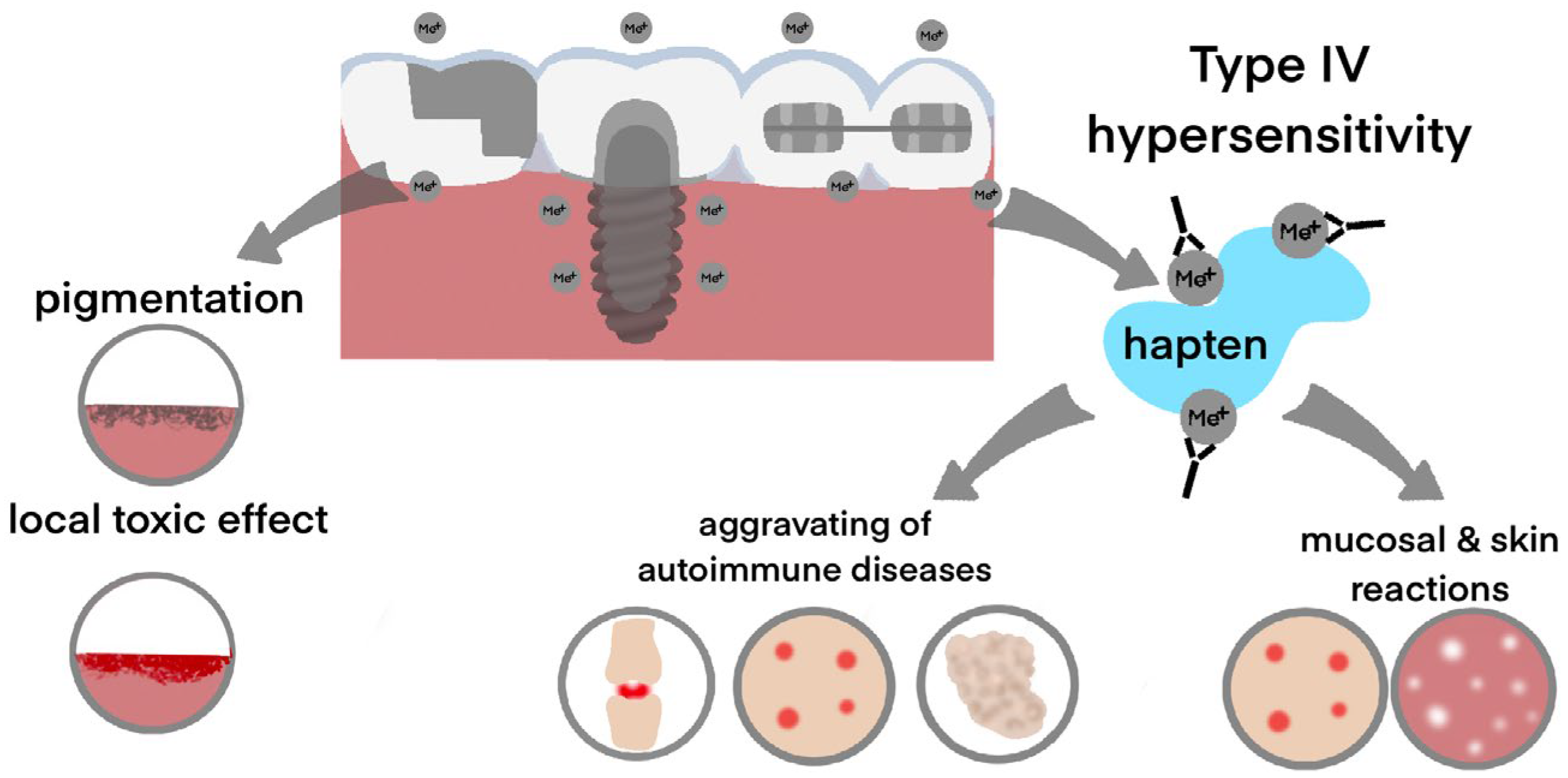
| Risk Factors | Mechanism of Action | |
|---|---|---|
| Local factors | Use of dental alloys with low corrosion resistance | Increased release of metal ions |
| Galvanic coupling of different dental alloys in the oral cavity | Increased release of metal ions, chronic irritation of the oral mucosa with the direct current | |
| Poor oral hygiene | Increased corrosion due to acidogenic flora activity | |
| Multiple caries Periodontal disease | Decreased salivary pH and increased corrosion | |
| Fluoride-containing oral hygiene products | The increase in galvanic current and corrosion | |
| Ethanol-based oral rinses | The increase in galvanic current and corrosion | |
| Abrasive toothpastes | Surface degradation of dental alloys | |
| Habitual factors | Smoking and hard alcohol consumption | Decreased resistance and healing potential of the oral mucosa |
| Regular intake of acidic foods and drinks | The increase in galvanic current and corrosion | |
| Systemic factors | Radiotherapy | Decreased resistance and healing potential of the oral mucosa, decreased salivary pH |
| Gastro-intestinal reflux disease | The increase in galvanic current and corrosion | |
| Post-menopausal period in women Diabetes mellitus Thyroid hypofunction | Decreased salivary pH, altered taste, increased sensitivity of the oral mucosa | |
| Renal disease | Increased electroconductivity of the whole saliva | |
| Autoimmune diseases | Potential hypersensitivity to metals |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arakelyan, M.; Spagnuolo, G.; Iaculli, F.; Dikopova, N.; Antoshin, A.; Timashev, P.; Turkina, A. Minimization of Adverse Effects Associated with Dental Alloys. Materials 2022, 15, 7476. https://doi.org/10.3390/ma15217476
Arakelyan M, Spagnuolo G, Iaculli F, Dikopova N, Antoshin A, Timashev P, Turkina A. Minimization of Adverse Effects Associated with Dental Alloys. Materials. 2022; 15(21):7476. https://doi.org/10.3390/ma15217476
Chicago/Turabian StyleArakelyan, Marianna, Gianrico Spagnuolo, Flavia Iaculli, Natalya Dikopova, Artem Antoshin, Peter Timashev, and Anna Turkina. 2022. "Minimization of Adverse Effects Associated with Dental Alloys" Materials 15, no. 21: 7476. https://doi.org/10.3390/ma15217476
APA StyleArakelyan, M., Spagnuolo, G., Iaculli, F., Dikopova, N., Antoshin, A., Timashev, P., & Turkina, A. (2022). Minimization of Adverse Effects Associated with Dental Alloys. Materials, 15(21), 7476. https://doi.org/10.3390/ma15217476








